Abstract
An eccentric contraction involves the active lengthening of muscle under an external load. The molecular and neural mechanisms underpinning eccentric contractions differ from those of concentric and isometric contractions and remain less understood. A number of molecular theories have been put forth to explain the unexplained observations during eccentric contractions that deviate from the predictions of the established theories of muscle contraction. Postulated mechanisms include a strain-induced modulation of actin-myosin interactions at the level of the cross-bridge, the activation of the structural protein titin, and the winding of titin on actin. Accordingly, neural strategies controlling eccentric contractions also differ with a greater, and possibly distinct, cortical activation observed despite an apparently lower activation at the level of the motor unit. The characteristics of eccentric contractions are associated with several acute physiological responses to eccentrically-emphasised exercise. Differences in neuromuscular, metabolic, hormonal and anabolic signalling responses during, and following, an eccentric exercise bout have frequently been observed in comparison to concentric exercise. Subsequently, the high levels of muscular strain with such exercise can induce muscle damage which is rarely observed with other contraction types. The net result of these eccentric contraction characteristics and responses appears to be a novel adaptive signal within the neuromuscular system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Eccentric contractions, whereby the muscle is actively lengthened under an external load, display a number of molecular and neural characteristics which distinguish them from isometric and concentric contractions. |
The distinct characteristics of eccentric contractions have a number of physiological implications for eccentrically-biased exercise, and, subsequently, the acute responses to exercise. |
The characteristic responses to eccentrically-biased exercise may be related to an adaptive signal within the neuromuscular system not observed with concentrically-biased exercise. |
1 Introduction
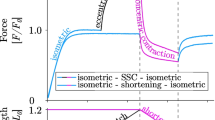
An eccentric muscle contraction refers to a muscle activity that occurs when the force applied to the muscle exceeds the momentary force produced by the muscle itself and results in a lengthening action (i.e. work is done on the muscle) [1]. The absorbed mechanical energy may be dissipated as heat in a dampening manner, or, alternatively, the energy may be recoverable and added to the active force produced during subsequent concentric action [1, 2]. In the latter manner the muscle–tendon system functions as a spring when active muscle lengthens before subsequent shortening. The coupling of eccentric with concentric muscle actions is referred to as the stretch shortening cycle (SSC); a phenomenon ubiquitous to efficient and powerful movements [1]. Despite the importance of eccentric muscle function to human movement, the mechanisms underpinning eccentric contractions remain to be determined. While concentric and isometric contractions are well described by the molecular theories of muscle contraction first described by Huxley and Niedergerke [3] and Huxley and Hanson [4], the mechanics of eccentric contractions are not [5]. A number of nuanced molecular mechanisms and neural strategies have been proposed to account for unexplained observations during eccentric contractions. A growing body of evidence indicates that eccentric-emphasised exercise can elicit acute responses which differ from concentric-only or traditional mixed exercise. In particular, eccentric resistance exercise which accounts for eccentric strength (i.e. is not constrained by concentric strength) appears to have a potent effect on a number of physiological variables underpinning a novel adaptive response. The purpose of this review is to describe the current theories which seek to explain the unique physiological characteristics of eccentric contractions. The novel responses to eccentric exercise are described, with particular reference to differences to concentric or traditional resistance training. Therefore, this review describes the specific molecular and neural characteristics of eccentric muscle actions and also provides the current state of knowledge regarding some of the acute responses to eccentric exercise, including neuromuscular, cardiorespiratory, hormonal and molecular aspects.
2 Characteristics of Eccentric Contractions
2.1 Molecular Characteristics
During muscle contraction the filaments actin and myosin remain at a constant length and a change in fibre length is achieved via a change in overlap between the two in a sliding motion; hence the sliding filament theory of muscle contraction [3, 4]. The driving force for the sliding motion is generated by myosin cross-bridges where the two filaments overlap. Myosin heads repeatedly interact with binding sites on actin, with each contact contributing to the force developed [6]. The performance of any one bridge is believed to remain uninfluenced by the activity of other bridges [7], and the number of cross-bridges formed is determined by the magnitude of contractile activation and amount of actin-myosin overlap (i.e. the length-tension relationship). This process effectively explains both concentric and isometric muscle actions [6]. In the case of isometric actions where there is no change in muscle length, cross-bridge turnover does still occur with bridges spontaneously dissociating and being replaced by new bridges maintaining the net cross-bridge formation, and energy expenditure occurs in the absence of external work [7, 8]. When the force applied is sufficient to overcome the external load, the muscle can shorten with thin filaments sliding towards the centre of thick filaments. With increasing speed this process decreases exposure time of myosin heads to actin binding sites [7], and thus reduces the number of cross-bridges that may be formed (i.e. a force–velocity relationship). Unfortunately, the cross-bridge theory alone is inadequate in explaining the greater force produced during active lengthening [9], the time-dependent residual force enhancement [10], and the reduced energy expenditure of eccentric contractions [8, 11].
The increased force production during lengthening contractions above isometric force capabilities may be related to differences in the number of attached cross-bridges and mechanical detachment of active cross-bridges. It has been proposed that the activation of the second (i.e. partner) head of a myosin molecule to actin increases the total number of active cross-bridges [9]. During isometric and concentric contractions only one myosin head is bound, whereas the increased strain on a single myosin head during lengthening contractions may facilitate the activation of the second head [9]. This mechanism would lead to twice the number of active cross-bridges during active lengthening and could be increasingly utilised with increasing contraction velocity [9]. It has further been postulated that cross-bridges do not complete a full cycle during eccentric contractions [12]; they become suspended in an active state bound to actin and become forcibly detached followed by a rapid re-attachment [13], because a full cross-bridge cycle is not completed less ATP is required to maintain force [13]. Linari and colleagues [12] also demonstrated that while fast myosin heavy chain (MHC) isoforms produced 40–70 % higher isometric force than slow isoforms, the slow isoform produced similar forces during lengthening. The kinetic and mechanical properties of actin-myosin interactions therefore appear to be independent of MHC isoform under lengthening conditions [12].
The greater force achieved during isometric contractions immediately following eccentric contractions indicates that passive factors beyond active cross-bridge mechanisms may underpin the observations that are not accounted for by the cross-bridge theory of contraction [14]. The passive component is postulated to be related to a change in stiffness of the molecular spring titin [15, 16]. Herzog [14] cites three theories to explain the residual force enhancement phenomenon: (1) an increase in active force of cross bridges, (2) a structural non-uniformity across the length-tension curve, and (3) the engagement of passive structures. Studies have demonstrated that the predictions of the first two theories fail [14, 17]. A passive mechanism for force enhancement remains plausible and may better reconcile experimental findings [14]. It is believed that a passive structural element within the sarcomere, and specifically the structural protein titin, is a key factor in the residual force enhancement with eccentric contractions [5]. Titin is the largest protein currently known in the natural world [18], and is an important structural component of the muscle cytoskeleton. Titin spans a half sarcomere inserting into a Z-band on one end and the M-line at the other, and has spring-like properties in the I-band region [14, 19] (Fig. 1). Titin’s passive force is directly related to sarcomeres and muscle length, is in parallel with the cross-bridge forces, is strengthened when cross-bridge forces become weak, and provides stability to sarcomeres [14, 18, 20, 21]. Therefore titin is logically posited as an important contributor to the regulation of muscle force [6].
A three-filament model of contraction has been proposed with actin and myosin retaining their established roles; but titin additionally acts as a spring that binds calcium upon activation and binds to actin upon cross-bridge attachment [14]. Calcium binding to certain regions of titin has been demonstrated to increase its stiffness and subsequently increase force upon lengthening [14, 18, 22, 23]. While the binding of titin to actin has not been directly demonstrated in situ, the proposition is supported by observations that even in the presence of calcium activation, increases in titin force are dependent on cross-bridge activation [18]. Further to this, Nishikawa and colleagues [24] propose a ‘winding filament’ hypothesis whereby cross bridges serve as rotors that wind titin on actin, storing elastic energy in the proline-glutamate-valine-lysine (PEVK) region of titin, which can subsequently contribute to the energy recovered during active shortening. Further research is necessary to verify both the three-filament model and the winding filament hypothesis, but both serve as promising avenues in explaining phenomena of eccentric contractions.
In summary, the sliding filament theory fails at a number of levels to reconcile aberrant experimental observations related to the greater force produced during active lengthening, residual force enhancement and lower energy expenditure of eccentric contractions. In addition to the theory of mechanical cross-bridge detachment, the three-filament model and winding filament hypothesis may provide additional insight into many of the unexplained observations associated with eccentric contractions, with titin probably being the long sought ‘skeletal muscle spring’.
2.2 Neural Characteristics
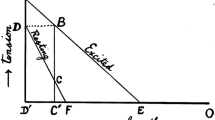
The neural strategies controlling eccentric contractions appear to be unique in comparison with concentric and isometric contractions [25, 26]. The differences between contraction types under maximal conditions have been investigated using three primary methods: surface electromyography (EMG), twitch interpolation and single motor unit assessment [27]. When muscle activity is inferred from surface EMG, the observed amplitude has been demonstrated to be lower during maximal eccentric contractions than maximal concentric and isometric contractions [28–31], although not in all cases [32–34]. This difference appears to be more pronounced in untrained individuals and may be attenuated with heavy load resistance training [28, 31]. The twitch interpolation technique has been used to assess the discrepancy between maximal voluntary contraction (MVC) and maximal muscle activation via superimposed electrical stimulation [35]. A greater voluntary activation deficit has been found in eccentric compared with concentric contractions [31, 36, 37], although this discrepancy can be removed with resistance training (Fig. 2) and may be muscle-group dependent [26, 27, 28, 31]. The assessment of single motor unit activity also indicates lower and more variable motor unit discharge rates during maximal eccentric versus concentric contractions [27, 38]. In effect, untrained individuals display an inhibited voluntary activation during maximal eccentric contractions, which is believed to be primarily constrained by mechanisms that establish motor unit discharge rates [27, 39].

Data from Amiridis et al. [31]
Representative torque-angular velocity curve during a single joint isokinetic movement (i.e. knee extension) for (a) an untrained and (b) a trained participant during a maximal voluntary contraction (MVC) and a MVC with superimposed electrical activation (MVC + SEA).
The greater intrinsic force capacity of muscle during eccentric contractions means fewer motor units are required to attain a given absolute force and a lower net activation is required for a given submaximal load [27]. The strategies of muscle control during submaximal eccentric contractions can also differ from concentric contractions. It has been demonstrated that high-threshold motor units can be selectively recruited during eccentric contractions, particularly at fast eccentric velocities [40]. It has further been shown that preferential recruitment of predominantly fast-twitch synergists can occur with increasing eccentric contraction velocities [41, 42]. However, most studies have found little difference in motor unit recruitment between contraction types [43–46]. Indeed, a progressive de-recruitment of the highest threshold motor units may occur during submaximal eccentric contractions. This observation aligns with Henneman’s size principle [47]. Fewer and/or smaller motor units are required to match the lower force demands during eccentric contractions. A reduced motor unit discharge rate in conjunction with progressive de-recruitment during eccentric contractions supports the notion of a general size-related recruitment strategy irrespective of contraction type [25, 27], although further research is necessary to elucidate the adjustments that can occur during fast eccentric contractions and differing mechanical conditions [39].
Mechanisms underpinning the unique neural strategies during eccentric contractions are not well understood, but are likely a combination of supraspinal and spinal factors [39]. Cortical excitability appears to be enhanced during eccentric contractions and a greater brain area is involved irrespective of the load condition and lower motor unit activity [48, 49]. Indeed, efferent motor output is not only regulated by central descending pathways, but also modulated by inflow from Golgi organs, muscle spindles, muscle afferents and recurrent inhibition from Renshaw cells [26]. Spinal inhibition is believed to be a primary mechanism underpinning reduced motor activity during eccentric contractions [27]. The enhanced cortical excitability and descending drive has been postulated as a compensatory response to such inhibition [50]. Motor evoked potentials in response to transcranial magnetic stimulation (TMS) have been found to be smaller during eccentric contractions [50–52], probably as a result of both pre- and post-synaptic mechanisms at the level of the motoneuron [27, 50]. Furthermore, depressed Hoffman reflex (H-reflex) amplitude is indicative of a disfacilitation of the motoneuron pool during eccentric contractions [32, 39]. Given a similar response across maximal and submaximal eccentric conditions [53], a tension-related Golgi tendon organ inhibition is unlikely to be a primary factor [39], while a clear reciprocal inhibition has also yet to be demonstrated [27]. Experimental data demonstrating recurrent inhibition are lacking, but this is a known mechanism for reducing motor unit discharge rates and stands as a possible mechanism in the reduced motor response during eccentric contractions, especially in untrained subjects [27, 39].
In summary, eccentric contractions exhibit unique neural strategies compared with concentric and isometric contractions under both maximal and submaximal conditions. Differences appear to be primarily mediated by spinal inhibition, although further research is necessary to determine the precise mechanisms. Heavy load resistance training has been established as an efficacious strategy in attenuating reflex inhibition during maximal eccentric contractions and can therefore induce improvements in neuromuscular activation and maximal eccentric strength [39].
3 Acute Responses to Eccentric Exercise
3.1 Neuromuscular Responses
The magnitude of joint moment is greatest during eccentric contractions, exceeding the isometric moment by 30–40 % [54]. The in vivo difference is smaller than previously demonstrated in single muscle fibres [55], and may be due to the greater voluntary activation deficit with eccentric contractions [30], particularly in untrained subjects [28, 31] (Fig. 2). Indeed, isometric force can occasionally exceed eccentric force at a given joint angle [56], probably due to difficulties with full activation during slow eccentric velocities and large ranges of motion [57]. When using dynamic isoinertial loads during conventional resistance exercises, individuals are 20–60 % stronger eccentrically than concentrically [58], which aligns with findings using isokinetic dynamometry [59]. Females can exhibit a greater difference between eccentric and concentric strength [58], possibly due to differences in elastic energy storage, motor unit recruitment and inhibition of maximal force [56, 60]. Unlike the force–velocity relationship during concentric contractions, force during eccentric contractions increases with velocity up to a certain point, after which it levels off, or declines slightly [54, 60, 61]. The ability to fully activate muscle in resistance-trained subjects may facilitate the increase in force with increasing eccentric contraction velocities [31]. It is presently unclear whether such a force–velocity relationship is influenced by sex, or the muscle group assessed [56, 60]. EMG activity does not appear to vary with eccentric contraction velocity, indicating that factors other than motor unit recruitment (e.g. viscoelastic properties and non-contractile elements) contribute to an increased force production capacity that can be observed with increased velocity [41]. The differences in force–velocity relationships between concentric and eccentric contractions mean that the discrepancy between moments becomes even greater with increasing angular velocities [54].
The unique neural strategies underpinning eccentric contractions can also affect the control of muscle force. The majority of research has investigated slow finger movements that may not translate to exercise-related tasks, but the control of knee extensor torque has been investigated in healthy young participants [62, 63]. Peak force variability (i.e. coefficient of variation of the % MVC) appears to be higher during anisometric contractions compared with isometric contractions [63], although in a follow-up study Christou and Carlton [62] found higher peak force variability during eccentric contractions at high contraction velocities. Furthermore, at low levels of force the time to peak force was demonstrated to be more variable during eccentric contractions [62]. The differences in motor output variability have been attributed to the greater motor unit discharge rate variability during eccentric contractions [38, 39] in conjunction with a possible selective recruitment of high threshold motor units [40]. Interestingly, the control of force during eccentric cycling has been found to be highly correlated with world ranking in elite alpine slalom skiers [2], which may have implications for the relevance and trainability of eccentric force control to the athletic population.
In summary, individuals are typically stronger eccentrically than concentrically and force production may not be impaired with increased contraction velocities. Age, sex and training history all appear to influence the eccentric to concentric strength ratio and force–velocity relationship [64]. The fine control of eccentric muscle contractions may be lower than concentric contractions, which could have implications in the performance of eccentric exercise modalities. It remains to be demonstrated whether this is a trainable quality, or relatively innate to elite athletes involved in eccentrically biased sports.
3.2 Cardiorespiratory and Fatigue Responses
For a given mechanical power output eccentric exercise is less metabolically demanding than concentric exercise [65, 66], with fewer motor units required for the same work rate [67]. Subsequently, oxygen consumption (\({\dot{\text{V}}\text{O}}_{ 2}\)) is lower during downhill walking versus uphill walking [68], downhill running versus uphill running at a given velocity [69], and during eccentric cycling versus concentric cycling at a given power output [65, 67, 70–72]. Furthermore, energy consumption during combined concentric and eccentric resistance exercise appears to be mostly related to the concentric component [73]. The ratio of \({\dot{\text{V}}\text{O}}_{ 2}\) for concentric versus eccentric exercise always exceeds 1.0, although the precise value will depend on the modality, movement velocity and method of assessment [54, 74]. The observation of an increased ratio with increased movement velocity [75] may be related to the differences in force–velocity relationships between contraction types [54]. Per unit of muscle activation (i.e. EMG), \({\dot{\text{V}}\text{O}}_{ 2}\) can be around three times lower during eccentric contractions [75]. Independent of any change in anaerobic metabolism [76], eccentric exercise (i.e. eccentric cycling and downhill walking) requires 4–5 times less oxygen [67], and a markedly lower cardiac output (\({\dot{\text{Q}}}\)) and heart rate (HR) response to concentric exercise at similar mechanical workloads [65]. The lower metabolic intensity of eccentric cycling has been demonstrated to result in lower perceived exertion, blood lactate accumulation, energy expenditure and carbohydrate oxidation, and higher fat oxidation than concentric cycling at a matched mechanical workload [66, 77]. To attain a similar \({\dot{\text{V}}\text{O}}_{ 2}\) during cycle ergometry, a substantially higher mechanical power output is necessary; under these conditions \({\dot{\text{Q}}}\) and HR are higher than at a similar \({\dot{\text{V}}\text{O}}_{ 2}\) for concentric cycling [65, 67]. Above a certain threshold (i.e. >1 L/min), \({\dot{\text{Q}}}\) can be 27 % higher and HR 17 % higher for a given \({\dot{\text{V}}\text{O}}_{ 2}\) during eccentric exercise [65], accompanied by a higher rating of perceived exertion [67]. Below this threshold eccentric and concentric cycling at similar metabolic intensities result in a similar HR response [78]. Pulmonary ventilation (\({\dot{\text{V}}}_{\text{E}}\)) may also be higher during eccentric exercise (i.e. downhill walking) for a given \({\dot{\text{V}}\text{O}}_{ 2}\) [79], which is probably due to larger muscle forces eliciting a higher neurogenic respiratory drive and \({\dot{\text{V}}}_{\text{E}}\) response [54]. Under such circumstances eccentric exercise is also more heat stressful (e.g. 2 °C higher muscle temperature), instigating a thermoregulatory response which can affect muscle metabolism and oxygen dissociation kinetics [54, 79, 80]. It should be noted that the relationship between \({\dot{\text{V}}}_{\text{E}}\) and \({\dot{\text{V}}\text{O}}_{ 2}\) may be modality dependent as eccentric cycling has been reported to elicit an equivalent \({\dot{\text{V}}}_{\text{E}}\) to concentric cycling for a given \({\dot{\text{V}}\text{O}}_{ 2}\) [71]. The higher mechanical loads with eccentric cycling require a postural bracing via activation of the upper extremities which may impair rib cage expansion, lung volume displacement and therefore \({\dot{\text{V}}}_{\text{E}}\) [71].
Fatigue seems to be less apparent during isokinetic eccentric exercise compared with isokinetic concentric exercise [81, 82]. Higher average mean and peak forces have been found across 100 maximal isokinetic eccentric contractions of the knee extensors in conjunction with a higher total work and lower fatigue index [82]. As with concentric fatigue there are a number of central and peripheral sites which can contribute to an impaired motor output [82], but fatigue resistance during eccentric actions may be particularly influenced by the capacity to maximally recruit muscle [81]. Aligning with this hypothesis, Hortobagyi et al. [83] found that increasing eccentric strength (i.e. a 42 % increase) somewhat attenuated (i.e. ~10 %) the fatigue resistance during an eccentric exercise protocol, albeit non-significantly. Irrespective of the lower energy expenditure and fatigue during eccentric exercise, it appears that the elevated energy expenditure following exercise (e.g. 48–72 h) is directly related to the eccentric contribution and may be due to muscle damage [74, 84, 85]. It should be noted that eccentric cycling exercise has been demonstrated to induce muscle damage without influencing resting energy expenditure in the days following the bout [66, 77], and therefore further research is necessary to draw firm conclusions.
In summary, eccentric exercise is less metabolically expensive at matched workloads and a substantially higher workload is necessary to elicit a comparable \({\dot{\text{V}}\text{O}}_{ 2}\) during downhill running and eccentric cycling. Furthermore, the energy expenditure during resistance exercise may be predominantly attributed to the concentric phase, while fatigue is substantially lower with eccentric versus concentric contractions. It may therefore be possible to attain higher session workloads with eccentric exercise in comparison to concentric or mixed exercise.
3.3 Hormonal Responses
Eccentric resistance exercise has been found to elicit comparable and lower testosterone and growth hormone (GH) responses, respectively, than concentric exercise at the same absolute workload [86]. Exercise at the same absolute load across contraction types precludes an equivalent relative intensity, and it was subsequently demonstrated that the GH response was similar during both concentric and eccentric resistance exercise when accounting for eccentric strength [87]. Kraemer and colleagues [88] reported that eccentric resistance exercise elicits similar GH and testosterone responses to concentric exercise at approximately equivalent relative intensities. Insulin-like growth factor-I (IGF-I) appears to be more responsive to the higher mechanical tension with eccentric contractions and has been found to be higher 48 h following eccentric exercise versus concentric exercise [89], although the body of evidence suggests little difference [88]. Most studies have compared concentric-only to eccentric-only protocols which may not, anecdotally at least, represent the diversity of approaches used within the field. Ojasto and Hakkinen [90] investigated the effects of an eccentric overload applied on top of a typical concentric load (i.e. with the addition of weight releasers which unhooked at the bottom portion of the lift) during a bench press. When eccentric and concentric loads were used equivalent to 90 and 70 % of the concentric one repetition maximum (1RM), respectively, the highest blood lactate and GH responses were observed [90]. It was proposed that this loading scheme allowed an optimal combination of intensity and volume as fewer repetitions were achieved with eccentric and concentric loads of 100 and 70 % 1RM with a concomitantly attenuated GH response [90]. Irrespective of contraction type, it appears that a slow contraction velocity maximises the GH response, while IGF-I and testosterone seem to be less influenced by time under tension [88]. Although much of the available data comes from untrained subjects [88], Calixto et al. [91] demonstrated in resistance-trained participants a higher GH response with slower eccentric velocities compared with fast eccentric velocities. The blood lactate response was also substantially higher with slow contractions, which implies a relationship between the GH response and the anaerobic glycolytic contribution to the exercise bout [91]. Indeed, less lactate accumulation has been observed following eccentric versus concentric resistance exercise at similar absolute workloads [86], and the magnitude of the difference is partly attenuated with matched relative intensities [87]. Eccentric contractions do not appear to elicit notably different insulin or cortisol responses from concentric contractions, although most studies have used matched absolute loads and therefore the influence of relative intensity remains less clear [88].
In summary, the hormonal response does not appear to be largely influenced by contraction type but rather a combination of load and time under tension. Whether the acute hormonal response to exercise mediates long-term adaptations remains contentious [92], and is perhaps less important than other transcriptional factors.
3.4 Molecular Responses
The upregulation of satellite cell activity, in conjunction with other transcriptional pathways, has an important role in the adaptive response to training [93]. Satellite cells are mitotically and metabolically quiescent precursor (i.e. stem) cells that reside between the basal lamina and the sarcolemmal membrane of skeletal muscle [94]. With an appropriate stimulus (i.e. muscle damage from injury or exercise), satellite cells are activated, proliferate and migrate to areas of damage, fusing to surrounding muscle [95, 96]. Satellite cells produce daughter cells and subsequently new myonuclei within muscle, which increases the capacity for protein synthesis [97]. Although resistance training has been well established to increase myonuclear and satellite cell content [94], Hyldahl et al. [95] showed that maximal (i.e. isokinetic) eccentric but not concentric resistance exercise elicited satellite cell proliferation acutely following exercise. They suggested that the muscle damage associated with the eccentric component may be the primary driver activating the satellite cell gene pool [95]. This aligns with the finding that the cytokine interleukin-6, a signalling molecule for satellite cell activation [96], increases in the acute period following eccentric exercise, with a role in the immediate immune response to muscle damage [98]. In the 24-h period following a single bout of maximal isokinetic eccentric exercise, satellite cell content can increase from 30 to 150 % [94, 96, 97], and while satellite cell activity has been demonstrated to increase from 24 to 72 h [95, 96], other markers (e.g. natural cell adhesion molecule and the fetal antigen 1) can be elevated for up to 8 days following an eccentric exercise bout [99]. There is also evidence indicating a preferential satellite cell increase in fast twitch muscle fibres. A single bout of maximal eccentric exercise was found to induce a significant increase in satellite cell activity in type II fibres in contrast to no apparent change in type I fibres [94].
Protein synthesis is a key variable regulated in the post-exercise period [93]. Maximising net protein accretion (i.e. protein synthesis − protein breakdown) will benefit the hypertrophic response to a given training protocol [100]. Force generation and stretch have been established to activate protein synthesis [93], and given that eccentric contractions involve both, it is plausible that there is an additive effect beyond what could be attained with each mechanism in isolation [101]. Indeed, Z-disk streaming with eccentric resistance exercise is proposed to be an important factor in the hypertrophic response due to the presence of phospholipase D, which may mediate stretch-induced anabolic signalling [102, 103]. Changes in protein synthesis rates are mediated by the activation of enzymes which control protein translation into muscle [101], and intracellular signalling has been found to be influenced by contraction type [104, 105]. Matched for total work, maximal isokinetic eccentric exercise can induce a more rapid rise in myofibrillar protein synthesis and subsequently a greater myofibrillar protein accretion in the post-exercise period (i.e. 8.5 h) compared with maximal concentric exercise [100]. A modest bout of eccentric exercise (i.e. 4 × 6 maximal isokinetic contractions) can upregulate p70 S6 kinase (p70S6k) activity and thus protein translation initiation in the absence of nutritional intake for at least 2 h, while maximal concentric exercise may not [101]. The activation of p70S6k is an important step in the P13 K/Akt/mTORCI/p70S6k muscle hypertrophy signalling pathway which is known to increase protein synthesis in response to mechano growth factor messenger RNA expression [102]. Variation in eccentric contraction velocity (i.e. 20°·s−1 vs. 210°·s−1) does not appear to influence the magnitude of p70S6k upregulation [102]. In alignment with other findings of a fibre-type specific response to maximal eccentric contractions, type I and II muscle fibres may exhibit pronounced differences in p70S6k upregulation following eccentric resistance exercise with a substantially greater increase in type II fibres [103]. The striated muscle activator Rho signalling pathway assists with the transcription of specific myofibrillar genes in response to an acute exercise bout and is also upregulated to a greater extent with maximal eccentric contractions [104]. Maximal eccentric contractions can increase markers of collagen expression (i.e. transforming growth factor-β-1 [TGF-β-1]) in skeletal muscle to a greater extent than concentric contractions in rats, although tendon collagen expression was reported to be less sensitive to contraction type than muscle [106].
In summary, muscle satellite cell activity and anabolic signalling pathways appear to be upregulated to a greater extent with maximal eccentric contractions, while there is evidence that type II fibres in particular benefit from these anabolic processes.
3.5 Exercise-induced Muscle Damage and the Repeated Bout Effect
Fewer motor units are recruited for a given submaximal load during eccentric versus concentric contractions, which implies that there will be greater force per active motor unit [74, 75]. This is related to exercise-induced muscle damage (EIMD) to recruited muscle fibres [64], while aspects of eccentric contractions not related to tension per se also appear to predispose to the occurrence of EIMD [107, 108]. The extent of EIMD from eccentric exercise appears to be greater with higher loads [109], fast contraction velocities [110], long muscle lengths during exercise [107] and in untrained participants [111]. EIMD is characterised by increased circulating intramuscular enzymes such as creatine kinase (CK), along with skeletal troponin I, myoglobin and MHCs [112], and is known to impair force and power production [74]. Reductions of 10–60 % of MVC have been reported for up to a week following eccentric exercise [64, 113]. The magnitude of MVC impairment appears to be directly related to the number of muscle fibres with myofibrillar disruption [114]. Power output at various cycling cadences can be substantially impaired (i.e. 11–15 %) for at least 48 h following an eccentric cycling bout [115], with the impairment being specific to the muscle group (i.e. knee extensors) that absorbs the most force during the particular task [108]. The reduced neuromuscular performance with EIMD may, at least partly, be underpinned by impaired sarcolemmal action potential conduction velocity [116] and transient changes (e.g. 24 h) in central nervous system activity [74]. Delayed onset-muscle soreness (DOMS) refers to the dull, aching pain felt during movement or upon palpation of the affected tissue and often accompanies EIMD [117]. Muscle soreness appears in the hours following eccentric exercise, peaks after 1–3 days and disappears after 7–10 days [118]. Interestingly, DOMS appears to be independent of other markers (e.g. MVC, range of motion and plasma CK) of EIMD [119]. The finding that DOMS can reflect connective tissue damage and inflammation more so than muscle fibre damage and inflammation, may partly explain this discrepancy [120–122].
Passive tension, swelling of muscle and increases in muscle hardness [113] may all contribute to a reduced range of joint motion often observed following eccentric exercise [117]. Sense of force and position can both be negatively affected following eccentric exercise [107], which may have implications for the performance of sporting tasks. Running economy following a bout of downhill running can be reduced for 3 days [123], and may be particularly impaired at higher intensities with increased muscle fibre recruitment [124]. Gait can be affected by EIMD in a muscle-specific manner [125]. Two days (i.e. 48 h) following damaging isokinetic eccentric knee extensor exercise, subjects exhibited reduced knee joint range of motion during both walking and running [125]. A lack of change in stride frequency or stride time in conjunction with altered pelvic kinematics suggests subjects modulated gait in an effort to minimise pain [125], and a stiffer leg spring has been observed during SSC activities in the presence of EIMD, which supports a possible compensatory stiffness modulation [126]. As noted, knee joint power is most affected following eccentric cycling (i.e. 19 %), but total power is reduced to a lesser extent (i.e. 11 %), indicating that multi-joint performance can be better maintained in the presence of EIMD than single-joint performance [108]. A number of metabolic consequences of EIMD have also been reported, including decreased glucose uptake and insulin sensitivity, impaired glycogen synthesis, elevated metabolic rate and a shift towards non-oxidative metabolism [112]. Symptoms of EIMD become prominent 12–48 h after intense or unfamiliar eccentric exercise, peaking between 24 and 72 h, and gradually disappearing in 5–7 days in concert with the restoration of neuromuscular capabilities [64, 74].
The pathophysiology of EIMD is not entirely understood and several theories have been proposed to explain the phenomenon. It has been disputed whether disruption to sarcomeres within the myofibrils or damage to the excitation–contraction coupling system is the primary event underpinning EIMD [127]. Proske and Morgan [127] have argued in favour of the former (e.g. the ‘popping sarcomere’ hypothesis), and proposed that a region of instability on the descending limb of the length-tension curve is the basis for EIMD with eccentric exercise. When myofibrils are stretched while contracting, sarcomeres with an overlap closer to their optimum value resist stretch more so than others, meaning that weaker sarcomeres take up most of the stretch. These sarcomeres become progressively weaker if this occurs on the descending limb of the length-tension curve, and upon reaching their yield point will lengthen uncontrollably (i.e. ‘popping’) to the point of no myofilament overlap [128], subsequently engaging passive structures to maintain active tension equivalent to adjacent sarcomeres [127]. Across a series of contractions, an increasing number of sarcomeres (i.e. from weakest to strongest) become overstretched and may not reinterdigitate during relaxation and subsequently become disrupted [129]. With a progressive increase in overstretched and disrupted fibres, damage may spread longitudinally to adjacent sarcomeres in the myofibril and transversely to adjacent myofibrils [107]. Overstretched sarcomeres become disorganised (e.g. Z-disk streaming), leading to lesions of the sarcolemma, transverse tubule dilation, sarcoplasmic reticulum fragmentation [64] and thus disruption to excitation–contraction coupling machinery [127, 130]. Extensive damage from repeated eccentric contractions can elicit symptoms of inflammation and necrosis [74], triggering nociceptor (i.e. type III and IV afferents) stimulation and subsequently DOMS [127]. Passive tension can rise with EIMD and muscle stiffness can double, remaining elevated for around 4 days [127]. The uncontrolled release of Ca2+ resulting from membrane damage may elicit a low-level muscle activation and subsequent rise in passive tension [127], although this process remains to be demonstrated. While both slow and fast twitch fibre types can be damaged with eccentric exercise, there is evidence to suggest that type II fibres are particularly susceptible to damage from intense eccentric exercise [131, 132]. Large fast-fatigable motor units may be more vulnerable due to their lack of oxidative capacity, a higher tension generating capacity and/or because they have a shorter optimum length for tension [127].
The repeated bout effect refers to the phenomenon whereby the magnitude of EIMD and DOMS is progressively attenuated with repeated exposures to the same eccentric exercise bout [133]. A second similar bout elicits substantially less EIMD and DOMS [134], with the protective effects lasting from several weeks and possibly up to 6 months [135, 136]. Trained individuals, and particularly those engaged in eccentric exercise, are less susceptible to EIMD and the associated pathophysiological symptoms [111]. The mechanisms underpinning the repeated bout effect are not entirely clear but it appears that neural, mechanical and cellular adaptations all contribute to the adaptive response [74]. Given the unique neural strategies during eccentric contractions, it is plausible that neural adjustments underpin the repeated bout effect [133]. Specifically, changes in activation may better distribute the fibre stresses to limit myofibrillar damage [137]. Supporting this are EMG data indicating a redistribution of stress across a greater number of fibres [83]. The observation of a protective effect on the contralateral limb with ipsilateral training is supportive of a neural component of the repeated bout effect [138], although these adjustments do not appear to completely account for the phenomenon [133]. The mechanical avenue postulates changes in passive and dynamic stiffness via adaptations to non-contractile elements of the musculoskeletal system [133]. As noted, damage to the cytoskeleton is believed to be an important determining factor in EIMD, and subsequently an increase in the structural protein desmin content has been demonstrated within 3–7 days following damaging eccentric exercise in rats [139]. The increase in desmin content is proposed to provide additional reinforcement against mechanical sarcomere strain [139]. Increases in intramuscular connective tissue may also be a protective mechanism by dissipating myofibrillar shear stresses [133]. However, the role of changes in tissue stiffness with the repeated bout effect remains to be clarified as it has been found that stiffer muscles can exacerbate markers of EIMD [140].
Changes at the cellular level of the contractile machinery and in the inflammatory response to exercise may also play a role in the repeated bout effect [133]. Aligning with the popping sarcomere hypothesis, longer muscle lengths during eccentric contractions have been demonstrated as an important determinant of the extent of muscle damage. Morgan (1990) suggested the number of sarcomeres in series increases as an adaptive response to eccentric training, which provides a protective effect against this mechanism of muscle damage by reducing sarcomere strain and mechanical disruption [128]. Indeed, a rightward shift of the length-tension curve can occur following damaging eccentric exercise and may be attributed to added sarcomeres in series [141, 142], although such morphological adaptations require a longer period of time to materialise (e.g. 7 days) indicating a biphasic mechanism underpinning length-tension changes [135]. Shorter term rightward shifts in the length-tension curve reflect popped sarcomeres on the descending limb, while the longer term shift is likely a protective adaptation [135]. Finally, the inflammatory response to eccentric EIMD can exacerbate damage (i.e. secondary damage) prior to any obvious recovery [143]. An attenuated inflammatory response to eccentric exercise was observed when preceded by a training intervention which elicited an initial inflammatory response [143]. Whether this reflects a decrease in secondary proliferation damage or a reduced insult to myofibrillar elements remains to be determined [133].
In summary, EIMD associated with eccentric modalities has a number of consequences for the performance of subsequent exercise within the short term (e.g. ≤7 days). Symptoms of EIMD can be attenuated by a progressive increase in eccentric loading, or via the incorporation of other preconditioning exercises. Trained athletes are less affected by EIMD, and thus a simple progressive overload will probably suffice to minimize the detrimental effects of muscle damage.
4 Conclusion
During eccentric contractions the external force exceeds that produced by the muscle [2]. The molecular mechanisms underpinning eccentric contractions have yet to be confidently elucidated, but recent theories have, at least partly, reconciled unexplained eccentric-related phenomena with established theories of muscular contraction. The increased force produced with lengthening contractions may be a function of mechanical detachment of cross bridges suspended in an actively bound state [9, 12], while residual force enhancement may be explained by the passive action of titin interacting with actin and myosin [5, 24]. Unique neural strategies are apparently involved during maximal and submaximal eccentric contractions [25]; cortical excitability seems to be greater with eccentric contractions yet motor unit activity is lower [27]. Lower motor unit discharge rates suggest spinal inhibition constrains eccentric force [39], particularly in untrained subjects [26, 31]. The unique characteristics of eccentric contractions have important implications for the acute responses during and following eccentric exercise bouts. Approximately 20–60 % more force can be generated during eccentric contractions compared with eccentric contractions [58, 59], yet eccentric exercise requires less energy per unit work and thus elicits a substantially lower cardiopulmonary response [65, 67]. It remains unclear whether contraction type influences hormonal responses [88], although there is some evidence indicating a larger increase in IGF-I following eccentric exercise [89]. Muscle satellite cell activity [95] and anabolic signalling pathways [100, 101, 104–106] are upregulated to a greater extent with eccentric contractions, while fast twitch fibres appear especially responsive to these anabolic stimuli [94, 103]. Eccentric contractions are damaging to muscle, and a host of consequences have been reported in the acute post-exercise period [64]. Type II fibres are seemingly most susceptible to EIMD [131, 132], which aligns with findings of increased anabolic signalling within these fibres. Repeated exposure to the same eccentric bout attenuates EIMD symptoms and has been termed the repeated bout effect [133]. The acute responses to eccentric exercise probably underpin many of the unique chronic adaptations observed with long-term eccentric training.
References
Lindstedt SL, LaStayo PC, Reich TE. When active muscles lengthen: properties and consequences of eccentric contractions. News Physiol Sci. 2001;16:256–61.
Vogt M, Hoppeler HH. Eccentric exercise: mechanisms and effects when used as training regime or training adjunct. J Appl Physiol. 2014;116(11):1446–54.
Huxley AF, Niedergerke R. Structural changes in muscle during contraction: interference microscopy of living muscle fibres. Nature. 1954;173:971–3.
Huxley HE, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173:971–3.
Herzog W. Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J Appl Physiol. 2014;116(11):1407–17.
Herzog W, Powers K, Johnston K, et al. A new paradigm for muscle contraction. Front Physiol. 2015;6(174):1–11.
Schmidtbleicher D. Training for power events. In: Komi PV, editor. Strength and power in sport: the encyclopaedia of sports medicine. Encyclopaedia of sports medicine. Oxford: Blackwell Science Ltd; 1992. p. 381–395.
Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318.
Linari M, Lucii L, Reconditi M, et al. A combined mechanical and X-ray diffraction study of stretch potentiation in single frog muscle fibers. J Physiol. 2000;526(3):589–96.
Edman KAP, Elzinga G, Noble MIM. Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol. 1982;80:769–84.
Curtin NA, Davies RE. Very high tension with very little ATP breakdown by active skeletal muscle. J Mechanochem Cell Motil. 1975;3(2):147–54.
Linari M, Bottinelli R, Pellegrino MA, et al. The mechanism of the force response to stretch in human skinned muscle fibers with different myosin isoforms. J Physiol. 2004;554(2):335–52.
Huxley AF. Biological motors: energy storage in myosin molecules. Curr Biol. 1998;8(14):R485–8.
Herzog W. The role of titin in eccentric muscle contraction. J Exp Biol. 2014;217:2825–33.
Herzog W, Leonard TR, Joumaa V, et al. Mysteries of muscle contraction. J Appl Biomech. 2008;24:1–13.
Menard MR, Penn AM, Lee JW, et al. Relative metabolic efficiency of concentric and eccentric exercise determined by 31P magnetic resonance spectroscopy. Arch Phys Med Rehab. 1991;72(12):976–83.
Rassier DE, Herzog W, Pollack GH. Stretch-induced force enhancement and stability of skeletal muscle myofibrils. Adv Exp Med Biol. 2003;538:501–15.
Leonard TR, Herzog W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am J Physiol. 2010;229:C14–20.
Kellermayer MSZ, Smith SB, Granzier HL, et al. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276:1112–6.
Horowits R, Podolsky RJ. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol. 1987;105:2217–23.
Herzog JA, Leonard TR, Jinha A, et al. Are titin properties reflected in single myofibrils? J Biomech. 2012;45:1893–9.
DuVall MM, Gifford JL, Amrein M, et al. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur Biophys J. 2013;42:301–7.
Labeit D, Watanabe K, Witt C, et al. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA. 2003;100:13716–21.
Nishikawa KC, Monroy JA, Uyeno TE, et al. Is titin a ‘winding filament’? A new twist on muscle contraction. Proc Biol Sci. 2012;279:981–90.
Enoka RM. Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol. 1996;81(6):2339–46.
Aagaard P. Training-induced changes in neural function. Exerc Sport Sci Rev. 2003;31(2):61–7.
Duchateau J, Baudry S. Insights into the neural control of eccentric contractions. J Appl Physiol. 2014;116(11):1418–25.
Aagaard P, Simonsen EB, Andersen JL, et al. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol. 2000;89:2249–57.
Kellis E, Baltzopoulos V. Muscle activation differences between eccentric and concentric isokinetic exercise. Med Sci Sports Exerc. 1998;30(11):1616–23.
Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol Occup Physiol. 1991;62(2):104–8.
Amiridis IG, Martin A, Morlon B, et al. Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur J Appl Physiol. 1996;73:149–56.
Duclay J, Martin A. Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol. 2005;94(5):3555–62.
Babault N, Pousson M, Ballay Y, et al. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91:2628–34.
Baudry S, Klass M, Pasquet B, et al. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–25.
Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–83.
Beltman JGM, Sargeant AJ, van Mechelen W, et al. Voluntary activation level and muscle fiber recruitment of human quadriceps during lengthening contractions. J Appl Physiol. 2004;97(2):619–26.
Westing SH, Seger JY, Thorstensson A. Effects of electrical stimulation on eccentric and concentric torque-velocity relationships during knee extension in man. Acta Physiol Scand. 1990;140:17–22.
Del Valle A, Thomas CK. Firing rates during strong dynamic contractions. Muscle Nerve. 2005;32:316–25.
Duchateau J, Enoka RM. Neural control of lengthening contractions. J Exp Biol. 2016;219:197–204.
Nardone A, Romano C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol. 1989;409(1):451–71.
Kulig K, Powers CM, Shellock FG, et al. The effects of eccentric velocity on activation of elbow flexors: evaluation by magnetic resonance imaging. Med Sci Sports Exerc. 2001;33(2):196–200.
Nardone A, Schieppati M. Shift of activity from slow to fast muscle during voluntary lengthening contractions of the triceps surae muscles in humans. J Physiol. 1988;395:363–81.
Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol. 2006;577(Pt 2):753–65.
Søgaard K, Christensen H, Jensen BR, et al. Motor control and kinetics during low level concentric and eccentric contractions in man. Electroencephalogr Clin Neurophysiol. 1996;101(5):453–60.
Stotz PJ, Bawa P. Motor unit recruitment during lengthening contractions of human wrist flexors. Muscle Nerve. 2001;24(11):1535–41.
Bawa P, Jones KE. Do lengthening contractions represent a case of reversal in recruitment order? Prog Brain Res. 1999;123:215–20.
Henneman E. Relationship between size of neurons and their susceptibility to discharge. Science. 1957;126(3287):1345–7.
Fang Y, Siemionow V, Sahgal V, et al. Distinct brain activation patterns for human maximal voluntary eccentric and concentric muscle actions. Brain Res. 2004;1023:200–12.
Fang Y, Siemionow V, Sahgal V, et al. Greater movement-related cortical potential during human eccentric versus concentric muscle contractions. J Neurophysiol. 2001;86(4):1764–72.
Gruber M, Linnamo V, Strojnik V, et al. Excitability at the motoneuron pool and motor cortex is specifically modulated in lengthening compared to isometric contractions. J Neurophysiol. 2009;101(4):2030–40.
Abbruzzese G, Morena M, Spadavecchia L, et al. Response of arm flexor muscles to magnetic and electrical brain stimulation during shortening and lengthening tasks in man. J Physiol. 1994;481(Pt 2):499–507.
Sekiguchi H, Kimura T, Yamanaka K, et al. Lower excitability of the corticospinal tract to transcranial magnetic stimulation during lengthening contractions in human elbow flexors. Neurosci Lett. 2001;312(2):83–6.
Pinniger GJ, Steele JR, Thorstensson A, et al. Tension regulation during lengthening and shortening actions of the human soleus muscle. Eur J Appl Physiol. 2000;81:375–83.
Prilutsky BI. Eccentric muscle action in sport and exercise. In: Zatsiorsky VM, editor. Biomechanics in sport: volume IX encyclopaedia of sports medicine. encyclopaedia of sports medicine. Oxford: Blackwell Science Ltd; 2000. p. 56–86.
Cavagna GA, Citterio G. Effects of stretching on the elastic characteristics and the contractile component of frog striated muscle. J Physiol. 1974;239:1–14.
Westing SH, Seger JY, Karlson E, et al. Eccentric and concentric torque-velocity characteristics of the quadriceps femoris in man. Eur J Appl Physiol Occup Physiol. 1988;58:100–4.
Linnamo V, Strojnik V, Komi PV. Maximal force during eccentric and isometric actions at different elbow angles. Eur J Appl Physiol. 2006;96(6):672–8.
Hollander DB, Kraemer RR, Kilpatrick MW, et al. Maximal eccentric and concentric strength discrepancies between young men and women for dynamic resistance exercise. J Strength Cond Res. 2007;21(3):34–40.
Hortobagyi T, Katch F. Eccentric and concentric torque velocity relationships during arm flexion and extension: influence of strength level. Eur J Appl Physiol. 1990;60:395–401.
Griffin JW, Tooms RE, Vander Zwaag R, et al. Eccentric muscle performance of elbow and knee muscle groups in untrained men and women. Med Sci Sports Exerc. 1993;25(8):936–44.
Reeves ND, Narici MV. Behavior of human muscle fascicles during shortening and lengthening contractions in vivo. J Appl Physiol. 2003;95(3):1090–6.
Christou EA, Carlton LG. Motor output is more variable during eccentric compared with concentric contractions. Med Sci Sports Exerc. 2002;34(11):1773–8.
Christou EA, Carlton LG. Age and contraction type influence motor output variability in rapid discrete tasks. J Appl Physiol. 2002;93:489–98.
Guilhem G, Cornu C, Guevel A. Neuromuscular and muscle-tendon system adaptations to isotonic and isokinetic eccentric exercise. Ann Phys Rehabil Med. 2010;53:319–41.
Dufour SP, Lampert E, Doutreleau S, et al. Eccentric cycle exercise: training application of specific circulatory adjustments. Med Sci Sports Exerc. 2004;36(11):1900–6.
Penailillo L, Blazevich A, Numazawa H, et al. Metabolic and muscle damage profiles of concentric versus repeated eccentric cycling. Med Sci Sports Exerc. 2013;45(9):1773–81.
Perrey S, Betik A, Candau R, et al. Comparison of oxygen uptake kinetics during concentric and eccentric cycle exercise. J Appl Physiol. 2001;91(5):2135–42.
Navalta JW, Sedlock DA, Park KS. Physiological responses to downhill walking in older and younger individuals. J Exerc Physiol. 2004;7(6):45–51.
Minetti AE, Moia C, Roi GS, et al. Energy cost of walking and running at extreme uphill and downhill slopes. J Appl Physiol. 2002;93:1039–46.
Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol. 1952;117:380–90.
Lechauve JB, Parrault H, Aguilaniu B, et al. Breathing patterns during eccentric exercise. Respir Physiol Neurobiol. 2014;202:53–8.
Knuttgen HG, Patton JF, Vogel JA. An ergometer for concentric and eccentric muscular exercise. J Appl Physiol. 1982;53(3):784–8.
Dudley GA, Tesch PA, Harris RT, et al. Influence of eccentric actions on the metabolic cost of resistance exercise. Aviat Space Environ Med. 1991;62(7):678–82.
Isner-Horobeti M, Dufour SP, Vautravers P, et al. Eccentric exercise training: modalities, applications and perspectives. Sports Med. 2013;43:483–512.
Bigland-Ritchie B, Woods JJ. Integrated electromyogram and oxygen uptake during positive and negative work. J Physiol. 1976;260(2):267–77.
Bonde-Petersen F, Knuttgen HG, Henriksson J. Muscle metabolism during exercise with concentric and eccentric contractions. J Appl Physiol. 1972;33(6):792–5.
Penailillo L, Blazevich A, Nosaka K. Energy expenditure and substrate oxidation during and after eccentric cycling. Eur J Appl Physiol. 2014;114:805–14.
Knuttgen HG, Klausen K. Oxygen debt in short-term exercise with concentric and eccentric muscle contractions. J Appl Physiol. 1971;30(5):632–5.
Davies CT, Barnes C. Negative (eccentric) work. II. Physiological responses to walking uphill and downhill on a motor-driven treadmill. Ergonomics. 1972;15:121–31.
Nielsen B, Nielsen SL, Petersen FB. Thermoregulation during positive and negative work at different environmental temperatures. Acta Physiol Scand. 1972;85:249–57.
Grabiner MD, Owings TM. Effects of eccentrically and concentrically induced unilateral fatigue on the involved and uninvolved limbs. J Electromyogr Kinesiol. 1999;9(3):185–9.
Baroni BM, Stocchero CMA, do Espírito Santo RC, et al. The effect of contraction type on muscle strength, work and fatigue in maximal isokinetic exercise. Isokinet Exerc Sci. 2011;19(3):215–20.
Hortobagyi T, Barrier J, Beard D, et al. Greater initial adaptations to submaximal muscle lengthening than maximal shortening. J Appl Physiol. 1996;81(4):1677–82.
Dolezal BA, Potteiger JA, Jacobsen DJ, et al. Muscle damage and resting metabolic rate after acute resistance exercise with an eccentric overload. Med Sci Sports Exerc. 2000;32(7):1202–7.
Hackney KJ, Engels HJ, Gretebeck RJ. Resting energy expenditure and delayed-onset muscle soreness after full-body resistance training with an eccentric concentration. J Strength Cond Res. 2008;22(5):1602–9.
Durand RJ, Castracane VD, Hollander DB, et al. Hormonal responses from concentric and eccentric muscle contractions. Med Sci Sports Exerc. 2003;35(6):937–43.
Kraemer RR, Hollander DB, Reeves GV, et al. Similar hormonal responses to concentric and eccentric muscle actions using relative loading. Eur J Appl Physiol. 2006;96:551–7.
Kraemer RR, Castracane VD. Endocrine alterations from concentric vs. eccentric muscle actions: a brief review. Metabolism. 2015;64(2):190–201.
Bamman MM, Shipp JR, Jiang J, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentration in humans. Am J Physiol Endocrinol Metab. 2001;280:E383–90.
Ojasto T, Hakkinen K. Effects of different accentuated eccentric loads on acute neuromuscular, growth hormone, and blood lactate responses during a hypertrophic protocol. J Strength Cond Res. 2009;23(3):946–53.
Calixto RD, Verlengia R, Crisp AH, et al. Acute effects of movement velocity on blood lactate and growth hormone responses after eccentric bench press exercise in resistance-trained men. Biol Sport. 2014;31(4):289–94.
Schoenfeld BJ. Postexercise hypertrophic adaptations: a reexamination of the hormone hypothesis and its applicability to resistance training program design. J Strength Cond Res. 2013;27(6):1720–30.
Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006;97:643–63.
Cermak NM, Snijders T, McKay BR, et al. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc. 2013;45(2):230–7.
Hyldahl RD, Olson T, Welling T, et al. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front Physiol. 2014;5(485):1–11.
McKay BR, De Lisio M, Johnston APW, et al. Association of interleukin-6 signalling with muscle stem cell response following muscle-lengthening contractions in humans. PLoS One. 2009;4(6):1–13.
Dreyer HC, Blanco CE, Sattler FR, et al. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33:242–53.
Willoughby DS, McFarlin B, Bois C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med. 2003;24:15–21.
Crameri RM, Langberg H, Magnusson P, et al. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004;558(1):333–40.
Moore DR, Phillips SM, Babraj JA, et al. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–9.
Eliasson J, Elfegoun T, Nilsson J, et al. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291:E1197–205.
Roschel H, Ugrinowistch C, Barroso R, et al. Effects of eccentric exercise velocity on akt/mtor/p70s6k signalling in human skeletal muscle. Appl Physiol Nutr Metab. 2011;36:283–90.
Tannerstedt J, Apró W, Blomstrand E. Maximal lengthening contractions induce different signalling responses in the type I and type II fibers of human skeletal muscle. J Appl Physiol. 2009;106:1412–8.
Vissing K, Rahbek SK, Lamon S, et al. Effect of resistance exercise contraction mode and protein supplementation on members of the STARS signalling pathway. J Physiol. 2013;591(15):3749–63.
Kostek MC, Chen YW, Cuthbertson DJ, et al. Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol Genomics. 2007;31:42–52.
Heinemeier KM, Olesen JL, Haddad F, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582(3):1303–16.
Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Med Sci Sports Exerc. 2005;33(2):98–104.
Elmer SJ, Martin JC. Joint-specific power loss after eccentric exercise. Med Sci Sports Exerc. 2010;42(9):1723–30.
McHugh MP, Tetro DT. Changes in the relationship between joint angle and torque production associated with the repeated bout effect. J Sport Sci. 2003;21:927–32.
Chapman D, Newton M, Sacco P, et al. Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med. 2006;27:591–8.
Newton MJ, Morgan GT, Sacco P, et al. Comparison of responses to strenuous eccentric exercise of the elbow flexors between resistance-trained and untrained men. J Strength Cond Res. 2008;22(2):597–607.
Tee JC, Bosch AN, Lambert MI. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007;37(10):827–36.
Murayama M, Nosaka K, Yoneda T, et al. Changes in hardness of the human elbow flexor muscles after eccentric exercise. Eur J Appl Physiol. 2000;82(5–6):361–7.
Raastad T, Owe SG, Paulsen G, et al. Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc. 2010;42(1):86–95.
Elmer SJ, McDaniel J, Martin JC. Alterations in neuromuscular function and perceptual responses following acute eccentric cycling exercise. Eur J Appl Physiol. 2010;110:1225–33.
Piitulainen H, Bottas R, Komi P, et al. Impaired action potential conduction at high force levels after eccentric exercise. J Electromyogr Kinesiol. 2010;20:879–87.
Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–20.
Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med. 2003;33(2):145–64.
Nosaka K, Newton M, Sacco P. Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand J Med Sci Sports. 2002;12:337–46.
Crameri RM, Aagaard P, Qvortrup K, et al. Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol. 2007;583(1):365–80.
Gibson W, Arendt-Nielsen L, Taguchi T, et al. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194(2):299–388.
Lau WY, Blazevich AJ, Newton MJ, et al. Changes in electrical pain threshold of fascia and muscle after initial and secondary bouts of elbow flexor eccentric exercise. Eur J Appl Physiol. 2015;115(5):959–68.
Chen TC, Nosaka K, Tu JH. Changes in running economy following downhill running. J Sport Sci. 2007;25(1):55–63.
Chen TC, Nosaka K, Lin MJ, et al. Changes in running economy at different intensities following downhill running. J Sport Sci. 2009;27(11):1137–44.
Paschalis V, Giakas G, Baltzopoulos V, et al. The effects of muscle damage following eccentric exercise on gait biomechanics. Gait Posture. 2007;25:236–42.
Harrison AJ, Gaffney SD. Effects of muscle damage on stretch-shortening cycle function and muscle stiffness control. J Strength Cond Res. 2004;18(4):771–6.
Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical adaptations. J Physiol. 2001;537:333–45.
Morgan DL. New insights into the behaviour of muscle during active lengthening. Biophys J. 1990;57:209–21.
Wood SA, Morgan DL, Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. Am J Physiol. 1993;265(3):C792–800.
Piitulainen H, Holobar A, Avela J. Changes in motor unit characteristics after eccentric elbow flexor exercise. Scand J Med Sci Sports. 2012;22:418–29.
Vijayan K, Thompson JL, Norenberg KM, et al. Fiber-type susceptibility to eccentric contraction-induced damage of hindlimb-unloaded rat AL muscles. J Physiol. 2001;90:770–6.
Fridén J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;04(3):170–6.
McHugh MP. Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports. 2003;13(2):88–97.
Morgan DL, Proske U. Popping sarcomere hypothesis explains stretch-induced muscle damage. Clin Exp Pharmacol Physiol. 2004;31:541–5.
Brughelli M, Cronin J. Altering the length-tension relationship with eccentric exercise: implications for performance and injury. Sports Med. 2007;37(9):807–26.
Nosaka K, Sakamoto K, Newton M, et al. How long does the protective effect on eccentric exercise-induced muscle damage last? Med Sci Sports Exerc. 2001;33(9):1490–5.
Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27:1263–9.
Howatson G, van Someren KA. Evidence of a contralateral repeated bout effect after maximal eccentric contractions. Eur J Appl Physiol. 2007;101(2):207–14.
Barash IA, Peters D, Friden J, et al. Desmin cytoskeletal modifications after a bout of eccentric exercise in the rat. Am J Physiol Regul Integr Comp Physiol. 2002;283:R958–63.
McHugh MP, Connolly DAJ, Eston RG, et al. The role of passive muscle stiffness in symptoms of exercise-induced muscle damage. Am J Sports Med. 1999;27(5):594–9.
Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exerc. 2001;33(5):783–90.
Morgan DL, Talbot JA. The addition of sarcomeres in series is the main protective mechanism following eccentric exercise. J Mech Med Biol. 2002;2(3&4):421–31.
Pizza FX, Koh TJ, McGregor SJ, et al. Muscle inflammatory cells after passive stretches, isometric contractions, and lengthening contractions. J Appl Physiol. 2002;92(5):1873–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflicts of interest
Jamie Douglas, Simon Pearson, Angus Ross and Mike McGuigan declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Douglas, J., Pearson, S., Ross, A. et al. Eccentric Exercise: Physiological Characteristics and Acute Responses. Sports Med 47, 663–675 (2017). https://doi.org/10.1007/s40279-016-0624-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0624-8