Abstract
Background
High-intensity interval training (HIIT) is promoted as a time-efficient strategy to improve body composition.
Objective
The aim of this meta-analysis was to assess the efficacy of HIIT in reducing total, abdominal, and visceral fat mass in normal-weight and overweight/obese adults.
Methods
Electronic databases were searched to identify all related articles on HIIT and fat mass. Stratified analysis was performed using the nature of HIIT (cycling versus running, target intensity), sex and/or body weight, and the methods of measuring body composition. Heterogeneity was also determined
Results
A total of 39 studies involving 617 subjects were included (mean age 38.8 years ± 14.4, 52% females). HIIT significantly reduced total (p = 0.003), abdominal (p = 0.007), and visceral (p = 0.018) fat mass, with no differences between the sexes. A comparison showed that running was more effective than cycling in reducing total and visceral fat mass. High-intensity (above 90% peak heart rate) training was more successful in reducing whole body adiposity, while lower intensities had a greater effect on changes in abdominal and visceral fat mass. Our analysis also indicated that only computed tomography scan or magnetic resonance imaging showed significant abdominal and/or visceral fat-mass loss after HIIT interventions.
Conclusion
HIIT is a time-efficient strategy to decrease fat-mass deposits, including those of abdominal and visceral fat mass. There was some evidence of the greater effectiveness of HIIT running versus cycling, but owing to the wide variety of protocols used and the lack of full details about cycling training, further comparisons need to be made. Large, multicenter, prospective studies are required to establish the best HIIT protocols for reducing fat mass according to subject characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
High-intensity interval training protocols are effective in decreasing fat-mass deposits, including abdominal and visceral fat mass. |
Comparison of running and cycling shows that running is potentially more successful in reducing total and visceral adipose tissues. |
1 Introduction
Currently, 2.1 billion individuals, approximately 30% of the world’s population, are overweight or obese [1]. The escalating obesity epidemic in the last decade has been accompanied by an increase in metabolic disorders such as insulin resistance, type 2 diabetes, and cardiovascular diseases. The World Health Organization defines overweight and obesity as an abnormal accumulation or excess of fat mass that can adversely affect health [2]. In addition, fat localization is a major determinant of the occurrence of metabolic disorders [3], with abdominal adipose tissue being particularly involved. It is also important to differentiate white adipose tissue in subcutaneous adipose tissue, which is characterized by high storage capacity, from visceral adipose tissue, which is metabolically more active. In visceral adipose tissue, greater lipolysis leads to higher free fatty acid secretion, which in turn results in ectopic deposits and/or direct transport to the liver by the portal vein. Visceral adipose tissue also releases several proinflammatory factors, including proinflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-6, IL-1β), hormones (leptin, resistin), and other molecules such as monocyte chemoattractant protein-1 (MCP-1), that participate in the establishment of chronic inflammation related to insulin resistance [4]. Visceral adipose tissue is therefore highly correlated with cardiovascular risks [5].
Against this background, effective fat-loss strategies, including dietary or physical activity interventions, or both, are required. In the short- and long-term, programs based on nutritional recommendations alone are less effective than those also including physical activity [6]. Current guidelines recommend moderate-intensity continuous training (MICT) [7], mainly because it can be maintained over a long period, thereby promoting fat mobilization and oxidation [8, 9]. MICT has positive cardiovascular and metabolic effects but it often leads to little or no fat loss [10, 11]. Conversely, emerging evidence on high-intensity interval training (HIIT) suggests that this exercise modality could lead to greater adipose tissue loss than low/moderate continuous training [12,13,14], and could more effectively reduce abdominal and visceral fat mass, which are the most dangerous fat deposits [15, 16]. Recent systematic reviews and meta-analyses have compared the effects of MICT versus HIIT on fat-mass loss; however, owing to the small number of comparative studies, abdominal and visceral fat mass have not been examined [13, 14]. Thus, the aim of the present meta-analysis was to assess the effectiveness of HIIT in reducing total, abdominal, and visceral fat mass in normal-weight and overweight/obese adults. We also analyzed the issues regarding the nature of HIIT (cycling versus running, target intensity), sex, body weight, and the methods used to measure body composition.
2 Methods
2.1 Literature Search Strategy
A systematic literature search of the PubMed and Google Scholar electronic databases, from January 1980 until July 2017, was conducted using the keywords ‘high-intensity interval training’, ‘high-intensity intermittent exercise’, and ‘aerobic interval training’. The reference list of the publications selected was also manually screened to detect references not found during the initial electronic search. Publications in English and French were retained for analysis.
2.2 Inclusion and Exclusion Criteria
2.2.1 Type of Interval Training
In the HIIT modality, short bursts of high-intensity exercise are alternated with periods of lower-intensity effort or complete rest for recovery [17]. In the last few years, HIIT has grown in popularity among athletes or as a strategy to counteract the adverse effects of metabolic disorders [18]. This has led to a wide range of terms to describe HIIT protocols, such as aerobic interval training (AIT) or high-intensity intermittent exercise (HIIE). Recently, Weston et al. suggested a simple classification of the different modalities based on exercise intensity [17]. Accordingly, the term HIIT should be used to design protocols with a target intensity ‘near the maximal’ effort (i.e. between 80 and 100% of the peak heart rate [PHR]), while sprint interval training (SIT) is more appropriate for ‘all out’ or ‘supramaximal’ efforts (≥ 100% maximal oxygen consumption, \({{\dot{V}}}\)O2max). In addition, physiological and metabolic adaptations are different in SIT and HIIT [19, 20]. For these reasons, we excluded from our analysis studies that involved SIT. When publications referred incorrectly to ‘SIT’ or ‘Wingate’ protocols (i.e. when subjects performed with an intensity level below 100% of the PHR), the data were nevertheless included. In our meta-analysis, only running, cycling and elliptical modalities were selected. There was no restriction regarding the duration of the protocol and the HIIT modality.
2.2.2 Type of Subjects
We decided to focus the review on adult subjects (age ≥ 18 years) because training adaptations and carbohydrate and lipid utilization in children and adolescents can be different. Subjects were not restricted by body mass index (BMI), sex, pathologies, or ethnic origins, but high-level athletes were not included.
2.2.3 Outcome Measures
The primary outcome was total body fat mass (kg), while secondary outcomes were abdominal and visceral fat mass (with different units as grams, percentages, cm2, or cm3).
2.3 Data Collection or Data Synthesis
The first author (FM) extracted data from studies, with advice from NB on selection criteria. First, the title and abstract were screened, and then, if data were missing or interesting, the full text was analyzed; if it met our criteria, data were extracted. A request for missing data (total fat mass, abdominal fat mass, visceral fat mass, number of male/female subjects before and after the protocol, BMI, and age at the beginning of the study) was sent to corresponding authors when appropriate.
2.4 Statistical Analysis
After extraction, the data were compiled into software designed specifically for meta-analyses (Comprehensive Meta-Analysis, version 2; Biostat, Englewood, NJ, USA). Data included were sample size, and pre- and post-intervention values. The standardized mean differences (paired SMD) were calculated to determine Cohen’s d for each study, and Hedges’ g was used to account for potential bias in small sample sizes. Effect sizes (ESs) were calculated using a random-effects model (DerSimonian and Laird approach) that accounts for true variation in effects occurring from study to study and for random errors within a single study. The random-effects model was preferred to a fixed-effect model as certain experimental parameters had wide variation. The ESs were interpreted according to Cohen, i.e. < 0.2 as trivial, 0.2–0.3 as small, 0.5 as moderate, and > 0.8 as large [21]. A negative ES value indicates that exercise decreased outcomes, while a positive ES indicates that exercise increased outcomes. The I 2 index was used to measure heterogeneity, with 25, 50, and 75% indicating low, moderate, and high heterogeneity, respectively. In stratified analysis, we arbitrarily chose high-intensity levels as target intensities above 90% of PHR (and low intensity below 90% PHR). To test sensitivity and whether results were biased by a particular study, the analyses were conducted by excluding one study at a time. Funnel plots were used to assess publication bias. In the absence of bias, studies should be distributed evenly around the mean ES because of random sampling error. A meta-regression was performed to measure the impact of sex on variation of parameters.
3 Results
3.1 Study Selection
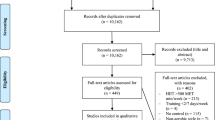
The search strategy identified 1156 articles from electronic databases, and six other articles were found manually. In total, 360 publications were excluded because of duplicate keywords or after title and/or abstract analysis. Of the remaining articles, 45 fulfilled our inclusion criteria. Among the 34 authors contacted for further details, 11 did not respond, therefore their publications were excluded. The final number of publications included in our meta-analysis was 39 (35 for total fat mass, 20 for abdominal fat mass, and 14 for visceral fat mass) (Fig. 1).
3.2 Subject Characteristics
Subject characteristics are summarized in Table 1. Overall, 617 subjects were included in the meta-analysis. Four studies gave no sex breakdown, and, in the remaining studies (n = 35) there were more women (321) than men (217). In accordance with the inclusion criteria, subjects were adults, with a mean age ranging from 19.8 ± 0.8 to 69 ± 2.8 years. All but two studies recruited overweight or obese subjects, whose BMI ranged from 25.4 ± 2.4 to 38.2 ± 7.9 kg/m2. Some subjects had conditions that could have influenced the effects of physical activity: type 2 diabetes (n = 6), polycystic ovary syndrome (n = 2), hormonal state (menopause; n = 2), non-alcoholic fatty liver disease (n = 1), metabolic syndrome (n = 5), and rheumatic disease (n = 1).
3.3 High-Intensity Interval Training Program Characteristics
The HIIT programs are summarized in Table 2. Of the HIIT studies, 26 used cycling and 13 used running, with 4 studies offering a choice between the two. Only one study tested an elliptical modality. The most widely used protocol consisted of alternate 4 min at high intensity followed by 3 min of recovery (n = 12). Other programs used shorter times (8 s or 1 min) at high intensity. When specified, recovery was active in all but one study. The programs ranged in duration from 4 weeks to 6 months but generally lasted at least 12 weeks. Except in nine protocols, there were three HIIT sessions a week. Half of the studies used high-intensity training, defined in this meta-analysis as exercise at intensities above 90% PHR.
3.4 Body Composition Assessments
Most of the studies (n = 30) used a dual-energy X-ray absorptiometry (DXA; the ‘gold standard’ method) to determine whole-body fat mass. Others used less accurate and/or repeatable methods, such as impedance (n = 11), plethysmography (n = 2) or skinfold measurements (n = 2), of which the last was recognized as the least reliable. Computed tomography (CT) scan and magnetic resonance imaging (MRI) were widely used to assess abdominal or visceral fat mass. The most recent DXA scans can also measure abdominal fat mass in different anatomical regions [22, 23] and estimate visceral fat-mass content [24, 25].
3.5 Meta-Analysis
3.5.1 Total Fat Mass
As shown in Fig. 2a, HIIT resulted in a reduction of total fat mass (ES − 0.2, 95% confidence interval [CI] − 0.31 to − 0.07, I 2 = 0.0%) of approximately 2 kg. Stratified analysis of exercise modalities showed that running (ES − 0.34, 95% CI − 0.56 to − 0.12, I 2 = 0.0%) was more effective than cycling (ES − 0.13, 95% CI − 0.3 to 0.04, I 2 = 0.0%) in decreasing total fat mass (Fig. 2b). The greatest HIIT effect was observed with protocols using high-intensity exercises (i.e. > 90% PHR) [ES − 0.21, 95% CI − 0.38 to − 0.04, I 2 = 0.0%]; however, a trend was observed for low-intensity programs (ES − 0.18, 95% CI − 0.37 to 0.01, I 2 = 14.5%) (Fig. 2c). Comparison of normal-weight and overweight/obese subjects showed that HIIT protocols decreased total fat mass only in patients with excess adiposity (ES 0.34, 95% CI − 0.29 to 0.96, I 2 = 77.7%; ES − 0.21, 95% CI − 0.34 to − 0.08, I 2 = 0.0%; respectively) (Fig. 2d). When normal-weight subjects were excluded from the analysis and only overweight/obese subjects were taken into consideration, significance persisted and was improved. There was no difference between male and female subjects in HIIT-induced fat-mass loss (p = 0.34).
Forest plot for the HIIT effect on (a) total fat mass (kg), (b) with stratified analysis of exercise modalities, (c) intensities, and (d) body weight. 1 and 2 represent the same study but different HIIT protocols. CI confidence interval, HIIT high-intensity interval training, NR not reported, Std standardized
3.5.2 Abdominal Fat Mass
Figure 3a shows that HIIT reduced abdominal fat mass (ES − 0.19, 95% CI − 0.32 to − 0.05, I 2 = 0.0%). The first stratified analysis of exercise modalities showed that, in contrast to results on total fat mass, cycling was more effective in decreasing abdominal fat mass (ES − 0.24, 95% CI − 0.40 to − 0.08, I 2 = 58.1%) than running (ES − 0.05, 95% CI − 0.41 to 0.31, I 2 = 0.0%) (Fig. 3b). The second stratified analysis, involving method assessment, showed that CT scan (ES − 0.33, 95% CI − 0.56 to − 0.1, I 2 = 0.0%) detected more reductions in abdominal fat mass after HIIT than DXA scan (ES − 0.12, 95% CI − 0.30 to 0.06, I 2 = 0.0%) and impedance (ES − 0.08, 95% CI − 0.51 to 0.35, I 2 = 0.0%) (Fig. 3c). Low-intensity training reduced abdominal adiposity (ES − 0.21, 95% CI − 0.40 to − 0.02, I 2 = 0.0%), but no effect was observed at higher intensities (ES − 0.18, 95% CI − 0.41 to 0.05, I 2 = 17.0%) (Fig. 3d). HIIT decreased abdominal fat mass in overweight/obese subjects only (ES − 0.19, 95% CI − 0.33 to − 0.05, I 2 = 0.0%) (Fig. 3e). When normal-weight subjects were excluded from the analysis and only overweight/obese subjects were taken into consideration, significance persisted and was improved. There was no difference between male and female subjects in HIIT-induced abdominal fat-mass loss (p = 0.70).
Forest plot for the HIIT effect on (a) abdominal fat mass, (b) with stratified analysis of exercise modalities, (c) methods of measuring body composition, (d) intensities, and (e) body weight. 1–4 represent the same study but different HIIT protocols. CI confidence interval, CT computed tomography, DXA dual-energy X-ray absorptiometry, HIIT high-intensity interval training, MRI magnetic resonance imaging, NR not reported, Std standardized
3.5.3 Visceral Fat Mass
As shown in Fig. 4a, HIIT decreased visceral fat mass (ES − 0.24, 95% CI − 0.44 to − 0.04, I 2 = 0.0%). A stratified analysis showed that only running reduced visceral fat mass (ES − 0.44, 95% CI − 0.86 to − 0.02, I 2 = 0.0%), while a trend was observed for cycling (ES − 0.21, 95% CI − 0.46 to 0.04, I 2 = 0.0%) (Fig. 4b). Another stratified analysis showed that no study with DXA scan (ES − 0.30, 95% CI − 0.57 to 0.51, I 2 = 0.0%) resulted in visceral fat-mass changes (ES − 0.30, 95% CI − 0.52 to − 0.07, I 2 = 0.0%) (Fig. 4c). As observed for abdominal fat mass, protocols using intensities <90% PHR decreased visceral fat mass (ES − 0.31, 95% CI − 0.57 to − 0.05, I 2 = 0.0%), but not at an intensity above 90% PHR (ES − 0.13, 95% CI − 0.47 to 0.22, I 2 = 0.0%) (Fig. 4d). HIIT was only successful in overweight/obese subjects (ES − 0.26, 95% CI − 0.47 to − 0.05, I 2 = 0.0%) (Fig. 4e). As reported for total and abdominal fat mass, when normal-weight subjects were excluded from the analysis and only overweight/obese subjects were taken into consideration, significance persisted and was improved. There was no difference between male and female subjects in HIIT-induced visceral fat-mass loss (p = 0.69).
Forest plot for the HIIT effect on (a) visceral fat mass, (b) with stratified analysis of exercise modalities, (c) methods of measuring body composition, (d) intensities, and (e) body weight. 1 and 2 represent the same study but different HIIT protocols. CI confidence interval, CT computed tomography, DXA dual-energy X-ray absorptiometry, HIIT high-intensity interval training, MRI magnetic resonance imaging, NR not reported, Std standardized
4 Discussion
The present study is the first meta-analysis to investigate the effect of HIIT interventions on total, abdominal, and visceral adipose tissues in non-athlete subjects. The review involved 617 subjects (48% male and 52% female, mean age 38.8 ± 14.4 years, mean BMI 30.3 ± 4.0 kg/m2) included in 39 studies (35 evaluating total fat mass, 19 abdominal fat mass, and 14 visceral fat mass). Only two studies were performed with normal-weight subjects, with the others involving overweight or obese patients. Our results showed that HIIT programs are effective in significantly reducing total, abdominal, and visceral fat mass in both males and females. These beneficial effects only occurred in overweight and obese subjects. Comparisons of running and cycling indicated that running is more effective in reducing total and visceral fat mass. High intensities (above 90% of PHR) seem more likely to reduce whole-body adiposity and lower intensities more successful in reducing abdominal and visceral fat mass. Finally, our analysis demonstrated that only CT scan or MRI studies showed significant abdominal and/or visceral fat-mass changes after HIIT interventions.
The HIIT modality is well tolerated, safe, and is a time-efficient strategy for improving patient health [26]; however, it should not be proposed to patients with uncontrolled type 2 diabetes or hypertension, or after recent cardiac events [17]. For these patients, and for individuals with a high level of sedentary life/inactivity, the American College of Sports Medicine (ACSM) recommends reaching a ‘base fitness level’ by 20- to 60-min sessions, three to five times prior to beginning any training program [7].
The primary finding of our analysis is that HIIT significantly reduces whole-body fat mass. This result is in agreement with the results of recent reviews by Wewege et al. [14], who reported a mean loss of approximately 2 kg after HIIT protocols, and Keating et al. [13], who reported a loss of approximately 6% of body weight. The main objective of these two meta-analyses was to compare the effects of HIIT versus MICT, or HIIT/SIT versus MICT, on whole-body fat mass; however, owing to the comparative nature of their reviews, the number of studies analyzed was much smaller (n = 13 and n = 28, respectively) than in our meta-analysis (n = 35). In addition, these reviews did not perform a meta-analysis of HIIT-induced abdominal and/or visceral fat-mass changes and included no specific results regarding the effects of sex and body adiposity.
HIIT or SIT protocols were prima facie used in high-level athletes for increasing \({\dot{V}}\)O2max and/or reducing the percentage of fat mass before a competition [27, 28]. The use of HIIT interventions in overweight/obese patients is more recent and interest in HIIT-induced fat-mass loss in normal-weight subjects is still limited. This probably explains the small number of studies (n = 2) in our meta-analysis dealing with normal-weight patients. With these limitations (only two HIIT modalities, 21 subjects tested), no significant effect emerged from our analysis of HIIT-induced total fat-mass loss in this population. The sensitivity test performed (excluding normal-weight subjects) confirmed the finding that HIIT is more likely to decrease total fat-mass loss in overweight/obese subjects (overall: p = 0.003; overweight/obese subjects only: p = 0.001).
The second finding was the impact of HIIT programs on abdominal and visceral fat mass. Documented reports have shown that effective abdominal/visceral fat-loss strategies should include a hypocaloric diet or physical activity, or both [29]; however, the best results are obtained when the two strategies are combined [6]. The recent meta-analysis of Verheggen et al. [30] confirmed that diet or training alone can significantly alter visceral fat mass, but generally to a greater extent overall with exercise (p = 0.08). In this review [30], 117 studies (4815 subjects) were included and, in the absence of weight loss, the authors showed that exercise is still related to a 6.1% decrease in visceral adipose tissue. In the literature, of the 12 publications that compared the effects of HIIT versus MICT on abdominal/visceral fat-mass loss, only 6 reported an effect of HIIT or a greater effect of this modality, 3 reported an equivalent effect, and 3 did not find any significant difference between the two modalities. In our review, we separately assessed the effects of HIIT on abdominal (18 studies) and visceral (14 studies) adipose tissues using different methods, including DXA, CT scan, and MRI. One drawback of the present meta-analysis with regard to the assessment of abdominal fat was the region chosen by the authors to represent ‘abdominal adiposity’. Three areas, designated ‘abdominal’ (n = 14), ‘trunk’ (n = 12), or ‘android region or area’ (n = 6), were used in the publication analyzed, but most of the time represented different anatomic regions. In relation to visceral fat mass, the data were more homogeneous since most of the studies analyzed the L4-L5 junction. Our results showed that HIIT significantly reduced abdominal (p = 0.007) and visceral (p = 0.018) fat mass, with no difference between males and females. When the statistical analysis was performed in only normal-weight subjects, the results did not show any effect of HIIT on abdominal/visceral adipose tissue. However, the analyses of abdominal and visceral fat mass related to only three and two publications, respectively. In conclusion, HIIT is an efficient method to reduce central adiposity, at least in overweight/obese patients, which suggests that it could favorably contribute to decreasing the risks of cardiovascular disease. Furthermore, the effects of HIIT, when compared with those of MICT, seem more likely to decrease abdominal/visceral adipose tissue than endurance training [16, 31]. However, additional research is required to fully understand the mechanisms underlying abdominal/visceral fat reduction induced by HIIT programs.
The third finding to emerge from this meta-analysis was that running is more effective than cycling in reducing total and visceral fat mass, and that cycling is more successful in decreasing total abdominal fat mass. Physiological, metabolic and ergogenic responses differ between running and cycling. Running promotes larger muscle mass than cycling and the type of muscle contraction during running (concentric and eccentric) contributes to greater fat oxidation at the same relative intensity [32, 33]. In addition, excess post-exercise oxygen consumption (EPOC) is greater (+ 37%) after a running session than after a cycling session, as shown in the study by Cunha et al. comparing HIIT and MICT treadmill protocol in overweight men (exercises performed at 75% of oxygen uptake reserve, running session corresponding to 400 kcal, and interval training including two series of 200 kcal) [34]. In addition, plasma lactate concentrations are higher in cycling [34], which reflects greater carbohydrate utilization [33]. Together, these results could explain the greater effect of running on decreasing total fat mass. However, it is more difficult to explain the impact of running and cycling on abdominal and visceral adipose tissues. One of the potential explanations could be the release of catecholamines. During high-intensity exercise (i.e. > 65% \({{\dot{V}O}}\) 2max), catecholamine responses significantly increase [35], which favors lipolysis via β-adrenergic receptors. The total abdominal area includes subcutaneous plus visceral adipose tissue. As the content of β-adrenergic receptors is higher in visceral than in subcutaneous adipose tissue [36], greater activation of the sympathetic nervous system (by noradrenaline release) during HIIT running could explain why there is a higher reliance on visceral adipose tissue than with a cycling protocol. However, little is known about the differences in catecholamine production during cycling and running performed at the same relative intensity, especially in overweight/obese subjects. Davies et al. [37] reported that catecholamine secretion was proportional to the muscle mass involved during exercise, a result at variance with the study of Nieman et al. [38], who observed no differences in catecholamine production between these two modalities. It is likely that the patients’ habits (whether they go cycling and/or running, or even walking, regularly or not) could interfere with the results, as indicated by the great heterogeneity of our meta-analysis results regarding HIIT running-induced abdominal fat-mass loss. To conclude on this point, while our statistical analysis of 35 studies indicates that running is more effective than cycling in reducing whole-body fat mass (in part owing to the greater muscle mass involved and the higher post-exercise oxygen consumption), the choice of ‘the best’ modality to achieve higher abdominal and/or visceral fat-mass loss remains to be elucidated and could be patient-dependent if related to catecholamine responses. The lack of information regarding cycling programs, such as cycle ergometer used, revolutions per minute, resistance, watts, and heart rate, and running protocols, such as speed, gradient treadmill, and heart rate, sometimes make it difficult to compare the two modalities or studies using the same modality. Future studies with fuller details of the method used are needed to establish the best HIIT protocol to achieve total and abdominal/visceral fat-mass loss.
Three other parameters were taken into account in this meta-analysis. The first was the potential influence of sex difference. No sex-related effect was found for HIIT-induced reduction in total and abdominal or visceral fat mass. A meta-analysis of Vissers et al. [39] showed that physical activity (resistance or endurance training) in general had a greater impact on total and visceral fat mass in males than females according to their obesity phenotype: abdominal obesity in men and gynoid obesity in females, at least before menopause. However, only one study is available regarding sex differences in HIIT-induced fat-mass loss. The authors found a sex-related effect with a greater effect observed in males, but body composition was determined by impedance, which is not the most reliable method [40]. Thus, additional studies are still necessary, particularly comparisons of pre- and postmenopausal women, to draw any meaningful conclusions.
The second parameter was the method used to assess abdominal and visceral fat mass. Our analysis showed that only CT scan or MRI showed significant abdominal and/or visceral fat-mass changes after HIIT interventions. DXA scan is a ‘gold-standard’ instrument for measuring total fat mass, but, as shown by Shuster et al. [41], it is probably not the best method for assessing abdominal and, more particularly, visceral fat mass. Harmonizing the methods for measuring abdominal and visceral fat mass would help in the future to determine the real impact of HIIT on ‘central obesity’.
The last parameter related to the intensity of the HIIT protocol. An HIIT program comprises eight main components: peak workload intensity, peak workload duration, recovery load, recovery duration, number of repetitions and series, and duration and intensity phases between series [42]. Endless combinations are possible and the isolated manipulation of each variable might differently affect the acute or chronic physiological responses [43]. Nevertheless, we arbitrarily chose to separate high and lower intensities using the threshold of 90% PHR. With this criterion, our meta-analysis showed that intensities above 90% PHR are more effective than lower intensities in reducing whole-body adiposity. In contrast, intensities below 90% PHR are more likely to decrease abdominal and visceral fat mass. In women aged 18–34 years, only a high intensity (defined as heart rate around 160 and 165) has been shown to decrease total fat mass, and no effect was observed with lower intensities (heart rate around 150 and 160) [44]. The same result was found in untrained, middle-aged Korean females performing high- (≥ 70% \({{\dot{V}O}}\) 2max) or low-intensity (50% \({{\dot{V}O}}\) 2max) exercises over 14 weeks [45]. With regard to abdominal and visceral fat mass, results in our study might appear surprising. The meta-analysis of Vissers et al. [39] on the effect of exercise on visceral fat mass in overweight adults suggested an intensity threshold and advised moderate (45–55% \({{\dot{V}O}}\) 2max) to vigorous (≥ 70% \({{\dot{V}O}}\) 2max) exercise intensity to significantly decrease visceral fat mass [39]. In our meta-analysis, no moderate-intensity training was taken into account (i.e. low- to moderate-intensity interval training), and the lower intensities were still between 80 and 90% PHR owing to the threshold chosen. This may partly explain the results observed for abdominal/visceral fat mass since the duration of HIIT was generally longer when the intensity was lower. At these intensities, catecholamine release is still high and promotes lipolysis during exercise and fat oxidation during the recovery period.
5 Conclusions
Variations in the intensity and duration of the active and recovery periods, number of repetitions and series of the HIIT protocols combined with the lack of details regarding the cycling or running HIIT protocol itself make it difficult to analyze HIIT-induced fat-mass loss. Nevertheless, the results obtained with a wide range of HIIT protocols involving normal-weight and overweight/obese subjects suggest that HIIT, especially running, is a time-efficient strategy to decrease fat-mass deposits, including abdominal and visceral fat mass. Large, multicenter, prospective studies are required to establish the optimal HIIT protocols to reduce fat mass according to subject characteristics, such as age, sex, body adiposity, and metabolic disorders.
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–81.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech Rep Ser. 2000;894:i–xii, 1–253.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738.
Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol Oxf. 2012;205:194–208.
Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
Johns DJ, Hartmann-Boyce J, Jebb SA, et al. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114:1557–68.
Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71.
Lazzer S, Tringali G, Caccavale M, et al. Effects of high-intensity interval training on physical capacities and substrate oxidation rate in obese adolescents. J Endocrinol Investig. 2017;40:217–26.
Maurie J, Brun J, Jean E, et al. Comparaison de deux modalités différentes d’activité physique (SWEET et Lipoxmax) chez des diabétiques de type 2. Sci Sports. 2011;26:92–6.
Shaw K, Gennat H, O’Rourke P, et al. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD003817.
Wu T, Gao X, Chen M, et al. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313–23.
Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes. 2011;2011:868305.
Keating SE, Johnson NA, Mielke GI, et al. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–64.
Wewege M, Van den berg R, Ward RE, et al. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18:635–46.
Trapp EG, Chisholm DJ, Freund J, et al. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes. 2005;2008(32):684–91.
Maillard F, Rousset S, Pereira B, et al. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab. 2016;42:433–41.
Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48:1227–34.
Azuma K, Matsumoto H. Potential universal application of high-intensity interval training from athletes and sports lovers to patients. Keio J Med. 2017;66:19–24.
Granata C, Oliveira RSF, Little JP, et al. Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J. 2016;30:959–70.
MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595:2915–30.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1998.
Gillen JB, Percival ME, Ludzki A, et al. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obes Silver Spring. 2013;21:2249–55.
Hutchison SK, Stepto NK, Harrison CL, et al. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:E48–56.
Fex A, Leduc-Gaudet J-P, Filion M-E, et al. Effect of elliptical high intensity interval training on metabolic risk factor in pre and type 2 diabetes patients a pilot study. J Phys Act Health. 2015;12:942–6.
Sawyer BJ, Tucker WJ, Bhammar DM, et al. Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. J Appl Physiol. 1985;2016(121):279–88.
Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42:489–509.
Gibala MJ, Jones AM. Physiological and performance adaptations to high-intensity interval training. Nestle Nutr Inst Workshop Ser. 2013;76:51–60.
Ribeiro RL, de Oliveira Ítalo, Silva J, Dantas M, et al. High-intensity interval training applied in Brazilian Jiu-jitsu is more effective to improve athletic performance and body composition. J Combat Sports Martial Arts. 2015;6:1–5.
Drenowatz C, Hand GA, Sagner M, et al. The prospective association between different types of exercise and body composition. Med Sci Sports Exerc. 2015;47:2535–41.
Verheggen RJHM, Maessen MFH, Green DJ, et al. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17:664–90.
Terada T, Friesen A, Chahal BS, et al. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract. 2013;99:120–9.
Capostagno B, Bosch A. Higher fat oxidation in running than cycling at the same exercise intensities. Int J Sport Nutr Exerc Metab. 2010;20:44–55.
Knechtle B, Müller G, Willmann F, et al. Fat oxidation in men and women endurance athletes in running and cycling. Int J Sports Med. 2004;25:38–44.
Cunha FA, Midgley AW, McNaughton LR, et al. Effect of continuous and intermittent bouts of isocaloric cycling and running exercise on excess postexercise oxygen consumption. J Sci Med Sport. 2016;19:187–92.
Zouhal H, Jacob C, Delamarche P, et al. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38:401–23.
Rebuffé-Scrive M, Andersson B, Olbe L, et al. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989;38:453–8.
Davies CT, Few J, Foster KG, et al. Plasma catecholamine concentration during dynamic exercise involving different muscle groups. Eur J Appl Physiol. 1974;32:195–206.
Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, et al. Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. J Appl Physiol. 1985;1998(84):1252–9.
Vissers D, Hens W, Taeymans J, et al. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PloS One. 2013;8:e56415.
Morikawa M, Okazaki K, Masuki S, et al. Physical fitness and indices of lifestyle-related diseases before and after interval walking training in middle-aged and older males and females. Br J Sports Med. 2011;45:216–24.
Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10.
Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle. Part II: anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013;43:927–54.
Gosselin LE, Kozlowski KF, DeVinney-Boymel L, et al. Metabolic response of different high-intensity aerobic interval exercise protocols. J Strength Cond Res. 2012;26:2866–71.
Bryner RW, Toffle RC, Ullrich IH, et al. The effects of exercise intensity on body composition, weight loss, and dietary composition in women. J Am Coll Nutr. 1997;16:68–73.
Lee M-G, Park K-S, Kim D-U, et al. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl Physiol Nutr Metab. 2012;37:1019–27.
Ahmadizad S, Avansar AS, Ebrahim K, et al. The effects of short-term high-intensity interval training vs. moderate-intensity continuous training on plasma levels of nesfatin-1 and inflammatory markers. Horm Mol Biol Clin Investig. 2015;21:165–73.
Almenning I, Rieber-Mohn A, Lundgren KM, et al. Effects of high intensity interval training and strength training on metabolic, cardiovascular and hormonal outcomes in women with polycystic ovary syndrome: apilot study. PloS One. 2015;10:e0138793.
Arad AD, DiMenna FJ, Thomas N, et al. High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J Appl Physiol. 1985;2015(119):352–62.
Cassidy S, Thoma C, Hallsworth K, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2016;59:56–66.
Coquart JBJ, Lemaire C, Dubart A-E, et al. Intermittent versus continuous exercise: effects of perceptually lower exercise in obese women. Med Sci Sports Exerc. 2008;40:1546–53.
Eimarieskandari R, Zilaeibouri S, Zilaeibouri M, et al. Comparing two modes of exercise training with different intensity on body composition in obese young girls. Sci Mov Health. 2012;12:473–8.
Fisher G, Brown AW, Bohan Brown MM, et al. High intensity interval vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PloS One. 2015;10:e0138853.
Gahreman D, Heydari M, Boutcher Y, et al. The effect of green tea ingestion and interval sprinting exercise on the body composition of overweight males: a randomized trial. Nutrients. 2016;8:510.
Guadalupe-Grau A, Fernández-Elías VE, Ortega JF, et al. Effects of 6-month aerobic interval training on skeletal muscle metabolism in middle-aged metabolic syndrome patients. Scand J Med Sci Sports. 2017;. https://doi.org/10.1111/sms.12881.
Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci Lond. 2015;129:1097–105.
Heydari M, Freund J, Boutcher SH. The effect of high-intensity intermittent exercise on body composition of overweight young males. J Obes. 2012;2012:480467.
Hornbuckle LM, McKenzie MJ, Whitt-Glover MC. Effects of high-intensity interval training on cardiometabolic risk in overweight and obese African–American women: a pilot study. Ethn Health. 2017;1:1–15.
Hwang C-L, Yoo J-K, Kim H-K, et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–9.
Karstoft K, Winding K, Knudsen SH, et al. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36:228–36.
Kong Z, Sun S, Liu M, et al. Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J Diabetes Res. 2016;2016:4073618.
Martins C, Kazakova I, Ludviksen M, et al. High-intensity interval training and isocaloric moderate-intensity continuous training result in similar improvements in body composition and fitness in obese individuals. Int J Sport Nutr Exerc Metab. 2016;26:197–204.
Matinhomaee H, Banaei J, Azarbayjani MA, et al. Effects of 12-week high-intensity interval training on plasma visfatin concentration and insulin resistance in overweight men. J Exerc Sci Fit. 2014;12:20–5.
Nikseresht M, Agha-Alinejad H, Azarbayjani MA, et al. Effects of nonlinear resistance and aerobic interval training on cytokines and insulin resistance in sedentary men who are obese. J Strength Cond Res. 2014;28:2560–8.
Panissa VLG, Julio UF, França V, et al. Sex-related differences in self-paced all out high-intensity intermittent cycling: mechanical and physiological responses. J Sports Sci Med. 2016;15:372–8.
Ramos JS, Dalleck LC, Borrani F, et al. The effect of different volumes of high-intensity interval training on proinsulin in participants with the metabolic syndrome: a randomised trial. Diabetologia. 2016;59:2308–20.
Sandstad J, Stensvold D, Hoff M, et al. The effects of high intensity interval training in women with rheumatic disease: a pilot study. Eur J Appl Physiol. 2015;115:2081–9.
Sasaki H, Morishima T, Hasegawa Y, et al. 4 weeks of high-intensity interval training does not alter the exercise-induced growth hormone response in sedentary men. SpringerPlus. 2014;3:336.
Schjerve IE, Tyldum GA, Tjønna AE, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci (Lond). 1979;2008(115):283–93.
Shepherd SO, Wilson OJ, Taylor AS, et al. Low-volume high-intensity interval training in a gym setting improves cardio-metabolic and psychological health. PloS One. 2015;10:e0139056.
Smith-Ryan AE, Melvin MN, Wingfield HL. High-intensity interval training: modulating interval duration in overweight/obese men. Phys Sports Med. 2015;43:107–13.
Steckling FM, Farinha JB, Santos DLD, et al. High intensity interval training reduces the levels of serum inflammatory cytokine on women with metabolic syndrome. Exp Clin Endocrinol Diabetes Off. 2016;124:597–601.
Stensvold D, Tjønna AE, Skaug E-A, et al. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 1985;2010(108):804–10.
Tjønna AE, Leinan IM, Bartnes AT, et al. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PloS One. 2013;8:e65382.
Wallman K, Plant LA, Rakimov B, et al. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight population. Sports Med. 2009;17:156–70.
Zhang H, Tong TK, Qiu W, et al. Effect of high-intensity interval training protocol on abdominal fat reduction in overweight Chinese women: a randomized controlled trial. Kinesiology. 2015;47:57–66.
Zhang H, Tong TK, Qiu W, et al. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res. 2017;2017:5071740.
Ziemann E, Grzywacz T, Łuszczyk M, et al. Aerobic and anaerobic changes with high-intensity interval training in active college-aged men. J Strength Cond Res. 2011;25:1104–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Florie Maillard, Bruno Pereira and Nathalie Boisseau declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Maillard, F., Pereira, B. & Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sports Med 48, 269–288 (2018). https://doi.org/10.1007/s40279-017-0807-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-017-0807-y