Abstract
Introduction
Biodentine™ has frequently been acknowledged in the literature as a promising material and serves as an important representative of tricalcium silicate based cements used in dentistry.
Aim
To provide an update on the physical and biological properties of Biodentine™ and to compare these properties with those of other tricalcium silicate cements namely, different variants of mineral trioxide aggregate (MTA) such as ProRoot MTA, MTA Angelus, Micro Mega MTA (MM-MTA), Retro MTA, Ortho MTA, MTA Plus, GCMTA, MTA HP and calcium enriched mixture (CEM), Endosequence and Bioaggregate™.
Study design
A comprehensive literature search for publications from November 20, 2013 to November 20, 2016 was performed by two independent reviewers on Medline (PubMed), Embase, Web of Science, CENTRAL (Cochrane), SIGLE, SciELO, Scopus, Lilacs and clinicaltrials.gov. Electronic and hand search was carried out to identify randomised control trials (RCTs), case control studies, case series, case reports, as well as in vitro and animal studies published in the English language.
Conclusions
The enhanced physical and biologic properties of Biodentine™ could be attributed to the presence of finer particle size, use of zirconium oxide as radiopacifier, purity of tricalcium silicate, absence of dicalcium silicate, and the addition of calcium chloride and hydrosoluble polymer. Furthermore, as Biodentine™ overcomes the major drawbacks of MTA it has great potential to revolutionise the different treatment modalities in paediatric dentistry and endodontics especially after traumatic injuries. Nevertheless, high quality long-term clinical studies are required to facilitate definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodentine™ (henceforth, referred to as Biodentine) has frequently been acknowledged in the literature as a promising material and serves as an important representative of tricalcium silicate based cements used in dentistry. Biodentine has earned positive reviews in the literature owing to its superior physical properties, better handling, increased biocompatibility and wide range of clinical applications (Malkondu et al. 2014).
The present review is a 3 year update of the previously published review (Rajasekharan et al. 2014) and aims to provide an updated analysis of the physical and biological properties of Biodentine and to compare these properties with those of other tricalcium silicate cements viz. different variants of mineral trioxide aggregate (ProRoot MTA, MTA Angelus, MM-MTA, Retro MTA, Ortho MTA, MTA Plus, GCMTA, MTA HP), calcium enriched mixture (CEM), Endosequence and Bioaggregate™.
Materials and methods
The previously published review of Biodentine (Rajasekharan et al. 2014) summarised the literature till November 20, 2013. In the present update, a comprehensive literature search for publications from November 20, 2013 to November 20, 2016 was performed by two independent reviewers (L. M and R. C) on Medline (PubMed), Embase, Web of Science, CENTRAL (Cochrane), System for Information on Grey Literature in Europe (SIGLE), SciELO, Scopus, Lilacs and clinicaltrials.gov. The following search terms Biodentine, “tricalcium silicate”, Ca3SiO5, “dentine substitute”, “dentin substitute” and Bioceramic were used. Only randomised control trials (RCT), case control studies, case series, case reports, as well as in vitro studies and animal studies in English language were considered for this review. The search was supplemented by checking citations of relevant articles and hand searching for articles published in journals not indexed on Medline.
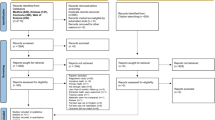
The electronic search resulted in 823 articles while hand search and citation search led to an additional five articles, giving a grand total of 828 articles of which 191 formed the basis of the present review, and of which the majority were in vitro studies. The detailed search methodology and selection criteria is illustrated in Fig. 1 and the physical characteristics of Biodentine compared with other materials is summarised in Table 1.
To facilitate a simple work flow the present paper is organised into four sections as follows. Section I: composition and setting, Section II: physical and mechanical properties, Section III: biological properties, and Section IV: potential clinical applications.
Review update
Section I: composition and setting
Composition
Two studies (Setbon et al. 2014; Gandolfi et al. 2014) employed an environmental scanning electron microscope—energy dispersive X-ray (ESEM-EDX) to analyse the elemental composition (wt%) of unhydrated Biodentine powder and reported the presence of carbon (4.34 and 9.7), oxygen (42 and 38.5), silicon (7.3 and 7.7), calcium (39 and 41.9) and zirconium (2.2 and 2.2), respectively.
Recent studies based on X-ray Energy Dispersive Analysis (EDX) suggest the absence of dicalcium silicate in Biodentine (Camilleri 2014; Setbon et al. 2014). This confirms previous study data (Camilleri et al. 2013) that used XRD to define that Biodentine is mainly composed of tricalcium silicate cement, which facilitates better purification during the fabrication process and may explain the more homogenous particle size. The difference in the chemical composition between Biodentine and ProRoot MTA does not affect the surface topography and both materials exhibited similar levels of surface roughness (Attik et al. 2014). The short setting time of Biodentine compared to all other materials is explained by the absence of dicalcium silicate, which is associated with a slower hydration reaction (Darvell and Wu 2011).
Setting time
The initial setting time of Biodentine as indicated by its manufacturer is about 12 min. However, some studies have reported the initial setting time of Biodentine to be 6.5 ± 1.7 min (Butt et al. 2014) and 30 ± 0 min (Alhodiry et al. 2014). These differences in the setting time may be explained by the different ISO standards used. According to the International Organisation for Standardisation guidelines, ISO 9917-1:2007, the setting time of Biodentine was assessed as 15 ± 1 min (Jang et al. 2014) and 13.1 ± 1.1 min (Dawood et al. 2015c). In the study by Kaup et al., the final setting time of Biodentine was 85.66 ± 6.03 min (Kaup et al. 2015b). Nevertheless, all the studies reported a shorter setting time for Biodentine when compared to ProRoot MTA.
Both saliva and blood contamination, increased the setting time of Biodentine by 1 ± 6.51 min and 16 ± 8.21 min respectively. While, the blood-contaminated group showed a significantly longer setting time compared to the non-contaminated Biodentine group (p < 0.01), there was no significant difference in the saliva-contaminated group (p > 0.05).
Section II: physical and mechanical properties
Radiopacity
The radiopacity of Biodentine was found to be significantly lower than ProRoot MTA (Kaup et al. 2015b), MTA Angelus, Micro Mega MTA (Tanalp et al. 2013), MTA Plus and Neo MTA Plus (Camilleri 2015). Furthermore, the radiopacity of Biodentine varied between studies with some studies reporting the radiopacity to be lower than the ISO 6876:2001 requirement (Tanalp et al. 2013; Kaup et al. 2015b). In a cone-beam computer tomography (CBCT) study, Biodentine and MTA generated fewer artefacts than amalgam. Therefore, it was concluded that the use of 84 or 96 kVp with metal artefact reduction (MAR) and low resolution reduced the artefacts and generated the lowest effective dose (Demirturk Kocasarac et al. 2016; Helvacioglu-Yigit et al. 2016).
Colour stability
Biodentine maintained colour stability up to 6 months and exhibited significantly less discolouration compared with ProRoot MTA (Valles et al. 2015; Marconyak et al. 2016), Ortho MTA (Shokouhinejad et al. 2016), gray MTA and white MTA (Kohli et al. 2015), Bioaggregate and MTA Angelus (Yoldas et al. 2016). The presence of bismuth oxide and uptake of blood components in the porosities of various Portland cement based products were considered to be a possible factor to induce discolouration (Lenherr et al. 2012; Marconyak et al. 2016). Conversely, Beatty et al. concluded that Biodentine discoloured significantly more than ProRoot MTA (Beatty and Svec 2015). Clinically perceptible discolouration was observed with Biodentine in the presence of sodium hypochlorite (Camilleri 2015; Keskin et al. 2015), chlorhexidine gluconate (Keskin et al. 2015) and blood (Shokouhinejad et al. 2016). Delayed tooth discolouration was detected in both ProRoot MTA and Biodentine at the one-year evaluation, but it was more evident for ProRoot MTA than Biodentine (Ramos et al. 2016).
Porosity and compressive strength
Biodentine and ProRoot MTA demonstrated lower average pore diameter, porosity, total pore area and higher bulk density when compared to Bioaggregate™, MTA Angelus and MTA Plus (Camilleri et al. 2014a; Gandolfi et al. 2014). Compressive strength of Biodentine was found to be significantly higher than MTA-Angelus, trial MTA (GCMTA) and calcium-enriched mixture (Kayahan et al. 2013; Butt et al. 2014; Dawood et al. 2015c; Natale et al. 2015). Biodentine did not show a significant reduction in its compressive strength when exposed to sodium hypochlorite (NaOCl) while ethylenediamenetetraacetic acid (EDTA) reduced the compressive strength of Biodentine (Govindaraju et al. 2016). Exposure to different pH (4.4, 5.4, 6.4 and 7.4) environments for 7 days led to Biodentine displaying significantly higher compressive strength than white MTA (Elnaghy 2014). In this study, white MTA appeared to be more sensitive to acidic pH than Biodentine. Conversely, another study reported that acid etching procedures after 7 days did not reduce Biodentine’s compressive strength (Kayahan et al. 2013), which supports the manufacturer’s recommendations to delay the placement of the final restoration for at least one week to obtain mature crystalline formation.
Hardness and flexural strength Biodentine exhibited significantly higher hardness, flexural strength and elastic modulus than ProRoot MTA, Angelus MTA and GCMTA (Dawood et al. 2015c; Kaup et al. 2015b; Natale et al. 2015). Vickers microhardness of Biodentine was identical to sound human dentine but 2-fold higher than that of ProRoot MTA (Kaup et al. 2015b). Surface hardness of Biodentine was not affected by moist or dry storage environment (Caronna et al. 2014) but decreased significantly in the presence of acidic pH of 6.4, 5.4 and 4.4 (Elnaghy 2014). Saliva and blood caused no significant difference in the bi-axial flexural strength of either Biodentine or Portland cement (Alhodiry et al. 2014).
Solubility
According to ISO 6876:2001, studies confirmed that Biodentine displayed solubility similar to ProRoot MTA up to 10-day exposure times. After 10 days, Biodentine demonstrated a marked increase in its solubility (Dawood et al. 2015c; Kaup et al. 2015b; Singh et al. 2015) which could be explained by the higher dissolution of calcium and silicon ions (Singh et al. 2015). Though Biodentine solubility values were higher than ProRoot MTA, this solubility occurred only at the surface which is exposed to the solution and caused negligible dimensional change (Singh et al. 2015). The solubility of Biodentine was higher in distilled water than in phosphate buffered saline (Kaup et al. 2015b). The increased solubility of Biodentine has been attributed to the use of water soluble polycarboxylate in the liquid component. This hydrosoluble polymer has a surfactant effect and thus leads to increased dissolution by applying a charge on it’s surfaces (Dawood et al. 2015c). Conflicting results were obtained in the study by Gandolfi et al. with significantly lower solubility values for Biodentine compared to ProRoot MTA, MTA Angelus and MTA Plus (Gandolfi et al. 2014).
Heavy metal release
Nine heavy metal ions released in distilled water (µg/L) were evaluated after 7 days in the study by Jang et al. Arsenic (9.3 ± 6.7), copper (31.7 ± 13.4), iron (711.7 ± 267.9), manganese (11.1 ± 3.7) and zinc (91.7 ± 17.2) were released significantly higher in Biodentine than MTA and Bioaggregate™. Cadmium (0.1 ± 01), chromium (46.2 ± 30.8), nickel (59.0 ± 58.3) and lead (1.1 ± 0.3) release was also higher in Biodentine but without any significant difference in comparison to Bioaggregate™ and MTA (Jang et al. 2014). This increased heavy metal release could be correlated to increased solubility.
Arsenic and lead released from Biodentine was well below the ISO (International Standardisation Organisation) recommendations of < 2 and 100 ppm, respectively. As there are no standards on other trace metals, the maximum allowable levels in drinking water as put forward by the United States Environmental Protection Agency (USEPA) may be compared. The overall concentration of heavy metals leached out from Biodentine in distilled water was below toxic levels and did not exceed 0.1 ppm in all materials, except for iron. USEPA also set the guidelines of the maximum allowable levels of iron to be 0.3 ppm (Kum et al. 2014).
Calcium ion release
Biodentine released significantly more calcium ions than MTA Angelus and GCMTA after 1, 3, 7 and 14 days (Dawood et al. 2015c). In a similar study, Biodentine showed significantly higher calcium ion release at short-term (3 h) than ProRoot MTA, MTA Angelus and MTA Plus. However, in the long-term (28 days) calcium ion release from Biodentine was significantly higher than MTA Plus alone (Gandolfi et al. 2014). Biodentine released more calcium ions in the early stages and the calcium ion release at 30 days was significantly lower than at day one (Li et al. 2016). The higher calcium ion release from Biodentine could be attributed to the presence of pure tricalcium silicate, calcium chloride, increased calcium hydroxide formation and high solubility (Camilleri 2014; Camilleri et al. 2014b; Gandolfi et al. 2014). When comparing calcium ion release in pH 5.5 and 7.0, Biodentine released significantly more calcium at neutral pH (Natale et al. 2015). In an attempt to compare the effect of leaching in different soaking solutions, it was found that Biodentine leached more calcium ions in Hank’s Balanced Salt Solution (HBSS) than in distilled water (Camilleri 2014). Also, Biodentine induced a rise in pH of the soaking water indicating alkalization (Gandolfi et al. 2014).
Microleakage
In an analysis of the sealing ability by fluid-filtration technique, Biodentine provided a valid and stable apical seal for the entire 12-week period tested. At 4- and 24-h period Biodentine provided a seal similar to ProRoot MTA, grey Portland cement and Tech Biosealer (Bani et al. 2015; El-Khodary et al. 2015) but significantly superior than MTA Angelus (Butt et al. 2014). Better adhesion of Biodentine to dentine may result from the physical process of crystal growth within the dentinal tubules leading to micromechanical bonding (Naik et al. 2015). Using the dye penetration technique, it was reported that Biodentine exhibited significantly lesser microleakage than MTA (Soundappan et al. 2014; Agrafioti et al. 2015; Mandava et al. 2015; Nanjappa et al. 2015) but significantly higher than ProRoot MTA, MM-MTA, Glass ionomer cement (Fuji IX GP) and Endosequence (Jeevani et al. 2014; Raju et al. 2014; Vemisetty et al. 2014). When the materials were stored in an acidic environment, no statistical significant difference was found between Biodentine and ProRoot MTA after 3 months (Agrafioti et al. 2015).
Also, in a comparison of various root-end cavity preparation techniques, Er:YAG laser preparation showed better sealing ability with Biodentine than ultrasonic preparation (Nanjappa et al. 2015). The removal of smear layer with MTAD™ irrigation significantly improved the apical seal of Biodentine (Naik et al. 2015). Upon assessing various manipulation techniques, increased microleakage was evident when Biodentine was manually manipulated as compared to machine trituration. This could be explained by the more homogenous mix obtained by mechanical trituration (Gupta et al. 2015). When different storage environments were tested, dry storage of Biodentine resulted in microstructural changes and cracks at the root dentine to Biodentine interface (Camilleri et al. 2014a). Biodentine samples stored in phosphate buffer solution (PBS) produced larger amounts of calcium phosphate precipitates and showed a higher percentage of marginal adaptation than MTA Angelus (Aggarwal et al. 2015). In the presence of simulated body fluid, Biodentine demonstrated the presence of an interfacial layer formation on root canal dentine indicating bioactivity (Kim et al. 2015). However, the thickness of the interfacial layer formed was significantly less than that of white ProRoot MTA. Furthermore, blood contamination did not affect the marginal adaptation of Biodentine, Bioaggregate™, MTA and CEM (Bolhari et al. 2015). In addition, it has also been established that Biodentine exhibited greater dentine mineralisation in totally demineralised dentine than Fuji IX glass ionomer cement (Atmeh et al. 2015).
Push-out bond strength
The capacity of Biodentine to resist dislodgement was greater than Bioaggregate™ (Alsubait et al. 2014; Ulusoy et al. 2015), ProRoot MTA (Nagas et al. 2016a), MTA Angelus (Elnaghy 2014; Dawood et al. 2015b; Centenaro et al. 2016; De-Deus et al. 2016; Silva et al. 2016), GCMTA (Dawood et al. 2015b) and MTA HP (high plasticity). The higher dislodgement resistance of Biodentine is speculated to be a result from smaller particle size that has the potential to enhance penetration of cement into dentinal tubules. This effect is further reinforced through formation of ‘mineral tags’ leading to increased micromechanical retention (Han and Okiji 2011; Nagas et al. 2016a). The micromechanical anchorage is also partly due to increased calcium and hydroxyl ion release responsible for improved apatite formation at the Biodentine-dentine interface (Silva et al. 2016a). Biodentine and ProRoot MTA were found to fill gaps between dentine and cement by calcium phosphate deposition without any chemical changes to the adjacent dentine. The thickness of the transition zone as measured by micro Raman spectroscopy was 7.5 ± 4.2 µm for Biodentine compared to 6.2 ± 5.4 µm for ProRoot MTA with no significant difference between the groups (Li et al. 2015). On the contrary, one study concluded that Biodentine showed significantly lower bond strength than ProRoot MTA and Retro MTA (Ustun et al. 2015).
Micro-push-out bond strength of Biodentine decreased significantly with decreasing pH form 7.4, 6.4, 5.4 to 4.4 (Elnaghy 2014). Conversely, blood contamination did not affect the dislocation resistance of Biodentine (Ustun et al. 2015). Conflicting results have been published regarding the effect of PBS on the push-out bond strength of Biodentine. Long-term PBS immersion (60 days) was found to positively influence the resistance to dislodgement in the study by De-Deus et al. whereas the study by Cechella et al., showed that the bond strength of Biodentine increased up to 3 days but reduced significantly after 28 days when exposed to PBS (Cechella et al. 2015; De-Deus et al. 2016). Regardless of the placement technique used (manual compaction or ultrasonic activation), Biodentine exhibited significantly higher bond strength values when compared with MTA or MTA + CaCl2 (calcium chloride) groups (Kucukkaya Eren et al. 2016).
Shear bond strength
Biodentine exhibited lower shear bond strength than MTA Angelus (Altunsoy et al. 2015), CEM (Altunsoy et al. 2015) and Fuji IX GP (Raju et al. 2014) but higher shear bond strength than ProRoot MTA (Cantekin and Avci 2014; Kaup et al. 2015a). Biodentine presented significantly lower shear bond strength values when immediately bonded to resin composite. Cohesive failure within Biodentine indicated a weak material in its early setting phase (Deepa et al. 2016). Therefore, several authors have concluded that a final resin composite restoration should best be delayed for more than 2 weeks to allow adequate setting and sufficient intrinsic maturation of Biodentine for withstanding contraction forces from the resin composite (Hashem et al. 2014; Deepa et al. 2016). Also, the placement of glass ionomer cement based materials prior to composite resin restorations decreased the shear bond strength (Cengiz and Ulusoy 2016). For immediate permanent restoration, a stainless steel crown loaded with glass ionomer cement could be seated on unset Biodentine after the third minute of mixing in pulpotomy cases (Dawood et al. 2015a).
No statistically significant differences were evident between different adhesive systems (Colak et al. 2016) but the 2-step self-etch adhesives exhibited higher shear bond strength to Biodentine in comparison to 1-step self-etch adhesives and etch-and-rinse adhesives (Odabas et al. 2013).
Fracture resistance
For the treatment of simulated immature teeth with open apices, Biodentine, White MTA Angelus, Calcium-enriched mixture (CEM) and Bioaggregate did not show any significant difference regarding root fracture resistance (Bayram and Bayram 2016; Elnaghy and Elsaka 2016; Evren et al. 2016). Subsequent inspection of the fractured tooth revealed fracture along the same plane as surrounding dentine and no areas of material de-bonding from dentine (Di Fiore et al. 2016). In the study by Zhabuawala et al. the fracture resistance of immature teeth with an apical plug of Biodentine followed by obturation with gutta-percha, composite resin, or Biodentine was similar when tested immediately. In the same study, after 3 months of aging, teeth obturated completely with Biodentine showed a drastic reduction in the fracture resistance whereas there was no significant reduction in fracture strength in teeth with an apical plug of Biodentine backfilled with gutta-percha and composite resin (Zhabuawala et al. 2016).
Alterations to the composition To improve the properties of Biodentine, CPP-ACP has been added to the original composition in varying concentrations (0.5, 1, 2 and 3%). The addition of up to 1% CPP-ACP did not affect the physical properties of Biodentine except for a significant increase in the setting time, calcium and phosphate ion release (Dawood et al. 2015c) and push-out bond strength (Dawood et al. 2015b). The incorporation of 3% CPP-ACP into Biodentine increased solubility but reduced the compressive strength and surface microhardness by 36 and 31% respectively. In another study by Nagas et al., addition of 5 wt% alkali resistant glass fibre powder to Biodentine resulted in higher compressive and diametrical tensile strength than Biodentine, ProRoot MTA or fibre-reinforced ProRoot MTA (Nagas et al. 2016b).
Section III: biological properties
Antimicrobial activity
Biodentine’s antibacterial activity was strongest against the Streptococcus sanguis strains, which was significantly higher than MTA Angelus, ProRoot MTA and intermediate restorative material (Poggio et al. 2014a, 2015a; Ceci et al. 2015). The weakest antibacterial activity of Biodentine was seen against Streptococcus mutans with Biodentine exhibiting minimal or almost no antibacterial activity (Poggio et al. 2014a; Ceci et al. 2015; Poggio et al. 2015a). Against Streptococcus salivarius, MTA Angelus and ProRoot MTA showed significantly higher antibacterial activity than Biodentine (Poggio et al. 2014a, 2015a; Ceci et al. 2015). Furthermore, Biodentine’s antibacterial activity was similar to MTA Angelus but significantly higher than ProRoot MTA and MTA Plus against Enterococcus faecalis (Bhavana et al. 2015; Hiremath et al. 2015; Koruyucu et al. 2015) and Escherichia coli (Bhavana et al. 2015). Antifungal activity of Biodentine against Candida albicans was similar to MTA Angelus and MTA Plus (Hiremath et al. 2015) but significantly higher than ProRoot MTA (Bhavana et al. 2015).
Gene expression
Biodentine exhibited the capacity to induce odontoblastic differentiation of human dental pulp stem cells (hDPSCs) obtained from impacted third molars via heme oxygenase-1 (HO-1), reactive oxygen species (ROS), nuclear factor-E2-related factor-2 (Nrf2), mitogen-activated protein kinase (MAPK) and calmodulin-dependent protein kinase (CAMKII) pathways (Chang et al. 2014; Luo et al. 2014b; Jung et al. 2015). Biodentine increased phosphorylation of extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (Jung et al. 2015). Like ProRoot MTA, Biodentine also induced the up-regulation of osteocalcin (OCN), dentine sialophosphoprotein (DSPP), dentine matrix acidic phosphoprotein 1(DMP1), collagen type I (COL1A1), runt related transcription factor (Runx2) and bone sialoprotein (BSP) upon exposure to hDPSCs (Chang et al. 2014; Luo et al. 2014b; Widbiller et al. 2016). Although Biodentine stimulated similar markers as MTA, the staining was more intense and spread over a larger area of the pulp tissue (Daltoe et al. 2016). Exposure of hDPSCs to Biodentine (0.2 mg/ml) for 24 hours showed a significantly increased mRNA expression of chemokines such as CXC chemokine receptor type 4 (CSCR4), monocyte chemoattractant protein 1 (MCP-1), stromal cell-derived factor-1 (SDF-1) and adhesion molecules such as fibronectin (FN), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and Integrinβ1 (Luo et al. 2014a). Osteogenic differentiation of hDPSCs were increased after elution of cytotoxic components from Biodentine.
Exposure to MTA and Biodentine stimulated expression of angiogenic genes such as vascular endothelial growth factor (VEGFA) and c-fos induced growth factor (FIGF/VEGFD) but significantly decreased the mRNA levels of angiopoietin 1, ANGPT1 and fibroblast growth factor 2 (FGF2). Based on these findings, Biodentine might enhance angiogenesis when used in direct contact with SCAP (Peters et al. 2015). Biodentine also elicited a favourable response on human mesenchymal stem cells (hMSCs) and human umbilical vein endothelial cells (HUVECs) but the osteogenic and angiogenic outcome was slightly lower than ProRoot MTA and MTA Plus (Costa et al. 2016). Biodentine induced mRNA expression of alkaline phosphatase (ALP), osteocalcin (OC) and bone sialoprotein (BSP) in hMSCs (Lee et al. 2014).
Cytotoxicity
After 24 h, cytotoxicity was in the order of CEM > Biodentine > ProRoot MTA on human stem cells from apical papilla (SCAP). At 48- and 72-h, the cytotoxicity was reported to be MTA > Biodentine > CEM (Saberi et al. 2016). Stem cells from apical papilla in contact with Biodentine exhibited increased cell viability than control group at day one but not at days 3 and 7. Biodentine showed significantly less cell viability (73%) after 24 h of incubation, whereas more than 90% cell viability was evident after 48- and 72-h of incubation with human periodontal ligament fibroblasts (Kucukkaya et al. 2016). In comparison to ProRoot MTA, Biodentine demonstrated significantly better results regarding cell survival and proliferation of periodontal ligament cells (Jung et al. 2014; Escobar-Garcia et al. 2016). The presence of a less toxic radiopacifier (zirconium oxide) in Biodentine could be responsible for these results (Kucukkaya et al. 2016). Contrasting results with ProRoot MTA exhibiting better human periodontal ligament cell viability than Biodentine and Endosequence has also been reported (Samyuktha et al. 2014). Biodentine and ProRoot MTA showed similar effects in terms of cytotoxicity and cytokine expression (interleukin-1α and interleukin-6) level in mouse embryonic fibroblast cells (Corral Nunez et al. 2014) and primary mouse embryonic Balb/c 3T3 fibroblasts (Silva et al. 2016b). The cytotoxic effect of Biodentine on hDPSCs were time and concentration dependent. The initial cytotoxicity of Biodentine could be attributed to the high pH values (Bortoluzzi et al. 2015), and when used in direct contact with the pulp can positively influence healing by enhancing proliferation, migration and adhesion of hDPSCs (Luo et al. 2014a).
Biodentine and ProRoot MTA showed lower cytotoxicity towards MDPC-23 murine odontoblasts cells (Poggio et al. 2015b). In other studies, with MDPC-23 cells, at 24- and 48-h, Biodentine, MTA Angelus and ProRoot MTA did not show any significant differences in the cytocompatibility but at 72 h, Biodentine demonstrated significantly higher cytocompatibility than MTA Angelus (Poggio et al. 2014a, b; Ceci et al. 2015). TRPA1 is an ion channel responsible for pain and inflammation. Its expression was induced in cultured odontoblast like cells by tumour necrosis factor alpha (TNF-α) and this expression was significantly reduced in the presence of Biodentine (El Karim et al. 2016).
Biodentine, ProRoot MTA and MTA Plus revealed dose-dependent cytotoxicity and time dependent cell viability of osteoblasts (Cornelio et al. 2015). There was no significant difference in the osteoblast cell viability between ProRoot MTA and Biodentine (Jung et al. 2014; Cornelio et al. 2015).
Animal model
Subcutaneous implantation of endodontic materials in 60 Holtzman adult male rats (da Fonseca et al. 2016), 45 white female Wistar rats (Simsek et al. 2015) and 15 male Wistar rats (Mori et al. 2014) concluded that Biodentine showed an initial inflammatory response that was quickly followed by acceptance of Biodentine by the tissue in contact. The reduction in inflammatory process and lymphocyte infiltration from 7 to 14 and 30 days was statistically significant (Mori et al. 2014). This process was coupled with the formation of collagen fibre bundles in the capsules of Biodentine and not harmful to the connective tissue after prolonged implantation in subcutaneous tissue (da Fonseca et al. 2016). Biodentine could be considered to be biocompatible as it allows for reduction in the inflammatory response over time (Mori et al. 2014) and the decline in inflammation is more rapid in Biodentine when compared to MM-MTA and Bioaggregate™ (Simsek et al. 2015).
In a pulp capping study performed in 18 Sprague–Dawley rats (9 weeks old), micro CT analysis revealed that Biodentine and ProRoot MTA showed significantly thicker hard tissue formation than Bioaggregate™. Hematoxylin and eosin staining illustrated formation of complete dentine bridge with normal pulp histology. In comparison to ProRoot MTA, Biodentine showed an irregular, heterogeneous distribution of mineralization nodules within a uniform thickness of hard tissue barrier. This could be a result of rapid initial disorganised formation of the reparative dentine (Kim et al. 2016). Similar results were obtained in the human model, with tomographic evaluation of direct pulp capping executed in 44 caries-free human third molars indicated for extraction. The dentine bridges in the Biodentine group were found to have highest average and maximum volumes in comparison to ProRoot white MTA (Nowicka et al. 2015).
Pulp capping study by Tziafa et al. on 34 teeth of three miniature swine revealed that the thickness of hard tissue bridges were significantly higher with Biodentine when compared to white MTA Angelus. In the Biodentine group, a thick zone of new osteodentinal matrix with cellular inclusions was consistently observed after 3 and 8 weeks. Ectopic formation of osteodentine far from the capping materials was also noted to be significantly higher in Biodentine than in white MTA Angelus (Tziafa et al. 2014). Pulpotomy performed in 30 teeth of 3 beagle dogs (12 months old) demonstrated mineralised tissue bridge formation in significantly more specimens treated with Biodentine (96.8%) than with ProRoot white MTA (72.2%). Radiographic visualisation of more bridges in Biodentine was related to the sensitivity of radiographic techniques to detect bridges thinner than 0.5 mm. The tissue bridges formed by both the cements had similar morphology but the thickness was significantly more in the Biodentine group (De Rossi et al. 2014).
The analyses of magnesium, aluminium, calcium, chromium, arsenic and lead accumulation in the brain, kidney and liver of 18 Wistar albino rats (3–5 months old) was detected after subcutaneous implantation of endodontic materials by inductively coupled plasma mass spectrometry (ICP-MS) with a sensitivity of 0.2 parts per billion (ppb). Elevated levels of these trace elements were identified in the different organs but they were below the toxic levels in all cases. Furthermore, there was no significant difference between the control groups and Biodentine, MM-MTA and Bioaggregate™ based on the concentration of aluminium, calcium, arsenic and lead in the rat organs (Simsek et al. 2016).
Section IV: clinical applications
A total of 43 clinical studies were identified, of which four were RCTs, three were case control studies and 36 were case reports. Very few high quality clinical trials with long follow-up periods were identified. Only seven clinical studies were based on treatment of the primary dentition and all of them evaluated Biodentine as a pulpotomy medicament. As case reports are considered to be of low evidence, the results of this section have to be interpreted with caution. However, the case reports included provided an overview of the possible clinical indications in which Biodentine™ could be used. In addition, 11 ongoing clinical trials were identified in the database of clinicaltrials.gov of which six were newly registered in the past 3 years and 5 were still in their recruitment phase.
Randomised controlled trial
A RCT evaluating Biodentine as a pulpotomy agent in 41 primary molars in children aged 4 to 9 years (Cuadros-Fernandez et al. 2015) and reported a 100% clinical and 94.9% radiographic success after 12 months. Similarly, another RCT of 25 primary molars treated with Biodentine, reported 95.2% clinical and 94.4% radiographic success after 18 months (Rajasekharan et al. 2016). In both RCTs, clinical and radiographic findings did not show any significant difference between Biodentine and MTA. However, another randomised, split-mouth, double blind, controlled clinical trial carried out in 56 primary molars showed 100% clinical and radiographic success with Biodentine after 6 months (Meligy et al. 2016).
A RCT evaluated the efficacy of Biodentine as an indirect pulp capping material was assessed in 18 to 76 year old adults (Hashem et al. 2015). Thirty-six teeth with reversible pulpitis were used in each group with 85% of the restorations placed in molars. After 12 months follow up, clinical success rates for Biodentine and Fuji IX GIC were 83.3%. Statistically, there was no significant difference in the dentine-pulp response between Biodentine and Fuji IX GIC.
Case–control studies
Three case–control studies evaluated Biodentine as a pulpotomy medicament in primary molars. In the study by Kusum et al. 25 primary molars in 3 to 10 year old children were treated with Biodentine (Kusum et al. 2015). MTA and Biodentine showed 92 and 80% radiographic success respectively after 9 months follow-up and 100% clinical success was observed in both the groups. Similarly, in the study by Niranjani et al., no statistically significant difference was observed between MTA and Biodentine as a pulpotomy medicament after 6 months follow-up (Niranjani et al. 2015). In this study, 25 primary molars in 5–9 year old children were treated with Biodentine. The study by Togaru et al., evaluated 90 decayed primary molars that required pulpotomy treatment with either Biodentine or MTA. Both the groups showed a 95.5% success rate at the end of 12 months (Togaru et al. 2016).
Case reports
A total of 36 case reports were published in the past 3 years and the use of Biodentine in various therapies such as direct pulp capping (Bhat et al. 2014), partial pulpotomy (Villat et al. 2013; Martens et al. 2015), pulpotomy (Borkar and Ataide 2015; Kenchappa et al. 2015; Martens et al. 2015; Soni 2016), palatogingival groove (Johns et al. 2014; Sharma et al. 2015), palatoradicular groove (Naik et al. 2014; Nadig et al. 2016), apexification (Khetarpal et al. 2014; Nayak and Hasan 2014; Sinha et al. 2014; Bajwa et al. 2015; Kenchappa et al. 2015; Martens et al. 2016; Niranjan et al. 2016; Vidal et al. 2016), apexogenesis (Kenchappa et al. 2015), single-visit pulp revascularization/regeneration (Aldakak et al. 2016; Topcuoglu and Topcuoglu 2016), internal resorption (Umashetty et al. 2015), invasive cervical resorption (Salzano and Tirone 2015; Baranwal 2016; Karypidou et al. 2016), perforation repair (Borkar and de Noronha de Ataide 2015; Kenchappa et al. 2015; Pruthi et al. 2015), incomplete vertical root fracture (Hadrossek and Dammaschke 2014), endodontic surgery (Caron et al. 2014) and retrograde restoration (Pawar et al. 2013) have been reported. All reported case reports have advocated the use of Biodentine as they demonstrated successful healing without adverse clinical and/or radiographic symptoms.
A summary of the treatment, type of study, number of teeth used, age of the patient and follow up period is listed in Table 2. The use of Biodentine has been reported to be successful in certain unconventional circumstances which include pulpotomy after several days of traumatic pulp exposure, single visit apexification, massive resorptive lesion with multiple perforations, combined endodontic-periodontic lesion and incomplete vertical root fracture. Although Biodentine has demonstrated successful outcomes in a variety of treatment scenarios, high quality clinical trials are still scarce.
Conclusion
In summary, recent studies have confirmed the absence of dicalcium silicate in Biodentine. The initial setting time times range between 6 and 30 min in various studies. Radiopacity of Biodentine was significantly lower than other tricalcium silicate based cements. Contrasting reports were published on whether the radiopacity values were in accordance with the ISO limits. Similarly, conflicting results were observed regarding the colour stability of Biodentine. The conflicting results could be due to the heterogeneity in the methodology used in respective studies.
On a positive note, Biodentine exhibited significantly superior compressive strength, microhardness, flexural strength, sealing ability, push-out bond strength and calcium ion release in comparison to other tricalcium silicate based cements. On the other hand, increased long-term solubility, higher heavy metal release and decreased shear bond strength were also observed with Biodentine.
Antimicrobial activity of Biodentine was significantly higher against certain strains such as Streptococcus sanguis, Enterococcus faecalis, Escherichia coli and Candida albicans whereas significantly lower antibacterial activity was observed against Streptococcus mutans and Streptococcus salivarius. Similair to other tricalcum silicate based cements, cytotoxicity of Biodentine was dose and time dependent.
In animal model studies, pulp capping experiments showed thicker hard tissue bridges and increased mineralised tissue bridge formation was observed in pulpotomy experiments. Randomised controlled trials and case control studies showed Biodentine to be a suitable alternative to MTA. A wide range of clinical indications have been published as case reports regarding the use of Biodentine but clinical studies of long term efficiency and high evidence are still lacking and that precludes a definitive conclusion.
The enhanced physical and biologic properties of Biodentine have been repeatedly emphasised in the literature. Due to its ability to overcome the drawbacks of MTA, Biodentine has great potential to revolutionise the different treatment modalities in paediatric dentistry and endodontics especially after traumatic injuries.
Change history
15 March 2018
Owing to a misunderstanding on the part of the authors, the name of the last author, Prof. R. M. H. Verbeeck, was omitted from this article.
10 September 2020
A Correction to this paper has been published: https://doi.org/10.1007/s40368-020-00553-7
References
Aggarwal V, Singla M, Yadav S, Yadav H, Ragini H. Marginal adaptation evaluation of Biodentine and MTA plus in “Open Sandwich” class II restorations. J Esthet Restor Dent. 2015;27:167–75.
Agrafioti A, Tzimpoulas N, Chatzitheodoridis E, Kontakiotis EG. Comparative evaluation of sealing ability and microstructure of MTA and Biodentine after exposure to different environments. Clin Oral Investig. 2015;20:1535–40.
Aldakak MM, Capar ID, Rekab MS, Abboud S. Single-visit pulp revascularization of a nonvital immature permanent tooth using Biodentine. Iran Endod J. 2016;11:246–9.
Alhodiry W, Lyons MF, Chadwick RG. Effect of saliva and blood contamination on the bi-axial flexural strength and setting time of two calcium-silicate based cements: Portland cement and Biodentine. Eur J Prosthodont Restor Dent. 2014;22:20–3.
Alsubait SA, Hashem Q, Alhargan N, Almohimeed K, Alkahtani A. Comparative evaluation of push-out bond strength of ProRoot MTA, bioaggregate and Biodentine. J Contemp Dent Pract. 2014;15:336–40.
Altunsoy M, Tanriver M, Ok E, Kucukyilmaz E. Shear bond strength of a self-adhering flowable composite and a flowable base composite to mineral trioxide aggregate, calcium-enriched mixture cement, and Biodentine. J Endod. 2015;41:1691–5.
Atmeh AR, Chong EZ, Richard G, et al. Calcium silicate cement-induced remineralisation of totally demineralised dentine in comparison with glass ionomer cement: tetracycline labelling and two-photon fluorescence microscopy. J Microsc. 2015;257:151–60.
Attik GN, Villat C, Hallay F, et al. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: Biodentine versus MTA((R)). Int Endod J. 2014;47:1133–41.
Bajwa NK, Jingarwar MM, Pathak A. Single visit apexification procedure of a traumatically injured tooth with a novel bioinductive material (Biodentine). Int J Clin Pediatr Dent. 2015;8:58–61.
Bani M, Sungurtekin-Ekci E, Odabas ME. Efficacy of Biodentine as an apical plug in nonvital permanent teeth with open apices: an in vitro study. Biomed Res Int. 2015;2015(2015):4.
Baranwal AK. Management of external invasive cervical resorption of tooth with Biodentine: a case report. J Conserv Dent. 2016;19:296–9.
Bayram E, Bayram HM. Fracture resistance of immature teeth filled with mineral trioxide aggregate, bioaggregate, and Biodentine. Eur J Dent. 2016;10:220–4.
Beatty H, Svec T. Quantifying coronal tooth discoloration caused by Biodentine and endosequence root repair material. J Endod. 2015;41:2036–9.
Bhat SS, Hegde SK, Adhikari F, Bhat VS. Direct pulp capping in an immature incisor using a new bioactive material. Contemp Clin Dent. 2014;5:393–6.
Bhavana V, Chaitanya KP, Gandi P, et al. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J Conserv Dent. 2015;18:44–6.
Bolhari B, Ashofteh Yazdi K, Sharifi F, Pirmoazen S. Comparative scanning electron microscopic study of the marginal adaptation of four root-end filling materials in presence and absence of blood. J Dent (Tehran). 2015;12:226–34.
Borkar S, De Noronha De Ataide I. Management of a massive resorptive lesion with multiple perforations in a molar: case report. J Endod. 2015;41:753–8.
Borkar SA, Ataide I. Biodentine pulpotomy several days after pulp exposure: four case reports. J Conserv Dent. 2015;18:73–8.
Bortoluzzi EA, Niu LN, Palani CD, et al. Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dent Mater. 2015;31:1510–22.
Butt N, Talwar S, Chaudhry S, et al. Comparison of physical and mechanical properties of mineral trioxide aggregate and Biodentine. Indian J Dent Res. 2014;25:692–7.
Camilleri J. Hydration characteristics of Biodentine and theracal used as pulp capping materials. Dent Mater. 2014;30:709–15.
Camilleri J. Staining potential of Neo MTA plus, MTA plus, and Biodentine used for pulpotomy procedures. J Endod. 2015;41:1139–45.
Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement. Biodentine and MTA angelus. Dent Mater. 2013;29:580–93.
Camilleri J, Grech L, Galea K, et al. Porosity and root dentine to material interface assessment of calcium silicate-based root-end filling materials. Clin Oral Investig. 2014a;18:1437–46.
Camilleri J, Laurent P, About I. Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod. 2014b;40:1846–54.
Cantekin K, Avci S. Evaluation of shear bond strength of two resin-based composites and glass ionomer cement to pure tricalcium silicate-based cement (Biodentine(R)). J Appl Oral Sci. 2014;22:302–6.
Caron G, Azerad J, Faure MO, Machtou P, Boucher Y. Use of a new retrograde filling material (Biodentine) for endodontic surgery: two case reports. Int J Oral Sci. 2014;6:250–3.
Caronna V, Himel V, Yu Q, Zhang JF, Sabey K. Comparison of the surface hardness among 3 materials used in an experimental apexification model under moist and dry environments. J Endod. 2014;40:986–9.
Cechella BC, De Almeida J, Felippe MC, et al. Influence of phosphate buffered saline on the bond strength of endodontic cement to dentin. Braz J Oral Sci. 2015;14:126–9.
Ceci M, Beltrami R, Chiesa M, Colombo M, Poggio C. Biological and chemical-physical properties of root-end filling materials: a comparative study. J Conserv Dent. 2015;18:94–9.
Cengiz E, Ulusoy N. Microshear bond strength of tri-calcium silicate-based cements to different restorative materials. J Adhes Dent. 2016;18:231–7.
Centenaro CF, Santini MF, Da Rosa RA, et al. Effect of calcium hydroxide on the bond strength of two bioactive cements and SEM evaluation of failure patterns. Scanning. 2016;38:240–4.
Chang SW, Lee SY, Ann HJ, Kum KY, Kim EC. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J Endod. 2014;40:1194–200.
Colak H, Tokay U, Uzgur R, et al. The effect of different adhesives and setting times on bond strength between Biodentine and composite. J Appl Biomater Funct Mater. 2016;14:e217–22.
Cornelio AL, Rodrigues EM, Salles LP, et al. Bioactivity of MTA Plus, Biodentine and experimental calcium silicate-based cements in human osteoblast-like cells. Int Endod J. 2015;50:39–47.
Corral Nunez CM, Bosomworth HJ, Field C, Whitworth JM, Valentine RA. Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J Endod. 2014;40:406–11.
Costa F, Sousa Gomes P, Fernandes MH. Osteogenic and angiogenic response to calcium silicate-based endodontic sealers. J Endod. 2016;42:113–9.
Cuadros-Fernandez C, Lorente Rodriguez AI, Saez-Martinez S, et al. Short-term treatment outcome of pulpotomies in primary molars using mineral trioxide aggregate and Biodentine: a randomized clinical trial. Clin Oral Investig. 2015;20:1639–45.
Da Fonseca TS, Da Silva GF, Tanomaru-Filho M, et al. In vivo evaluation of the inflammatory response and IL-6 immunoexpression promoted by Biodentine and MTA Angelus. Int Endod J. 2016;49:145–53.
Daltoe MO, Paula-Silva FWG, Faccioli LH, et al. Expression of mineralization markers during pulp response to Biodentine and mineral trioxide aggregate. J Endod. 2016;42:596–603.
Darvell BW, Wu RC. “MTA”-an hydraulic silicate cement: review update and setting reaction. Dent Mater. 2011;27:407–22.
Dawood AE, Manton DJ, Parashos P, Wong RH. The effect of working time on the displacement of Biodentine beneath prefabricated stainless steel crown: a laboratory study. J Investig Clin Dent. 2015a;7:391–5.
Dawood AE, Manton DJ, Parashos P, et al. Push-out bond strength of CPP-ACP-modified calcium silicate-based cements. Dent Mater J. 2015b;34:490–4.
Dawood AE, Manton DJ, Parashos P, et al. The physical properties and ion release of CPP-ACP-modified calcium silicate-based cements. Aust Dent J. 2015c;60:434–44.
De-Deus G, Ferreira CB, Oliveira Dda S, et al. Resistance of hydraulic calcium silicate cements to dislodgment in short- and long-term assessment. J Adhes Dent. 2016;18:157–60.
De Rossi A, Silva LA, Gaton-Hernandez P, et al. Comparison of pulpal responses to pulpotomy and pulp capping with Biodentine and mineral trioxide aggregate in dogs. J Endod. 2014;40:1362–9.
Deepa VL, Dhamaraju B, Bollu IP, Balaji TS. Shear bond strength evaluation of resin composite bonded to three different liners: TheraCal LC, Biodentine, and resin-modified glass ionomer cement using universal adhesive: An in vitro study. J Conserv Dent. 2016;19:166–70.
Demirturk Kocasarac H, Helvacioglu Yigit D, et al. Contrast-to-noise ratio with different settings in a CBCT machine in presence of different root-end filling materials: an in vitro study. Dentomaxillofac Radiol. 2016;45:20160012.
Di Fiore PM, Reyes A, Dorn SO, Cron SG, Ontiveros JC. Evaluation of a calcium silicate-based cement as a root reinforcement material for endodontically treated maxillary anterior teeth. J Prosthet Dent. 2016;115:35–41.
El-Khodary HM, Farsi DJ, Farsi NM, Zidan AZ. Sealing ability of four calcium containing cements used for repairing furcal perforations in primary molars: an in vitro study. J Contemp Dent Pract. 2015;16:733–9.
El Karim IA, Mccrudden MT, Mcgahon MK, et al. Biodentine reduces tumor necrosis factor alpha-induced TRPA1 expression in odontoblastlike cells. J Endod. 2016;42:589–95.
El Meligy OA, Allazzam S, Alamoudi NM. Comparison between Biodentine and formocresol for pulpotomy of primary teeth: a randomized clinical trial. Quintessence Int. 2016;47:571–80.
Elnaghy AM. Influence of acidic environment on properties of Biodentine and white mineral trioxide aggregate: a comparative study. J Endod. 2014;40:953–7.
Elnaghy AM, Elsaka SE. Fracture resistance of simulated immature teeth filled with Biodentine and white mineral trioxide aggregate—an in vitro study. Dent Traumatol. 2016;32:116–20.
Escobar-Garcia DM, Aguirre-Lopez E, Mendez-Gonzalez V, Pozos-Guillen A. Cytotoxicity and initial biocompatibility of endodontic biomaterials (MTA and Biodentine) used as root-end filling materials. Biomed Res Int. 2016;2016:7926961.
Evren OK, Altunsoy M, Tanriver M, et al. Fracture resistance of simulated immature teeth after apexification with calcium silicate-based materials. Eur J Dent. 2016;10:188–92.
Gandolfi MG, Siboni F, Botero T, et al. Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater. 2014;13:43–60.
Govindaraju L, Neelakantan P, Gutmann JL (2016) Effect of root canal irrigating solutions on the compressive strength of tricalcium silicate cements. Clin Oral Investig [Epub ahead of print].
Gupta PK, Garg G, Kalita C, et al. Evaluation of sealing ability of Biodentine as retrograde filling material by using two different manipulation methods: an in vitro study. J Int Oral Health. 2015;7:111–4.
Hadrossek PH, Dammaschke T. New treatment option for an incomplete vertical root fracture—a preliminary case report. Head Face Med. 2014;10:9.
Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J. 2011;44:1081–7.
Hashem DF, Foxton R, Manoharan A, Watson TF, Banerjee A. The physical characteristics of resin composite-calcium silicate interface as part of a layered/laminate adhesive restoration. Dent Mater. 2014;30:343–9.
Hashem D, Mannocci F, Patel S, et al. Clinical and radiographic assessment of the efficacy of calcium silicate indirect pulp capping: a randomized controlled clinical trial. J Dent Res. 2015;94:562–8.
Helvacioglu-Yigit D, Demirturk Kocasarac H, Bechara B, Noujeim M. Evaluation and reduction of artifacts generated by 4 different root-end filling materials by using multiple cone-beam computed tomography imaging settings. J Endod. 2016;42:307–14.
Hiremath GS, Kulkarni RD, Naik BD. Evaluation of minimal inhibitory concentration of two new materials using tube dilution method: an in vitro study. J Conserv Dent. 2015;18:159–62.
Jang YE, Lee BN, Koh JT, et al. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod. 2014;39:89–94.
Jeevani E, Jayaprakash T, Bolla N, et al. Evaluation of sealing ability of MM-MTA, endosequence, and Biodentine as furcation repair materials: UV spectrophotometric analysis. J Conserv Dent. 2014;17:340–3.
Johns DA, Shivashankar VY, Shobha K, Johns M. An innovative approach in the management of palatogingival groove using Biodentine and platelet-rich fibrin membrane. J Conserv Dent. 2014;17:75–9.
Jung JY, Woo SM, Lee BN, et al. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J. 2015;48:177–84.
Jung S, Mielert J, Kleinheinz J, Dammaschke T. Human oral cells’ response to different endodontic restorative materials: an in vitro study. Head Face Med. 2014;10:55.
Karypidou A, Chatzinikolaou ID, Kouros P, Koulaouzidou E, Economides N. Management of bilateral invasive cervical resorption lesions in maxillary incisors using a novel calcium silicate-based cement: a case report. Quintessence Int. 2016;47:637–42.
Kaup M, Dammann CH, Schafer E, Dammaschke T. Shear bond strength of Biodentine, ProRoot MTA, glass ionomer cement and composite resin on human dentine ex vivo. Head Face Med. 2015a;11:14.
Kaup M, Schafer E, Dammaschke T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. 2015b;11:16.
Kayahan MB, Nekoofar MH, Mccann A, et al. Effect of acid etching procedures on the compressive strength of 4 calcium silicate-based endodontic cements. J Endod. 2013;39:1646–8.
Kenchappa M, Gupta S, Gupta P, Sharma P. Dentine in a capsule: clinical case reports. J Indian Soc Pedod Prev Dent. 2015;33:250–4.
Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. 2015;41:409–11.
Khetarpal A, Chaudhary S, Talwar S, Verma M. Endodontic management of open apex using Biodentine as a novel apical matrix. Indian J Dent Res. 2014;25:513–6.
Kim JR, Nosrat A, Fouad AF. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J Dent. 2015;43:241–7.
Kim J, Song YS, Min KS, et al. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor Dent Endod. 2016;41:29–36.
Kohli MR, Yamaguchi M, Setzer FC, Karabucak B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J Endod. 2015;41:1862–6.
Koruyucu M, Topcuoglu N, Tuna EB, et al. An assessment of antibacterial activity of three pulp capping materials on Enterococcus faecalis by a direct contact test: an in vitro study. Eur J Dent. 2015;9:240–5.
Kucukkaya S, Gorduysus MO, Zeybek ND, Muftuoglu SF. In vitro cytotoxicity of calcium silicate-based endodontic cement as root-end filling materials. Scientifica. 2016;2016(2016):5.
Kucukkaya Eren S, Aksel H, Serper A. Effect of placement technique on the push-out bond strength of calcium-silicate based cements. Dent Mater J. 2016;35:742–7.
Kum KY, Kim EC, Yoo YJ, et al. Trace metal contents of three tricalcium silicate materials: MTA Angelus, Micro Mega MTA and Bioaggregate. Int Endod J. 2014;47:704–10.
Kusum B, Rakesh K, Richa K. Clinical and radiographical evaluation of mineral trioxide aggregate, Biodentine and propolis as pulpotomy medicaments in primary teeth. Restor Dent Endod. 2015;40:276–85.
Lee BN, Lee KN, Koh JT, et al. Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J Endod. 2014;40:1217–22.
Lenherr P, Allgayer N, Weiger R, et al. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012;45:942–9.
Li X, Pongprueksa P, Van Landuyt K, et al. Correlative micro-Raman/EPMA analysis of the hydraulic calcium silicate cement interface with dentin. Clin Oral Investig. 2015;20:1663–73.
Li X, Yoshihara K, De Munck J, et al. (2016) Modified tricalcium silicate cement formulations with added zirconium oxide. Clin Oral Investig [Epub ahead of print].
Luo Z, Li D, Kohli MR, et al. Effect of Biodentine (TM) on the proliferation, migration and adhesion of human dental pulp stem cells. J Dent. 2014a;42:490–7.
Luo ZR, Kohli MR, Yu Q, et al. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J Endod. 2014b;40:937–42.
Malkondu O, Karapinar Kazandag M, Kazazoglu E. A review on Biodentine, a contemporary dentine replacement and repair material. Biomed Res Int. 2014;2014:160951.
Mandava P, Bolla N, Thumu J, Vemuri S, Chukka S. Microleakage evaluation around retrograde filling materials prepared using conventional and ultrasonic techniques. J Clin Diagn Res. 2015;9:43–6.
Marconyak LJ Jr, Kirkpatrick TC, Roberts HW, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod. 2016;42:470–3.
Martens L, Rajasekharan S, Cauwels R. Pulp management after traumatic injuries with a tricalcium silicate-based cement (Biodentine): a report of two cases, up to 48 months follow-up. Eur Arch Paediatr Dent. 2015;16:491–6.
Martens L, Rajasekharan S, Cauwels R. Endodontic treatment of trauma-induced necrotic immature teeth using a tricalcium silicate-based bioactive cement. A report of 3 cases with 24-month follow-up. Eur J Paediatr Dent. 2016;17:24–8.
Mori GG, Teixeira LM, De Oliveira DL, Jacomini LM, Da Silva SR. Biocompatibility evaluation of Biodentine in subcutaneous tissue of rats. J Endod. 2014;40:1485–8.
Nadig PP, Agrawal IS, Agrawal VS, Srinivasan SC. Palato-radicular groove: a rare entity in maxillary central incisor leading to endo-perio lesion. J Clin Diagn Res. 2016;10:14–5.
Nagas E, Cehreli ZC, Uyanik MO, Vallittu PK, Lassila LV. Effect of several intracanal medicaments on the push-out bond strength of ProRoot MTA and Biodentine. Int Endod J. 2016a;49:184–8.
Nagas E, Cehreli ZC, Uyanik O, Vallittu PK, Lassila LV. Reinforcing effect of glass fiber-incorporated ProRoot MTA and Biodentine as intraorifice barriers. J Endod. 2016b;42:1673–6.
Naik M, De Ataide Ide N, Fernandes M, Lambor R. Treatment of combined endodontic: periodontic lesion by sealing of palato-radicular groove using Biodentine. J Conserv Dent. 2014;17:594–7.
Naik MM, De Ataide Ide N, Fernandes M, Lambor R. Assessment of apical seal obtained after irrigation of root end cavity with MTAD followed by subsequent retrofilling with MTA and Biodentine: an in vitro study. J Conserv Dent. 2015;18:132–5.
Nanjappa AS, Ponnappa KC, Nanjamma KK, et al. Sealing ability of three root-end filling materials prepared using an erbium: yttrium aluminium garnet laser and endosonic tip evaluated by confocal laser scanning microscopy. J Conserv Dent. 2015;18:327–30.
Natale LC, Rodrigues MC, Xavier TA, et al. Ion release and mechanical properties of calcium silicate and calcium hydroxide materials used for pulp capping. Int Endod J. 2015;48:89–94.
Nayak G, Hasan MF. Biodentine-a novel dentinal substitute for single visit apexification. Restor Dent Endod. 2014;39:120–5.
Niranjan B, Shashikiran ND, Dubey A, Singla S, Gupta N. Biodentine-A new novel bio-inductive material for treatment of traumatically injured tooth (single visit apexification). J Clin Diagn Res. 2016;10:3–4.
Niranjani K, Prasad MG, Vasa AA, et al. Clinical evaluation of success of primary teeth pulpotomy using mineral trioxide aggregate((R)), laser and Biodentine(TM)—an in vivo study. J Clin Diagn Res. 2015;9:35–7.
Nowicka A, Wilk G, Lipski M, Kolecki J, Buczkowska-Radlinska J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca(OH)2, MTA, Biodentine, and dentin bonding system in human teeth. J Endod. 2015;41:1234–40.
Odabas ME, Bani M, Tirali RE. Shear bond strengths of different adhesive systems to Biodentine. Sci World J. 2013;2013:626103.
Pawar AM, Kokate SR, Shah RA. Management of a large periapical lesion using Biodentine() as retrograde restoration with eighteen months evident follow up. J Conserv Dent. 2013;16:573–5.
Peters OA, Galicia J, Arias A, et al. Effects of two calcium silicate cements on cell viability, angiogenic growth factor release, and related gene expression in stem cells from the apical papilla. Int Endod J. 2015;49:1132–40.
Poggio C, Arciola CR, Beltrami R, et al. Cytocompatibility and antibacterial properties of capping materials. Sci World J. 2014a;2014:181945.
Poggio C, Ceci M, Beltrami R, et al. Biocompatibility of a new pulp capping cement. Ann Stomatol (Roma). 2014b;5:69–76.
Poggio C, Beltrami R, Colombo M, et al. In vitro antibacterial activity of different pulp capping materials. J Clin Exp Dent. 2015a;7:e584–8.
Poggio C, Ceci M, Dagna A, et al. In vitro cytotoxicity evaluation of different pulp capping materials: a comparative study. Arh Hig Rada Toksikol. 2015b;66:181–8.
Pruthi PJ, Dharmani U, Roongta R, Talwar S. Management of external perforating root resorption by intentional replantation followed by Biodentine restoration. Dent Res J (Isfahan). 2015;12:488–93.
Rajasekharan S, Martens LC, Cauwels RG, Verbeeck RM. Biodentine material characteristics and clinical applications: a review of the literature. Eur Arch Paediatr Dent. 2014;15:147–58.
Rajasekharan S, Martens L, Vandenbulcke J, et al. Efficacy of three different pulpotomy agents in primary molars—a randomised control trial. Int Endod J. 2016;50:215–28.
Raju VG, Venumbaka NR, Mungara J, et al. Comparative evaluation of shear bond strength and microleakage of tricalcium silicate-based restorative material and radioopaque posterior glass ionomer restorative cement in primary and permanent teeth: an in vitro study. J Indian Soc Pedod Prev Dent. 2014;32:304–10.
Ramos JC, Palma PJ, Nascimento R, et al. 1-year in vitro evaluation of tooth discoloration induced by 2 calcium silicate-based cements. J Endod. 2016;42:1403–7.
Saberi EA, Karkehabadi H, Mollashahi NF. Cytotoxicity of various endodontic materials on stem cells of human apical papilla. Iran Endod J. 2016;11:17–22.
Salzano S, Tirone F. Conservative nonsurgical treatment of class 4 invasive cervical resorption: a case series. J Endod. 2015;41:1907–12.
Samyuktha V, Ravikumar P, Nagesh B, et al. Cytotoxicity evaluation of root repair materials in human-cultured periodontal ligament fibroblasts. J Conserv Dent. 2014;17:467–70.
Setbon HM, Devaux J, Iserentant A, Leloup G, Leprince JG. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent Mater. 2014;30:1291–303.
Sharma S, Deepak P, Vivek S, Ranjan Dutta S. Palatogingival groove: recognizing and managing the hidden tract in a maxillary incisor: a case report. J Int Oral Health. 2015;7:110–4.
Shokouhinejad N, Nekoofar MH, Pirmoazen S, Shamshiri AR, Dummer PM. Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: an ex vivo study. J Endod. 2016;42:140–4.
Silva EJ, Carvalho NK, Zanon M, et al. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz Oral Res. 2016a;30(1). https://doi.org/10.1590/1807-3107BOR-2016.vol30.0084
Silva EJ, Senna PM, De-Deus G, Zaia AA. Cytocompatibility of Biodentine using a three-dimensional cell culture model. Int Endod J. 2016b;49:574–80.
Simsek N, Alan H, Ahmetoglu F, et al. Assessment of the biocompatibility of mineral trioxide aggregate, bioaggregate, and Biodentine in the subcutaneous tissue of rats. Niger J Clin Pract. 2015;18:739–43.
Simsek N, Bulut ET, Ahmetoglu F, Alan H. Determination of trace elements in rat organs implanted with endodontic repair materials by ICP-MS. J Mater Sci Mater Med. 2016;27:46.
Singh S, Podar R, Dadu S, Kulkarni G, Purba R. Solubility of a new calcium silicate-based root-end filling material. J Conserv Dent. 2015;18:149–53.
Sinha N, Singh B, Patil S. Cone beam-computed topographic evaluation of a central incisor with an open apex and a failed root canal treatment using one-step apexification with Biodentine: a case report. J Conserv Dent. 2014;17:285–9.
Soni HK. Biodentine pulpotomy in mature permanent molar: a case report. J Clin Diagn Res. 2016;10:9–11.
Soundappan S, Sundaramurthy JL, Raghu S, Natanasabapathy V. Biodentine versus mineral trioxide aggregate versus intermediate restorative material for retrograde root end filling: an invitro study. J Dent (Tehran). 2014;11:143–9.
Tanalp J, Karapinar-Kazandag M, Dolekoglu S, Kayahan MB. Comparison of the radiopacities of different root-end filling and repair materials. Sci World J. 2013;2013:594950.
Togaru H, Muppa R, Srinivas N, et al. Clinical and radiographic evaluation of success of two commercially available pulpotomy agents in primary teeth: an in vivo study. J Contemp Dent Pract. 2016;17:557–63.
Topcuoglu G, Topcuoglu HS. Regenerative endodontic therapy in a single visit using platelet-rich plasma and Biodentine in necrotic and asymptomatic immature molar teeth: a report of 3 cases. J Endod. 2016;42:1344–6.
Tziafa C, Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Dentinogenic responses after direct pulp capping of miniature swine teeth with Biodentine. J Endod. 2014;40:1967–71.
Ulusoy OI, Paltun YN, Guven N, Celik B. Dislodgement resistance of calcium silicate-based materials from root canals with varying thickness of dentine. Int Endod J. 2015;49:1188–93.
Umashetty G, Hoshing U, Patil S, Ajgaonkar N. Management of inflammatory internal root resorption with Biodentine and thermoplasticised gutta-percha. Case Rep Dent. 2015;2015:452609.
Ustun Y, Topcuoglu HS, Akpek F, Aslan T. The effect of blood contamination on dislocation resistance of different endodontic reparative materials. J Oral Sci. 2015;57:185–90.
Valles M, Roig M, Duran-Sindreu F, Martinez S, Mercade M. Color stability of teeth restored with Biodentine: a 6-month in vitro study. J Endod. 2015;41:1157–60.
Vemisetty H, Reddy SJ, et al. Comparative evaluation of marginal adaptation of Biodentine(TM) and other commonly used root end filling materials-an in vitro study. J Clin Diagn Res. 2014;8:243–5.
Vidal K, Martin G, Lozano O, et al. Apical closure in apexification: a review and case report of apexification treatment of an immature permanent tooth with Biodentine. J Endod. 2016;42:730–4.
Villat C, Grosgogeat B, Seux D, Farge P. Conservative approach of a symptomatic carious immature permanent tooth using a tricalcium silicate cement (Biodentine): a case report. Restor Dent Endod. 2013;38:258–62.
Widbiller M, Lindner SR, Buchalla W, et al. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin Oral Investig. 2016;20:237–46.
Yoldas SE, Bani M, Atabek D, Bodur H. Comparison of the potential discoloration effect of bioaggregate, Biodentine, and white mineral trioxide aggregate on bovine teeth In vitro research. J Endod. 2016;42:1815–8.
Zhabuawala MS, Nadig RR, Pai VS, Gowda Y. Comparison of fracture resistance of simulated immature teeth with an open apex using Biodentine and composite resin: an in vitro study. J Indian Soc Pedod Prev Dent. 2016;34:377–82.
Funding
The authors declare that no funding was received for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The research group received material support from Septodont, Paris, France for educational programmes intended for general dentists.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s40368-018-0335-y.
Rights and permissions
About this article
Cite this article
Rajasekharan, S., Martens, L.C., Cauwels, R.G.E.C. et al. Biodentine™ material characteristics and clinical applications: a 3 year literature review and update. Eur Arch Paediatr Dent 19, 1–22 (2018). https://doi.org/10.1007/s40368-018-0328-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40368-018-0328-x