Abstract
The objective of the study was to systematically review published data and perform a meta-analysis about the discordance rate between radiolabelled choline PET/CT and bone scintigraphy (BS) in detecting bone metastases in patients with prostate cancer (PCa). A comprehensive literature search of studies or subsets of studies published through November 2014 including information on the comparison among radiolabelled choline PET/CT and bone scintigraphy in PCa patients was carried out. A meta-analysis was performed in order to calculate the pooled discordance rate among these methods in detecting bone metastases on a per patient-based analysis. Twelve articles were selected. The pooled discordance rate among radiolabelled choline PET/CT and BS in detecting bone metastases was 10.9 % (95 % confidence interval 6.3–16.7 %). Discordant findings were due to radiolabelled choline positive and BS negative or inconclusive findings, but BS positive and radiolabelled choline-negative findings also occurred. We discuss the possible causes of discordant findings. Discordance rate between radiolabelled choline PET/CT and BS in detecting bone metastases in PCa patients is not negligible and both methods are useful in this setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most frequently diagnosed malignancy in men and its incidence has been increasing in the last decades [1]. The clinical outcome of PCa is highly variable. In some patients the tumor can grow so slowly that it may never be life-threatening, while in other patients it can exhibit an aggressive pattern implying early spread to the skeleton and death [2, 3].
The detection of bone metastases is of paramount importance in the management of patients with PCa, in particular for selecting an appropriate therapy, determining tumor staging, assessing prognosis, and evaluating the efficacy of treatments. Patients with bone metastases may not need local treatment such as surgery or local radiotherapy, but may be eligible for hormone therapy or chemotherapy [2–4]. Furthermore, the extent of bone metastatic disease is an independent prognostic factor in patients with PCa [5].
Bone scintigraphy (BS) using technetium-99m-diphosphonates (99mTc-DPs) and radiolabelled choline positron emission tomography/computed tomography (PET/CT) are both useful to detect bone metastases in PCa patients [6, 7].
BS is used as a standard technique for the assessment of bone metastases of PCa because of its entire skeleton screening at once and widespread availability. 99mTc-DPs accumulate in bone structures depending on local blood flow and osteoblastic activity. Planar scintigraphic images are obtained with a gamma-camera; tomographic images may be obtained by single photon emission computed tomography (SPECT) or SPECT/CT. Sites of increased 99mTc-DPs uptake imply accelerated bone turnover and may indicate metastatic disease. Osseous metastatic disease may be diagnosed based on the overall pattern of activity, or in conjunction with anatomic imaging [4, 6, 8].
In recent years, PET/CT using choline radiolabelled with carbon-11 (11C) or fluorine-18 (18F) has been shown to be useful for staging or restaging PCa patients with biochemical failure after radical prostatectomy or radiation therapy [9–12]. Radiolabelled choline is biochemically indistinguishable from natural choline (an essential component of the phospholipids in the cell membrane), thus it can be considered as a true tracer of cancer cell metabolism. As tumor cells present a high metabolic rate, choline uptake increases in tumor tissue to keep up with the demands of the synthesis of phospholipids in cellular membranes [13–15]. The greatest advantage of radiolabelled choline PET/CT lies in its ability to assess disease at multiple anatomical sites at a single time while preserving an accuracy similar to or greater than that of other conventional imaging techniques [7]. The most remarkable difference between 11C-choline and 18F-choline is the half-life of the isotopes (20 min for 11C vs. 110 min for 18F). In addition, urinary excretion of 18F-choline is comparatively higher than that of 11C-choline, but the overall imaging findings are similar between the different radiolabelled choline agents [8, 9, 13].
The aim of this article is to systematically review the literature and perform a meta-analysis about the discordance rate among BS and radiolabelled choline PET/CT in detecting bone metastases in PCa patients and to discuss the causes of the discordant findings.
Methods
Search strategy
A comprehensive computer literature search of the PubMed/MEDLINE database was conducted to find relevant published articles on the comparison between radiolabelled choline PET/CT and BS in detecting bone metastases in PCa patients. We used a search algorithm that was based on a combination of the terms: (a) “PET” or “positron emission tomography” and (b) “choline” and (c) “scan” or “scintigraphy” and (d) “bone” or “osseous” or “skeleton” or “skeletal” and (e) “prostate”. No beginning date limit and language restriction were used; the search was updated until November 30th, 2014. To expand our search, references of the retrieved articles were also screened for additional studies.
Study selection
Studies or subsets in studies comparing radiolabelled choline PET/CT and BS findings in PCa patients were eligible for inclusion. The exclusion criteria were: (a) articles not within the field of interest of this review; (b) review articles, editorials or letters, comments, conference proceedings; (c) case reports or small case series; (d) studies performing PET only (to reduce the heterogeneity of the results derived by pooled analysis of PET and PET/CT findings together); (e) studies performing radiolabelled choline PET/CT only in PCa patients with negative or inconclusive BS (possible selection bias for the calculation of the discordance rate among these methods); (f) articles from the same group (possible data overlap; in such cases the most complete article was selected); (g) absence of data for assessing the discordance rate on a per patient-based analysis (i.e., only per lesion-based analysis available).
Two researchers (GT and CV) independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and exclusion criteria mentioned above. Articles were rejected if they were clearly ineligible. The same researchers then independently reviewed the full-text version of the remaining articles to determine their eligibility for inclusion.
Data extraction
For each included study, information was collected concerning basic study (authors, journal and year of publication, country of origin, study design), number of patients performing both methods, PET radiopharmaceutical used, number of patients with discordant findings (radiolabelled choline PET/CT positive and BS negative or vice versa).
Statistical analysis
A pooled analysis of the discordance rate between radiolabelled choline PET/CT and BS in PCa patients was performed using data retrieved by the selected studies. A random-effects model was used for statistical pooling of the data taking into account the heterogeneity between studies. The different weight of each study in the pooled analysis was related to the different sample size. Pooled data were presented with their respective 95 % confidence interval (95 % CI) and data were displayed using a forest plot. A I 2 index was used to test for heterogeneity between studies. Publication bias was evaluated by using Egger’s test [16].
Statistical analyses were performed using StatsDirect statistical software version 3.0 (StatsDirect Ltd; Altrincham, UK).
Results
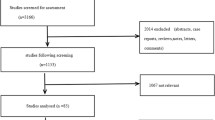
The comprehensive computer literature search from PubMed/MEDLINE database revealed 111 articles. Reviewing titles and abstracts 102 articles were excluded: (a) 87 because not within the field of interest or as review articles, editorials or letters; (b) 7 as case reports or small case series (<10 patients performing both methods); (c) 2 studies performing PET only (no PET/CT) [17, 18]; (d) 3 studies performing radiolabelled choline PET/CT only in PCa patients with negative or inconclusive BS [19–21]; (e) one for possible partial data overlap with other articles of the same group [22]; (f) 2 for absence of data for assessing the discordance rate on a per patient-based analysis (only per lesion-based analysis available) [23, 24]. Nine articles were selected and the full-text was retrieved [25–33]. Other three articles were included screening the references of the selected studies [34–36]. Two articles of the same group were included because no data overlap was evident [31, 36].
Finally, 12 articles including 740 PCa patients were selected and were included in the meta-analysis [25–36] (Fig. 1). The characteristics of the included studies are shown in Table 1.
Most of the selected studies were performed at European centers and half of them were prospective. Heterogeneity about characteristics of the patients included (i.e., evaluation of PCa patients in staging or restaging, evaluation of hormone refractory PCa patients) and type of PET radiopharmaceutical used (18F-choline in 8 studies and 11C-choline in 4 articles) was evident.
Discordant findings between radiolabelled choline PET/CT and BS were reported by most of the studies included in the meta-analysis.
Cimitan et al. [33] reported 8/68 discrepant results between 18F-choline PET/CT and BS, including 2 cases of BS positive and 18F-choline PET/CT negative and 6 cases of 18F-choline PET/CT positive and BS negative or inconclusive.
In the retrospective study of Castellucci et al. [35] 11C-choline PET/CT findings were positive for bone lesions in 31 of 130 patients who had a BS performed before PET/CT: 22 were negative at BS, and 9 patients had shown a single lesion at BS but multiple bone lesions at PET/CT.
Picchio et al. in their retrospective study reported concordant findings between 11C-choline PET/CT and BS in 55 of 78 (71 %) cases. In particular, 18 of 55 (33 %) patients concordantly had true-positive results and 37 of 55 (67 %) had true-negative findings with both methods. In the remaining 23 cases, 11C-choline PET/CT and BS findings were discordant. In particular, of the 21 BS equivocal findings, the results of 11C-choline PET/CT were true-negative in 13 of 21 (62 %), false-negative in 2 of 21 (9 %) and true-positive in 6 of 21 (29 %) patients. In one of three 11C-choline PET/CT false-negative patients the BS result was true-positive. The single 11C-choline PET/CT equivocal finding was true-negative at BS [26].
In a recent article by Garcia et al. on 169 patients with biochemical recurrence of PCa, on a per patient-based analysis, 11C-choline PET/CT and BS were both negative for bone metastases in 118 patients (69.8 %). 11C-choline PET/CT and/or BS were positive for bone lesions in 51 patients (30.2 %), being concordant in 30 patients and discordant in 21 cases. On a per lesion-based analysis, BS detected 38 blastic, 2 lytic and 10 non-CT-evident lesions, whereas 11C-choline PET/CT detected 41 blastic, 4 lytic and 29 non-CT-evident lesions [25].
Overall, the discordance rate among radiolabelled choline PET/CT and BS in detecting bone metastases in PCa patients ranged from 0 to 29.5 % in the articles included in this meta-analysis, with a pooled estimate of 10.9 % (95 % CI 6.3–16.7 %) (Fig. 2). The heterogeneity between the included studies was significant (I 2 = 75 %) but the presence of significant publication bias was not demonstrated by the Egger’s test (Egger’s bias = 2; 95 % CI −0.2 to 4; p = 0.07).
Discussion
Functional imaging techniques such as BS and radiolabelled choline PET/CT are both useful in the evaluation of PCa patients.
National Comprehensive Cancer Network (NCCN) guidelines suggest the use of BS in the initial evaluation of patients at high risk for skeletal metastases (T1 disease and PSA ≥ 20 ng/ml, T2 disease and PSA ≥10 ng/ml, Gleason score ≥8, T3/T4 disease, any stage disease with symptoms suggestive of osseous metastatic disease) [4]. BS can be considered for the evaluation of the post-prostatectomy patient when there is failure of PSA to fall to undetectable levels, or when there is undetectable PSA after radical prostatectomy with a subsequent detectable PSA that increases on two or more consecutive determinations. BS can be considered for the evaluation of patients with an increasing PSA or positive digital rectal exam after radiation therapy if the patient is a candidate for additional local therapy or systemic therapy. Low- and intermediate-risk patients with low serum PSA levels postoperatively have a very low risk of positive BS [4].
Radiolabelled choline PET/CT has been used to staging and restaging PCa patients [9–12]. In particular, radiolabelled choline PET/CT may be useful to detect metastases or relapse of PCa as stated in the last NCCN guidelines [4]. Several studies have shown that the positive detection rate (DR) of the technique increases with increasing PSA levels. Furthermore, due to the strong relationship between PSA kinetics and DR of radiolabelled choline PET/CT, PSA doubling time and PSA velocity should be taken into account in the selection of PCa patients who should undergo radiolabelled choline PET/CT for restaging [37].
To the best of our knowledge, this meta-analysis is the first to evaluate the discordance rate between BS and radiolabelled choline PET/CT in detecting bone metastases in patients with PCa. Several studies have reported data about the discordance rate of these methods (Table 1). In order to derive more robust estimates in this regard, we have pooled published studies. A systematic review process was adopted in ascertaining studies, thereby avoiding selection bias [38].
We found a relevant pooled discordance rate among these imaging methods on a per patient-based analysis. Discordant findings were due to radiolabelled choline positive and BS negative or inconclusive results but BS positive and radiolabelled choline-negative findings also occurred.
Beyond the different diagnostic performance of BS and PET/CT (a higher sensitivity is usually expected by using PET/CT compared to planar scintigraphy or SPECT [8]), discordant findings are likely related to the different mechanism of uptake of 99mTc-DPs and radiolabelled choline, respectively. In fact the uptake of 99mTc-DPs is based on the osteoblastic response to metastatic lesions, whereas radiolabelled choline aims to detect malignant cells directly [39, 40].
Bone metastases from PCa are mainly osteoblastic lesions and these lesions are usually well detected by BS, because most of them are accompanied by an osteoblastic reaction. False-negative BS findings can result from the absence of reactive bone changes or rapid growing such as in pure osteolytic metastases [8, 39, 40].
On the other hand, radiolabelled choline PET/CT may detect bone metastatic lesions without abnormalities at the co-registered CT which could be bone marrow lesions sometimes negative at BS [19, 25, 39]. The early detection of these bone marrow lesions may have therapeutical and clinical implications in PCa patients.
As previously demonstrated, densely sclerotic bone metastases may show reduced radiolabelled choline uptake and increased uptake at BS [39, 41]. In fact osteoblastic proliferation results in a bone matrix increase with a relative decrease in cell density, thus determining the decrease of tumor activity and radiolabelled choline uptake [40]. However, it is not clear if the decrease in radiolabelled choline uptake in the sclerotic lesions is due to therapy response (with prognostic implications) or lower sensitivity of radiolabelled choline PET [41]. More studies with histopathological verification of bone metastases could be needed to clarify this issue. Serial BS or radiolabelled choline PET/CT in patients undergoing therapy would also help answer this question without necessarily requiring biopsy proof of viability.
It is also important to mention that degenerative changes in the skeleton usually show no increased tracer uptake on radiolabelled choline PET, whereas they are usually cause of equivocal findings at BS. This point emphasizes the higher specificity of radiolabelled choline PET/CT compared to BS [25, 41]. In fact, the main deficiency of BS is its relative low specificity, because the tracer uptake is not tumor-specific [8]. On the other hand, possible radiolabelled choline PET/CT false-positive findings for bone metastases could not be excluded when there is no confirmatory biopsy or follow-up data.
However, it should be underlined that radiolabelled choline PET/CT is not only able to detect “early marrow-based” metastases, but it may also provide relevant information in staging and restaging of PCa (such as the detection of local recurrence or lymph nodal metastases) compared to BS. Radiolabelled choline PET/CT has thus the potential to be a “one-stop diagnostic procedure” in the assessment of PCa patients [41]. However, its cost-effectiveness in detecting bone metastases in PCa patients compared to BS is unclear warranting further evaluation in future studies. In fact BS is more accessible and less expensive, but with a higher number of equivocal findings compared to radiolabelled choline PET/CT [25, 26] determining a significant number of additional diagnostic tests for confirmation, with cost- and time-consuming consequences.
To date, there are not sufficient data to recommend replacement of BS by radiolabelled choline PET/CT for detecting bone metastases in all those cases when BS is conventionally indicated according to international guidelines [4, 26]. However, when PSA serum measurements is <20 ng/ml, radiolabelled choline PET/CT could detect a higher number of bone metastases in PCa patients than BS, thus providing the possibility to identify bone metastatic involvement earlier than BS [25, 26].
Possible limitations of our analysis should be underlined. We have focused our analysis on the prevalence of discordant findings among BS and radiolabelled choline PET/CT in detecting bone metastases in PCa. The aim of our article was not to define the most sensitive method in this setting. A recent meta-analysis on this topic found that on a per-patient basis the pooled sensitivities for detection of bone metastases of PCa by using choline PET/CT, magnetic resonance imaging (MRI), and BS were 91 % (95 % CI 83–96 %), 97 % (95 % CI 91–99 %), and 79 % (95 % CI 73–83 %), respectively, and the pooled specificities were 99 % (95 % CI 93–100 %), 95 % (95 % CI 90–97 %), and 82 % (95 % CI 78–85 %), respectively [8]. On a per-lesion basis, the pooled sensitivities of choline PET/CT, bone SPECT, and BS were 84 % (95 % CI 81–87 %), 90 % (95 % CI 86–93 %), 59 % (95 % CI 55–63 %), respectively, and the pooled specificities were 93 % (95 % CI 89–96 %), 85 % (95 % CI 80–90 %) and 75 % (95 % CI 71–79 %), respectively [8].
Most studies included in our analysis compared a tomographic modality such as PET/CT, which combines functional and morphological data, with planar BS only. In our opinion, more studies comparing radiolabelled choline PET/CT and BS with tomographic and hybrid modality (SPECT/CT) are needed for a better comparison of these techniques. In fact, it is expected that SPECT/CT may reduce the number of equivocal lesion at planar BS improving the specificity of this method. On the other hand, whole-body BS should be compared to a whole-body radiolabelled choline PET/CT including the skull [41].
We chose to perform a meta-analysis on a per patient-based analysis only, because this analysis was adopted by most of the studies. We could not retrieve sufficient data from the included articles to perform a pooled per lesion-based analysis. Moreover, in the included studies there was not histological validation of all bone findings, because of its ethical infeasibility. A major limitation in determining the diagnostic accuracy of imaging methods for assessing bone spread is the gold standard. There are very few histopathological confirmations, therapeutic interventions reduce the validity of clinical and imaging follow-up, and all in all, MRI is sometimes considered as the gold standard, in spite of its own limitations.
Heterogeneity among the included studies could be another limitation. This heterogeneity likely derives from the baseline differences among the included patients, such as previous treatment and different PSA values, and technical aspects (Table 1). However, the heterogeneity between studies was accounted for using a random-effects model in our pooled analysis.
Conclusions
The discordance rate among BS and radiolabelled choline PET/CT in detecting bone metastases in PCa patients is not negligible and both methods are useful in this setting. Cost-effectiveness analyses and more studies comparing BS with SPECT/CT acquisition to radiolabelled choline PET/CT are warranted.
References
Marta GN, Hanna SA, Fernandes da Silva JL, Carvalho Hde A (2013) Screening for prostate cancer: an updated review. Expert Rev Anticancer Ther 13:101–108
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479
NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer. Version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
Rigaud J, Tiguert R, Le Normand L, Karam G, Glemain P, Buzelin JM, Bouchot O (2002) Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol 168:1423–1426
Caldarella C, Treglia G, Giordano A, Giovanella L (2013) When to perform positron emission tomography/computed tomography or radionuclide bone scan in patients with recently diagnosed prostate cancer. Cancer Manag Res 5:123–131
Evangelista L, Cervino AR, Burei M, Gregianin M, Saladini G, Marzola MC, Chondrogianis S, Rubello D (2013) Comparative studies of radiolabeled choline positron emission tomography, histology of primary tumor and other imaging modalities in prostate cancer: a systematic review and meta-analysis. Clin Transl Imaging 1:99–109
Shen G, Deng H, Hu S, Jia Z (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 43:1503–1513
von Eyben FE, Kairemo K (2014) Meta-analysis of (11)C-choline and (18)F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun 35:221–230
Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM (2013) The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 64:106–117
Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F (2013) Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol 63:1040–1048
Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, Rubello D (2013) Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med 38:305–314
Treglia G, Giovannini E, Di Franco D, Calcagni ML, Rufini V, Picchio M, Giordano A (2012) The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: a systematic review. Ann Nucl Med 26:451–461
Podo F (1999) Tumour phospholipid metabolism. NMR Biomed 12:413–439
DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, Reiman R, Price DT, Coleman RE (2001) Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med 42:1805–1814
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Kotzerke J, Prang J, Neumaier B, Volkmer B, Guhlmann A, Kleinschmidt K, Hautmann R, Reske SN (2000) Experience with carbon-11 choline positron emission tomography in prostate carcinoma. Eur J Nucl Med 27:1415–1419
de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ (2003) 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol 44:32–38
Fuccio C, Castellucci P, Schiavina R, Guidalotti PL, Gavaruzzi G, Montini GC, Nanni C, Marzola MC, Rubello D, Fanti S (2012) Role of 11C-choline PET/CT in the re-staging of prostate cancer patients with biochemical relapse and negative results at bone scintigraphy. Eur J Radiol 81:e893–e896
Fuccio C, Castellucci P, Schiavina R, Santi I, Allegri V, Pettinato V, Boschi S, Martorana G, Al-Nahhas A, Rubello D, Fanti S (2010) Role of 11C-choline PET/CT in the restaging of prostate cancer patients showing a single lesion on bone scintigraphy. Ann Nucl Med. 24:485–492
Poulsen MH, Bouchelouche K, Høilund-Carlsen PF, Petersen H, Gerke O, Steffansen SI, Marcussen N, Svolgaard N, Vach W, Geertsen U, Walter S (2012) [18F]fluoromethylcholine (FCH) positron emission tomography/computed tomography (PET/CT) for lymph node staging of prostate cancer: a prospective study of 210 patients. BJU Int 110:1666–1671
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, Cozzarini C, Di Muzio N, Rigatti P, Fazio F, Messa C (2010) Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging 37:301–309
Tuncel M, Souvatzoglou M, Herrmann K, Stollfuss J, Schuster T, Weirich G, Wester HJ, Schwaiger M, Krause BJ (2008) [(11)C]Choline positron emission tomography/computed tomography for staging and restaging of patients with advanced prostate cancer. Nucl Med Biol 35:689–695
Poulsen MH, Petersen H, Høilund-Carlsen PF, Jakobsen JS, Gerke O, Karstoft J, Steffansen SI, Walter S (2014) Spine metastases in prostate cancer: comparison of technetium-99m-MDP whole-body bone scintigraphy, [(18) F]choline positron emission tomography(PET)/computed tomography (CT) and [(18) F]NaF PET/CT. BJU Int 114:818–823
Garcia JR, Moreno C, Valls E, Cozar P, Bassa P, Soler M, Alvarez-Moro FJ, Moragas M, Riera E (2014) Diagnostic performance of bone scintigraphy and (11)C-Choline PET/CT in the detection of bone metastases in patients with biochemical recurrence of prostate cancer. Rev Esp Med Nucl Imagen Mol. doi:10.1016/j.remn.2014.08.001
Picchio M, Spinapolice EG, Fallanca F, Crivellaro C, Giovacchini G, Gianolli L, Messa C (2012) [11C]Choline PET/CT detection of bone metastases in patients with PSA progression after primary treatment for prostate cancer: comparison with bone scintigraphy. Eur J Nucl Med Mol Imaging 39:13–26
Schillaci O, Calabria F, Tavolozza M, Caracciolo CR, Finazzi Agrò E, Miano R, Orlacchio A, Danieli R, Simonetti G (2012) Influence of PSA, PSA velocity and PSA doubling time on contrast-enhanced 18F-choline PET/CT detection rate in patients with rising PSA after radical prostatectomy. Eur J Nucl Med Mol Imaging 39:589–596
McCarthy M, Siew T, Campbell A, Lenzo N, Spry N, Vivian J, Morandeau L (2011) 18F-Fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: prospective comparison with standard imaging. Eur J Nucl Med Mol Imaging 38:14–22
Beauregard JM, Williams SG, Degrado TR, Roselt P, Hicks RJ (2010) Pilot comparison of F-fluorocholine and F-fluorodeoxyglucose PET/CT with conventional imaging in prostate cancer. J Med Imaging Radiat Oncol 54:325–332
Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, Nader M, Gruy B, Janetschek G, Langsteger W (2010) 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology 254:925–933
Kwee SA, Coel MN, Ly BH, Lim J (2009) (18)F-Choline PET/CT imaging of RECIST measurable lesions in hormone refractory prostate cancer. Ann Nucl Med 23:541–548
Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, Cservenyàk T, Hany TF (2008) Evaluation of [(18)F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging 35:253–263
Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, Borsatti E, Drigo A, Trovò MG (2006) [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging 33:1387–1398
Rinnab L, Mottaghy FM, Blumstein NM, Reske SN, Hautmann RE, Hohl K, Möller P, Wiegel T, Kuefer R, Gschwend JE (2007) Evaluation of [11C]-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int 100:786–793
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, Franceschelli A, Martorana G, Manferrari F, Fanti S (2009) Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med 50:1394–1400
Kwee SA, Coel MN, Lim J (2012) Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med 26:501–507
Treglia G, Ceriani L, Sadeghi R, Giovacchini G, Giovanella L (2014) Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med 52:725–733
Sadeghi R, Treglia G (2013) Meta-analyses and systematic reviews on PET and PET/CT in oncology: the state of the art. Clin Transl Imaging 1:73–75
Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J, Loidl W, Pirich C, Fogelman I, Langsteger W (2009) The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol 11:446–454
Beheshti M, Langsteger W, Fogelman I (2009) Prostate cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med 39:396–407
Beheshti M, Langsteger W (2012) Choline PET/CT compared with bone scintigraphy in the detection of bone metastases in prostate cancer patients. Eur J Nucl Med Mol Imaging 39:910–911
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal studies
For this type of study formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Treglia, G., Vigneri, C., Sadeghi, R. et al. Discordance rate between radiolabelled choline PET/CT and bone scintigraphy in detecting bone metastases in patients with prostate cancer: a meta-analysis. Clin Transl Imaging 3, 133–140 (2015). https://doi.org/10.1007/s40336-015-0107-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-015-0107-1