Abstract
Chimeric antigen receptor T-cell therapies have transformed the management of hematologic malignancies but have not yet demonstrated consistent efficacy in solid tumors. Glioblastoma is the most common primary malignant brain tumor in adults and remains a major unmet medical need. Attempts at harnessing the potential of chimeric antigen receptor T-cell therapy for glioblastoma have resulted in glimpses of promise but have been met with substantial challenges. In this focused review, we discuss current and future strategies being developed to optimize chimeric antigen receptor T cells for efficacy in patients with glioblastoma, including the identification and characterization of new target antigens, reversal of T-cell dysfunction with novel chimeric antigen receptor constructs, regulatable platforms, and gene knockout strategies, and the use of combination therapies to overcome the immune-hostile microenvironment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key challenges for chimeric antigen receptor T-cell therapy in glioblastoma include tumor heterogeneity, intrinsic and iatrogenic T-cell dysfunction, and severe immunosuppression mediated through both the local tumor microenvironment and systemically. |
Novel strategies for optimizing chimeric antigen receptor T-cell therapy for glioblastoma include the identification and characterization of new target antigens, reversal of T-cell dysfunction with novel chimeric antigen receptor constructs, regulatable platforms, and gene knockout strategies, and the use of combination therapies to overcome the immune-hostile microenvironment. |
1 Introduction
Chimeric antigen receptor (CAR) T cells are patient-derived lymphocytes transfected with a gene encoding a chimeric transmembrane receptor that incorporates an extracellular antigen-recognition domain, a transmembrane and hinge domain to anchor the receptor on the cell surface and project the antigen-targeting moiety out to the extracellular space, and an intracellular T-cell signaling domain [1, 2]. The extracellular domain enables recognition of target cell-surface antigens with high specificity in a non-major histocompatibility complex restricted manner [3]. This is accomplished through the inclusion of a single-chain variable fragment of a tumor antigen-specific antibody that contains the VH and VL chains joined by a peptide linker of approximately 15 amino acids [4]. Upon antigen engagement, two discrete signaling events are mediated by the cell surface receptors [5]. The primary “activation” signal is produced by ligation of the T-cell receptor with a major histocompatibility complex-peptide complex. The second “co-stimulatory” signal is generated by ligation of a co-stimulatory molecule on the T-cell surface with its cognate ligand on the surface of an antigen-presenting cell. Several T-cell co-stimulatory molecules have been identified, including members of the immunoglobulin superfamily (CD28) and members of the tumor necrosis factor superfamily (e.g., CD40L, CD134 [OX-40], and CD137 [4-1BB]) [6]. Once these two signaling events have occurred, the CAR’s intracellular signaling domain is triggered, promoting numerous T-cell effector functions. Overall, the unique construct of a CAR enables the combination of the cytotoxic functions of T lymphocytes with the ability to recognize predefined surface membrane tumor antigens in a manner that is both highly specific and independent of antigen processing or human leukocyte antigen expression.
The clinical efficacy of CAR T cells (CAR-T) targeting CD19 and BCMA has been firmly established in B-cell and plasma cell malignancies, respectively. As of July 2023, six different CAR-T products have been approved by the US Food and Drug Administration for use in patients with numerous hematologic cancers based on reports demonstrating the potential for single doses of CAR-T to induce deep and durable responses in patients with otherwise treatment-refractory disease [7,8,9,10,11,12,13,14,15,16]. Despite the impressive efficacy data and routine clinical implementation of CAR-T therapies for hematologic cancers over the past 5 years, progress for patients with solid tumors has been slower and more challenging [17]. No regulatory approvals have been obtained for CAR-T in any non-hematologic cancer. Barriers to effective CAR-T therapy for solid tumors have been reviewed extensively elsewhere [17, 18], with key challenges including the identification of optimal target antigens, heterogeneity and tumor antigen escape, impaired T-cell trafficking solid tumors, the highly immunosuppressive tumor microenvironment, and balancing the anti-tumor effects of CAR-T with their unique toxicities. Although these issues and others have thus far impeded progress in solid tumors relative to hematologic malignancies, early signals of efficacy for CAR-T against solid tumors have recently been detected in several small studies, particularly in pediatric oncology. For example, objective responses and associated clinical benefit have been reported for CAR-T targeting the disialoganglioside GD2 in children with both relapsed/refractory high-risk neuroblastoma [19] as well as recurrent H3K27M-mutated diffuse midline glioma [20]. In addition, B7-H3 targeted CAR-T have shown clinical activity in a child with diffuse intrinsic pontine glioma [21]. These studies have galvanized the cellular therapy field, providing proof of principle that CAR-T have the potential for treating some of the most challenging solid tumors.
One such cancer is glioblastoma (GBM), the most common primary brain cancer in adults and an incurable and aggressive malignancy carrying a median overall survival typically less than 2 years [22]. Current standard of care treatment for GBM includes maximal safe surgical resection followed by adjuvant radiotherapy and temozolomide chemotherapy, with or without tumor-treating fields [23]. Because of its diffusely infiltrative nature and a significant treatment-refractory tumor stem cell population, GBM invariably recurs following this regimen. Over the past two decades, bevacizumab has been the only systemic therapy approved by the Food and Drug Administration for recurrent GBM [24], and neither bevacizumab nor any other treatment has ever been shown to improve overall survival versus best supportive care in this setting. Accordingly, there is no widely accepted standard of care for recurrent glioblastoma, and novel effective treatments are desperately needed.
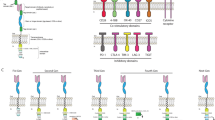
In this review, we summarize the state of the field regarding the development of novel strategies for optimizing CAR-T therapy for GBM, including the identification and characterization of new target antigens, reversal of T-cell dysfunction, and the use of combination therapies to address the immunosuppressive microenvironment (Fig. 1). We do not provide a comprehensive summary of previously conducted CAR-T trials in GBM, as these have been reviewed recently by our group [17, 25] and others [26, 27].
Potential methods for optimization of chimeric antigen receptor (CAR) T cells for glioblastoma. (1) Novel targeting monovalent CAR; (2) bispecific T-cell engager (BiTE); (3) tandem bivalent CARs; (4) parallel bivalent CARs; (5) oncovirus-delivered gene therapy (OV); (6) immune checkpoint inhibition (ICI); (7) CRISPR; and (8) combination with radiotherapy. IFNy interferon-γ, PD1 programmed cell death protein-1
2 New Target Antigens
2.1 Overview
Completed CAR-T trials have been conducted for five different therapeutic targets (epidermal growth factor receptor variant III [EGFRvIII], erythropoietin-producing hepatocellular carcinoma A2 [EphA2], GD2, human epidermal growth factor receptor 2 [HER2], and interleukin [IL]-13Rα2; Table 1) [28,29,30,31,32,33,34,35,36]. In addition, four additional targets are the subject of presently ongoing trials: B7-H3, CD147, chlorotoxin, and IL-7Rα (Table 2). Moreover, additional candidate targets have been assessed with some evidence of efficacy in the preclinical setting. Here, we briefly summarize the five targets that have been evaluated in previously reported clinical trials and, subsequently, dedicate individual sections to the four novel targets against which CAR-T are currently being evaluated in patients with GBM.
Among previously studied targets, EGFRvIII is a tumor-specific EGFR splice variant found in 30% of newly diagnosed GBM cases and is the second most common EGFR alteration frequency behind wild-type EGFR amplification [37,38,39]. This variant results in a constitutively active receptor resistant to EGFR inhibitors and is a negative prognostic marker [37,38,39]. It has attracted interest as an antigen target for immunotherapy owing to both its extracellular location and the presence of a novel glycine residue to abnormal splicing [37,38,39]. EGFRvIII has been the target for three clinical trials, including two in the setting of recurrent GBM and one for primary GBM [28,29,30].
The EphA2 receptor has been shown to mediate tumorigenic functions including cellular motility, invasion, and angiogenesis, and has accordingly been associated with poorer outcomes for GBM [40,41,42]. It is overexpressed in GBM but frequently not expressed in normal brain tissue, making it a viable target for immunotherapy [31]. EphA2 has been the subject of one clinical trial involving peripheral CAR infusion in three patients [31].
The disialoganglioside GD2 is a plasma membrane component that has been demonstrated to be overexpressed in GBM, while comprising just 1–2% of total gangliosides in the central nervous system [43,44,45]. It has been the subject of one trial involving peripheral infusion in five patients and a combined peripheral and post-resection intracavitary infusion in three patients [32].
HER2 is a tumor-associated antigen expressed in up to 80% of GBM cases, correlating with a degree of anaplasia in glial tumors, but not by normal brain tissue [33, 46,47,48]. HER2 signaling has been shown to mediate cell proliferation and inhibition of apoptosis [33, 49]. It has been targeted in one prior clinical trial, which utilized peripheral infusion in 17 patients [33, 49].
Finally, IL-13Rα2 is an IL-13 signaling receptor found to be expressed in several different human tumors, including approximately 82% of GBM, but is not expressed in any normal tissue, except for the adult testes [50,51,52]. IL-13 signaling through this receptor has been documented to mediate tumor migration and invasion [50,51,52]. IL-13Rα2 has been the target in three prior clinical trials involving a post-resection intracavitary or intratumoral infusion [34,35,36].
2.2 B7-H3
B7-H3 is a transmembrane protein with putative co-stimulatory and co-inhibitory functions on different T-cell subsets, which has also served as an antitumor target in several solid tumor preclinical models [53]. B7-H3 expression, found on immunohistochemistry in 50–64% of GBM samples without expression in adjacent normal cerebral tissue, has been documented to be associated with a higher tumor grade and poorer survival among patients with GBM, and survival was extended following treatment with anti-B7-H3 CAR-T across several orthotopic mouse models [54]. Expression of B7-H3 has additionally been documented in pediatric central nervous system malignancies, including medulloblastoma and diffuse intrinsic pontine glioma [55]. In vivo studies in murine models determined that an anti-B7-H3 CAR construct induced complete regression of GBM for an average of 2 months, with recurrence posited by the authors to be due to target antigen heterogeneity [54]. Accordingly, B7-H3 is presently the target for five ongoing clinical trials for recurrent GBM, all utilizing intratumoral, postresection intracavitary, and/or intraventricular delivery. Two trials are additionally assessing the safety of administering B7-H3-directed CAR-T between temozolomide cycles (Table 2).
2.3 CD147
CD147, also known as basigin or extracellular matrix metalloproteinase inducer (EMMPRIN), is a glycoprotein in the immunoglobulin superfamily, found to be expressed on the surface of tumor cells or released through microvesicles. In one analysis of 206 GBM cases, strong positive staining for CD147 was observed in 23.8%. It is implicated in tumor invasion and metastasis by promotion of astrocyte-mediated matrix metalloproteinase activity [56, 57], with CD147 knockout shown to decrease the secretion of active matrix metalloproteinase 9 and tumor invasion [58, 59]. Expression of CD147 has been shown to prognosticate reduced overall survival for patients with GBM [60]. While CD147 is expressed at low levels of normal epithelial tissue, earlier in vivo studies in the setting of hepatocellular carcinoma have suggested that off-target toxicity on normal tissue is minimal [61]. One clinical trial is presently evaluating the safety and tolerability of anti-CD147 CAR-T, with up to three infusions administered intracranially (Table 2).
2.4 Chlorotoxin
Chlorotoxin is a 36-amino acid peptide with extensive GBM-binding properties but minimal cross-reactivity with non-malignant cells in the central nervous system or body, which was studied initially in conjugation with the I132 radioisotope [62, 63]. While chlorotoxin does not have any intrinsic cytotoxic properties, including to normal tissue, studies have suggested that chlorotoxin binding may impair GBM migration [64]. Chlorotoxin has been studied across a range of clinical applications, including intraoperative visualization of GBM and as a medium to traffic delivery of chemoradiotherapy and other cytotoxic agents [65,66,67]. This target has additionally gained interest because of potentially addressing limitations in therapeutic efficacy due to tumor heterogeneity, as chlorotoxin-targeting CAR-T can mediate antitumor activity even in GBM cell populations lacking other characteristics of tumor antigens, such as EGFRvIII, IL-13Rα2, or HER2 [68]. For example, in one analysis of 15 patients, 80% of freshly dissociated GBM cells exhibited chlorotoxin binding [68]. A CAR construct utilizing chlorotoxin as the tumor-targeting domain has been demonstrated to induce tumor regression in orthotopic mouse models without off-target effector activity, although binding activity required the surface expression of metalloproteinase 2 [68]. Two trials are presently studying the use of chlorotoxin-bearing CAR-T, infused via a post-resection intracavitary and/or intraventricular approach (Table 2).
2.5 Targets Under Preclinical Study
Integrin receptors have emerged as an area of preclinical study owing to their mediation of cell migration, tissue invasion, and angiogenesis [69]. Integrin alphav beta3 is expressed on GBM tumor cells and associated vasculature in a manner correlated with tumor grade [70]. Following treatment with a second-generation construct against alphav beta3, an orthotopic mouse model exhibited GBM regression as well as increased progression-free survival and overall survival [71]. Another receptor implicated in tumor proliferation and angiogenesis is fibroblast growth factor-inducible 14, which is similarly associated with a higher tumor grade and poorer prognosis [72]. A second-generation anti-fibroblast growth factor-inducible 14 construct was demonstrated to induce GBM regression in an orthotopic mouse model, with recurrence reduced further by concomitant use of bispecific T-cell engagers (BiTEs) or IL-15 to promote adoptive transfer of central memory T cells [73]. Finally, CAR-T therapy directed against P32, a receptor most commonly localized to the mitochondrial matrix but also documented to be expressed on the surface of GBM cells, has been shown to extend survival and exert anti-angiogenic activity in orthotopic mouse models [74].
Markers for cancer stem cells in GBM such as CD70 and CD133, which have been documented to correlate with poorer clinical outcomes, constitute another group of therapeutic targets of interest [75]. CD70, a member of the tumor necrosis factor superfamily, may be constitutively expressed in GBM and has been shown to mediate tumor progression and immune escape, including recruitment of immunosuppressive T regulatory (Treg) cells and induction of T-cell exhaustion [76, 77]. In an orthotopic mouse model of recurrent GBM, anti-CD70 CAR-T was demonstrated to extend survival [78]. CD133 has been demonstrated to be a marker of cancer stem cell resistance to chemotherapy and radiotherapy as well as a prognosticator of earlier GBM recurrence [79, 80]. In an orthotopic mouse model with post-natal engraftment of the human hematopoietic system via an injection of human fetal CD34+ cord blood, anti-CD133 CAR-T prolonged survival without off-target effects on normal CD133+ hematopoietic stem cells [81]. An additional target of study is natural killer group 2 member D ligand, which is overexpressed across several solid tumors, including glioma cancer stem cells [82]. Treatment with anti-natural killer group 2 member D ligand CAR-T in a subcutaneous xenograft mouse model resulted in tumor regression, with no evidence of cytotoxicity [83].
Finally, other preclinical studies have attempted to target markers enriched in the GBM tumor microenvironment. For example, carbonic anhydrase IX has been documented to play an important homeostatic role for GBM cells in maintaining intracellular pH, given increased tumor glycolytic activity and the hypoxic tumor microenvironment [84]. A third-generation CAR-T directed against carbonic anhydrase IX has been shown to prolong survival in an orthotopic mouse model, with additional benefit conferred by pharmacologic induction of hypoxia via anti-angiogenic agents, such as bevacizumab [85].
In summary, prior clinical trials of CAR-T therapy for adults with GBM have primarily targeted EGFRvIII, IL-13Rα2, and HER2. Taken together, the data from these trials have suggested that CAR-T targeting these antigens can successfully traffic to the tumor and reduce target antigen expression, and both tumor regression [34] and prolonged survival [86] have been reported in rare individual cases. However, these therapies have not demonstrated clinical efficacy in the vast majority of cases. While the choice of target antigen(s) may be part of the problem, there are also opportunities to improve T-cell function and address the immunosuppressive GBM microenvironment, both of which we cover in the following sections.
3 Modulating Native T-Cell and CAR-T Function
3.1 Novel CAR-T Constructs
Chimeric antigen receptor targeting of multiple antigens has been evaluated as a potential method to overcome antigen loss and heterogeneity as barriers to CAR-T efficacy. Whereas tandem (or bivalent) CAR constructs incorporate two separate antigen-binding sites on the same extracellular domain, bicistronic CAR products utilize two distinct antigen-binding sites on two separate extracellular motifs. In two murine GBM studies assessing tandem CAR-T, one evaluating dual HER2 and IL-13Rα2 targeting and the other studying dual IL-13Rα2 and EphA2 targeting, tandem therapy achieved greater glioma regression, relative to single antigen targeting [87, 88]. For bicistronic CAR-T therapy, an ongoing trial is evaluating the efficacy of a single peripheral infusion of a bicistronic anti-EGFR and anti-IL-13Rα2 CAR-T construct for recurrent GBM (NCT05168423).
Moreover, BiTEs have emerged as a strategy for overcoming antigen loss and heterogeneity as well as off-tumor activity. A BiTE comprises two tandem single-chain variable fragments, with one capable of binding to the T-cell complex, such as the CD3 subunit, in order to increase linkage and engagement between T cells and targeted tumor cells [89]. For GBM, BiTE-secreting bivalent CAR-T targeting EGFRvIII and IL-13Rα2 was found to have a superior tumor response to counterparts without BiTE secretion [90]. Additionally, in another study assessing a BiTE directed against EGFR, anti-EGFRvIII CAR-T with BiTE secretion notably induced recruitment of normal bystander T cells against EGFR-bearing GBM cells [91]. An ongoing trial is evaluating intracranial administration EGFRvIII-directed CAR-T in tandem with a BiTE targeting wild-type EGFR (NCT05660369).
Another CAR-T strategy used to address the barriers of off-tumor cytotoxic activity due to poor specificity of antigen targeting as well as antigen heterogeneity is the synNotch receptor system, which requires recognition of both a “priming” antigen that is tumor or organ specific and a “killing” antigen that represents the actual therapeutic target. In one analysis of a tandem CAR construct targeting EphA2 and IL-13Rα2, two antigens expressed on a wide range of normal tissue, a synNotch construct priming with either EGFRvIII (a tumor-specific antigen) or myelin oligodendrocyte glycoprotein (a tissue-specific antigen) improved antitumor efficacy without evidence of off-tumor activity [92]. This system has additionally been shown to confer the advantage of avoiding T-cell exhaustion resultant from tonic signaling and activity [92].
Finally, other CAR-T constructs have focused on inducing transgenic expression to enhance immune function. For example, constructs co-expressing proinflammatory cytokines IL-12, interferon (IFN)-α2, or IL-15 have been demonstrated to achieve improved anti-glioma activity relative to the analogous constructs alone [73, 93, 94]. A similar rationale was applied towards another study generating CAR-T with secretion of Clostridium perfringens neuraminidase, a compound with known cytotoxic and mitogenic effects. To modulate T-cell chemotaxis, another study assessing tandem co-expression of an IL-8 receptor (CXCR1 or CXCR2) with anti-CD70 CAR determined that modification conferred improved intratumoral CAR-T migration and persistence [95]. Finally, CAR modifications have also been evaluated to ameliorate mechanisms of immunosuppression, such as an anti-EGFRvIII construct incorporating a TGF-beta type II receptor (TGFRII) ectodomain as a TGF-β “trap” to increase TGF-β resistance, which was shown to extend survival in murine GBM models [96]. Other dominant-negative CAR constructs have focused on directly targeting mediators of T-cell dysfunction or exhaustion, such as programmed cell death protein-1 (PD-1) or TGF-β, although such constructs have yet to be assessed in the setting of GBM [97, 98].
3.2 Modulating Extrinsic Causes of T-Cell Dysfunction
Identifying and reducing drivers of T-cell dysfunction in patients with GBM is important given the autologous nature of currently available CAR-T therapies. Accordingly, earlier studies for CAR-T therapy in the setting of hematologic malignancies have hypothesized that differences in therapeutic success may be attributed to baseline interpatient variation in immune system deficiencies and intrinsic T-cell characteristics, such as the elevated frequency of specific T-cell populations that may be associated with a higher likelihood of response [99, 100]. In GBM, lymphopenia has been documented even in treatment-naïve patients, in large part due to bone marrow sequestration [101]. Moreover, through both local and systemic mechanisms, GBM has been found to elicit distinct modes of T-cell dysfunction, including senescence, tolerance, anergy, and exhaustion [102, 103].
The glioma tumor microenvironment (TME) promotes the recruitment of immunosuppressive Treg cells through upregulation of cytokines promoting Treg cell persistence, including indoleamine 2,3-dioxygenase, and TGF-β [104]. Accordingly, higher proportions of circulating Treg cells have been documented in patients with glioma relative to healthy controls [105]. In addition, local infiltration of Treg cells is present within the tumor mass, with depletion being associated with improved survival in murine models of GBM [105, 106]. Accordingly, prior research has focused on modulating Treg cell activity to improve the efficacy of immunotherapy for GBM, such as co-administration of CAR-T with intratumoral IL-12, which has been found to decrease Treg cell numbers and improve CAR-T cytotoxicity [107, 108]. Another immune cell subpopulation implicated in GBM-mediated T-cell immunosuppression is myeloid-derived suppressor cells, a heterogenous subset of immature myeloid cells upregulated across several malignancies [109, 110]. Reduced IFNγ production by T cells obtained from patients with GBM and restoration of production with removal of the myeloid-derived suppressor cell population have been previously documented [110]. In-human inhibition of myeloid-derived suppressor cells for GBM with the antimetabolite capecitabine has been trialed and shown to increase cytotoxic infiltration into the TME, although this strategy has yet to be tested in tandem with CAR-T therapy [111].
Moreover, the glioma TME is also characterized by an unfavorable metabolic landscape that impairs T-cell function, including hypoxia [112,113,114], low glucose availability [25, 115], and low levels of amino acids needed for effector T-cell functions [116, 117], which promotes T-cell exhaustion and apoptosis. Accordingly, prior research in the preclinical setting has demonstrated evidence of improved immune activity through modulation of these factors, such as inhibition of hypoxia-inducible factor-1α [118]. Indoleamine 2,3-dioxygenase, an enzyme that increases amino acid unavailability by catalyzing tryptophan metabolism, was found to be upregulated in the GBM TME following anti-EGFRvIII CAR-T therapy, suggesting another possible target to improve CAR-T function and efficacy [29, 117].
3.3 Modulating Intrinsic Causes of Dysfunction
Recent research has focused on elucidating genetic and epigenetic dependencies of CAR-T effector function. In the setting of hematologic malignancies, epigenetic disruption of known drivers of CAR-T dysfunction, such as TET2 or DNMT3A, have been documented to improve therapeutic efficacy [119, 120]. For GBM, potential targets have been identified via approaches such as genome-wide CRISPR knockout screen. In one study querying regulators of CAR-T cytotoxic activity, Transducin Like Enhancer of Split 4 and Ikaros Family Zinc Finger Protein 2 were identified as targets of interest, with CRISPR-mediated knockout conferring increased expansion, killing potency, and resistance to exhaustion in vitro [121]. Knockout of Ikaros Zinc Finger Transcription Factor 3, another known modulator of cytokine signaling, via a single guide RNA pair targeting the IZFT3 gene locus in CD133-directed CAR-T was also associated with potentiated cytotoxicity and cytokine release in vitro [122]. In the setting of EGFR-directed CAR-T, inhibition of the epigenetic regulator BRD4 inhibition improved survival in a murine GBM model following CAR-T [123]. Another strategy for genetic knockout in CAR-T is the use of zinc finger nucleases, which were used to disrupt expression of the glucocorticoid receptor in one series of six patients with unresectable GBM requiring maintenance on systemic dexamethasone, which conferred steroid-resistant CAR-T activity [36].
Reciprocal screening has also been conducted on GBM cells, including GSCs, to identify dependencies for tumor susceptibility to CAR activity. Prior research has elucidated that one potential explanation for the relatively limited efficacy of CAR-T for solid tumors, relative to hematologic malignancies, is a dependency on IFNγ receptor signaling activity for CAR-T-mediated killing, with knockout in GBM cells resulting in downregulation of CAR-T adhesion [124]. Conversely, CRISPR screening of GSCs has additionally identified targets whose deletion was associated with increased susceptibility to CAR activity, such as V-Rel Reticuloendotheliosis Viral Oncogene Homolog A and Nuclear Protein Localization Protein 4 Homolog [121].
Finally, other studies have attempted to reduce CAR-T dysfunction by targeting mediators of checkpoint inhibition, such as PD-1. In two studies using CRISPR-mediated approaches to disrupt PD-1 expression, with one adopting a triple an approach that also inhibited endogenous T-cell receptor (TRAC) and beta-2 microglobulin expression, PD-1 inhibition was associated with improved antitumor activity in vitro [125, 126]. Nevertheless, a prior analysis of data for the first in-human trial data of anti-EGFRvIII CAR-T for recurrent GBM has determined that higher PD-1 expression in the CAR infusion product was associated with increased peripheral engraftment and progression-free survival [127]. Given an expanding body of research documenting evidence that PD-1 may mediate physiological functions beyond exhaustion, such as memory and specific stages of T-cell activation, further research on the impact of PD-1 inhibition on CAR-T therapy for GBM, especially with in-human use, is warranted [128,129,130,131]. Another strategy for subverting checkpoint inhibition has been the utilization of chimeric switch receptors, which involve constructs pairing an extracellular domain that recognizes a normally inhibitory stimulus, such as PD-1 or cytotoxic T-lymphocyte-associated protein 4, with an intracellular co-stimulation domain to convert inhibitory signals into stimulatory activity [132, 133]. In the setting of GBM, one in-human trial utilizing a chimeric switch receptor responsive to PD-1 was documented to increase levels of proinflammatory cytokines and T-cell levels in the cerebrospinal fluid [134].
4 Combination Therapies
4.1 Overview
Significant interest exists in combining CAR-T therapies with other approaches, both approved and experimental, in order to both maximize CAR-T activity and provide disease control. Several of these combinations have reached clinical trials, with varying efficacy, while many more are at the investigational stage.
4.2 Clinical Stage
Three clinical trials, one completed and two currently enrolling, have employed combination therapy incorporating CAR-T designed to synergize directly with CAR-T functionality. The first combination was an EGFRvIII-targeting CAR combined with pembrolizumab, blocking PD-1 signaling (NCT03726515). This approach was based on preclinical demonstration of CAR-T combined with immune checkpoint-targeting antibodies against PD-1, cytotoxic T-lymphocyte-associated protein 4, or TIM3 [52]. This finding led to the first in-human solid tumor concurrent combination trial of a CAR and immune checkpoint blockade. Preliminary results from the trial did not demonstrate the same synergistic or additive effect in patients that was seen in the laboratory [30]. The second clinical trial (NCT04003649), opened in 2019, combines an IL-13Rα2-targeting zetakine CAR with either nivolumab or both nivolumab and ipilimumab. This combination approach blocks binding of both PD-1 (nivolumab) and cytotoxic T-lymphocyte-associated protein 4 (ipilimumab). The last trial, opened in 2023, uses a combination of EGFRvIII-targeting CAR-T with T-cell engagers, designed to help recruit naïve T cells in the vicinity of the tumor [135]. The T-cell engager, termed a TEAM, is based on cetuximab, an EGFR-targeting antibody with cross-reactivity to multiple EGFR mutations [136].
Ongoing clinical trials have additionally varied other characteristics related to the method of CAR-T administration, such as the route of administration and the number or frequency of injections. While intratumoral trafficking in the brain in one trial of anti-EGFRvIII CAR-T was found to correlate with the timeframe for peak engraftment in the peripheral blood [29], the peripheral blood does not represent the site of therapeutic action for solid tumors. Accordingly, of 16 ongoing CAR-T clinical trials for GBM (Table 2), 15 are utilizing intracranial methods of CAR-T administration, such as intratumoral, postresection intracavitary, or intraventricular. An additional consideration related to CAR-T delivery is the use of lymphodepleting preconditioning, which has been documented to improve CAR-T efficacy for hematologic malignancies owing to factors including augmenting space for CAR-T peripheral expansion, depleting Treg cells, and enhancing innate immune system activity [25, 137, 138]. While the impact of lymphodepleting chemotherapy on the efficacy of CAR-T for GBM remains uncertain, it is likely less important for studies utilizing direct/local central nervous system delivery of the cells via the cerebrospinal fluid (i.e., Ommaya reservoir) than in studies administering cells through the peripheral blood.
4.3 Preclinical Stage
Preclinical experimentation on combinations with CAR-T fall into two categories: combinations that intend to create synergistic effects and combinations that serve to enhance the CAR-T activity. Synergistic effects have often focused on antibody-mediated approaches to block immune checkpoint markers or mechanisms of immunosuppression. CAR-T enhancement strategies often involve cytokine and chemokine secretion, either from the CAR-T themselves or through oncolytic virus-mediated infection of tumor cells.
Combinations of CAR-T with immune checkpoint blockade, such as anti-PD-1, anti- cytotoxic T-lymphocyte-associated protein 4, and anti-TIM3 antibodies, have seen significant work in the preclinical arena [52, 139, 140]. While CAR and immune checkpoint blockade pairing was not found to be synergistic, the EGFRvIII-targeting CAR 2173 showed increased activity in murine models specifically when followed by sequential administration of PD-1 and TIM3 blockade [52]. Similar results were observed when EGFRvIII-targeting CAR-T and anti-PD-1 antibodies were administered concurrently, with mice surviving significantly longer when treated with the combination versus CAR-T alone [140]. In a separate study, in vitro co-culture of GBM cells with anti-PD-1 antibodies and HER2-targeting CAR-T led to enhanced killing when compared with CAR-T alone [139]. Despite the extensive preclinical work performed thus far to explore CAR-T and immune checkpoint blockade combinations, the optimal sequencing and timing of these therapies together remain uncertain. Another strategy for addressing immunosuppression is modulating tumor-associated myeloid cells, such as macrophages. For example, toosendanin, a small-molecule compound shown to reduce the immunosuppressive activity of tumor-related macrophages, has been demonstrated to sensitize GBM murine models to anti-EGFRvIII CAR-T [141].
Beyond the immune checkpoint blockade, other combinatorial approaches aimed at enhancing CAR-T function have utilized a wide variety of strategies, taking advantage of numerous T-cell-inherent pathways. Blocking inhibitors of apoptosis proteins led to sensitization of target cells to T-cell-induced apoptosis through tumor necrosis factor-α signaling [142]. This effect was demonstrated to impact tumor target-negative cells, providing a potential strategy to address target heterogeneity and tumor escape. Combination of the bacterial enzyme C. perfringens neuroaminidase with galactose oxidase in CAR-T showed reduced T-cell differentiation and led to enhanced tumor control in GBM as well as other solid and liquid tumor models [143]. Pharmacologic blockade of protein phosphatase-2A led to increased intracellular cytokine production and tumor killing, shown to occur via mTORC1 activation in the CAR-T [144]. This effect was demonstrated in vivo, using local administration of anti-carbonic anhydrase IX CAR-T combined with systemic administration of LB-100, a protein phosphatase-2A antagonist.
The use of bevacizumab in tandem with CAR-T has also been studied in the preclinical setting. Prior studies demonstrating vascular normalization following inhibition of vascular endothelial growth factor signaling have suggested that this process improves T-cell delivery and intratumoral trafficking [145]. One analysis of GBM murine models demonstrated that bevacizumab co-administration with anti-EGFR CAR-T increased CAR-T distribution in the GBM tumor microenvironment and survival [146].
Last, oncolytic viruses (OVs) have also been used in several preclinical combination studies, given both their potential to infect and kill tumor cells and to stimulate an immune response through cytokine production in the infected cells. Combination of OVs with CD70-targeting CAR-T led to enhanced antitumor activity, driven by IFNγ secreted from the tumor cells and acting on the CAR-T [147]. In another study, use of a tumor-specific podoplanin-targeting CAR construct combined with an OV in xenograft models demonstrated significantly enhanced survival when compared with CAR or OV alone [148]. Finally, co-administration of a CXCL11-armed OV and B7H3 CAR-T led to enhanced tumor killing and increased CAR-T infiltration in an immunocompetent syngeneic model [149].
5 Summary
Development of effective CAR-T therapy for GBM will require employment of novel strategies that account for the unique features of this disease, including its extensive molecular heterogeneity, highly immunosuppressive and T-cell hostile microenvironment, systemic immune barriers including T-cell dysfunction, and its location in the central nervous system with resultant challenges related to blood–brain barrier penetration and management of neurotoxicity. Despite these tremendous obstacles, recent signals of efficacy in pediatric brain tumors and exponential growth of translational research in the field of solid tumor cell therapy point to a bright future for CAR-T in GBM. In addition to addressing the key challenges described above, the path to success will also need to include a better understanding of the optimal management of CAR neurotoxicity, improved ability to monitor tumor response and progression beyond standard magnetic resonance imaging, and broad collaboration and sharing of data across centers currently using CAR-T to treat GBM.
References
Pinthus JH, Waks T, Kaufman-Francis K, Schindler DG, Harmelin A, Kanety H, et al. Immuno-gene therapy of established prostate tumors using chimeric receptor-redirected human lymphocytes. Cancer Res. 2003;63(10):2470–6.
Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86(24):10024–8.
Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60.
Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–98.
Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167(11):6123–31.
Paterson AM, Vanguri VK, Sharpe AH. SnapShot: B7/CD28 costimulation. Cell. 2009;137(5):974-4.e1.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–39.
Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–32.
Laetsch TW, Maude SL, Rives S, Hiramatsu H, Bittencourt H, Bader P, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664–9.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–54.
Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–42.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491–502.
Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141(14):1675–84.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–24.
Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388(11):1002–14.
Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: lessons learned and future directions. Pharmacol Ther. 2020;205: 107419.
Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021;20(7):531–50.
Del Bufalo F, De Angelis B, Caruana I, Del Baldo G, De Ioris MA, Serra A, et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N Engl J Med. 2023;388(14):1284–95.
Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–41.
Vitanza NA, Wilson AL, Huang W, Seidel K, Brown C, Gustafson JA, et al. Intraventricular B7–H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2023;13(1):114–31.
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl. 5):v1-95.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Bagley SJ, Desai AS, Linette GP, June CH, O’Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20(11):1429–38.
Huang Z, Dewanjee S, Chakraborty P, Jha NK, Dey A, Gangopadhyay M, et al. CAR T cells: engineered immune cells to treat brain cancers and beyond. Mol Cancer. 2023;22(1):22.
Pant A, Lim M. CAR-T therapy in GBM: current challenges and avenues for improvement. Cancers (Basel). 2023;15(4):1249.
Goff SL, Morgan RA, Yang JC, Sherry RM, Robbins PF, Restifo NP, et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother. 2019;42(4):126–35.
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984.
Bagley SJ, Binder ZA, Desai AS, Nasrallah MP, Maloney E, Brem S, et al. Phase I study of repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab in patients with newly diagnosed, MGMT-unmethylated glioblastoma. Research Square. 2023. https://doi.org/10.21203/rs.3.rs-2742648/v1
Lin Q, Ba T, Ho J, Chen D, Cheng Y, Wang L, et al. First-in-human trial of EphA2-redirected CAR T-cells in patients with recurrent glioblastoma: a preliminary report of three cases at the starting dose. Front Oncol. 2021;11: 694941.
Liu Z, Zhou J, Yang X, Liu Y, Zou C, Lv W, et al. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol Cancer. 2023;22(1):3.
Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–101.
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–9.
Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, et al. Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–72.
Brown CE, Rodriguez A, Palmer J, Ostberg JR, Naranjo A, Wagner JR, et al. Off-the-shelf, steroid-resistant, IL13Ralpha2-specific CAR T cells for treatment of glioblastoma. Neuro Oncol. 2022;24(8):1318–30.
Chistiakov DA, Chekhonin IV, Chekhonin VP. The EGFR variant III mutant as a target for immunotherapy of glioblastoma multiforme. Eur J Pharmacol. 2017;5(810):70–82.
Li G, Wong AJ. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev Vaccines. 2008;7(7):977–85.
Padfield E, Ellis HP, Kurian KM. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol. 2015;5:5.
Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7(8):717–22.
Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66(22):10815–23.
Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–51.
Traylor TD, Hogan EL. Gangliosides of human cerebral astrocytomas. J Neurochem. 1980;34(1):126–31.
Doronin II, Vishnyakova PA, Kholodenko IV, Ponomarev ED, Ryazantsev DY, Molotkovskaya IM, et al. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer. 2014;28(14):295.
Golinelli G, Grisendi G, Prapa M, Bestagno M, Spano C, Rossignoli F, et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020;27(7–8):558–70.
Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 2004;64(14):4980–6.
Ozaki M, Kishigami S, Yano R. Expression of receptors for neuregulins, ErbB2, ErbB3 and ErbB4, in developing mouse cerebellum. Neurosci Res. 1998;30(4):351–4.
Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Hernan R, Fasheh R, Calabrese C, Frank AJ, Maclean KH, Allard D, et al. ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res. 2003;63(1):140–8.
Bartolome RA, Garcia-Palmero I, Torres S, Lopez-Lucendo M, Balyasnikova IV, Casal JI. IL13 receptor alpha2 signaling requires a scaffold protein, FAM120A, to activate the FAK and PI3K pathways in colon cancer metastasis. Cancer Res. 2015;75(12):2434–44.
Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60(5):1168–72.
Yin Y, Boesteanu AC, Binder ZA, Xu C, Reid RA, Rodriguez JL, et al. Checkpoint blockade reverses anergy in IL13Rα2 humanized scFv based CAR T cells to treat murine and canine gliomas. Mol Ther Oncolytics. 2018;11:20–38.
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR T cells targeting B7–H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–74.
Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, et al. B7–H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncol. 2019;27(14):279–87.
Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–50.
Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, et al. Expression of emmprin (CD147), a cell surface inducer of matrix metalloproteinases, in normal human brain and gliomas. Int J Cancer. 2000;88(1):21–7.
Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56(7):359–67.
Colangelo NW, Azzam EI. Extracellular vesicles originating from glioblastoma cells increase metalloproteinase release by astrocytes: the role of CD147 (EMMPRIN) and ionizing radiation. Cell Commun Signal. 2020;18(1):21.
Liang Q, Xiong H, Gao G, Xiong K, Wang X, Zhao Z, et al. Inhibition of basigin expression in glioblastoma cell line via antisense RNA reduces tumor cell invasion and angiogenesis. Cancer Biol Ther. 2005;4(7):759–62.
Yang M, Yuan Y, Zhang H, Yan M, Wang S, Feng F, et al. Prognostic significance of CD147 in patients with glioblastoma. J Neurooncol. 2013;115(1):19–26.
Tseng HC, Xiong W, Badeti S, Yang Y, Ma M, Liu T, et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020;11(1):4810.
Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58(21):4871–9.
Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39(2):162–73.
Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278(6):4135–44.
Mamelak AN, Rosenfeld S, Bucholz R, Raubitschek A, Nabors LB, Fiveash JB, et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol. 2006;24(22):3644–50.
Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67(14):6882–8.
Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, et al. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. 2010;31(31):8032–42.
Wang D, Starr R, Chang WC, Aguilar B, Alizadeh D, Wright SL, et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med. 2020;12(533):eaaw2672.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22.
Schnell O, Krebs B, Wagner E, Romagna A, Beer AJ, Grau SJ, et al. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18(3):378–86.
Cobb DA, de Rossi J, Liu L, An E, Lee DW. Targeting of the alphav beta3 integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma. J Immunother Cancer. 2022;10(2): e003816.
Willis AL, Tran NL, Chatigny JM, Charlton N, Vu H, Brown SA, et al. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res. 2008;6(5):725–34.
Li G, Zhang Z, Cai L, Tang X, Huang J, Yu L, et al. Fn14-targeted BiTE and CAR-T cells demonstrate potent preclinical activity against glioblastoma. Oncoimmunology. 2021;10(1):1983306.
Rousso-Noori L, Mastandrea I, Talmor S, Waks T, Globerson Levin A, Haugas M, et al. P32-specific CAR T cells with dual antitumor and antiangiogenic therapeutic potential in gliomas. Nat Commun. 2021;12(1):3615.
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14(1):123–9.
Wischhusen J, Jung G, Radovanovic I, Beier C, Steinbach JP, Rimner A, et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62(9):2592–9.
Chahlavi A, Rayman P, Richmond AL, Biswas K, Zhang R, Vogelbaum M, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65(12):5428–38.
Seyfrid M, Maich WT, Shaikh VM, Tatari N, Upreti D, Piyasena D, et al. CD70 as an actionable immunotherapeutic target in recurrent glioblastoma and its microenvironment. J Immunother Cancer. 2022;10(1): e003289.
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;2(5):67.
Shibahara I, Sonoda Y, Saito R, Kanamori M, Yamashita Y, Kumabe T, et al. The expression status of CD133 is associated with the pattern and timing of primary glioblastoma recurrence. Neuro Oncol. 2013;15(9):1151–9.
Vora P, Venugopal C, Salim SK, Tatari N, Bakhshinyan D, Singh M, et al. The rational development of CD133-targeting immunotherapies for glioblastoma. Cell Stem Cell. 2020;26(6):832-44.e6.
Fluh C, Chitadze G, Adamski V, Hattermann K, Synowitz M, Kabelitz D, et al. NKG2D ligands in glioma stem-like cells: expression in situ and in vitro. Histochem Cell Biol. 2018;149(3):219–33.
Yang D, Sun B, Dai H, Li W, Shi L, Zhang P, et al. T cells expressing NKG2D chimeric antigen receptors efficiently eliminate glioblastoma and cancer stem cells. J Immunother Cancer. 2019;7(1):171.
Proescholdt MA, Merrill MJ, Stoerr EM, Lohmeier A, Pohl F, Brawanski A. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol. 2012;14(11):1357–66.
Cui J, Zhang Q, Song Q, Wang H, Dmitriev P, Sun MY, et al. Targeting hypoxia downstream signaling protein, CAIX, for CAR T-cell therapy against glioblastoma. Neuro Oncol. 2019;21(11):1436–46.
Durgin JS, Henderson F Jr, Nasrallah MP, Mohan S, Wang S, Lacey SF, et al. Case report: prolonged survival following EGFRvIII CAR T cell treatment for recurrent glioblastoma. Front Oncol. 2021;11: 669071.
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–52.
Muhammad N, Wang R, Li W, Zhang Z, Chang Y, Hu Y, et al. A novel TanCAR targeting IL13Ralpha2 and EphA2 for enhanced glioblastoma therapy. Mol Ther Oncolytics. 2022;17(24):729–41.
Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290–6.
Yin Y, Rodriguez JL, Li N, Thokala R, Nasrallah MP, Hu L, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022;30(7):2537–53.
Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–58.
Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. 2021;13(591):eabe7378.
Gargett T, Ebert LM, Truong NTH, Kollis PM, Sedivakova K, Yu W, et al. GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J Immunother Cancer. 2022;10(9): e005187.
Meister H, Look T, Roth P, Pascolo S, Sahin U, Lee S, et al. Multifunctional mRNA-based CAR T cells display promising antitumor activity against glioblastoma. Clin Cancer Res. 2022;28(21):4747–56.
Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019;10(1):4016.
Li Y, Wu H, Chen G, Wei X, Wang C, Zhou S, et al. Arming anti-EGFRvIII CAR-T with TGFbeta trap improves antitumor efficacy in glioma mouse models. Front Oncol. 2020;10:1117.
Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26(7):1855–66.
Liu X, Zhang Y, Li K, Liu Y, Xu J, Ma J, et al. A novel dominant-negative PD-1 armored anti-CD19 CAR T cell is safe and effective against refractory/relapsed B cell lymphoma. Transl Oncol. 2021;14(7): 101085.
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71.
Garfall AL, Dancy EK, Cohen AD, Hwang WT, Fraietta JA, Davis MM, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019;3(19):2812–5.
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–68.
Grabowski MM, Sankey EW, Ryan KJ, Chongsathidkiet P, Lorrey SJ, Wilkinson DS, et al. Immune suppression in gliomas. J Neurooncol. 2021;151(1):3–12.
Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018;24(16):3792–802.
Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110–21.
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–302.
El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8(3):234–43.
Agliardi G, Liuzzi AR, Hotblack A, De Feo D, Nunez N, Stowe CL, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12(1):444.
Poirier MD, Haban H, El Andaloussi A. A combination of systemic and intracranial anti-CD25 immunotherapy elicits a long-time survival in murine model of glioma. J Oncol. 2009;2009: 963037.
Raychaudhuri B, Rayman P, Huang P, Grabowski M, Hambardzumyan D, Finke JH, et al. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J Neurooncol. 2015;122(2):293–301.
Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–9.
Peereboom DM, Alban TJ, Grabowski MM, Alvarado AG, Otvos B, Bayik D, et al. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight. 2019;4(22): e130748.
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–75.
Shay JE, Celeste SM. Hypoxia-inducible factors: crosstalk between inflammation and metabolism. Semin Cell Dev Biol. 2012;23(4):389–94.
Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS ONE. 2011;6(1): e16195.
Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016;45(2):358–73.
Howie D, Waldmann H, Cobbold S. Nutrient sensing via mTOR in T cells maintains a tolerogenic microenvironment. Front Immunol. 2014;5:409.
Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21(24):5427–33.
Mangraviti A, Raghavan T, Volpin F, Skuli N, Gullotti D, Zhou J, et al. HIF-1alpha-targeting acriflavine provides long term survival and radiological tumor response in brain cancer therapy. Sci Rep. 2017;7(1):14978.
Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558(7709):307–12.
Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021;13(620):eabh0272.
Wang D, Prager BC, Gimple RC, Aguilar B, Alizadeh D, Tang H, et al. CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 2021;11(5):1192–211.
Zou Y, Liu B, Li L, Yin Q, Tang J, Jing Z, et al. IKZF3 deficiency potentiates chimeric antigen receptor T cells targeting solid tumors. Cancer Lett. 2022;1(524):121–30.
Xia L, Liu JY, Zheng ZZ, Chen YJ, Ding JC, Hu YH, et al. BRD4 inhibition boosts the therapeutic effects of epidermal growth factor receptor-targeted chimeric antigen receptor T cells in glioblastoma. Mol Ther. 2021;29(10):3011–26.
Larson RC, Kann MC, Bailey SR, Haradhvala NJ, Llopis PM, Bouffard AA, et al. CAR T cell killing requires the IFNgammaR pathway in solid but not liquid tumours. Nature. 2022;604(7906):563–70.
Choi BD, Yu X, Castano AP, Darr H, Henderson DB, Bouffard AA, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7(1):304.
Nakazawa T, Natsume A, Nishimura F, Morimoto T, Matsuda R, Nakamura M, et al. Effect of CRISPR/Cas9-mediated PD-1-disrupted primary human third-generation CAR-T cells targeting EGFRvIII on in vitro human glioblastoma cell growth. Cells. 2020;9(4):998.
Tang OY, Tian L, Yoder T, Xu R, Kulikovskaya I, Gupta M, et al. PD1 expression in EGFRvIII-directed CAR T cell infusion product for glioblastoma is associated with clinical response. Front Immunol. 2022;13: 872756.
Davidson TB, Lee A, Hsu M, Sedighim S, Orpilla J, Treger J, et al. Expression of PD-1 by T cells in malignant glioma patients reflects exhaustion and activation. Clin Cancer Res. 2019;25(6):1913–22.
Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS ONE. 2013;8(3): e60186.
Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–46.
Wei J, Luo C, Wang Y, Guo Y, Dai H, Tong C, et al. PD-1 silencing impairs the anti-tumor function of chimeric antigen receptor modified T cells by inhibiting proliferation activity. J Immunother Cancer. 2019;7(1):209.
Kobold S, Grassmann S, Chaloupka M, Lampert C, Wenk S, Kraus F, et al. Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst. 2015;107(8):djv146.
Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76(6):1578–90.
Guo JX, Wu CX, Wang PF, Li ZJ, Han S, Jin W, et al. Bioactivity and safety of chimeric switch receptor T cells in glioblastoma patients. Front Biosci (Landmark Ed). 2019;24(6):1158–66.
Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci USA. 2013;110(1):270–5.
Thokala R, Binder ZA, Yin Y, Zhang L, Zhang JV, Zhang DY, et al. High-affinity chimeric antigen receptor with cross-reactive scFv to clinically relevant EGFR oncogenic isoforms. Front Oncol. 2021;10(11): 664236.
Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26(2):111–7.
Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139.
Shen L, Li H, Bin S, Li P, Chen J, Gu H, et al. The efficacy of third generation anti-HER2 chimeric antigen receptor T cells in combination with PD1 blockade against malignant glioblastoma cells. Oncol Rep. 2019;42(4):1549–57.
Song Y, Liu Q, Zuo T, Wei G, Jiao S. Combined antitumor effects of anti-EGFR variant III CAR-T cell therapy and PD-1 checkpoint blockade on glioblastoma in mouse model. Cell Immunol. 2020;352: 104112.
Yang F, Zhang D, Jiang H, Ye J, Zhang L, Bagley SJ, et al. Small-molecule toosendanin reverses macrophage-mediated immunosuppression to overcome glioblastoma resistance to immunotherapy. Sci Transl Med. 2023;15(683):eabq3558.
Song EZ, Wang X, Philipson BI, Zhang Q, Thokala R, Zhang L, et al. The IAP antagonist birinapant enhances chimeric antigen receptor T cell therapy for glioblastoma by overcoming antigen heterogeneity. Mol Ther Oncol. 2022;15(27):288–304.
Durgin JS, Thokala R, Johnson L, Song E, Leferovich J, Bhoj V, et al. Enhancing CAR T function with the engineered secretion of C. perfringens neuraminidase. Mol Ther. 2022;30(3):1201–14.
Cui J, Wang H, Medina R, Zhang Q, Xu C, Indig IH, et al. Inhibition of PP2A with LB-100 enhances efficacy of CAR-T cell therapy against glioblastoma. Cancers (Basel). 2020;12(1):139.
Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109(43):17561–6.
Dong X, Ren J, Amoozgar Z, Lee S, Datta M, Roberge S, et al. Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice. J Immunother Cancer. 2023;11(3): e005583.
Zhu G, Zhang J, Zhang Q, Jin G, Su X, Liu S, et al. Enhancement of CD70-specific CAR T treatment by IFN-gamma released from oHSV-1-infected glioblastoma. Cancer Immunol Immunother. 2022;71(10):2433–48.
Chalise L, Kato A, Ohno M, Maeda S, Yamamichi A, Kuramitsu S, et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Delta combination therapy against glioblastoma. Mol Ther Oncolytics. 2022;15(26):265–74.
Wang G, Zhang Z, Zhong K, Wang Z, Yang N, Tang X, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31(1):134–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of interest/competing interests
Zev A. Binder has an inventorship interest in intellectual property owned by the University of Pennsylvania and has received royalties related to CAR-T therapy in solid tumors. Donald M. O’Rourke is an inventor of intellectual property (US patent numbers 7,625,558 and 6,417,168 and related families) and has received royalties related to targeted ErbB therapy in solid cancers previously licensed by the University of Pennsylvania. Stephen J. Bagley has an inventorship interest in intellectual property owned by Novartis and the University of Pennsylvania: US Patent 62/809,245: “Combination therapies of EGFRvIII chimeric antigen receptors and PD-1 inhibitors”.
Authors’ Contributions
Literature review: Z.A.B., O.Y.T, and S.J.B. Initial draft of manuscript: Z.A.B., O.Y.T, and S.J.B. Critical review and editing of manuscript: Z.A.B., O.Y.T, D.M.O, and S.J.B.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, O.Y., Binder, Z.A., O’Rourke, D.M. et al. Optimizing CAR-T Therapy for Glioblastoma. Mol Diagn Ther 27, 643–660 (2023). https://doi.org/10.1007/s40291-023-00671-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-023-00671-0