Abstract
Background
No previous systematic review has quantitatively examined the association between muscular fitness during childhood and adolescence and health parameters later in life.
Objective
The aim was to systematically review and meta-analyze the current evidence for a prospective association between muscular fitness in childhood and adolescence and future health status.
Methods
Two authors systematically searched MEDLINE, EMBASE and SPORTDiscus electronic databases and conducted manual searching of reference lists of selected articles. Relevant articles were identified by the following criteria: apparently healthy children and adolescents aged 3–18 years with muscular fitness assessed at baseline (e.g., handgrip, standing long jump, sit-ups, among others), and a follow-up period of ≥ 1 year. The outcome measures were anthropometric and adiposity measurements and cardiometabolic, bone and musculoskeletal health parameters. Two authors independently extracted data.
Results
Thirty studies were included in the meta-analysis, yielding a total of 21,686 participants. The meta-analysis found a significant, moderate-large (p < 0.05) effect size between muscular fitness at baseline and body mass index (r = − 0.14; 95% confidence interval (CI) − 0.21 to − 0.07), skinfold thickness (r = − 0.32; 95% CI − 0.40 to − 0.23), homeostasis model assessment estimated insulin resistance (r = − 0.10; 95% CI − 0.16 to − 0.05), triglycerides (r = − 0.22; 95% CI − 0.30 to − 0.13), cardiovascular disease risk score (r = − 0.29; 95% CI − 0.39 to − 0.18), and bone mineral density (r = 0.166; 95% CI 0.086 to 0.243) at follow-up.
Conclusion
A prospective negative association was observed between muscular fitness in childhood/adolescence and adiposity and cardiometabolic parameters in later life, together with a positive association for bone health. There is inconclusive evidence for low back pain benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A high level of physical fitness in childhood and adolescence is associated with lower body mass index, skinfold thickness, homeostasis model assessment estimated insulin resistance, triglycerides, cardiovascular disease risk score and higher bone mineral density values later in life. |
There is no convincing evidence linking a high level of muscular fitness with low back pain later in life. |
The effect sizes reported using endurance (push-ups, sit-ups, bent arm hang, etc.) or strength tests (handgrip, standing long jump, vertical jump, etc.) were similar. |
1 Introduction
Physical fitness in childhood and adolescence is considered an important health indicator [1]. The fitness components that have been shown to directly relate to improvements in health include cardiorespiratory fitness (CRF), muscular fitness, local muscular endurance, and body composition [2, 3]. Specifically, muscular fitness is an important marker of health throughout life [1], and a valuable indicator for monitoring child [4] and adolescent health [5]. The World Health Organization and the United States Department of Health and Human Services support the promotion of muscle-strengthening activities in addition to aerobic activity as part of their physical activity guidelines for children and adolescents [6, 7]. In spite of this, however, recent evidence indicates that the muscular fitness levels of school-age youth are decreasing [8, 9], and it has been proposed that monitoring temporal trends in muscular fitness could support the development of health-promotion strategies.
Low muscular fitness is recognized as a strong marker of poor metabolic profile during childhood and adolescence and is associated with several non-communicable diseases [1], and also with mortality in adulthood [10]. Indeed, several cross-sectional studies support a strong inverse relationship between low muscular fitness and cardiovascular disease (CVD) risk factors and metabolic syndrome in young people [5, 11]. Observational studies have shown that youth with low levels of muscular fitness are at increased risk of maintaining a low muscular fitness level into adulthood [12]. Despite these recent findings, the importance of muscle fitness and its association with future health is less clear. A previous systematic review by Ruiz et al. [3] found that muscular fitness is strongly associated with overall adiposity, but also found inconclusive evidence for cardiometabolic outcomes. In a similar vein, another systematic review reported a strong association between muscular fitness, total and central adiposity, cardiometabolic outcomes and bone health among youth [13]; however, this study included cross-sectional and longitudinal data.
Although there have been reviews of the benefits of health-related fitness in youth [3, 13], to our knowledge, no previous systematic review has quantitatively examined (meta-analyzed) the association between muscular fitness during childhood and adolescence and health parameters later in life. Therefore, our aim was to systematically review and meta-analyze the current evidence for a prospective association between muscular fitness in childhood and adolescence and future health status.
2 Methods
A systematic review and meta-analysis was conducted following the guidelines of the Cochrane Collaboration [14]. Findings were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15]. The review was registered in PROSPERO (registration number: CRD42018111352).
2.1 Search Strategy
Two authors (AG-H and RR-C) systematically searched MEDLINE, EMBASE, and SPORTDiscus electronic databases from inception until 1 October 2018 (Electronic Supplementary Material (ESM) Appendix S1). The following terms were used: ‘muscles’ OR ‘muscle strength’ OR ‘muscular fitness’ OR ‘muscular’ OR ‘strength’ AND ‘longitudinal’ OR ‘prospective’. Searching was restricted to published articles in the English language.
2.2 Selection Criteria
The a priori inclusion criteria for this meta-analysis were as follows: (1) exposure: muscular fitness measured using a muscular fitness test (e.g., handgrip, standing long jump, sit-ups, etc.) and assessed at baseline; (2) participants: generally healthy population and at baseline were aged 3 up to 18 years (mean age); and (3) study design: prospective cohort studies with a follow-up period of ≥ 1 year. The outcomes measures included were classified into the following four categories: (1) anthropometric and adiposity parameters; (2) cardiometabolic parameters; (3) bone health parameters; and (4) musculoskeletal parameters. By protocol, we did not restrict inclusion to a specific primary or secondary outcome. Two authors (AG-H and RR-C) independently assessed the electronic search results. When an article title seemed relevant, the abstract was reviewed for eligibility. When more information was required, the full text of the article was retrieved and appraised. Any differences in the assessments between the two authors were discussed and, if necessary, a third author was involved in decision making (MI). Reasons for exclusion of identified articles were recorded in all cases.
2.3 Data Collection Process and Data Items
Two authors (AG-H and RR-C) independently extracted data including the following information: the first author’s name, year of publication, enrollment year, duration of follow-up, study location, sample size, age at baseline examination, results, adjusted variables, method of muscular fitness assessment, and outcome of interest and number of cases. The outcome measures were as follows: body mass index (BMI), waist circumference (WC), body fat, waist–height ratio, waist-to-hip ratio, skinfold thickness, total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, fasting glucose, fasting insulin, homeostatic model assessment-insulin resistance (HOMA-IR) index, HOMA-beta cell function (HOMA-β), blood pressure, metabolic syndrome, bone mineral density (BMD), bone mineral content (BMC), tension neck, knee injury and low back pain (LBP) (Table 1). When there was insufficient information, the respective corresponding author was contacted [16,17,18,19].
2.4 Risk of Bias in Individual Studies
An assessment of risk of bias in selected studies was made using an adjusted format of the Newcastle–Ottawa quality assessment scale [20] by two authors (AG-H and RR-C) independently. This scale contains eight items categorized into three domains (selection, comparability, and exposure). A star system is used to enable semi-quantitative assessment of study quality, such that the highest quality studies are awarded a maximum of one star per item with the exception of the comparability domain, which allows allocating two stars. Thus, the scores ranged from zero to nine stars.
2.5 Summary Measures
Meta-analyses were conducted if at least three studies provided effect sizes for the same health parameter [21]. Two types of effect size estimates were reported: the main meta-analysis was based on correlation coefficients (r) as a well-known effect size estimate, and the secondary analysis reported pooled odds ratio (OR). Several of the studies used multivariate linear regression, and so we converted the unstandardized regression coefficients (β) to r with a series of transformations [22, 23]. Detailed description of the basic input data is available in ESM Appendix S2. Correlation coefficients and ORs were entered along with the corresponding standard errors or sample size, and the software was set to produce pooled r or with 95% confidence interval (CI) using random effects models. The likelihood approach with random effects was used to better account for the inaccuracy in the estimate of between-study variance [24]. The pooled effect size for r was classified as small (≤ 0.1), moderate (0.1–0.29) or large (≥ 0.30) [25]. All analyses were carried out using the Comprehensive Meta-Analysis program (version 2; Biostat, Englewood, NJ, USA).
2.6 Synthesis of Results
The percentage of total variation across the studies due to heterogeneity (Cochran’s Q-statistic) [26] was used to calculate the I2 statistics, considering I2 values of < 25%, 25–75%, and > 75% as small, moderate, and high heterogeneity, respectively [26, 27]. Sensitivity analyses were conducted to assess the robustness of the summary estimates in order to determine whether a particular study accounted for the heterogeneity. Thus, each study was deleted from the model once in order to analyze the influence of each study on the overall results.
2.7 Risk of Bias Across Studies
Small-study effects bias was assessed using the extended Egger’s test [28].
2.8 Additional Analysis
We examined potential sources of heterogeneity including sex, muscular fitness constructs (endurance or strength), and time for assessment at follow-up (childhood/adolescence or adulthood) by stratifying meta-analyses by each of these factors. A p value of < 0.05 was considered a threshold for statistical significance.
3 Results
3.1 Study Selection
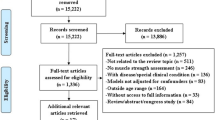
The electronic search strategy retrieved 4112 articles. After removal of duplicate references, and screening of titles and abstracts, we excluded 3884 articles. Of the remaining 228 articles, and after full-text screening and checking the reference lists of included studies and previous reviews for additional relevant articles, a total of 59 studies were read in full. The reasons for exclusion based on full text were (1) inappropriate outcome measurement (18 articles); (2) inappropriate study population (10 articles); and (3) inappropriate study design (1 article) (ESM Appendix S3). Finally, 30 studies met all the inclusion criteria and were included in the systematic review, and of these, 26 studies were included in the meta-analysis (Fig. 1).
3.2 Study Characteristics
Table 1 summarizes the characteristics of the 30 included studies in the systematic review [17,18,19, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The 30 studies included 21,686 participants, with sample sizes ranging from 36 [31] to 6297 [43]. Participants included only boys [33], only girls [31, 51] or both [17,18,19, 28, 32, 34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] sexes. Most studies involved adolescents at baseline (≥ 13 years [17,18,19, 29,30,31,32,33,34, 38, 39, 42, 45, 46, 48,49,50, 53, 54]). In four studies [40, 41, 44, 52], the mean age of the included participants at baseline was 12 years or younger; these were considered children. In the nine remaining studies [16, 35,36,37,38, 43, 47, 51, 55], both children and adolescents were included. The length of follow-up ranged from 1 year [34, 43] to 27 years [33] (mean 8.6 years).
3.3 Muscular Fitness Measurement
Muscular fitness was measured in a variety of ways (Table 1). Most of the studies assessed muscular endurance using the following tests: sit-ups test [19, 32,33,34, 38, 40, 43,44,45, 49], bent arm hang test [19, 38, 40, 43, 46], pull-ups test [33, 40, 43, 44], push-ups test [16, 33, 37], and the back muscle endurance test [47, 49, 50]. Other studies assessed muscular strength using the handgrip strength test [23, 29, 32, 33, 37, 38, 41, 52, 54] and the standing long jump (SLJ) test [19, 29, 37, 38, 52, 54]. Finally, six studies used a muscular fitness score [17, 18, 29, 36, 39, 42] and other tests different from those mentioned.
3.4 Risk of Bias Within Studies
All 30 studies met at least five Newcastle–Ottawa quality assessment scale criteria and were considered to have moderate methodological quality. The average score was 6.3/9.0 (ESM Appendix S4).
3.5 Meta-Analysis
Table 2 shows the synthesis of results and subgroup analysis. Forest plots are shown in ESM Appendix S5. BMI at follow-up was related to muscular fitness at baseline with a moderate effect size (r = − 0.14; 95% CI − 0.21 to − 0.07; p < 0.001; I2 = 27.04); similar results were obtained when analyzing muscular endurance tests (BMI: r = − 0.14; 95% CI − 0.22 to − 0.07, p < 0.001; I2 = 0%). Skinfold thickness was also related to muscular fitness at baseline with a large effect size (r = − 0.32; 95% CI − 0.40 to − 0.23; p < 0.001; I2 = 81.15%), and showed similar results in boys and girls. The effect sizes were higher when analyzing muscular endurance tests (r = − 0.34; 95% CI − 0.45 to − 0.22; p < 0.001; I2 = 82.33%), strength tests (r = − 0.41; 95% CI − 0.50 to − 0.31; p < 0.001; I2 = 76.14%), and analyzing only studies that included the assessment in childhood.
Regarding cardiometabolic parameters, HOMA-IR (r = − 0.10; 95% CI − 0.16 to − 0.05; p < 0.001; I2 = 67.12%), triglycerides (r = − 0.22; 95% CI − 0.30 to − 0.13; p < 0.001; I2 = 74.41%), and CVD risk score (r = − 0.29; 95% CI − 0.39 to − 0.18; p < 0.001; I2 = 84.56%) at follow-up showed a moderate relationship with muscular fitness at baseline.
BMD at follow-up was related to muscular fitness at baseline with a medium effect size (r = 0.17; 95% CI 0.09–0.24; p < 0.001) and low heterogeneity (I2 = 24.92%). The effect sizes were slightly higher when we analyzed only girls and muscular endurance tests (girls: r = 0.24; 95% CI 0.09–0.38; p = 0.002; I2 = 33.06%; muscular endurance tests: r = 0.20; 95% CI 0.10–0.29; p < 0.001; I2 = 0%). However, analyzing only studies that included the assessment at adulthood, the relationship decreased slightly and the heterogeneity increased (r = 0.12; 95% CI 0.05–0.19; p = 0.001; I2 = 73.63%).
Finally, muscular fitness was not significantly related to other musculoskeletal parameters (Table 2).
3.6 Publication Bias and Sensitivity Analysis
Egger’s linear regression tests provided evidence that there was no indication of study bias. In the sensitivity analysis, with each study deleted from the model once, the results remained consistent across all deletions.
4 Discussion
We here summarize the evidence for a prospective relationship between muscular fitness and health parameters in youth. The evidence for a prospective association between muscular fitness at baseline and lower BMI, skinfold thickness, HOMA-IR, triglycerides, CVD risk score or higher BMD later in life is consistent, and is supported by the meta-analysis. Accordingly, muscular fitness should be developed during childhood and adolescence in order to promote healthier adiposity, cardiometabolic and bone health outcomes later in life. The evidence for LBP outcomes is inconclusive. That said, the results of the present meta-analysis should be interpreted with caution because of (1) the variety of tests used to assess muscular fitness (strength, power or endurance); (2) the outcome measures; (3) the follow-up time (from 1 year to 25 years); (4) the age of the participants; and (5) the role of potential confounders.
4.1 Anthropometric and Adiposity Parameters
Our findings provide evidence of an inverse moderate association between muscular fitness and some anthropometric parameters (BMI and skinfold thickness) later in life. In line with the present study, a systematic review published in 2014 suggested an inverse association between muscular fitness and adiposity (r = − 0.25) [13]; however, this study included cross-sectional results and, therefore, the association is probably bidirectional with increases in fitness or adiposity likely to impact on each other. Another systematic review gave support to the notion that muscular fitness is highly influenced by body weight in children aged 6–17 years [3], especially with regards to weight-bearing tests. However, individual studies included in our meta-analysis have shown that muscular fitness measured both in absolute terms [38] and relative to body weight [19, 39, 41] is inversely associated with adiposity later in life. Also, the effect sizes reported using endurance (push-ups, sit-ups, bent arm hang, etc.) or strength tests (handgrip, SLJ or vertical jump) were similar.
The inverse relationship between muscular fitness at baseline and anthropometric parameters at follow-up may occur through both physiological and psycho-behavioral mechanisms. For example, higher muscular fitness performance favors greater participation in physical activity [56], larger work capacity amongst youth [57], and is hence more enjoyable [58]. Also, because skeletal muscle is a highly energetic tissue that contributes substantially to basal metabolic rate [59], high muscular fitness levels at baseline may reflect larger skeletal muscle mass, higher metabolic efficiency of muscle (i.e., lipid oxidation and glucose transport capacity), or both, resulting in greater overall daily energy expenditure [60].
The pooled effect size of four studies [18, 30, 42, 43] revealed a non-significant relationship between low muscular fitness level at baseline and overweight/obesity at follow-up, also by sex and analyzing only the upper-body muscular tests. However, Hruby et al. [40] showed, in a large sample of 2793 American children, that both achieving and maintaining adequate muscular fitness over a 4-year period resulted in significantly greater odds of a healthy weight at 4-year follow-up. By contrast, Barnekow-Bergkvist et al. [30] reported that higher performance in the bench press and two-hand lift was associated with greater odds of high BMI for both males and females at age 34 (20 years later). Due to the heterogeneity of the results, further investigations are warranted to clarify the relationship between muscular fitness in children and future body weight.
4.2 Cardiometabolic Parameters
A low muscular fitness level is recognized as a marker of poor metabolic profile during childhood and adolescence [5]. In their systematic review, Smith et al. [13] provided strong evidence for the importance of muscular fitness during childhood and adolescence for cardiometabolic risk. Despite individual studies demonstrating that muscular fitness during childhood and adolescence was inversely associated with cardiometabolic risk/metabolic syndrome (several risk factors) [18, 48, 52], lipids [18, 42, 52], blood pressure [18, 29, 37], and fasting glucose and insulin [17, 36] in later life, our pooled analysis revealed only an inverse association between muscular fitness and HOMA-IR, with a small effect size and moderate-high heterogeneity. Because muscle-strengthening activities are strongly related to gains in muscle strength in youth [61, 62], these observations support our analysis and suggest that low muscle fitness is causally related to development of unfavorable levels of insulin resistance. A possible mechanism through which high muscular fitness may influence insulin resistance is by stimulating proteins in the insulin-signaling cascade [63]. Consistent with this, several experimental studies support the biological plausibility of our findings, suggesting that increased muscular fitness via resistance training favors increased insulin sensitivity [64]. For example, Fraser et al. [36] found that muscular endurance and muscular strength (in males) are associated with measures of insulin resistance and beta cell function in adulthood, independent of the CRF phenotype and WC. These authors also suggested that WC attenuates the association between childhood muscular fitness and adult insulin resistance outcomes, which could explain our findings (i.e., we reported an inverse relationship between muscular fitness and some anthropometric parameters, which in turn may be related to lower levels of insulin resistance). Therefore, moderate heterogeneity in our results could be due to covariates included in the analysis.
Although the present meta-analysis did not include pooled data for metabolic syndrome or cardiometabolic risk (due to the limited number of studies), several single-study reports yielded inconclusive results, with a negative [18, 37, 48] or no [39, 42, 48] significant association with muscular fitness. In a recent study of 235 American adolescents, Peterson et al. [48] suggested that greater baseline body-mass–normalized handgrip strength was associated with both cardiometabolic health maintenance (no risk factors identified at either time point) and health improvements (presence of ≥ 1 baseline risk factor and fewer or no risk factors at follow-up) over a 2-year period. By contrast, Jekal et al. [42] studied 1006 Korean adolescents and reported that low muscular fitness during adolescence was not related to metabolic syndrome prevalence 22 years later.
4.3 Bone Health
There is scientific evidence to indicate that childhood may be the opportune time to build bone mass and enhance bone structure through participation in weight-bearing physical activities [65]. The majority of adult bone mass is laid down before 17 years of age [66] and is determined, to a large extent, by genetics [67], and also by a number of modifiable determinants such as physical activity, nutritional factors [68] and muscular fitness [69]. The findings of our meta-analysis support the role of muscular fitness during youth and its association with BMD later in life, with a medium effect size. Notwithstanding that bone mass is, in part, racially determined [70], our meta-analysis included youth from diverse ethnic groups (Asian and Caucasian) and the results showed low heterogeneity. Consistent with this, Foley et al. [35] reported modest but significant beneficial relationships between childhood SLJ and adult quantitative ultrasound index. Moreover, two longitudinal studies included in this systematic review reported an association between upper- and lower-body strength and BMC [33, 51].
Regarding sub-group analysis, the present findings reported a slightly higher effect size for upper-body muscular tests compared with overall pooled results, but there was no significant association between BMD and lower-body muscular tests (Table 2). As suggested by Foley et al. [35], the relationship between childhood and adolescent SLJ and adult bone mass could be attenuated after adjustment for adulthood SLJ measure, suggesting that muscular power is only an important determinant of adult bone mass if sustained into adulthood. Overall, it would seem to be worthwhile to promote exercise combining strength and impact training (e.g., plyometric training) [71] in growing children to favor bone mass response [72].
4.4 Musculoskeletal Disorders
Musculoskeletal disorders such as LBP, neck pain and osteoarthritis are highly prevalent in the adult population [73]. LBP is among the most commonly reported health problems in the United States and up to 80% of individuals are expected to consult their physician about LBP at some point in their lifetime [74]. In addition, back pain impacts over 100 million individuals in the US and costs more than US$200 billion per year due to job absenteeism, medical and legal fees, disability payments, worker’s compensation, and long-term disability insurance [75]. When considering muscle strength in youth as a potential risk factor in the longer term, previous systematic reviews suggest that its association with future LBP is relatively unknown [3, 13], despite the fact that strength training seems to be important for secondary and tertiary prevention of LBP [76]. Also, Mikkelsson et al. [45] showed that good flexibility in boys and good endurance strength in girls may contribute to a decreased risk of neck tension, and that high endurance in boys may indicate an increased risk of knee injury. The present meta-analysis reinforces these findings with inconclusive evidence, indicating that low muscular fitness in childhood and adolescence might not be a predictor of LBP later in life, together with a moderate heterogeneity (OR 1.29; 95% CI 0.96–1.75; p = 0.094; I2 = 55.07%). A large study of 5489 young adult men (mean age 18.2 years) from Sweden seems to confirm our findings and does not provide evidence in support of a theoretical model in which low muscle fitness in young adult men is associated with an increased risk of musculoskeletal pain later in life [77]. Another two studies included in this systematic review [47, 49] confirm this non-association. By contrast, the study by Sjölie and Ljunggren [50] indicated that low lumbar extension strength may be a risk factor for LBP later in life.
4.5 Strength and Limitations
To the best of our knowledge, this is the first meta-analysis to provide a quantitative and comprehensive evaluation of the range of future health parameters associated with muscular fitness during childhood and adolescence. Furthermore, our meta-analysis provides an update of the evidence reported within earlier reviews [3, 13].
There are some limitations that warrant discussion. First, the included studies were heterogeneous with respect to methodology, measurement of muscular strength, outcomes, length of follow-up, ethnicity, and potential confounders such as adiposity and CRF, which might explain the inconsistent findings. However, only a few pooled parameters (4/13: WC, skinfold thickness, diastolic blood pressure, and CVD risk score) in our meta-analysis showed high heterogeneity (i.e., I2 ≥ 75%). Second, only two studies adjusted the outcome variable of interest for baseline values, a key issue with great implications for the interpretation of the temporal sequence and thus causality [78]. Third, most of the studies included children and adolescents in their analysis, and therefore sexual maturation could have affected baseline muscular fitness [78]. Fourth, due to sample loss, individuals examined at follow-up could have been unrepresentative of those at baseline, which could have led to overestimated or underestimated associations. Fifth, our pooled analysis included a variety of tests used to assess muscular fitness (i.e., strength, power or endurance) and that may lead to bias; however, the heterogeneity in most of the results was low-moderate. Finally, only three studies [17, 18, 48] used ‘relative’ muscular fitness (i.e., divided by body mass) to determine their relationship with health outcomes, and therefore the association can change and even reverse in comparison with ‘absolute’ muscular fitness (many weight-bearing muscular strength tests are correlated with body mass and/or adiposity) [79].
5 Conclusion
The present results show moderate-large relationships between muscular fitness in childhood and adolescence and future levels of BMI, skinfold thickness, HOMA-IR, triglycerides, CVD risk score, and BMD, but evidence for LBP was unconvincing. Therefore, the early identification and treatment of youth with low levels of muscular fitness could improve long-term health outcomes, since the prevention of chronic diseases should start as early in life as possible. Recommendations for future research include the exploration of whether sustained high levels or improving muscular fitness in children and adolescents leads to fewer health problems later in life [19, 29, 39].
Data availability statement
The data that support the findings of this review are available on reasonable request from the corresponding author (Antonio García-Hermoso).
References
Ortega F, Ruiz J, Castillo M, et al. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond). 2008;32(1):1.
Mintjens S, Menting MD, Daams JG, et al. Cardiorespiratory fitness in childhood and adolescence affects future cardiovascular risk factors: a systematic review of longitudinal studies. Sports Med. 2018;48(11):2577–605.
Ruiz JR, Castro-Piñero J, Artero EG, et al. Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med. 2009;43(12):909–23.
Garcia-Hermoso A, Vegas-Heredia ED, Fernández-Vergara O, et al. Independent and combined effects of handgrip strength and adherence to a Mediterranean diet on blood pressure in Chilean children. Nutrition. 2019;60:170–4.
Ramírez-Vélez R, Peña-Ibagon JC, Martínez-Torres J, et al. Handgrip strength cutoff for cardiometabolic risk index among Colombian children and adolescents: the FUPRECOL Study. Sci Rep. 2017;7:42622.
Committee PAGA. Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health and Human Services; 2018.
World Health Organization. Global health estimates: deaths by cause, age, sex and country, 2000–2012. Geneva: WHO; 2014. p. 9.
Sandercock GR, Cohen DD. Temporal trends in muscular fitness of English 10-year-olds 1998–2014: an allometric approach. J Sci Med Sport. 2019;22(2):201–5.
Moliner-Urdiales D, Ruiz J, Ortega F, et al. Secular trends in health-related physical fitness in Spanish adolescents: the AVENA and HELENA studies. J Sci Med Sport. 2010;13(6):584–8.
García-Hermoso A, Cavero-Redondo I, Ramírez-Vélez R, et al. Muscular strength as a predictor of all-cause mortality in apparently healthy population: a systematic review and meta-analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil. 2018;99(10):2100–13.
Steene-Johannessen J, Anderssen SA, Kolle E, et al. Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc. 2009;41(7):1361–7.
Fraser BJ, Schmidt MD, Huynh QL, et al. Tracking of muscular strength and power from youth to young adulthood: longitudinal findings from the Childhood Determinants of Adult Health Study. J Sci Med Sport. 2010;20(10):927–31.
Smith JJ, Eather N, Morgan PJ, et al. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med. 2014;44(9):1209–23.
Green S, Higgins J. Cochrane handbook for systematic reviews of interventions. Version; 2005.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):65–94.
Aires L, Andersen LB, Mendonça D, et al. A 3-year longitudinal analysis of changes in fitness, physical activity, fatness and screen time. Acta Paediatr. 2010;99(1):140–4.
Grøntved A, Ried-Larsen M, Ekelund U, et al. Independent and combined association of muscle strength and cardiorespiratory fitness in youth with insulin resistance and β-cell function in young adulthood: the European Youth Heart Study. Diabetes Care. 2013;36(9):2575–81.
Grøntved A, Ried-Larsen M, Møller NC, et al. Muscle strength in youth and cardiovascular risk in young adulthood (the European Youth Heart Study). Br J Sports Med. 2015;49(2):90–4.
Toriola OO, Monyeki MA, Toriola AL. Two-year longitudinal health-related fitness, anthropometry and body composition status amongst adolescents in Tlokwe Municipality: the PAHL Study. Afr J Prim Health Care Fam Med. 2015;7(1):896.
Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Oct 20, 2018.
Skrede T, Steene-Johannessen J, Anderssen S, et al. The prospective association between objectively measured sedentary time, moderate-to-vigorous physical activity and cardiometabolic risk factors in youth: a systematic review and meta-analysis. Obes Rev. 2019;20(1):55–74.
Nieminen P, Lehtiniemi H, Vähäkangas K, et al. Standardised regression coefficient as an effect size index in summarising findings in epidemiological studies. Epidemiol Biostat Public Health. 2013;10(4):1–15.
Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90(1):175.
Hardy RJ, Thompson SG. A likelihood approach to meta-analysis with random effects. Stat Med. 1996;15(6):619–29.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Erlbaum; 1988.
Higgins J, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Agostinis-Sobrinho C, Ruiz JR, Moreira C, et al. Changes in muscular fitness and its association with blood pressure in adolescents. Eur J Pediatr. 2018;177(7):1101–9.
Barnekow-Bergkvist M, Hedberg G, Janlert U, et al. Adolescent determinants of cardiovascular risk factors in adult men and women. Scand J Public Health. 2001;29(3):208–17.
Barnekow-Bergkvist M, Hedberg G, Pettersson U, et al. Relationships between physical activity and physical capacity in adolescent females and bone mass in adulthood. Scand J Med Sci Sports. 2006;16(6):447–55.
Cheng J, Maffulli N, Leung S, et al. Axial and peripheral bone mineral acquisition: a 3-year longitudinal study in Chinese adolescents. Eur J Pediatr. 1999;158(6):506–12.
Delvaux K, Lefevre J, Philippaerts R, et al. Bone mass and lifetime physical activity in Flemish males: a 27-year follow-up study. Med Sci Sports Exerc. 2001;33(11):1868–75.
Feldman DE, Shrier I, Rossignol M, et al. Risk factors for the development of low back pain in adolescence. Am J Epidemiol. 2001;154(1):30–6.
Foley S, Quinn S, Dwyer T, et al. Measures of childhood fitness and body mass index are associated with bone mass in adulthood: a 20-year prospective study. J Bone Miner Res. 2008;23(7):994–1001.
Fraser BJ, Blizzard L, Schmidt MD, et al. Childhood cardiorespiratory fitness, muscular fitness and adult measures of glucose homeostasis. J Sci Med Sport. 2018;21(9):935–40.
Fraser BJ, Huynh QL, Schmidt MD, et al. Childhood muscular fitness phenotypes and adult metabolic syndrome. Med Sci Sports Exerc. 2016;48(9):1715–22.
Freitas D, Beunen G, Maia J, et al. Tracking of fatness during childhood, adolescence and young adulthood: a 7-year follow-up study in Madeira Island, Portugal. Ann Hum Biol. 2012;39(1):59–67.
Hasselstrøm H, Hansen S, Froberg K, et al. Physical fitness and physical activity during adolescence as predictors of cardiovascular disease risk in young adulthood. Danish Youth and Sports Study. An eight-year follow-up study. Int J Sports Med. 2002;23(1):27–31.
Hruby A, Chomitz VR, Arsenault LN, et al. Predicting maintenance or achievement of healthy weight in children: the impact of changes in physical fitness. Obesity. 2012;20(8):1710–7.
Janz K, Dawson J, Mahoney L. Increases in physical fitness during childhood improve cardiovascular health during adolescence: the Muscatine study. Int J Sports Med. 2002;23(S1):15–21.
Jekal Y, Kim Y, Yun JE, et al. The association of adolescent fatness and fitness with risk factors for adult metabolic syndrome: a 22-year follow-up study. J Phys Act Health. 2014;11(4):823–30.
Kim J, Must A, Fitzmaurice GM, et al. Relationship of physical fitness to prevalence and incidence of overweight among schoolchildren. Obes Res. 2005;13(7):1246–54.
Lopes VP, Maia JA, Rodrigues LP, et al. Motor coordination, physical activity and fitness as predictors of longitudinal change in adiposity during childhood. Eur J Sport Sci. 2012;12(4):384–91.
Mikkelsson LO, Nupponen H, Kaprio J, et al. Adolescent flexibility, endurance strength, and physical activity as predictors of adult tension neck, low back pain, and knee injury: a 25 year follow up study. Br J Sports Med. 2006;40(2):107–13.
Minck M, Ruiter L, Van Mechelen W, et al. Physical fitness, body fatness, and physical activity: the Amsterdam Growth and Health Study. Am J Hum Biol. 2000;12(5):593–9.
Newcomer K, Sinaki M. Low back pain and its relationship to back strength and physical activity in children. Acta Paediatr. 1996;85(12):1433–9.
Peterson MD, Gordon PM, Smeding S, et al. Grip strength is associated with longitudinal health maintenance and improvement in adolescents. J Pediatr. 2018;202:226–30.
Salminen JJ, Erkintalo M, Laine M, et al. Low back pain in the young. A prospective three-year follow-up study of subjects with and without low back pain. Spine. 1995;20(19):2101–7.
Sjölie AN, Ljunggren AE. The significance of high lumbar mobility and low lumbar strength for current and future low back pain in adolescents. Spine. 2001;26(23):2629–36.
Wang Q, Alén M, Nicholson P, et al. Weight-bearing, muscle loading and bone mineral accrual in pubertal girls—a 2-year longitudinal study. Bone. 2007;40(5):1196–202.
Zaqout M, Michels N, Bammann K, et al. Influence of physical fitness on cardio-metabolic risk factors in European children. The IDEFICS study. Int J Obes (Lond). 2016;40(7):1119–25.
Welten D, Kemper H, Post G, et al. Weight-bearing activity during youth is a more important factor for peak bone mass than calcium intake. J Bone Miner Res. 1994;9(7):1089–96.
Castro-Piñero J, Perez-Bey A, Cuenca-Garcia M, et al. Muscle fitness cut points for early assessment of cardiovascular risk in children and adolescents. J Pediatr. 2019;206:134–41.
Monyeki K, Kemper H, Makgae P. Relationship between fat patterns, physical fitness and blood pressure of rural South African children: Ellisras Longitudinal Growth and Health Study. J Hum Hypertens. 2008;22(5):311.
Garcia-Hermoso A, Correa-Bautista JE, Olloquequi J, et al. Health-related physical fitness and weight status in 13-to 15-year-old Latino adolescents. A pooled analysis. J Pediatr (Rio J). 2018. https://doi.org/10.1016/j.jped.2018.04.002.
Cattuzzo MT, dos Santos Henrique R, Ré AHN, et al. Motor competence and health related physical fitness in youth: a systematic review. J Sci Med Sport. 2016;19(2):123–9.
Castelli DM, Valley JA. Chapter 3: The relationship of physical fitness and motor competence to physical activity. J Teach Phys Educ. 2007;26(4):358–74.
Zurlo F, Larson K, Bogardus C, et al. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Investig. 1990;86(5):1423–7.
Moliner-Urdiales D, Ruiz JR, Vicente-Rodriguez G, et al. Associations of muscular and cardiorespiratory fitness with total and central body fat in adolescents: the HELENA study. Br J Sports Med. 2011;45(2):101–8.
García-Hermoso A, Ramírez-Vélez R, Ramírez-Campillo R, et al. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):161–6.
Moran J, Sandercock G, Ramirez-Campillo R, et al. A meta-analysis of resistance training in female youth: its effect on muscular strength, and shortcomings in the literature. Sports Med. 2018;48(7):1661–71.
Holten MKZM, Gaster M, Juel C, et al. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes Care. 2004;53(2):294–305.
Álvarez C, Ramírez-Campillo R, Ramírez-Vélez R, et al. Metabolic effects of resistance or high-intensity interval training among glycemic control-nonresponsive children with insulin resistance. Int J Obes (Lond). 2018;42(1):79.
Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14–27.
Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75(4):1060–5.
Kelly P, Eisman J, Sambrook P. Interaction of genetic and environmental influences on peak bone density. Osteoporos Int. 1990;1(1):56–60.
Babaroutsi E, Magkos F, Manios Y, et al. Body mass index, calcium intake, and physical activity affect calcaneal ultrasound in healthy Greek males in an age-dependent and parameter-specific manner. J Bone Miner Metab. 2005;23(2):157–66.
Vicente-Rodriguez G, Dorado C, Perez-Gomez J, et al. Enhanced bone mass and physical fitness in young female handball players. Bone. 2004;35(5):1208–15.
Gilsanz V, Roe TF, Mora S, et al. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med. 1991;325(23):1597–600.
Ramirez-Campillo R, Álvarez C, García-Hermoso A, et al. Methodological characteristics and future directions for plyometric jump training research: a scoping review. Sports Med. 2018;48(5):1059–81.
Vlachopoulos D, Barker AR, Ubago-Guisado E, et al. A 9-month jumping intervention to improve bone geometry in adolescent male athletes. Med Sci Sports Exerc. 2018;50(12):2544–54.
Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602.
Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a US national survey. Spine. 1995;20(1):11–9.
Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986–95.
Wewege M, Booth J, Parmenter B. Aerobic vs. resistance exercise for chronic non-specific low back pain: a systematic review and meta-analysis. J Back Musculoskelet Rehabil. 2018;35:889–99.
Timpka S, Petersson IF, Zhou C, et al. Muscle strength in adolescent men and future musculoskeletal pain: a cohort study with 17 years of follow-up. BMJ Open. 2013;3(5):e002656.
Hill AB. The environment and disease: association or causation? J R Soc Med. 1965;58:295–300.
Moran J, Sandercock GR, Ramírez-Campillo R, et al. A meta-analysis of maturation-related variation in adolescent boy athletes’ adaptations to short-term resistance training. J Sports Sci. 2017;35(11):1041–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
AGH is a Miguel Servet Fellow (Instituto de Salud Carlos III—CP18/0150). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Antonio García-Hermoso, Rodrigo Ramírez-Campillo, and Mikel Izquierdo declare that they have no conflicts of interest relevant to the content of this review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García-Hermoso, A., Ramírez-Campillo, R. & Izquierdo, M. Is Muscular Fitness Associated with Future Health Benefits in Children and Adolescents? A Systematic Review and Meta-Analysis of Longitudinal Studies. Sports Med 49, 1079–1094 (2019). https://doi.org/10.1007/s40279-019-01098-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-019-01098-6