Abstract
Background
Resistance training is the gold standard exercise mode for accrual of lean muscle mass, but the isolated effect of resistance training on body fat is unknown.
Objectives
This systematic review and meta-analysis evaluated resistance training for body composition outcomes in healthy adults. Our primary outcome was body fat percentage; secondary outcomes were body fat mass and visceral fat.
Design
Systematic review with meta-analysis.
Data Sources
We searched five electronic databases up to January 2021.
Eligibility Criteria
We included randomised trials that compared full-body resistance training for at least 4 weeks to no-exercise control in healthy adults.
Analysis
We assessed study quality with the TESTEX tool and conducted a random-effects meta-analysis, with a subgroup analysis based on measurement type (scan or non-scan) and sex (male or female), and a meta-regression for volume of resistance training and training components.
Results
From 11,981 records, we included 58 studies in the review, with 54 providing data for a meta-analysis. Mean study quality was 9/15 (range 6–15). Compared to the control, resistance training reduced body fat percentage by − 1.46% (95% confidence interval − 1.78 to − 1.14, p < 0.0001), body fat mass by − 0.55 kg (95% confidence interval − 0.75 to − 0.34, p < 0.0001) and visceral fat by a standardised mean difference of − 0.49 (95% confidence interval − 0.87 to − 0.11, p = 0.0114). Measurement type was a significant moderator in body fat percentage and body fat mass, but sex was not. Training volume and training components were not associated with effect size.
Summary/Conclusions
Resistance training reduces body fat percentage, body fat mass and visceral fat in healthy adults.
Study Registration
osf.io/hsk32.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Resistance training elicits average reductions of 1.4% body fat percentage and 0.55kg body fat mass compared with non-exercise control. |
Resistance training elicits moderate reductions in visceral fat compared with non-exercise control. |
Measurement type (scan or non-scan) may influence the magnitude of body composition changes, but not sex (male or female) or total training volume |
The magnitude of reduction in adiposity observed in this review is similar to previous reviews utilising different exercise modalities. |
1 Introduction
Resistance training (RT) is a popular mode of exercise with numerous benefits not as readily obtained through other modes of exercise (e.g. aerobic exercise), such as preserving bone mineral density [1] and increasing lean body mass [2]. It also plays a role in the prevention or symptom management of many chronic diseases [3, 4], and therefore physical activity guidelines recommend participating in RT 2–3 days per week [5].
While it is clear RT elicits improvements in lean body mass [6, 7], effects on other body composition outcomes (such as subcutaneous fat and visceral fat) are less clear. The American College of Sports Medicine position stand on physical activity for weight loss and prevention of weight regain [8] states that RT will not promote clinically significant weight loss, and may increase losses of body fat mass only when combined with aerobic exercise [8]. Importantly, this position stand is made in the context of weight loss, not participation in RT for health or performance. When considering the effect of RT on subcutaneous fat, the evidence is varied [8]. This may be because of differences in the study design such as the inclusion of concurrent interventions [9], or owing to the use of differing methodologies when analysing body composition [10]. Interestingly, RT appears to preferentially mobilise visceral fat in overweight and obese individuals [11] but the magnitude of this effect is unknown. Visceral fat exerts more negative metabolic and health consequences than subcutaneous fat [12]; therefore, any intervention that reduces visceral fat may have clinical relevance and may be important in cohorts of both normal weight and those overweight or obese.
It is generally accepted in the industry, but less so in the scientific literature, that RT may promote body composition changes in addition to accrual of lean mass. The concept of concomitantly reducing body fat mass and gaining lean mass has been termed body ‘recomposition’ [13]. Given that higher levels of lean mass are associated with a lower risk of all-cause mortality [14], body recomposition is arguably more important than simply reducing body mass in healthy individuals. As such, this review aimed to determine whether RT changes whole-body fat and visceral fat in healthy adults.
2 Methods
2.1 Protocol, Registration and Data Availability
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [15]. The PRISMA checklist is available in the Electronic Supplementary Material (ESM). We pre-registered the review protocol on the Open Science Framework on 4 June, 2020, prior to commencing the literature search (osf.io/hsk32). The data and R script used in the meta-analysis are also available on the Open Science Framework.
2.1.1 Deviations from Protocol
We conducted an additional post-hoc meta-regression to examine the relationship between the baseline value for each outcome and the effect size. Further details are provided in Sect. 2.8.
2.2 Eligibility Criteria
2.2.1 Study Design
We included English-language parallel randomised trials and the first phase of crossover trials published in peer-reviewed academic journals. We excluded cluster trials.
2.2.2 Participants
We included studies that examined apparently healthy adults with no known medical conditions/injuries who were not currently participating in RT and were not performing additional structured physical activity outside of the intervention (e.g. athletic populations with concurrent training). We included studies of overweight/obese adults provided other chronic diseases or risk factors were not present. If people with chronic disease or risk factors were incidentally recruited, we excluded studies where > 20% of participants presented with a given condition (e.g. hypertension). Medication usage was also considered as a surrogate for the presence of a chronic disease risk factor when judging eligibility.
2.2.3 Intervention and Comparator
We included studies that compared a whole-body RT intervention conducted for at least 4 weeks to a non-exercise control. Resistance training interventions must have used a dynamic machine or free weight-based constant, external loads, and included at least one upper and one lower body exercise in the overall programme. We excluded studies that exclusively utilised bodyweight exercises and studies with concurrent nutritional interventions or additional exercise (e.g. aerobic exercise or team sport training).
2.3 Outcomes
Our pre-specified primary outcome was the change in body fat percentage (measured in %) from baseline to the closest timepoint following the intervention. Our secondary outcomes were change in body fat mass (measured in kg) and change in visceral fat (measured in cm2, cm3, kg).
For body fat percentage and body fat mass, we considered measurements in the following hierarchy: dual-energy x-ray absorptiometry (DXA), magnetic resonance imaging (MRI), computerised tomography (CT), hydro-densitometry or whole-body air plethysmography. We excluded other measurements (e.g. bioimpedance analysis or skinfolds) because of the lack of measurement reliability and the lack of sensitivity to detect change [16]. For visceral fat, we considered measurements from DXA, MRI, or CT because hydro-densitometry and plethysmography cannot estimate visceral fat.
2.4 Literature Search
We searched five electronic databases (MEDLINE, Embase, Cochrane CENTRAL, SPORTDiscus and CINAHL) up to January 2021. The complete search strategy for MEDLINE was the following: (“Resistance exercise” OR “Resistance training” OR “Strength training” OR “Strength Exercise” OR “Weightlifting” OR “Weight training”) AND (“RCT” OR “randomised” OR “random”). We also looked for trials from relevant previous systematic reviews.
All authors screened records. Following duplicate deletion, two authors independently screened each record for eligibility by title/abstract and, if required, full text using Covidence [17]. Discrepancies were resolved via discussion, and arbitration from the senior author (ADH) as required. We collated multiple records reporting on the same data into a single study. For every included study in our review, we also searched the reference list and conducted forward citation tracking with Google Scholar to ensure a thorough literature search.
2.5 Data Extraction
All authors extracted data. Two authors independently extracted descriptive and outcome data from each study into custom spreadsheets. Discrepancies were resolved via discussion. For all outcomes, we preferentially extracted the change from baseline for intervention and control as mean and standard deviation (SD). We converted the percentage change from baseline into appropriate values in four studies [18,19,20,21]. We converted other measures of variability (e.g. standard error or 95% confidence intervals [CI]) to SD following guidance from section 6.5.2.3 of the Cochrane Handbook [22]. We converted median and interquartile for two studies [23, 24] to mean and SD using published equations [25]. If a study comprised multiple eligible resistance interventions, we split the control group sample proportionately [22].
If a change from baseline data was not reported, we extracted the baseline and post-intervention data and calculated the change from the baseline as post-intervention mean minus pre-intervention mean. We calculated SD for change using an estimated pre-/post-intervention correlation, following guidance from section 6.5.2.8 of the Cochrane Handbook [22]. As correlations are seldom reported, we identified all studies in our review that reported information sufficient to calculate a correlation, then imputed the median correlation derived for each outcome. We used a median correlation from four studies of 0.96 for body fat percentage (mean = 0.96, range 0.91–0.97), a median correlation from five studies of 0.95 for body fat mass (mean = 0.95, range 0.89–0.97) and a correlation from one study of 0.97 for visceral adipose tissue (range 0.97–0.98).
We did not extract any data from figures. We contacted the corresponding authors of four studies that reported insufficient data for extraction [26,27,28,29]. We received no response following author contact; therefore, we did not use any data from these studies in the meta-analysis. We contacted the corresponding authors of 15 studies for sex-disaggregated data and received data from five (33%).
2.6 Quality Assessment
We evaluated the quality of each included study using the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX) [30]. Two authors independently assessed each study across 15 criteria, each worth one point, with discrepancies were resolved via discussion. We did not classify scores as “high” or “low” (or some other arbitrary grouping) because of the limitations associated with these approaches [31].
2.7 Data Synthesis
We performed a random-effects meta-analysis in R (version 4.0.4) using the metafor package (version 2.4) [32]. For body fat percentage and absolute body fat, we calculated the pooled mean difference between RT and control groups with 95% CIs. For visceral adipose tissue, we calculated the standardised mean difference with 95% CIs to account for the variety of measurement units. We quantified heterogeneity using a restricted estimate maximum likelihood model with the 95% prediction interval, Cochran Q, tau (τ2) and I2. We used conventional and contour-enhanced funnel plots to illustrate possible publication asymmetry/bias, and if more than ten studies were available we conducted Egger’s regression test (α < 0.10 indicating the presence of asymmetry).
2.8 Additional Analyses
We conducted pre-planned subgroup analyses to examine the moderating effects of measurement type (scan: DXA, MRI, CT; or non-scan: hydro-densitometry, whole-body air plethysmography) and sex (male or female). We also conducted a pre-planned meta-regression to examine the moderating effect of total training volume during an intervention, with the hypothesis that increased training volume would be related to greater reductions in body fat and visceral fat. We considered total training volume as:
Study duration (weeks) multiplied by weekly training volume (calculated as frequency [days per week] × intensity [% 1 repetition maximum (RM)] × number of sets per session × number of repetitions per set).
If a range of values was used (e.g. 2–3 days per week, or 8–12 repetitions per set), we used the mean value (e.g. 2.5 and 10, respectively). When intensities were reported as a RM, we converted to %1RM via an estimated repetition at a %1RM chart [33]. If prescriptive parameters were unclear, we imputed the mean value from other studies: number of exercises = 8 (required for four studies [34,35,36]); number of sets = 2.6 (required for one study [37]). We excluded two studies [38, 39] (three comparisons) from this meta-regression because both studies completed as many repetitions as possible in each set.
We also conducted a post-hoc meta-regression to examine the moderating effect of baseline values, hypothesising that higher values at baseline would be associated with greater reductions in body fat and visceral fat. We combined the baseline means from the intervention and control groups using the formula from Table 6.5a in section 6.5.2.10 of the Cochrane Handbook [22]. Based on recommendations during peer review, we also conducted a post-hoc meta-regression to examine the moderating effect of study duration (weeks), frequency of sessions (days/week), intensity (%1RM) and total sets per week (number of sets × number of exercises × frequency).
3 Results
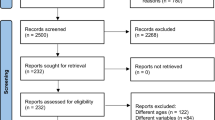
Our literature search is illustrated in Fig. 1. We screened 11,981 records and assessed 463 full-text articles for eligibility. Seventy-five included records formed 56 included studies following record collation. We identified two additional studies during citation tracking, ultimately resulting in 58 studies included in the review [18,19,20,21, 23, 24, 26,27,28,29, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81] (Table 1 of the ESM). Four of these studies failed to respond to e-mail requests for data [26,27,28,29]. Therefore, 54 studies were included in the meta-analysis [18,19,20,21, 23, 24, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. Fourteen studies investigated multiple resistance training interventions.
The number of participants randomised was 3058 (reported across 56 studies): 1232 male patients (40.3%), 1722 female patients (56.3%) and 104 with sex not reported (3.4%). Participants were on average 51.2 years of age (mean ages ranged from 19 to 72.1 years). No participants were experienced in RT, with baseline activity levels from sedentary to recreationally active.
Resistance training interventions (Table 2 of the ESM) were typically conducted at universities and supervised by qualified instructors. They were conducted for an average of 20.5 weeks (range 6–104 weeks), with an average frequency of 2.7 sessions per week (range 1–4 weeks). The average number of exercises per session was 8.1 (range 4–15), with most studies outlining the specific exercises; sets averaged 2.6 per session per exercise (range 1– 5) and repetitions averaged nine per set (range 5–15). Intensities were typically quantified through %1RM. Almost every study clearly reported progression through some means.
3.1 Study Quality
Included studies scored an average of 9 (SD = 2) out of 15 on the TESTEX (median = 8; range 6–15). As illustrated in Fig. 2, few studies included adequate reporting of allocation concealment (6%), intention-to-treat analysis (6%), assessor blinding (15%), random sequence generation (19%), control group monitoring (26%) and adverse events (32%). The majority of studies adequately reported point estimates (95%), between-group comparisons for the primary outcome (94%) and a secondary outcome (92%), consistent exercise intensity (90%), baseline similarity (89%), inclusion criteria (84%), sufficient prescriptive detail for the calculation of exercise volume (79%), adherence > 85% (69%) and session attendance (53%).
3.2 Body Fat Percentage
Data for body fat percentage were available from 41 studies (52 comparisons with 1506 participants: RT = 875; control = 631). We excluded six studies [26,27,28,29, 45, 77] (ten comparisons) with insufficient data. Resistance training reduced body fat percentage by − 1.46% (95% CI − 1.78 to − 1.14, p < 0.0001) (Fig. 3). Significant heterogeneity was apparent (Q = 155.19, p < 0.0001; I2 = 83%), with the 95% prediction interval spanning − 3.20 to 0.29. Our subgroup analysis demonstrated both scan and non-scan subgroups were associated with reductions in body fat percentage; non-scan displayed a significantly larger effect than scan (between-subgroup difference = 1.4% [95% CI 2.0–0.8], p < 0.0001). Heterogeneity was not evident in the scan subgroup (Q = 35.15, p = 0.65; I2 = 12%), but substantial heterogeneity remained in the non-scan subgroup (Q = 60.57, p < 0.0001; I2 = 97%).
Sex-disaggregated data were available from 23 female-only comparisons (648 participants: resistance training = 368; control = 280) and 24 male-only comparisons (591 participants: resistance training = 332; control = 259). As shown in Fig. 1 of the ESM, RT reduced body fat percentage in female-only comparisons by − 1.53% (95% CI − 2.14 to − 0.91, p < 0.0001) and in male-only comparisons by − 1.46% (95% CI − 1.79 to − 1.12, p < 0.0001). There was no difference between subgroups (0.13% [95% CI − 0.58 to 0.84], p = 0.72).
Our pre-planned meta-regression demonstrated no association between total training volume and effect size: β = 0.0 (95% CI 0.0–0.0), p = 0.31. Our post-hoc meta-regression also demonstrated no association between baseline value and effect size: β = 0.03 (95% CI 0.0–0.06), p = 0.07. Additional meta-regression analyses did not find associations for study duration, session frequency, intensity or total sets per week (Table 3 of the ESM).
3.3 Body Fat Mass
Data for body fat mass were available from 36 studies (53 comparisons with 1638 participants: resistance training = 960; control = 668). We excluded three studies [26, 28, 29] (four comparisons) with insufficient data. Resistance training reduced body fat mass by − 0.55 kg (95% CI − 0.75 to − 0.34, p < 0.0001) (Fig. 4). No heterogeneity was apparent (Q = 51.20, p = 0.51; I2 = 2.6%), with the 95% prediction interval spanning − 0.86 to − 0.26. Our subgroup analysis demonstrated both scan and non-scan subgroups were associated with reductions in body fat mass; non-scan displayed a significantly larger effect than scan (between-subgroup difference = 1.15 kg [95% CI 0.29–2.0], p = 0.009).
Sex-disaggregated data were available from 28 female-only comparisons (847 participants: resistance training = 497; control = 350) and 23 male-only comparisons (593 participants: resistance training = 329; control = 264). As shown in Fig. 2 of the ESM, RT reduced body fat mass in female-only comparisons by − 0.35 kg (95% CI − 0.60 to − 0.09, p = 0.008) and in male-only comparisons by − 0.69 kg (95% CI − 1.03 to − 0.34, p < 0.0001). There was no difference between subgroups (− 0.29 [95% CI − 0.69 to 0.11], p = 0.16).
Our pre-planned meta-regression demonstrated no association between total training volume and effect size: β = 0.0 (95% CI 0.0–0.0), p = 0.63. Our post-hoc meta-regression also demonstrated no association between baseline value and effect size: β = 0.0 (95% CI 0.0–0.0), p = 0.27. Additional meta-regression analyses did not find associations for study duration, session frequency, intensity or total sets per week (Table 3 of the ESM).
3.4 Visceral Fat
Data for visceral fat were available from four studies (four comparisons with 216 participants: resistance training = 111; control = 105). There were no missing data. Measurement units included volume (cm3), mass (kg) and area (cm2). Resistance training reduced visceral fat by a standardised mean difference of − 0.49 (95% CI − 0.87 to − 0.11, p = 0.011) (Fig. 5). Minimal heterogeneity was apparent (Q = 4.53, p = 0.21; I2 = 32.5%), with the 95% prediction interval spanning − 1.07 to 0.09.
Sex-disaggregated data were available for all studies: three female-only comparisons (212 participants: resistance training = 109; control = 103) and one male-only comparison (four participants: resistance training = two; control = two). As shown in Fig. 3 of the ESM, resistance training reduced visceral fat in female-only comparisons by a standardised mean difference of − 0.48 (95% CI − 0.86 to − 0.10, p = 0.0142). We did not assess between-subgroup differences because of a lack of data.
Our pre-planned meta-regression demonstrated no association between total training volume and effect size: β = 0.0 (95% CI 0.0–0.0), p = 0.78. Our post-hoc meta-regression also demonstrated no association between baseline value and effect size: β = 0.0 (95% CI 0.0–0.0), p = 0.79. We did not perform additional meta-regression analyses because of limited data.
3.5 Publication Bias
Conventional and contour-enhanced funnel plots are available in Figs. 4–6 of the ESM. Visual inspection did not indicate publication asymmetry, which was also indicated by Egger’s regression test for fat mass percentage (z = 0.51, p = 0.61) and body fat mass (z = − 1.30, p = 0.19).
4 Discussion
Our results show that RT elicits significant reductions in body fat percentage, body fat mass and visceral fat in healthy adults. This provides evidence for the notion of body recomposition and adds to the previous literature demonstrating benefits of RT [6, 7]. Body recomposition, rather than simply decreases in body mass, may therefore be the more appropriate goal of an RT exercise programme for healthy individuals. A clinically meaningful decrease of 3–5% of body mass is used in overweight and obese individuals and relates to health outcomes [8, 82]. However, when considering healthy individuals (particularly those within a healthy range for body mass), the components of body composition become more relevant.
Resistance training reduced body fat percentage by 1.4% in our review, a magnitude that is comparable to other modes of exercise. A previous meta-analysis of aerobic exercise modalities by Keating et al. [83] determined that high-intensity/sprint interval training and moderate-intensity continuous training reduced body fat percentage by 1.26% and 1.4%, respectively. However, the reduction in body fat mass of 0.55 kg in our review is smaller than the 1.38-kg or 0.91-kg reductions determined by Keating et al. for high-intensity/sprint interval training and moderate-intensity continuous training, respectively, suggesting that different types of training elicit different body composition adaptations. Previous reviews have shown that RT typically increases lean mass by approximately 1.5 kg [6, 7], although several factors can influence the magnitude of adaptation such as the intake of protein [84] and creatine [85]. Therefore, although not examined in our review, individuals participating in RT interventions in our review probably gained muscle mass and the changes in body fat percentage may reflect the accrual of lean mass unlikely to be experienced in the aerobic-based modalities. Similarly, RT participation during a weight loss programme conserves lean mass [86, 87], while no exercise [86] or aerobic exercise [86, 87] leads to losses in lean mass. Therefore, given RT appears to elicit similar reductions in adiposity as other exercise modes, yet has the added benefit of lean mass accrual, it should be considered a valuable component of weight loss interventions in overweight or obese individuals.
Importantly, we excluded studies with concurrent weight loss interventions (caloric restriction, dietary alteration, supplementation or concurrent aerobic exercise), suggesting our findings are ‘incidental’ body composition changes, as they were not the intent of the intervention. The long-term average weight gain in adulthood has been estimated to be 0.5–0.8 kg per year, primarily due to increases in fat mass [88]. Therefore, RT may be sufficient to offset some age-related alterations in body composition, but this effect would likely be even greater if combined with additional interventions (e.g. dietary interventions).
Visceral fat is important because of its negative association with many facets of health [12]. Our review found that RT conferred moderate reductions in visceral fat. Our findings contrast with those of Ismail et al. [89], who found that aerobic exercise, but not RT, significantly reduced visceral fat, and Maillard et al. [90], who found that high-intensity interval training reduced visceral fat in overweight or obese adults, but not in individuals with normal weight. There are numerous differences between the reviews that may explain the discrepant findings. For example, both Maillard et al. [90] and Ismail et al. [89] included cohorts with disease who may be performing different exercise interventions from those applied to healthy populations, and thus experiencing differing levels of adaptation. When we consider the notion of body recomposition, our findings suggest RT may elicit more favourable changes for healthy adults than participating in only aerobic exercise (in terms of both accrual of lean mass, and reductions in fat mass including visceral fat).
It is interesting to note effect sizes in body fat percentage and body fat mass were associated with measurement type, with effect sizes from scan measurements significantly smaller than effects from non-scan measurements. Studies with non-scan measurements in our review also appeared to drive the heterogeneity in the outcomes of body fat percentage and body fat mass. Scan measurements (DXA, MRI and CT) are more accurate than other types [16], which suggests body composition assessment methods must be considered in meta-analyses of exercise (and possibly other intervention types). Previous reviews have included non-scan measurements (bioelectrical impedance, skinfolds, hydro-densitometry or air displacement plethysmography) [83] and therefore their results may, to some extent, be an overestimation. Our strict inclusion criterion for outcome measurement increases the confidence in the accuracy of our effect and is a strength of our review because we excluded measurement tools with large variability. However, we noted that testing standardisation was poorly reported in our included studies and there is a possibility that some of the observed changes may be due to measurement error. Acute changes due to food and fluid intake do not influence scan-based assessments of body fat mass but do influence lean muscle mass, and therefore would influence the body fat percentage [91]. It is imperative that future studies adhere to testing standardisation protocols and adequately report on these protocols to ensure confidence in data interpretation. The other subgroup analysis in our review suggests no difference between male and female patients, and our meta-regressions did not find effect sizes were associated with training volume or baseline values.
The exact mechanisms by which RT elicits fat loss in our review are unclear. The concept of excess post-exercise oxygen consumption (EPOC) is related to the excess energy required to return the body to its normal resting state following exercise and has been postulated to contribute to body composition changes. The magnitude and duration of EPOC appear to be related to exercise duration and intensity [92]. Excess post-exercise oxygen consumption following RT has typically been shown to be negligible, and only lasts approximately 60 min following RT [93]. However, this is not always the case, with some research demonstrating that EPOC remains elevated for up to 38 h following RT in male patients [94]. There is also a wide variability in the duration of time in which EPOC may remain elevated with the largest decline towards baseline values occurring in the first 14 h following exercise [94]. Multiple prescriptive factors may influence the magnitude of EPOC observed. The amount of active muscle is likely to contribute to EPOC [95], and to the overall energy expenditure of the RT session. When RT volume is matched, EPOC has been shown to be greater following high-intensity RT compared with low-intensity RT despite similar within-exercise energy consumptions [96]. However, this difference was negligible by 20 min and, as such, is unlikely to contribute to alterations in body composition. Similarly, the volume of work done during a RT session affects the energy expenditure during the actual session, but does not influence post-exercise energy expenditure [97]. It has been suggested that many RT studies that report high EPOC values may overestimate its true effect due to methodologies employing acute exercise bouts [98] aiming to elicit a large degree of muscle damage that are not reflective of an ecologically valid RT programme [93, 98]. While EPOC is indeed a recognised physiological phenomenon, at present, there is insufficient evidence demonstrating EPOC as a key determinant in altering body composition [99]. The effect of EPOC on weight loss is suggested to be negligible, with the effect of exercise likely due to the energy expended during the exercise sessions [100, 101].
Resting metabolic rate (RMR) is the largest contributor to total daily energy expenditure [102] and, as such, it is plausible that RMR may influence body composition. A recent systematic review and meta-analysis examined the effect of differing exercise interventions on RMR [103]. This study found that combined aerobic and resistance training interventions, and aerobic-only interventions, did not increase RMR. The authors found that RT increased RMR compared with controls by a mean difference of 96.17 kcal per day [103]. While these differences were deemed statistically significant, the clinical relevance of an increase of this magnitude is less clear. In a cohort of overweight women, following 20 weeks of RT, the women gained an average of 1.9 kg of fat-free mass and increased their RMR by a corresponding 44 kcal per day [104]. However, although exercise can elicit increases in fat-free mass, a change in RMR does not always occur [102]. It is important to differentiate here that the concept of ‘maintaining’ RMR via RT during a weight loss programme is different from an RT programme conducted for other outcomes, as was the focus of the studies included in this review. Similar to the influence of EPOC on weight control and fat loss, the effect of RMR also appears to be negligible, with the benefit likely also arising predominantly from the exercise bout itself.
Perhaps, if fat loss is the goal of the exercise programme, then prescriptive modifications could focus on maximising energy expenditure within a given session. For example, super slow RT has been shown to be less metabolically demanding and create a lower overall energy expenditure than traditional RT [105]. The energy cost of traditional RT ranges significantly from 179 cal (inclusive of the 30-min session plus 15 min of recovery) [105] to approximately 300 cal following a body pump or high-intensity lifting session lasting approximately 1 h [98].
At this point, our understanding of ‘how’ resistance training should be prescribed to maximise fat loss is not defined. Our meta-regressions demonstrated no influence from the training variables duration, frequency, intensity or weekly set volume. From a pragmatic standpoint, it is likely that the interplay of these prescriptive parameters will influence the metabolic demands of the RT programme, and the subsequent body composition alterations. Farinatti et al. [106] conducted a systematic review examining the effect of RT variables on EPOC and found that RT volume influenced the magnitude and duration of EPOC, yet load influenced the magnitude. These authors recommended that high-intensity RT, performed in a circuit-style manner, would likely have the biggest influence on EPOC [106]. Again, it must be noted that this study focused on the prescriptive variables’ influence on EPOC, which does not necessarily translate to weight loss. However, this type of prescription may indeed elicit a greater energy expenditure in a shorter time period, i.e. be more efficient than traditional heavy RT with long rest periods. Ultimately, the prescription will be based on the individuals’ goals for the training programme.
Our review is not without limitations. We conducted a thorough literature search but did exclude languages other than English; however, but based on previous research we believe this will have minimal impact on our results [107]. We were also limited by the need to impute variance estimates for several studies and exclude data from four studies as a last resort. However, we undertook a valid approach to overcome these issues and attempted to adhere to principles of open science. To improve the confidence in their results, future studies should focus on better conduct and reporting of allocation concealment, intention-to-treat analysis, assessor blinding, random sequence generation, control group monitoring and adverse events.
5 Conclusions
In summary, we found that RT moderately decreased body fat percentage, fat mass and visceral fat in healthy adults. The changes after RT are incidental in nature and are likely combined with greater body recomposition benefits including gains of lean mass. A 3–5% recomposition may have importance and relevance for normal weight individuals participating in an RT programme.
References
Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: a randomized controlled trial. JAMA. 1994;272(24):1909–14. https://doi.org/10.1001/jama.1994.03520240037038.
Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24(10):2857–72. https://doi.org/10.1519/JSC.0b013e3181e840f3.
Gordon BA, Benson AC, Bird SR, et al. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2009;83(2):157–75. https://doi.org/10.1016/j.diabres.2008.11.024.
MacDonald HV, Johnson BT, Huedo-Medina TB, et al. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5(10): e003231. https://doi.org/10.1161/jaha.116.003231.
Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. https://doi.org/10.1249/MSS.0b013e318213fefb.
Benito PJ, Cupeiro R, Ramos-Campo DJ, et al. A systematic review with meta-analysis of the effect of resistance training on whole-body muscle growth in healthy adult males. Int J Environ Res Public Health. 2020;17(4):1285. https://doi.org/10.3390/ijerph17041285.
Hagstrom AD, Marshall PW, Halaki M, et al. The effect of resistance training in women on dynamic strength and muscular hypertrophy: a systematic review with meta-analysis. Sports Med. 2020;50(6):1075–93. https://doi.org/10.1007/s40279-019-01247-x.
Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71.
Glowacki SP, Martin SE, Maurer A, et al. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med Sci Sports Exerc. 2004;36(12):2119–27. https://doi.org/10.1249/01.mss.0000147629.74832.52.
Broeder CE, Burrhus KA, Svanevik LS, et al. Assessing body composition before and after resistance or endurance training. Med Sci Sports Exerc. 1997;29(5):705–12. https://doi.org/10.1097/00005768-199705000-00019.
Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes. 2011;2011: 482564. https://doi.org/10.1155/2011/482564.
Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49(6):883–8. https://doi.org/10.2337/diabetes.49.6.883.
Barakat C, Pearson J, Escalante G, et al. Body recomposition: can trained individuals build muscle and lose fat at the same time? Strength Cond J. 2020;42(5):7–21. https://doi.org/10.1519/ssc.0000000000000584.
Heitmann BL, Erikson H, Ellsinger BM, et al. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old Swedish men-a 22-year follow-up: the study of men born in 1913. Int J Obes Relat Metab Disord. 2000;24(1):33–7. https://doi.org/10.1038/sj.ijo.0801082.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. https://doi.org/10.1136/bmj.b2700.
Borga M, West J, Bell JD, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med. 2018;66(5):1–9. https://doi.org/10.1136/jim-2018-000722.
Veritas Health Innovation. Covidence systematic review software. Melbourne: Veritas Health Innovation.
Irving BA, Lanza IR, Henderson GC, et al. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab. 2015;100(4):1654–63. https://doi.org/10.1210/jc.2014-3081.
Schmitz KH, Hannan PJ, Stovitz SD, et al. Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am J Clin Nutr. 2007;86(3):566–72. https://doi.org/10.1093/ajcn/86.3.566.
Timmons JF, Minnock D, Hone M, et al. Comparison of time-matched aerobic, resistance, or concurrent exercise training in older adults. Scand J Med Sci Sports. 2018;28(11):2272–83. https://doi.org/10.1111/sms.13254.
Whiteford J, Ackland TR, Dhaliwal SS, et al. Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos Int. 2010;21(9):1529–36. https://doi.org/10.1007/s00198-009-1132-6.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions, version 6.0 (updated July 2019). Cochrane; 2011.
Croymans DM, Krell SL, Oh CS, et al. Effects of resistance training on central blood pressure in obese young men. J Hum Hypertens. 2014;28(3):157–64. https://doi.org/10.1038/jhh.2013.81.
Roberts CK, Croymans DM, Aziz N, et al. Resistance training increases SHBG in overweight/obese, young men. Metabolism. 2013;62(5):725–33. https://doi.org/10.1016/j.metabol.2012.12.004.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. https://doi.org/10.1186/1471-2288-14-135.
Fernandez-Garcia JC, Galvez-Fernandez I, Mercade-Mele P, et al. Longitudinal study of body composition and energy expenditure in overweight or obese young adults. Sci Rep. 2020;10(1):5305. https://doi.org/10.1038/s41598-020-62249-8.
Fragala MS, Fukuda DH, Stout JR, et al. Muscle quality index improves with resistance exercise training in older adults. Exp Gerontol. 2014;53:1–6. https://doi.org/10.1016/j.exger.2014.01.027.
Villanueva MG, He JX, Schroeder ET. Periodized resistance training with and without supplementation improve body composition and performance in older men. Eur J Appl Physiol. 2014;114(5):891–905. https://doi.org/10.1007/s00421-014-2821-1.
Vincent KR, Braith RW, Vincent HK. Influence of resistance exercise on lumbar strength in older, overweight adults. Arch Phys Med Rehabil. 2006;87(3):383–9. https://doi.org/10.1016/j.apmr.2005.11.030.
Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13(1):9–18. https://doi.org/10.1097/xeb.0000000000000020.
Jüni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054–60. https://doi.org/10.1001/jama.282.11.1054.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Haff GG, Triplett NT. Essentials of strength training and conditioning. 4th ed. Champaign: Human Kinetics; 2015.
Fernandez-Lezaun E, Schumann M, Makinen T, et al. Effects of resistance training frequency on cardiorespiratory fitness in older men and women during intervention and follow-up. Exp Gerontol. 2017;95:44–53. https://doi.org/10.1016/j.exger.2017.05.012.
Kukuljan S, Nowson CA, Sanders K, et al. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol. 2009;107(6):1864–73. https://doi.org/10.1152/japplphysiol.00392.2009.
Olson TP, Dengel DR, Leon AS, et al. Moderate resistance training and vascular health in overweight women. Med Sci Sports Exerc. 2006;38(9):1558–64. https://doi.org/10.1249/01.mss.0000227540.58916.0e.
Sillanpaa E, Hakkinen A, Nyman K, et al. Body composition and fitness during strength and/or endurance training in older men. Med Sci Sports Exerc. 2008;40(5):950–8. https://doi.org/10.1249/MSS.0b013e318165c854.
Anton MM, Cortez-Cooper MY, DeVan AE, et al. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol. 2006;101(5):1351–5. https://doi.org/10.1152/japplphysiol.00497.2006.
Tanimoto M, Sanada K, Yamamoto K, et al. Effects of whole-body low-intensity resistance training with slow movement and tonic force generation on muscular size and strength in young men. J Strength Cond Res. 2008;22(6):1926–38. https://doi.org/10.1519/JSC.0b013e318185f2b0.
Abe T, DeHoyos DV, Pollock ML, et al. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol. 2000;81(3):174–80. https://doi.org/10.1007/s004210050027.
Ades PA, Ballor DL, Ashikaga T, et al. Weight training improves walking endurance in healthy elderly persons. Ann Intern Med. 1996;124(6):568–72. https://doi.org/10.7326/0003-4819-124-6-199603150-00005.
Andersen TR, Schmidt JF, Pedersen MT, et al. The effects of 52 weeks of soccer or resistance training on body composition and muscle function in +65-year-old healthy males: a randomized controlled trial. PLoS ONE. 2016;11(2): e0148236. https://doi.org/10.1371/journal.pone.0148236.
Bouchard DR, Soucy L, Senechal M, et al. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16(1):66–72. https://doi.org/10.1097/gme.0b013e31817dacf7.
Broeder CE, Burrhus KA, Svanevik LS, et al. The effects of either high-intensity resistance or endurance training on resting metabolic-rate. Am J Clin Nutr. 1992;55(4):802–10.
Cavalcante EF, Ribeiro AS, do Nascimento MA, et al. Effects of different resistance training frequencies on fat in overweight/obese older women. Int J Sports Med. 2018;39(7):527–34. https://doi.org/10.1055/a-0599-6555.
Dao E, Davis JC, Sharma D, et al. Change in body fat mass is independently associated with executive functions in older women: a secondary analysis of a 12-month randomized controlled trial. PLoS ONE. 2013;8(1): e52831. https://doi.org/10.1371/journal.pone.0052831.
DeVallance E, Fournier S, Lemaster K, et al. The effects of resistance exercise training on arterial stiffness in metabolic syndrome. Eur J Appl Physiol. 2016;116(5):899–910. https://doi.org/10.1007/s00421-016-3348-4.
do Nascimento MA, Gerage AM, Januario RS, et al. Resistance training with dietary intake maintenance increases strength without altering body composition in older women. J Sports Med Phys Fit. 2018;58(4):457–64. https://doi.org/10.23736/S0022-4707.16.06730-X.
dos Santos L, Ribeiro AS, Nunes JP, et al. Effects of pyramid resistance-training system with different repetition zones on cardiovascular risk factors in older women: a randomized controlled trial. Int J Environ Res Public Health. 2020;17(17):6115. https://doi.org/10.3390/ijerph17176115.
Flack KD, Davy BM, DeBerardinis M, et al. Resistance exercise training and in vitro skeletal muscle oxidative capacity in older adults. Physiol Rep. 2016;4(13): e12849. https://doi.org/10.14814/phy2.12849.
Gettman LR, Ayres JJ, Pollock ML, et al. The effect of circuit weight training on strength, cardiorespiratory function, and body composition of adult men. Med Sci Sports. 1978;10(3):171–6.
Gettman LR, Ward P, Hagan RD. A comparison of combined running and weight training with circuit weight training. Med Sci Sports Exerc. 1982;14(3):229–34.
Gregory SM, Spiering BA, Alemany JA, et al. Exercise-induced insulin-like growth factor I system concentrations after training in women. Med Sci Sports Exerc. 2013;45(3):420–8. https://doi.org/10.1249/MSS.0b013e3182750bd4.
Henwood TR, Riek S, Taaffe DR. Strength versus muscle power-specific resistance training in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63(1):83–91. https://doi.org/10.1093/gerona/63.1.83.
Keating SE, Hackett DA, Parker HM, et al. Effect of resistance training on liver fat and visceral adiposity in adults with obesity: a randomized controlled trial. Hepatol Res. 2017;47(7):622–31. https://doi.org/10.1111/hepr.12781.
Kirk EP, Donnelly JE, Smith BK, et al. Minimal resistance training improves daily energy expenditure and fat oxidation. Med Sci Sports Exerc. 2009;41(5):1122–9. https://doi.org/10.1249/MSS.0b013e318193c64e.
Kirk EP, Washburn RA, Bailey BW, et al. Six months of supervised high-intensity low-volume resistance training improves strength independent of changes in muscle mass in young overweight men. J Strength Cond Res. 2007;21(1):151–6. https://doi.org/10.1519/00124278-200702000-00027.
LeMura LM, von Duvillard SP, Andreacci J, et al. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur J Appl Physiol. 2000;82(5–6):451–8. https://doi.org/10.1007/s004210000234.
Levinger I, Goodman C, Hare DL, et al. The effect of resistance training on functional capacity and quality of life in individuals with high and low numbers of metabolic risk factors. Diabetes Care. 2007;30(9):2205–10. https://doi.org/10.2337/dc07-0841.
Lo MS, Lin LL, Yao WJ, et al. Training and detraining effects of the resistance vs. endurance program on body composition, body size, and physical performance in young men. J Strength Cond Res. 2011;25(8):2246–54. https://doi.org/10.1519/JSC.0b013e3181e8a4be.
Malin SK, Hinnerichs KR, Echtenkamp BG, et al. Effect of adiposity on insulin action after acute and chronic resistance exercise in non-diabetic women. Eur J Appl Physiol. 2013;113(12):2933–41. https://doi.org/10.1007/s00421-013-2725-5.
Marcinik EJ, Potts J, Schlabach G, et al. Effects of strength training on lactate threshold and endurance performance. Med Sci Sports Exerc. 1991;23(6):739–43.
Marston KJ, Peiffer JJ, Rainey-Smith SR, et al. Resistance training enhances delayed memory in healthy middle-aged and older adults: a randomised controlled trial. J Sci Med Sport. 2019;22(11):1226–31. https://doi.org/10.1016/j.jsams.2019.06.013.
Marx JO, Ratamess NA, Nindl BC, et al. Low-volume circuit versus high-volume periodized resistance training in women. Med Sci Sports Exerc. 2001;33(4):635–43. https://doi.org/10.1097/00005768-200104000-00019.
Miller T, Mull S, Aragon AA, et al. Resistance training combined with diet decreases body fat while preserving lean mass independent of resting metabolic rate: a randomized trial. Int J Sport Nutr Exerc Metab. 2018;28(1):46–54. https://doi.org/10.1123/ijsnem.2017-0221.
Nichols JF, Omizo DK, Peterson KK, et al. Efficacy of heavy-resistance training for active women over sixty: muscular strength, body composition, and program adherence. J Am Geriatr Soc. 1993;41(3):205–10. https://doi.org/10.1111/j.1532-5415.1993.tb06692.x.
Pipes TV. Variable resistance versus constant resistance strength training in adult males. Eur J Appl Physiol Occup Physiol. 1978;39(1):27–35. https://doi.org/10.1007/BF00429676.
Pipes TV, Wilmore JH. Isokinetic vs isotonic strength training in adult men. Med Sci Sports. 1975;7(4):262–74.
Poehlman ET, Dvorak RV, DeNino WF, et al. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000;85(7):2463–8. https://doi.org/10.1210/jcem.85.7.6692.
Ribeiro AS, Schoenfeld BJ, Dos Santos L, et al. Resistance training improves a cellular health parameter in obese older women: a randomized controlled trial. J Strength Cond Res. 2020;34(10):2996–3002. https://doi.org/10.1519/JSC.0000000000002773.
Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334–40. https://doi.org/10.1016/j.exger.2013.01.007.
Saremi A, Parastesh M. Twelve-week resistance training decreases myostatin level and improves insulin sensitivity in overweight-obese women. Int J Diabetes Metab. 2011;19:63–8.
Scanlon TC, Fragala MS, Stout JR, et al. Muscle architecture and strength: adaptations to short-term resistance training in older adults. Muscle Nerve. 2014;49(4):584–92. https://doi.org/10.1002/mus.23969.
Schmitz KH, Ahmed RL, Yee D. Effects of a 9-month strength training intervention on insulin, insulin-like growth factor (IGF)-I, IGF-binding protein (IGFBP)-1, and IGFBP-3 in 30–50-year-old women. Cancer Epidemiol Biomark Prev. 2002;11(12):1597–604.
Sillanpää E, Laaksonen DE, Häkkinen A, Karavirta L, Jensen B, Kraemer WJ, et al. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur J Appl Physiol. 2009;106(2):285–96. https://doi.org/10.1007/s00421-009-1013-x.
Sun X, Ma XK, Zhang L, et al. Effects of resistance training on serum 25(OH) D concentrations in young men: a randomized controlled trial. Nutr Metab. 2020;17:59. https://doi.org/10.1186/s12986-020-00480-w.
Taaffe DR, Duret C, Wheeler S, et al. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–14. https://doi.org/10.1111/j.1532-5415.1999.tb05201.x.
Taaffe DR, Pruitt L, Reim J, et al. Effect of sustained resistance training on basal metabolic rate in older women. J Am Geriatr Soc. 1995;43(5):465–71. https://doi.org/10.1111/j.1532-5415.1995.tb06090.x.
Ucan Y. Effects of circuit resistance training on body composition and bone status in young males. Sport J. 2014.
Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc. 2002;50(6):1100–7. https://doi.org/10.1046/j.1532-5415.2002.50267.x.
Washburn RA, Kirk EP, Smith BK, et al. One set resistance training: effect on body composition in overweight young adults. J Sports Med Phys Fit. 2012;52(3):273–9.
Stevens J, Truesdale KP, McClain JE, et al. The definition of weight maintenance. Int J Obes. 2006;30(3):391–9. https://doi.org/10.1038/sj.ijo.0803175.
Keating SE, Johnson NA, Mielke GI, et al. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–64. https://doi.org/10.1111/obr.12536.
Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. https://doi.org/10.1136/bjsports-2017-097608.
Volek JS, Duncan ND, Mazzetti SA, et al. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Ex. 1999;31(8):1147–56. https://doi.org/10.1097/00005768-199908000-00011.
Bryner RW, Ullrich IH, Sauers J, et al. Effects of resistance vs. aerobic training combined with an 800 calorie liquid diet on lean body mass and resting metabolic rate. J Am Coll Nutr. 1999;18(2):115–21. https://doi.org/10.1080/07315724.1999.10718838.
Hunter GR, Byrne NM, Sirikul B, et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity. 2008;16(5):1045–51.
Dutton GR, Kim Y, Jacobs DR Jr, et al. 25-year weight gain in a racially balanced sample of U.S. adults: the CARDIA study. Obesity. 2016;24(9):1962–8. https://doi.org/10.1002/oby.21573.
Ismail I, Keating SE, Baker MK, et al. A systematic review and meta-analysis of the effect of aerobic vs resistance exercise training on visceral fat. Obes Rev. 2012;13(1):68–91. https://doi.org/10.1111/j.1467-789X.2011.00931.x.
Maillard F, Pereira B, Boisseau N. Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Med. 2018;48(2):269–88.
Horber FF, Thomi F, Casez JP, et al. Impact of hydration status on body composition as measured by dual energy X-ray absorptiometry in normal volunteers and patients on haemodialysis. Br J Radiol. 1992;65(778):895–900. https://doi.org/10.1259/0007-1285-65-778-895.
Børsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003;33(14):1037–60.
Arney BE, Foster C, Porcari J. EPOC: is it real and does it matter? ACSMs Health Fit J. 2019;23(4):9–13.
Schuenke MD, Mikat RP, McBride JM. Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: implications for body mass management. Eur J Appl Physiol. 2002;86(5):411–7.
Elliot DL, Goldberg L, Kuehl KS. Effect of resistance training on excess post-exercise oxygen consumption. J Strength Cond Res. 1992;6(2):77–81.
Thornton MK, Potteiger JA. Effects of resistance exercise bouts of different intensities but equal work on EPOC. Med Sci Sports Exerc. 2002;34(4):715–22.
Haddock BL, Wilkin LD. Resistance training volume and post exercise energy expenditure. Int J Sports Med. 2006;27(2):143–8.
Rustaden AM, Gjestvang C, Kari B, et al. Similar energy expenditure during BodyPump and heavy load resistance exercise in overweight women. Front Physiol. 2020;11:570.
Donnelly JE, Smith B, Jacobsen D, et al. The role of exercise for weight loss and maintenance. Best Pract Res Clin Gastroenterol. 2004;8(6):1009–29.
Laforgia J, Withers RT, Shipp NJ, et al. Comparison of energy expenditure elevations after submaximal and supramaximal running. J Appl Physiol (1985). 1997;82(2):661–6.
Laforgia J, Withers RT, Gore CJ. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J Sports Sci. 2006;24(12):1247–64.
Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36(3):239–62.
MacKenzie-Shalders K, Kelly JT, So D, et al. The effect of exercise interventions on resting metabolic rate: a systematic review and meta-analysis. J Sports Sci. 2020;38(14):1635–49.
Byrne HK, Wilmore JH. The effects of a 20-week exercise training program on resting metabolic rate in previously sedentary, moderately obese women. Int J Sport Nutr Exerc Metab. 2001;11(1):15–31.
Hunter GR, Seelhorst D, Snyder S. Comparison of metabolic and heart rate responses to super slow vs. traditional resistance training. J Strength Cond Res. 2003;17(1):76–81.
Farinatti P, Castinheiras Neto AG, da Silva NA. Influence of resistance training variables on excess postexercise oxygen consumption: a systematic review. ISRN Physiol. 2013. https://doi.org/10.1155/2013/825026.
Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–44. https://doi.org/10.1017/s0266462312000086.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this project. Michael Wewege was supported by a Postgraduate Scholarship from the National Health and Medical Research Council of Australia, a School of Medical Sciences Top-Up Scholarship from the University of New South Wales and a PhD Supplementary Scholarship from Neuroscience Research Australia. Imtiaz Desai was supported by a Scientia PhD Scholarship from the University of New South Wales. Hayley Leake was supported by an Australian Government Research Training Program Scholarship.
Conflict of interest
Michael Wewege, Imtiaz Desai, Cameron Honey, Brandon Coorie, Matthew Jones, Briana Clifford, Hayley Leake and Amanda Hagstrom declare that they have no conflicts of interest relevant to the content of this review.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data used in this study are available on the Open Science Framework (osf.io/hsk32). All R packages are available via the Comprehensive R Archive Network.
Code availability
The R script used in this study is available on the Open Science Framework (osf.io/hsk32).
Author contributions
ADH was responsible for the review design and team management, the literature search and drafting of the manuscript. All authors participated in screening and data extraction. MAW conducted the statistical analysis and reported its results. All authors approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wewege, M.A., Desai, I., Honey, C. et al. The Effect of Resistance Training in Healthy Adults on Body Fat Percentage, Fat Mass and Visceral Fat: A Systematic Review and Meta-Analysis. Sports Med 52, 287–300 (2022). https://doi.org/10.1007/s40279-021-01562-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01562-2