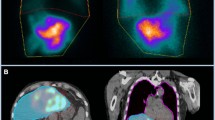
Abstract
Purpose
Yttrium 90-labeled intra-arterial liver therapy is an effective treatment for patients with unresectable primary or metastatic liver malignancy. Optimal radioisotope dose calculation is dependent upon lung shunt fraction (LSF) which is typically estimated by planar scintigraphy. The goal of this systematic review was to compare LSF using 2D planar scintigraphy and 3D SPECT/CT. A secondary outcome was to assess the impact on lung dosimetry.
Methods
PubMed, SCOPUS and Web of Science database were searched for studies in English language related to lung shunt fraction quantification utilizing technetium 99m-labeled macroaggregated albumin (99mTc-MAA) planar scintigraphy and SPECT/CT, published between January 2010 and November 2020. The review was conducted using the PRISMA statement and QUADAS-2 criteria.
Results
A total of 8 studies (one prospective, 4 retrospective studies and 3 abstracts from national conferences) with a sample size of 552 were included in this review. There were 456 patients (82.6%) with hepatocellular carcinoma and 95 patients (17.4%) with hepatic metastasis. A wider range of LSF percentages was noted in planar scintigraphy methodology (range 1.2–33.3%) when compared to SPECT/CT (range 0.4–21.7%). The median LSF percentages were 6.7 and 2.9% using planar scintigraphy and SPECT/CT, respectively.
Conclusion
The current clinical assessment of LSF is substantially overestimated by 2D planar scintigraphy when compared to 3D SPECT/CT. However, unclearness of blinding between the index test and reference standard was an area of quality concern. Hence, further randomized or prospective studies are needed to strengthen the role of SPECT/CT in lung shunt fraction estimation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interventional oncology is a rapidly growing sub-specialty with advancing technologies and disease-modifying treatments resulting in a significant impact on patients with cancer diagnosis. Selective internal radiation therapy (SIRT), also termed as Transarterial radioembolization (TARE), is a unique hepatic artery-based brachytherapy using yttrium 90 (90Y)-loaded microspheres for oncological management. SIRT is a safe and well-established treatment for unresectable hepatocellular carcinoma (HCC) and metastasis to the liver [1,2,3,4]. Currently, there are two commercially available radioactive microspheres for clinical use, namely, resin-based SIR-Spheres (Sirtex Medical Limited, North Sydney, Australia) and glass-based spheres (TheraSphere; Boston Scientific). The tumoricidal effects of 90Y are due to β-particles emission (933.7 keV) from the radioactive decay of 90Y (half-life = 64.2 h) to nonradioactive, Zirconium-90 [5].
Patient selection and success for TARE is dependent upon many clinical and technical aspects, for example, vascular anatomy, patients underlying health status, radiation-induced injury from non-targeted delivery of 90Y which can result in irreversible damage. Although less frequent than liver toxicity, radiation-induced pneumonitis due to hepatopulmonary shunting of 90Y particles remains a major concern. The biological effect of radiation is dependent upon the relation between the amount of radioactivity administered (measured in Giga becquerel; GBq) and absorbed dose into a specific volume of a tissue (measured in Gray; Gy). Radiation dose reduction is recommended when the lung dose limit exceeds 30 Gy for an individual treatment or cumulative dose of 50 Gy for multiple treatments.
As a standard clinical practice, before TARE, a mapping angiography is performed to evaluate the vascular supply to the tumor, perform prophylactic embolization of selected vessels as needed, determine appropriate catheter position to deliver the particles, and identify shunt vessels which may result in non-targeted delivery of radioisotope. Subsequently, a surrogate particle, 99mTc-macroaggregated albumin (99mTc-MAA), is injected into the liver to simulate the biodistribution of microspheres in the tumor, healthy liver and lungs to quantify the lung shunt fraction (LSF) in terms of percentage. This is an important step as the amount of 90Y administered is adjusted based on LSF calculations. According to the package insert for SIR-spheres, a LSF < 10% does not warrant a dose reduction whereas a LSF > 20% is considered a relative contraindication to TARE [6]. As per the TheraSphere manufacturer, an upper limit of 30 Gy for absorbed dose to the lungs based on the lung shunt fraction resulting from a delivery of greater than 16.5 mCi of yttrium 90 to the lungs is considered as contraindication [7].
At many institutions, the image analysis of LSF is typically performed by 2D planar gamma camera scintigraphy with simultaneously acquired 3D single-photon emission computed tomography (SPECT)/computed tomography (CT) images, primarily to identify extrahepatic radiotracer distribution. Several limitations have been noted with planar scintigraphy such as lack of anatomic references, operator dependence, attenuation difference between the lung and liver tissue. Another, significant limiting factor in planar imaging is the spill over of activity from the hepatic dome into the lung bases. Hence, the use of 3D SPECT/CT was proposed to overcome these limitations for an accurate estimate of LSF and radiation risk to the lungs. The primary purpose of this study was to systematically review the literature regarding the application of SPECT/CT for quantification of LSF.
Materials and methods
Search strategy and selection criteria
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a comprehensive search on medical database, PubMed, SCOPUS and Web of Science, for studies in English language from January 1, 2010 to November 30, 2020. The following combination of terms were used to search—“lung shunt fraction”, “hepatopulmonary shunt”, “lung dosimetry”, “radiation pneumonitis” AND “yttrium”, “radioembolization”, “Y90”, “Transarterial radioembolization”, and “SPECT/CT”.
Inclusion and exclusion criteria
The inclusion criteria were as follows:
-
1.
Observational studies, clinical studies and abstracts from national conferences in English language with a minimum sample size of ten patients.
-
2.
Adult (> 18 years old) male or female patients diagnosed with unresectable primary or metastatic liver tumors with planned yttrium 90 radioembolization.
-
3.
Studies that quantify lung shunt fraction using planar scintigraphy and SPECT/CT.
Review articles, animal studies, laboratory investigations, letters to the editor, case series, case reports and any duplicated clinical studies were excluded from the study.
Primary outcome
Identify the differences in quantitative LSF assessment using conventional planar scintigraphy versus SPECT/CT.
Secondary outcome
Evaluate the subsequent impact on yttrium 90 lung dosimetry.
Data collection and analysis
The reviewers independently performed data extraction and identified all potentially relevant studies. Data collected included the type of article (e.g., prospective or retrospective), country of origin, year of study, sample size, demographics (e.g., age, sex, etc.), clinical characteristics (e.g., primary malignancy or metastasis), LSF quantification and methodology (SPECT/CT and 99mTc-MAA whole-body planar imaging). Studies were classified into three levels of evidence as follows: level I, randomized controlled trials (RCTs); level II, non-RCTs or well-designed cohort studies; and level III, observational studies, as described by the U.S. Preventive Services Task Force.
Study quality appraisal and risk of bias
The authors independently evaluated the methodological assessment using the tool provided by the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), modified by removing the question “If a threshold was used, was it prespecified?” [8]. The QUADAS-2 form is composed of four domains: (1) patient selection, (2) index test, (3) reference standard and (4) flow and timing. 3D SPECT/CT was considered as the index test (intervention) and 2D planar scintigraphy was considered as a reference test (comparison).
Results
Following the PRISMA diagram, the initial search yielded 184 potential citations published between 2014 and 2019. Finally, one prospective study, four retrospective studies and three abstracts from national conferences were included in the study (Fig. 1).
Table 1 summarizes the data related to the year of publication, methodology, level of evidence, sample size, mean age, and pathology for each included study. Out of 552 patients, 456 patients had hepatocellular carcinoma and 95 patients had hepatic metastasis primarily from colorectal cancer (n = 24), cholangiocarcinoma (n = 15) and neuroendocrine cancer (n = 6). The data included from the three abstracts did not provide information regarding subtype of tumors.
Table 2 summarizes the studies according to 90Y spheres, SPECT/CT vendor, LSF quantification using planar scintigraphy and SPECT/CT with statistical coefficients. Five studies reported mean LSF percentages and four studies documented a wider range of LSF with planar technique (1.2–33.3%) when compared to SPECT/CT (range 0.4–21.7%). The median LSF percentages were 6.7% using planar imaging and 2.9% using SPECT/CT. Figure 2 shows results of LSF from each included study.
As a secondary outcome, we evaluated the impact of LSF quantification on the lung dosimetry using the Medical Internal Radiation dose (MIRD) formula [17, 18]:
The methodological quality is summarized in Fig. 3. In general, the patient population was highly selected by including patients with unresectable primary or metastatic liver malignancy. However, yttrium 90 radiotherapy can only be performed in this select patient population, hence, the applicability was judged to be good. All patients included in the study underwent both the index and reference test during pre-treatment evaluation. A major concern was the lack of clarity whether the investigator was blinded to the reference standard results while interpreting the index test.
Discussion
Image-guided oncological interventions have significantly increased over the past decade. Studies have shown that 90Y can prolong recurrence-free survival, overall survival and potentially curative therapy for liver cancer [19, 20]. The optimal threshold dose of 190 Gy has been shown to achieve complete tumoricidal effect using pathologic correlation [21]. Hence, it is vital to administer an appropriate radiopharmaceutical activity for local tumor control while minimizing non-targeted delivery, which is conditional upon LSF quantification. In the era of modern medicine, many authors have questioned the current clinical practice for calculating LSF using 2D planar scintigraphy [9,10,11,12,13,14,15,16].
In this systematic review, the included data suggests that planar scintigraphy overestimates the LSF when compared to SPECT/CT. Based on the data, there is a wider range of LSF percentages using planar imaging (range 1.2–33.3%) when compared to SPECT/CT (range 0.4–21.7%). The median LSF percentages were 6.7 and 2.9% using planar scintigraphy and SPECT/CT, respectively. These results are in accordance with prior anthropomorphic phantom studies suggesting SPECT/CT as a precise methodology [12, 22]. This is of important clinical significance, especially in patients with higher LSF (> 10%) which could result in dose reduction, potentially additional treatment procedure or even contraindication, according to current clinical practice [9, 10]. Higher LSF’s have been reported in hepatocellular malignancy than other hepatic tumors and may even serve as a biomarker for survival [23, 24]. Elsayed et al. suggested that there is a greater discrepancy of LSF in patients with worse Child–Pugh score and tumors larger than 5 cm [10]. It is important to acknowledge that a very small number of patients, 13 out of 552 (0.02%), had planar LSF lower than SPECT/CT LSF [10, 14, 15]. These patients had focal hepatic lesions near the diaphragm resulting in possible erroneous delineation of liver activity leading to underestimation of planar LSF.
Optimal lung dosimetry calculations require accurate LSF estimations. An initial study by Yu et al. concluded that “99mTc-MAA SPECT/CT provides a more accurate estimation of radiation risk to lungs”, particularly in patients with large LSF from planar imaging [25]. A standard lung mass value of 1000 g is often assumed for every patient irrespective of underlying lung disease, prior surgery or irradiation, which risks inaccurate absorbed lung dose. Kao et al. and Lopez et al. detailed a novel approach to overcome this limitation using a preprocedural diagnostic CT to estimate patient specific lung mass and volume, thereby emphasizing the need of precise and personalized dosimetry for therapeutic guidance [11, 13]. A prospective study of 50 patients by Dittmann et al. corroborated these results by stating that absorbed lung doses may be higher when calculated by planar methodology [9]. However, further clinical studies are required to establish safety of SPECT/CT derived lung dose limits.
The hybrid SPECT-CT scanners overcome the drawbacks of planar scintigraphy by three-dimensional anatomic localization and allowing for better quantification of radiotracer distribution by incorporating parameters such as photon attenuation, scatter attenuation and reconstruction algorithms. Hence, scanners from different vendors can have a certain degree of variability when using these quantification variables. The included studies were performed on different scanners manufactured by General Electric, Siemens and Phillips. A pilot study by Peter et al. showed that absolute SPECT quantification is feasible in multi-vendor settings which would be beneficial in dosimetry aimed at personalized radionuclide therapy [26].
Despite the advantages of SPECT/CT, there remains concern regarding additional radiation exposure associated with the CT portion of SPECT/CT. In addition to acquisition parameters such as pitch, rotation time, tube voltage (kV), and tube current (mA), CT dose optimization is also patient dependent. In general, a low-dose acquisition CT protocol is suggested for concurrent use with SPECT, resulting in an additional effective dose range of 1–4 mSv [27, 28]. It would be reasonable to state that a low-dose CT scan is justified for the advantage of attenuation correction and anatomical mapping.
A major limitation for lung shunt or dose calculation is the misregistration of SPECT/CT images from free breathing, particularly near the diaphragm, which results in a spillover of liver activity into the right lung base. Volumes of interest (VOIs) are drawn separately for the entire lung and liver using vendor-specific software. To avoid liver spill over activity, Yu et al. proposed to use the left lung for quantification whereas other investigators have suggested excluding 1.5–2 cm of the right lung base [12, 13, 24]. However, these approaches are based upon the assumption of homogenous perfusion of the lungs which may not be attained in every case [29]. Dittmann et al. delineated the entire right lung outside the liver activity using CT Hounsfield units [9].
Limitations of this review include small number of low-quality studies and limited overall level of evidence II/III. Another significant limitation was heterogenous patient population within the included studies, hence, metaanalysis could not be performed. There is a lack of true gold standard to validate LSF independent of 99mTc-MAA distribution, which is an imperfect surrogate s due to slightly dissimilar physical properties [30]. 99mTc-MAA contains smaller diameter particles (< 20 μm) which are more prone to shunting than the therapeutic microspheres. This was evaluated by Elschot et al. by comparing 99mTc-MAA with a novel radiotracer 166Holmium and concluded that Holmium microspheres enable a more accurate assessment of lung doses [31]. Larger prospective studies would be required to yield a higher quality of evidence.
Conclusion
In the recent decade, there have been many publications emphasizing the need to reassess the current methodology for estimating LSF and lung dosimetry with growing consensus regarding the use of SPECT/CT. Despite differences in the SPECT/CT-based methodology, there is general agreement that 2D planar scintigraphy substantially overestimates the LSF. SPECT/CT strategy is clinically feasible, more precise and can be incorporated into treatment planning for optimal tumoricidal response. In the era of personalized medicine, clinicians should provide advanced imaging-based methods for 90Y radiotherapy planning.
References
Salem R, Lewandowski RJ, Kulik L et al (2011) Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140(2):497–507
Hendlisz A, Van den Eynde M, Peeters M et al (2010) Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 28:3687–3694
Riaz A, Gates VL, Atassi B et al (2011) Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 79(1):163–171
Jia Z, Wang W (2018) Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: a systematic review. Eur J Radiol 100:23–29
Kritzinger J, Klass D, Ho S et al (2013) Hepatic embolotherapy in interventional oncology: technology, techniques, and applications. Clin Radiol 68:1–15
https://www.sirtex.com/media/169247/ssl-us-14-sir-spheres-microspheres-ifu-us.pdf. Accessed Sept 2020
https://www.bostonscientific.com/en-US/products/cancer-therapies/therasphere-y90-glass-microspheres/therasphere-y90-microspheres-brief-summary.html. Accessed Sept 2020
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Dittmann H, Kopp D, Kupferschlaeger J, Feil D, Groezinger G, Syha R, Weissinger M, la Fougère C (2018) A prospective study of quantitative SPECT/CT for evaluation of lung shunt fraction before SIRT of liver tumors. J Nucl Med 59(9):1366–1372
Elsayed M, Cheng B, Xing M, Sethi I, Brandon D, Schuster DM, Bercu Z, Galt J, Barron B, Kokabi N (2020) Comparison of Tc-99m MAA planar versus SPECT/CT imaging for lung shunt fraction evaluation prior to Y-90 radioembolization: are we overestimating lung shunt fraction? Cardiovasc Intervent Radiol. 44(2):254–260
Lopez B, Mahvash A, Lam MGEH, Kappadath SC (2019) Calculation of lung mean dose and quantification of error for 90Y-microsphere radioembolization using 99mTc-MAA SPECT/CT and diagnostic chest CT. Med Phys 46(9):3929–3940
Allred JD, Niedbala J, Mikell JK, Owen D, Frey KA, Dewaraja YK (2018) The value of 99mTc-MAA SPECT/CT for lung shunt estimation in 90Y radioembolization: a phantom and patient study. EJNMMI Res 8(1):50
Kao YH, Magsombol BM, Toh Y, Tay KH, Chow PKh, Goh AS, Ng DC (2014) Personalized predictive lung dosimetry by technetium-99m macroaggregated albumin SPECT/CT for yttrium-90 radioembolization. EJNMMI Res 29(4):33
Georgiou M, Kuker R, Portelance L, Studenski M, Lorenzo A (2018) Comparison of 2D planar versus 3D SPECT/CT lung shunt fraction calculation from 99mTc MAA pre-90Y imaging for hepatic microsphere radioembolization. J Nucl Med 59(supplement 1):601
Poon-iad N, Teyateeti A, Chaudakshetrin P (2019) Selective internal radiotherapy dose calculation: a pilot study to compare lung shunt fraction on planar and SPECT/CT imaging. International Symposium on Standards, Applications and Quality Assurance in Medical Radiation Dosimetry (18–21 June 2019), IAEA, Vienna
Gill H, Bian J, Gabriel M, Molvar C, Wagner R, Halama J (2019) 99mTc-MAA SPECT/CT imaging for quantitative assessment of lung shunt fraction prior to 90Y transarterial radioembolization. J Nucl Med 60(supplement 1):265
Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK (1997) Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med 24:293–298
Dezarn WA, Cessna JT, DeWerd LA et al (2011) American Association of Physicists in Medicine. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys 38(8):4824–4845
Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA et al (2018) Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology 287(3):1050–1058
Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K et al (2020) Liver transplantation following yttrium-90 radioembolization: 15-year experience in 207-patient cohort. Hepatology. https://doi.org/10.1002/hep.31318
Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D et al (2014) Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 60(1):192–201. https://doi.org/10.1002/hep.27057
Zaharakis A, Leveque F, Backiel J, Tursi G, Palestro C, Nichols K (2014) SPECT/CT for estimating hepatopulmonary shunting in selective internal radiotherapy: a phantom study. J Nucl Med 55(supplement 1):1496
Gaba RC, Zivin SP, Dikopf MS, Parvinian A, Casadaban LC, Lu Y, Bui JT (2014) Characteristics of primary and secondary hepatic malignancies associated with hepatopulmonary shunting. Radiology 271(2):602–612
Ludwig JM, Ambinder EM, Ghodadra A, Xing M, Prajapati HJ, Kim HS (2016) Lung shunt fraction prior to yttrium-90 radioembolization predicts survival in patients with neuroendocrine liver metastases: single-center prospective analysis. Cardiovasc Interv Radiol 39(7):1007–1014
Yu N, Srinivas SM, Difilippo FP et al (2013) Lung dose calculation with SPECT/CT for 90yttrium radioembolization of liver cancer. Int J Radiat Oncol Biol Phys 85:834–839
Peters SMB, van der Werf NR, Segbers M, van Velden FHP, Wierts R, Blokland KJAK, Konijnenberg MW, Lazarenko SV, Visser EP, Gotthardt M (2019) Towards standardization of absolute SPECT/CT quantification: a multi-center and multi-vendor phantom study. EJNMMI Phys 6(1):29
Buck AK, Nekolla S, Ziegler S, Beer A, Krause BJ, Herrmann K, Scheidhauer K, Wester HJ, Rummeny EJ, Schwaiger M, Drzezga A (2008) SPECT/CT. J Nucl Med 49:1305–1319
Roach PJ, Schembri GP, Ho Shon IA et al (2006) SPECT/CT imaging using a spiral CT scanner for anatomical localization: impact on diagnostic accuracy and reporter confidence in clinical practice. Nucl Med Commun 27:977–987
Salem R, Parikh P, Atassi B et al (2008) Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 31:431–438
Van de Wiele C, Maes A, Brugman E, D’Asseler Y, De Spiegeleer B, Mees G, Stellamans K (2012) SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imaging 39:1646–1655
Elschot M, Nijsen JF, Lam MG et al (2014) 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with 166Ho-microspheres. Eur J Nucl Med Mol Imaging 41:1965–1975
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization and idea: HG; methodology: HG and JH; literature search and data analysis: HG and JH; writing—original draft preparation: HG; writing—review and editing: JH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gill, H., Hiller, J. Systematic review of lung shunt fraction quantification comparing SPECT/CT and planar scintigraphy for yttrium 90 radioembolization planning. Clin Transl Imaging 9, 181–188 (2021). https://doi.org/10.1007/s40336-021-00417-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-021-00417-0