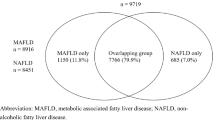
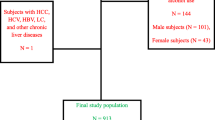
Abstract
Background and aim
Changing terminology of non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated fatty liver disease (MAFLD) is recently proposed by expert panels based on metabolic dysregulations. However, clinical evidences for the risk of cardiovascular disease (CVD) in MAFLD are limited. The aim of this study is evaluating the association of cardiovascular risk in these two terminology and subgroups of MAFLD.
Methods
A total of 2133 individuals who underwent ultrasound and cardiac computed tomography contemporaneously were included at a single medical checkup center. Ultrasound was used to define fatty liver, and coronary artery calcification (CAC) defined a coronary artery calcium score above 0 was used to estimate the cardiovascular risk.
Results
Overall, 911 participants were diagnosed with fatty liver. In the unadjusted analysis, NAFLD (OR = 1.4, 95% confidence interval [CI] = 1.05–1.85, p = 0.019) and MAFLD (OR = 1.55, 95% CI = 1.29–1.86, p = 0.046) were significantly associated with CAC. However, in sex and age-adjusted analyses, only MAFLD was associated with CAC (adjusted OR [aOR] = 1.38, 95% CI = 1.14–1.69, p = 0.001). Of the three subgroups of MAFLD (diabetic, nondiabetic overweight/obese, and nondiabetic normal weight/lean with at least two metabolic abnormalities), only diabetic MAFLD was associated with CAC (aOR = 2.65, 95% CI = 1.98–3.55, p < 0.001). When the minimal number of metabolic risk abnormalities increased to three, nondiabetic normal-weight/lean MAFLD was associated with CAC (aOR = 1.35, 95% CI = 1.02–1.77, p = 0.034).
Conclusion
Diabetic MAFLD predicted high-risk CVD phenotypes the best. Metabolic risk abnormalities in nondiabetic MAFLD patients were independently associated with the risk of CVD. The proposed diagnostic criteria for nondiabetic MAFLD need further investigation in terms of CVD risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD), characterized by ≥ 5% hepatic fat accumulation with no evidence of secondary causes of hepatic steatosis, manifests as simple steatosis, steatohepatitis, liver fibrosis, and cirrhosis [1]. Furthermore, it has been closely associated with metabolic dysfunction including type 2 diabetes mellitus (T2DM), dyslipidemia, and obesity, which can result in the development of liver fibrosis, cardiovascular disease (CVD), and increased liver-related and cardiovascular mortality [2,3,4].
In 2020, a panel of international experts proposed a new definition called metabolic dysfunction-associated fatty liver disease (MAFLD), which includes causes of chronic liver disease, such as viruses and excessive alcohol intake [5]. The definition of MAFLD is based on the presence of hepatic steatosis as a prerequisite and one of the following three features, including: (1) T2DM, (2) overweight or obesity, or (3) lean or normal weight with at least two metabolic risk abnormalities [5]. Based on the new nomenclature of MAFLD, comparative studies on clinical implication with NAFLD have been active recently [6]. In several studies, patients with MAFLD were more likely to have metabolic comorbidities, fibrosis progression, and incident cardiovascular disease risk analyzed using the traditional risk-scoring models [7,8,9,10].
Coronary artery calcium scoring with computed tomography (CT) is a noninvasive, reliable marker of coronary atherosclerotic burden [11]. The association between coronary artery calcification (CAC) and the risk of CVD has been well evaluated in the general population as well as in patients with NAFLD [12]. Additionally, the progression of coronary artery calcium scores was also associated with worsening coronary atherosclerosis, which predicts future CVD events, including myocardial infarction, ischemic stroke, and/or cardiovascular mortality, even in patients with NAFLD [13, 14]. However, little is known about the association between CAC and MAFLD.
This study aimed to investigate the association of the risk of CVD estimated by CAC with NAFLD and MAFLD. Furthermore, the association of CVD risk was investigated according to the three subgroups of MAFLD.
Patients and methods
Patients
This cross-sectional, retrospective study included individuals who underwent a medical checkup, including abdominal ultrasound and cardiac CT, from January 2017 to December 2021 at a health center in South Korea. The exclusion criterion was documented history of significant CVD, such as acute coronary syndrome, stable angina, history of angioplasty or stent placement, cerebrovascular disease, and peripheral vascular disease. However, no patient had significant CVD, because all relatively healthy individuals underwent medical checkups.
Data collection
We obtained medical records, including demographic variables, anthropometric measurements, laboratory findings, abdominal ultrasound, and coronary calcium scan at the time of the medical checkup. Variables, such as age, sex, comorbidities, history of alcohol, smoking, and medication use, were obtained based on self-reporting and direct interviews using standardized health questionnaires. Anthropometric assessments, including height, weight, waist circumference (WC), and blood pressure, were performed and recorded by trained nurses. Overweight and obesity were defined as a body mass index (BMI) ≥ 23 kg/m2 and ≥ 25 kg/m2 based on the Asia–Pacific region criteria, respectively [15]. Hypertension and T2DM were defined as current guideline [16, 17]. Metabolic syndrome was defined as the presence of three or more of the following factors by proposed criteria [18].
Blood biochemical tests and abdominal ultrasound were performed after overnight fasting. The patients’ liver profiles, including serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, and albumin levels; lipid profiles, including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), HDL-C, and TG levels; and platelet count, FPG, and HbA1c levels were measured.
The AST to platelet ratio index (APRI), fibrosis-4 (FIB-4) index, NAFLD fibrosis score (NFS), and noninvasive fibrosis score were calculated according to previous publications, and low cut-off values of APRI ≥ 0.5, FIB-4 ≥ 1.3, and NFS ≥ -1.455 were used for dichotomous analysis[19, 20].
Diagnosis of NAFLD and MAFLD
The fatty liver was evaluated by experienced radiologists using abdominal ultrasonography based on the standard criteria [21]. A diagnosis of NAFLD was made by the clinical practice guidelines of European Association for the Study of Liver Diseases and American Association for the Study of Liver Diseases for the Management of NAFLD [22, 23]. A diagnosis of MAFLD was based on the criteria proposed by an international expert panel [5]. The criteria include evidence of fatty liver on ultrasonography in addition to one of the following three criteria: overweight/obesity, presence of T2DM, or evidence of metabolic dysregulation. Metabolic dysregulation was defined as the presence of at least two metabolic risk abnormalities defined by an international expert panel (supplementary file 1) [5]. BMI and WC were determined using cut-off values for Asians, as all patients were Asian. Although the homeostasis model assessment of insulin resistance score ≥ 2.5 is one of the metabolic risk abnormalities, it was excluded in our study, because values of fasting insulin were unavailable.
Cardiac computed tomography for CAC
Non-contrast cardiac prospective electrocardiogram-triggered volumetric CT was performed using a 320-slice CT scanner (SOMATOM Force, Siemens Healthineers). At the end of inspiration, the patients held their breath as the scan ranged from the base of the heart to the carina; the field of view was 220 mm, whereas the scan collimation was 320 × 0.75 mm. As determined by the CARE Dose4D scanner software, a tube current ranging from 288 mA to 100–120 kVp was administered. The rotation time was 0.25 s. Using five filter revolutions, 3-mm-thick reconstruction slices were obtained. The Agatston scoring method, previously described by a fellowship-trained cardiac radiologist using independent post-processing software (Syngo.via, Siemens Healthineers), was used to quantify the coronary artery calcium scores. The presence of CAC was defined as a coronary artery calcium score > 0.
Statistical analysis
Data are presented as median with interquartile range (IQR) or number (%), as appropriate. No imputation was conducted for the missing data. Categorical variables were compared using the Chi-square test (or Fisher’s exact test), whereas the Student’s t test (or Mann–Whitney U test) after Shapiro–Wilk normality testing was used to compare continuous variables. Factors associated with CAC were identified using logistic regression analysis with stepwise backward elimination. Odds ratios (OR) and 95% confidence intervals (CI) were also calculated. A probability value of two-tailed p < 0.05 was considered statistically significant. Statistical analyses were performed using the R software (version 3.0, http://cran.r-project.org/,install.packages(“devtools’)). Logistic regression model-based plotting for the probability of CAC presence was generated using ggplot2.
Results
Baseline characteristics
A total of 2133 individuals who underwent cardiac CT for coronary artery calcium and ultrasonography were included in this study. All cardiac CT and ultrasound examinations were performed on the same day. The median age and body mass index were 58 years and 22.0 kg/m2, respectively. Overall, 911 (42.7%) individuals had fatty liver disease, and 794 (37.2%) had CAC with a median coronary artery calcium score of 46.4 [9.5–164.5]. The baseline characteristics of patients with and without fatty liver are shown in Table 1. Compared to individuals without fatty liver, those with fatty liver were predominantly male and more likely to have hypertension, T2DM, metabolic syndrome, and higher metabolic abnormality counts. Positivity of anti-HCV was not different with individuals with fatty liver and those without. The positivity of HBsAg is higher in individuals without fatty liver compared to those with it. However, ALT and FIB-4 is higher in those had HBsAg but not fatty liver compared to those had HBsAg and fatty liver (supplementary file 2).
Association of CAC with NAFLD and MAFLD
Prevalence of CAC in patients with NAFLD and MAFLD were 40.6% and 43.6%, respectively (Fig. 1a). The association between CAC and the type of fatty liver disease is shown in Table 2. In the unadjusted analysis, NAFLD (OR = 1.4, 95% CI = 1.05–1.85) and MAFLD (OR = 1.55, 95% CI = 1.29–1.86) were significantly associated with CAC. However, fatty liver without MAFLD was not associated with CAC. In sex- and age-adjusted analyses, only MAFLD was independently associated with CAC (adjusted OR [aOR] = 1.38, 95% CI = 1.14–1.69, p = 0.001).
Coronary artery calcification and coronary calcium score: a presence of coronary artery calcification according to presence of different fatty liver diseases, b presence of coronary artery calcification in patients with nondiabetic metabolic dysfunction-associated fatty liver disease, and c coronary artery calcium score in patients with nondiabetic metabolic dysfunction-associated fatty liver disease
Association of CAC with the metabolic subgroups of MAFLD
The baseline characteristics of MAFLD subgroups are shown in Table 3. Each subgroup had different clinical characteristics and values in the noninvasive fibrosis test. Of these, diabetic MAFLD had higher coronary artery calcium and noninvasive fibrosis test scores. Prevalence of CAC in patients with diabetic MAFLD was 57.0% (Fig. 1a). Though prevalence of CAC in patients with nondiabetic MAFLD was increasing up to three (Fig. 1b), mean coronary artery calcium score was constantly increasing according to number of metabolic risk abnormalities (Fig. 1c) in patients with nondiabetic MAFLD. In the unadjusted analysis, the subgroups of diabetic MAFLD and nondiabetic normal-weight/lean MAFLD with at least two and three metabolic risk abnormalities were significant factors associated with CAC, but nondiabetic overweight/obesity MAFLD was not (Table 2). In the sex- and age-adjusted analysis, the subgroup of diabetic MAFLD (aOR = 1.93, 95% CI = 1.42–2.64, p < 0.001) and nondiabetic normal-weight/lean MAFLD with at least three metabolic risk abnormalities were independently associated with CAC (aOR = 1.35, 95% CI = 1.02–1.77, p = 0.034), but those with at least two metabolic risk abnormalities were not (aOR = 1.18, 95% CI = 0.94–1.49, p = 0.156).
Association between CAC and number of metabolic risk abnormalities in individuals without T2DM
Sex- and age-adjusted analyses were performed to evaluate the association between CAC and the number of metabolic risk abnormalities among the control and nondiabetic MAFLD groups. In this analysis, the number of metabolic risk abnormalities was independently associated with CAC, irrespective of MAFLD (aOR = 1.23, 95% CI = 1.12–1.34, p < 0.001, Fig. 2).
Association between CAC and liver fibrosis in patients with MAFLD
To evaluate the association between CAC and liver fibrosis by FIB-4, multivariable-adjusted analysis, including values of the Framingham risk score, was performed in patients with MAFLD. In this analysis, liver fibrosis was independently associated with CAC (aOR = 2.08, 95% CI = 1.55–2.79, p < 0.001; Table 4). After further adjusted by positivity of HBsAg or anti-HCV, the association of CAC and liver fibrosis is not different (supplementary file 3).
Discussion
In this relatively healthy cohort, we demonstrated that MAFLD was independently associated with CAC using sex- and age-adjusted analyses. Among the three subgroups in the diagnostic criteria for MAFLD, only diabetic MAFLD was an independent predictor of CAC. However, when the minimal number of metabolic risk abnormalities increased to three, nondiabetic normal/lean MAFLD was associated with CAC, unlike the proposed diagnostic criteria of nondiabetic normal/lean MAFLD. Additionally, in nondiabetic MAFLD, the number of metabolic risk abnormalities was an independent predictor of CAC. In addition to these associations, liver fibrosis by FIB-4 was still an independent predictor of CAC using adjusted analysis of multiple cardiovascular risk factors.
These findings demonstrate that MAFLD is a better predictor of high-risk CVD phenotypes than NAFLD. However, most of these effects do not come from nondiabetic MAFLD but from diabetic MAFLD. We found that two metabolic risk abnormalities, which were defined as metabolic dysregulation in the proposed definition, were insufficient to determine the high-risk CVD phenotype in nondiabetic normal-weight/lean MAFLD, but instead three metabolic risk abnormalities were required. Thus, in terms of CVD risk, the number of metabolic risk abnormalities in normal-weight/lean nondiabetic MAFLD in the proposed diagnostic criteria may need to be further investigated. Furthermore, the addition of metabolic risk abnormalities in overweight/obese nondiabetic MAFLD may be considered. Consistent with a previous study on NAFLD, liver fibrosis remains an independent predictor of high-risk CVD phenotypes in MAFLD. As patients with diabetic MAFLD had higher scores on noninvasive fibrosis tests, liver fibrosis may independently increase the risk of CVD in diabetic MAFLD, which is consistent with a previous study on NAFLD.
Several studies have reported an association between NAFLD and CAC [14, 24, 25]. Although this association has been well evaluated in relatively large cohorts, it was not shown after adjusting for sex and age in the current study. A recent study reported that the association between NAFLD and CAC could be affected by sex and obesity [26]. This indicates that the impact of NAFLD on the risk of CAC could vary in cohorts with different BMIs. Unlike in other cohorts (mean or median BMI of 23–26 kg/m2), patients in our study cohort were relatively lean (median BMI was about 22 kg/m2) [14, 24,25,26]. Especially, among patients with nondiabetic MAFLD, only 1.5% of patients were diagnosed with nondiabetic overweight/obese MAFLD. Thus, we believe that these differences may have been due to the relatively lean cohort in this study.
Some recent studies have reported that in terms of prediction of the risk of CVD, the definition of MAFLD is better than that of NAFLD [10, 27]. In these studies, patients with MAFLD were more likely to have higher CVD risk as assessed by the Korean 10-year atherosclerotic cardiovascular disease (ASCVD) risk score, Siuta score, or Framingham risk score (FRS). In addition, the incidence of CVD events, including myocardial infarction, ischemic stroke, heart failure, and CVD-related death assessed by reimbursement claim data, was higher in MAFLD than in NAFLD [10]. However, no study has evaluated the clinical characteristics of relevant subgroups. Thus, to overcome the heterogeneity of the disease, further studies are required to precisely define subgroups of MAFLD. A large-scale cohort study demonstrated that regardless of BMI, metabolically unhealthy individuals had higher CVD risk than did healthy individuals [28]. However, regardless of metabolic health, overweight/obese individuals had higher CVD risk than did lean individuals, and the hazard ratio of metabolic factors was much greater than that of related factor [28]. Furthermore, the association between metabolic dysregulation and severity of NAFLD has also been demonstrated in a biopsy-proven NAFLD cohort study [29]. In this previous study, the number of metabolic risk factors, including impaired fasting glucose or T2DM, hypertension (HTN), hypertriglyceridemia, and low HDL-C, were also associated with the NAFLD activity score and fibrosis stage [29]. A recent large-scale retrospective cohort study demonstrated that at least two metabolic traits among obesity, dyslipidemia, HTN, and T2DM were associated with cirrhosis or HCC in patients with NAFLD [30]. However, the definition of metabolic health is heterogeneous and is not the same as factors in metabolic syndrome or traditional CVD risk assessments, such as FRS or ACC/AHA ASCVD risk estimator in various studies. In our study, patients with nondiabetic MAFLD had a relatively lower cardiovascular risk than those with diabetic MAFLD. According to a recently proposed definition of nondiabetic MAFLD, only normal-weight/lean MAFLD includes criteria for metabolic risk dysregulation, and overweight/obese MAFLD also have a relatively lower cardiovascular risk. Thus, we believe that additional metabolic criteria to define nondiabetic overweight/obese MAFLD are needed to determine the high-risk CVD phenotype.
The association between liver fibrosis and the risk of CVD in NAFLD has been reported in multiple studies [31,32,33,34,35,36]. The association between CAC and the risk of CVD has also been reported in many cohort studies; therefore, it can be an alternative option to assess the risk of CVD when traditional risk-scoring models, such as the FRS, are unclear [12, 37]. Additionally, because CAC is a highly specific feature of subclinical CVD, these associations are shown in early stage (stage ≥ 2) than in advanced fibrosis (stage ≥ 3) in patients with NAFLD regardless of their FRS [36]. In the current study, CAC was still an independent risk factor after adjusting for multiple risk factors, which were included in the FRS, and was consistent with the findings of a previous study. Recently, several studies have reported that changing NAFLD to MAFLD identifies more patients with significant liver fibrosis [7,8,9, 21]. In these previous studies, non-overlapping MAFLD had higher FIB-4 levels than non-overlapping NAFLD. Furthermore, one study reported higher CVD-related mortality in non-overlapping MAFLD than in non-overlapping NAFLD [8]. Thus, we think that liver fibrosis is an important risk factor for predicting CAC in MAFLD, as this study showed.
The strength of this study is that the risk of CVD and diagnosis of fatty liver were not assessed by a score-based test, such as the FRS and fatty liver index, but by imaging modalities, such as ultrasound and cardiac CT [10, 27]. Furthermore, although this was a single-center retrospective study, a well-organized cohort with no missing value was used to analyze the data. In this cohort, all values in the definition of MAFLD were included except fasting insulin levels and current medications, including antihypertensive, antidiabetic, and lipid-lowering agents. The present definition of nondiabetic lean/normal-weight MAFLD is relatively complicated and includes nonclinical friendly variables, such as fasting insulin and hsCRP; therefore, most previous studies did not include these metabolic risk abnormalities [10, 27].
This study has some limitations. First, almost all participants in this study were Korean. Thus, further studies are required to delineate our results in a multi-ethnic, multi-racial cohort. Second, the study population was relatively healthy and had normal weight or were lean, because this study was conducted using data from a health checkup center. Therefore, further studies are required in the general population.
In conclusion, changing from NAFLD to MAFLD could better predict the high-risk CVD phenotype. Of the three subgroups of MAFLD, diabetic MAFLD best predicted an increased risk of CVD. The number of metabolic risk abnormalities in patients with nondiabetic MAFLD was independently associated with the risk of CVD. The proposed diagnostic criteria for nondiabetic MAFLD may need to be further investigated in terms of CVD risk.
Data availability
The data supporting findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- APRI:
-
AST to platelet ratio index
- AST:
-
Serum aspartate aminotransferase
- CAC:
-
Coronary artery calcification
- CVD:
-
Cardiovascular disease
- FIB-4:
-
Fibrosis-4
- FPG:
-
Fasting plasma glucose
- HDL-C:
-
High-density lipoprotein cholesterol
- hsCRP:
-
High-sensitivity C reactive protein
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- NAFLD:
-
Non-alcoholic fatty liver disease
- NFS:
-
NAFLD fibrosis score
- T2DM:
-
Type 2 diabetes mellitus
- WC:
-
Waist circumference
References
Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27:363–401
Muthiah MD, Sanyal AJ. Burden of disease due to nonalcoholic fatty liver disease. Gastroenterol Clin North Am. 2020;49:1–23
Toh JZK, Pan XH, Tay PWL, Ng CH, Yong JN, Xiao J, et al. A meta-analysis on the global prevalence, risk factors and screening of coronary heart disease in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;20:2462–2473
Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209
Kang SH, Cho Y, Jeong SW, Kim SU, Lee JW, Korean NSG. From nonalcoholic fatty liver disease to metabolic-associated fatty liver disease: big wave or ripple? Clin Mol Hepatol. 2021;27:257–269
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21(619–629): e7
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(2172–2181): e2176
Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41:1290–1293
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19(2138–2147): e2110
Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging. Council Clin Cardiol Circ. 2006;114:1761–1791
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447
McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–1622
Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66:323–329
Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566
Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 Suppl 1:S62–69. https://doi.org/10.2337/dc10-S062.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–2752
Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736
Tamaki N, Kurosaki M, Huang DQ, Loomba R. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol Res. 2022;52(6):497–507. https://doi.org/10.1111/hepr.13764
Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40:3018–3030
EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–357
Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613
Chang Y, Ryu S, Sung KC, Cho YK, Sung E, Kim HN, et al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut. 2019;68:1667–1675
Kim SH, Park HY, Lee HS, Jung KS, Lee MH, Jhee JH, et al. Association between non-alcoholic fatty liver disease and coronary calcification depending on sex and obesity. Sci Rep. 2020;10:1025
Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res. 2021;51:1115–1128
Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J. 2018;39:397–406
Ampuero J, Aller R, Gallego-Duran R, Banales JM, Crespo J, Garcia-Monzon C, et al. The effects of metabolic status on non-alcoholic fatty liver disease-related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48:1260–1270
Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71:808–819
Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554
Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51:728–736
Moreno-Del Castillo MC, Sanchez-Rodriguez A, Hernandez-Buen Abad JJ, Aguirre-Valadez J, Ruiz I, Garcia-Buen Abad R, et al. Importance of evaluating cardiovascular risk and hepatic fibrosis in patients with newly diagnosed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:997–999
Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020;18(2324–2331): e2324
Niikura T, Imajo K, Ozaki A, Kobayashi T, Iwaki M, Honda Y, et al. Coronary artery disease is more severe in patients with non-alcoholic steatohepatitis than fatty liver. Diagnostics (Basel). 2020;10:129
Park JG, Jung J, Verma KK, Kang MK, Madamba E, Lopez S, et al. Liver stiffness by magnetic resonance elastography is associated with increased risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;53:1030–1037
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959
Funding
JGP receives funding supported by the 2022 Yeungnam University Research Grant. RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019).
Author information
Authors and Affiliations
Contributions
JGP and SYP is guarantor of integrity of the entire study. JGP designed the study and partially supervised by RL. All authors except RL collected data, which were reviewed by GJP, and analyzed by GJP based on the statistical analysis plan. GJP, MKG, and YLR drafted the manuscript, which was critically revised by SYP. All authors were responsible for collecting and interpretation of the data and approved the final version of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
RL serves as a consultant for Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharm, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer, and Sonic Incytes. He is also co-founder of Liponexus, Inc.
Ethical approval
This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki as revised in 2013. The study protocol was approved by the institutional review board of the study center (IRB No. KNUH-2021-07-057-001). The requirement for informed consent from the study participants was waived by the ethics committee because of the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, M.K., Lee, Y.R., Jang, S.Y. et al. Impact of metabolic factors on risk of cardiovascular disease in nondiabetic metabolic dysfunction-associated fatty liver disease. Hepatol Int 17, 626–635 (2023). https://doi.org/10.1007/s12072-023-10517-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10517-w