Abstract
Background
Evidence suggests that leisure-time physical activity (LTPA) during pregnancy is associated with a reduced risk of preeclampsia, gestational diabetes mellitus (GDM), and preterm birth. However, these results are inconsistent when comparing cohort studies and randomized controlled trials (RCTs).
Objective
The purpose of our study was to compare the associations between LTPA in pregnancy and maternal (GDM, preeclampsia, and weight gain during pregnancy) and child health outcomes (preterm birth, birthweight, and fetal growth) between RCTs and cohort studies.
Methods
We performed a systematic search in PubMed, Web of Science, and EBSCO up to 31 August 2015. Inclusion criteria for experimental studies required randomized trials with a control group and exposure to a physical activity structured program. The inclusion criteria for cohort studies required information on LTPA during pregnancy as an exposure and at least one maternal–child health outcome. We assessed the methodological quality of all studies and performed a meta-analysis to produce summary estimates of the effects using random models.
Results
We included 30 RCTs and 51 cohort studies. The meta-analysis of RCTs indicated that participation in LTPA was associated with lower weight gain during pregnancy, lower likelihood of GDM, and lower likelihood of delivering a large-for-gestational-age infant. Cohort studies indicated that participation in LTPA was associated with lower weight gain during pregnancy, lower likelihood of GDM, and lower risk of preterm delivery.
Conclusions
Our findings support the promotion of LTPA in pregnancy as a strategy to improve maternal and child health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The aim of this systematic review and meta-analysis was to compare the associations between leisure-time physical activity (LTPA) in pregnancy on maternal and child health outcomes between randomized controlled trials and cohort studies. |
Our analysis revealed that women who were active during pregnancy were less likely to have excessive weight gain, to have gestational diabetes mellitus, and to deliver a preterm infant or a baby large for gestational age. |
The findings presented in our study provide additional evidence of the positive effects of LTPA during pregnancy and support the promotion of LTPA in pregnancy as a strategy to improve maternal and child health. |
1 Introduction
Pregnancy is a period characterized by intense physical changes, in which morphological adaptations occur to create an ideal environment for the development of the fetus; such rapid changes produce short- and long-term impacts on health [1]. In addition, the gestational period is an opportunity to promote positive health behaviors, considering that women are concerned with the child’s well-being. In this context, the health effects of physical activity during pregnancy have been extensively investigated in the literature. The current evidence suggests potential benefits of leisure-time physical activity (LTPA) during pregnancy on maternal and child health [2–5].
Studies have reported that physical activity performed during pregnancy is related to a lower incidence of gestational diabetes mellitus (GDM) [3], preeclampsia [6], and excessive weight gain [2]. In addition, a decreased incidence of preterm birth [7] and obesity in adult life have been linked to maternal physical activity in pregnancy [8]. The prevention of these complications during pregnancy becomes necessary as the development of diabetes and gestational hypertensive disorders, as well as fetal growth restriction and premature birth, are associated with increased risk of cardiovascular disease and mortality in adulthood [9].
Despite substantial advances in the scientific knowledge and evolution of the guidelines to promote physical activity in pregnancy [10], most pregnant women do not reach the current recommendations of at least 150 min of moderate-intensity aerobic exercise per week and continue to be inactive before and after pregnancy [11, 12]. Furthermore, physical activity levels tend to decline during pregnancy [13, 14]. Previous studies from different countries have shown low levels of physical activity during pregnancy, especially in the third trimester [13–16]. Data from Norway showed that the proportion of women who performed regular exercise before pregnancy was 46.4 %, with sharp declines to 28 and 20 % at the 17th and 30th weeks of gestation, respectively [15]. Other studies conducted in the USA [17] and Denmark [16] showed a similar reduction in physical activity with advancing pregnancy, while one study in South Brazil showed that only 4 % of mothers were active in leisure time during the entire pregnancy [13].
Recent systematic reviews have summarized the associations of physical activity during pregnancy with specific maternal and child health outcomes [3–6]. Some reviews focused on experimental studies only [2, 4, 18], whereas others evaluated observational studies [3, 6, 7]. A series of methodological differences mean observational and experimental designs can lead to different findings. For example, whereas the results of previous meta-analyses of cohort studies showed positive associations between LTPA and maternal–child health [3, 6], most RCTs reported no associations [18–20]. An exploration of the different findings of cohort and experimental studies is a key literature gap.
The aim of our systematic review was to compare the associations between LTPA in pregnancy and three maternal (GDM, preeclampsia, and weight gain during pregnancy) and three child (preterm birth, birthweight, and fetal growth) health outcomes between randomized controlled trials (RCTs) and cohort studies.
2 Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21] and the Cochrane Handbook for Systematic Reviews of Interventions [22].
2.1 Search Strategy
We searched PubMed, Web of Science, and EBSCO up to 31 August 2015 for eligible studies. The following search terms were used in all databases: (physical activity OR exercise OR sports OR motor activity OR leisure time physical activity OR recreational activities OR fitness OR walking) AND (pregnancy OR pregnant woman OR gestation). The terms were entered individually and combined on the advanced search field on each database. We searched the reference lists of included studies and selected reviews for further analysis. In addition, we conducted searches in the Clinical Trials and Cochrane Database Controlled Trials websites. Only publications in English, Spanish, and Portuguese were included. The screening process was carried out independently by two researchers, and disagreements were solved by consensus. If disagreement persisted, a third reviewer resolved the disagreement.
2.2 Inclusion and Exclusion Criteria
The inclusion criteria for experimental studies were (1) RCT; (2) the intervention had to include at least one physical activity component in a structured program; (3) the study had to include a control group; and (4) outcomes had to be presented separately for the intervention and the control groups. Intervention studies were excluded from this review if the sample comprised only women with comorbidities, such as diabetes, preeclampsia, or obesity.
The inclusion criteria for cohort studies were (1) for studies using self-reported physical activity as the exposure variable, only information on LTPA was extracted; (2) studies using accelerometry as the exposure variable; (3) only physical activity during pregnancy was considered (studies evaluating exclusively physical activity before pregnancy were excluded); and (4) the study had to include one of the following three maternal (GDM, preeclampsia, gestational weight gain) or child (birth weight, preterm birth, fetal growth) health outcomes. Cohort studies were excluded if the sample was selected among a specific group of women with a high risk of developing a given outcome.
2.3 Definitions of Outcomes
Excessive gestational weight gain (EWG) was defined in accordance with the recommendations from the US Institute of Medicine (IOM), which establishes proper weight gain parameters according to categories of body mass index [23]. GDM is a specific disorder of pregnancy, defined as “any degree of glucose intolerance with onset or first recognition during pregnancy” [24]. Preeclampsia is also a specific disorder of pregnancy characterized by hypertension (blood pressure ≥140/90 mmHg) and the presence of protein in the urine from the 20th week of pregnancy in previously normotensive women [25]. Low birth weight was defined as birth weight <2500 g in term babies [26]. Preterm birth was defined as births occurring before the 37th week of gestation [27]. Fetal growth was divided into two separate outcome variables: [24] large for gestational age (LGA) was defined as a fetus or infant larger or more developed than expected for the baby’s sex and gestational age [28]; small for gestational age (SGA) was defined as a fetus or infant smaller or less developed than expected for the baby’s sex and gestational age [28].
2.4 Data Extraction
Data from selected studies were screened by two reviewers separately. From experimental studies, we extracted the characteristics of the study (author, year, country), participants (number in each group, total number), intervention (type, duration, frequency, and intensity of physical activity intervention), and findings. From cohort studies, we extracted characteristics of the study (author, year, country); cohort characteristics (gestational period, number of participants, measurement methods of physical activity), and findings.
2.5 Quality Assessment
We used the Jadad Scale [29] to evaluate the quality of experimental studies. The scale comprised three main topics: randomization, blinding, and dropouts. Points were also given for appropriate use and description of the randomization and blinding method. Because double blinding was not possible for exercise interventions, the final score ranged from 0 (worst) to 4 (best) points. For the quality assessment of cohort studies, we used the Newcastle–Ottawa Scale [30], comprising eight items on sampling methods, comparability, and outcome accuracy. Two researchers conducted the evaluation process independently. The proportion of disagreement was <10 %, and the two reviewers agreed by consensus in these instances.
2.6 Statistical Analysis
Statistical analyses were conducted using Stata version 13.0. We performed a meta-analysis to assess a summary estimate of the effects in each article by calculating a random model. I 2 was used to test heterogeneity. Usually I 2 values of <25, 25–50, and >50 % are considered to represent small, medium, and large levels of inconsistency. For binary outcomes, we calculated the odds ratios (OR) and 95 % confidence intervals (CIs) for the categorical outcomes of GDM, preeclampsia, and fetal growth (SGA vs. others, and LGA vs. others).
For studies using continuous outcomes, such as gestational weight gain (kg), birth weight (g), and gestational age (weeks), we calculated mean differences (MDs) and difference in standard error (DSE). In cohort studies, gestational weight gain and preterm births (<37 weeks) were calculated as binary outcomes. For the cohort studies in which birth weight was the outcome variable, we calculated regression coefficients (β) and 95 % CIs.
We used a random-effects meta-analysis to pool the estimates, which takes into account between-study heterogeneity, since the study design and exposure, definition of physical activity, and intensity were not uniform across cohort studies. We chose the DerSimonian and Laird method for estimating random effects to distribute weights evenly, since this method evaluates the contribution of studies with small sample size as well as heterogeneity between studies [22].
To allow for comparability of exposure across cohort studies, we analyzed the OR for the highest physical activity category versus the lowest (reference) category. All analyses were conducted separately for experimental and cohort studies.
3 Results
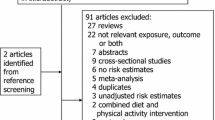
The search strategy resulted in 17,925 titles to be examined, of which 1119 were selected for abstract review. After reading the abstracts, we excluded 1001 articles, and therefore read 118 full texts. After we applied the inclusion and exclusion criteria, our review included 30 randomized trials and 51 cohort studies (Fig. 1).
3.1 Randomized Controlled Trials
Table 1 presents the characteristics of the 30 RCTs included in our review. The countries with more studies were Spain (n = 10), Brazil (n = 4), Norway (n = 4), and the USA (n = 3). A large variability was found in the number of participants for each intervention, ranging from nine to 481 individuals in the intervention group and six to 481 in the control group. Only the study by de Oliveria Melo [31] included three groups, as described in Table 1. All interventions comprised a structured exercise program. Most included moderate-intensity physical activities three times per week. The duration of sessions varied between 20 and 70 min. Exercise strategies varied widely, although the majority included aerobic exercises and muscle resistance or strength training. In terms of methodological quality according to the Jadad scale [29], the mean score was 2.7 points, ranging from 1 (worst) to 4 (best) points.
In most included studies, the dropout rate was low (<15 %) [1, 8, 32–42], four studies had a dropout rate between 15 and 20 % [31, 43–45], eight studies between 20 and 25 % [46–53], and five studies had a dropout rate >25 % [54–58]. The main reasons for dropouts in the intervention group were discontinued intervention [1, 34, 38, 40, 46–48, 55–57], risk of premature birth [42, 50, 51], pregnancy-induced hypertension [35, 39], logistical difficulties [54], and lack of flexibility in test scheduling [53]. Regarding compliance with the LTPA intervention protocol, nine studies reported a high rate (>90 %) [32, 36, 39–41, 43, 44, 48, 49], eight had a rate between 80 and 90 % [8, 34, 35, 44, 46, 47, 50, 51], and six studies had compliance <80 % [1, 33, 38, 42, 54, 58]. In six other studies, the adherence rate could not be identified [37, 52, 53, 55–57]. The main reasons to quit the LTPA programs were unwillingness to do the exercise [41], personal reasons [8, 43–45], transport issues and logistical difficulties [54]. The main barrier reported by women in these studies was related to the difficulties the women had in regularly attending the scheduled programs sessions. Lack of blinding was a methodological flaw recognized in most interventions; however, it is difficult to overcome this problem in physical activity programs for pregnant women.
3.2 Cohort Studies
Table 2 displays the characteristics of the cohort studies included. Most studies came from the USA (n = 24), followed by Denmark (n = 6), Norway (n = 5), and Brazil (n = 4). Sample sizes ranged from 44 to 87,232 participants. We observed a clear heterogeneity of physical activity definitions, including continuous scores in metabolic equivalents (METs) multiplied by time, counts from accelerometry, and duration. Most instruments used to assess physical activity were self-reported questionnaires; four studies included accelerometry [59–62]. The quality assessment resulted in a mean of 6.6 points in the Newcastle–Ottawa Scale, ranging from 4 (worst) to 9 (best) points. The main methodological problems detected were the use of self-reported information on physical activity instead of accelerometry and the lack of a detailed characterization of non-respondents.
3.3 Meta-Analysis: Weight Gain During Pregnancy
Figures 2, 3, 4, 5, 6, 7 and 8 display the meta-analysis graphs comparing experimental and cohort studies. For gestational weight gain (GWG), the meta-analysis of the RCTs included 1605 women in the control groups and 1598 in the exercise groups. The meta-analysis resulted in a mean difference in GWG of −1.11 kg (DSE −1.53; −0.69). Women exposed to exercise interventions gained less weight during pregnancy than those not taking part in an exercise intervention. There was no heterogeneity across the trials (I 2 = 0 %; p = 0.868; Fig. 2a).
Meta-analysis of the effect of physical activity on gestational weight gain in randomized controlled trials and cohort studies: a mean difference and difference in standard error between intervention group and control group (continuous analysis); b odds ratio ± 95 % confidence interval for exceeding the gestational weight gain guidelines from the US Institute of Medicine [23] (binary analysis). Schlaff et al. [63] (a) = effect measure for moderate physical activity; Schlaff et al. [63] (b) = effect measure for vigorous physical activity; Chasan-Taber et al. [64] (c) and Jiang et al. [65] (c) = effect measure for physical activity during early pregnancy; Chasan-Taber et al. [64] (d) and Jiang et al. [65] (d) = effect measure for physical activity during mid pregnancy; Chasan-Taber et al. [64] (e) and Jiang et al. [65] (e) = effect measure for physical activity during late pregnancy. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, GWG gestational weight gain, IG intervention group
Meta-analysis of the effect of physical activity on gestational diabetes mellitus: a relative risk ± 95 % confidence interval in randomized controlled trials; (b) odds ratio ± 95 % confidence interval in cohort studies. Barakat et al. [43] (a) = analysis with World Health Organization criteria; Barakat et al. [43] (b) = analysis with International Association for Diabetes in Pregnancy Study Group criteria. Chasan-Taber et al. [66] (c) = effect measure for physical activity during early pregnancy; Chasan-Taber et al. [66] (d) = effect measure for physical activity during mid pregnancy. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, GDM gestational diabetes mellitus, IG intervention group, RR relative risk
Meta-analysis of the effect of physical activity on pre-eclampsia: a relative risk ± 95 % confidence interval in randomized controlled trials; b odds ratio ± 95 % confidence interval in cohort studies. De Oliveria Melo et al. [31] (a) = exercise initiated at 13 weeks; De Oliveria Melo et al. [31] (b) = exercise initiated at 20 weeks; Vollebregt et al. [67] (a) = effect measure for moderate physical activity; Vollebregt et al. [67] (b) = effect measure for vigorous physical activity. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, IG intervention group, RR relative risk
Meta-analysis of the effect of physical activity on difference in mean birthweight: a mean difference and difference in standard error in randomized controlled trials; b adjusted regression coefficient (β) ± 95 % confidence interval in cohort studies. De Oliveria Melo et al. [31] (a) = exercise initiated at 13 weeks; De Oliveria Melo et al. [31] (b) = exercise initiated at 20 weeks; Hegaard et al. [16] (a), Sternfeld et al. [68] (f), and Hatch et al. [69] (i) = effect measure for physical activity during mid pregnancy; Fleten et al. [71] (c), Sternfeld et al. [68] (e), and Hatch et al. [69] (h) effect measure for physical activity during early pregnancy; Hegaard et al. [16] (b), Sternfeld et al. [71] (g), Fleten et al. [71] (d), and Hatch et al. [69] (j) = effect measure for physical activity during late pregnancy. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, IG intervention group
Meta-analysis of the effect of physical activity on gestational age: a mean difference and difference in standard error in randomized controlled trials (continuous analysis); b odds ratio ± 95 % confidence interval in cohort studies (binary analysis). Owe et al. [72] (a), Evenson et al. [73] (d) = effect measure for physical activity during mid pregnancy; Owe et al. [72] (b) = effect measure for physical activity during late pregnancy; Evenson et al. [73] (c) = effect measure for physical activity during early pregnancy. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, IG intervention group
Meta-analysis of the effect of physical activity on being born small for gestational age (defined as birth weight below the 10th centile or 2500 g): a relative risk ± 95 % confidence interval in randomized controlled trials; b odds ratio ± 95 % confidence interval in cohort studies. De Oliveria Melo et al. [31] (a) = exercise initiated at 13 weeks; De Oliveria Melo et al. [31] (b) = exercise initiated at 20 weeks; Harrod et al. [74] (a), Gollenberg et al. [75] (d) = effect measure for physical activity during early pregnancy; Harrod et al. [74] (b), Gollenberg et al. [75] (e) = effect measure for physical activity during mid pregnancy; Harrod et al. [74] (c) = effect measure for physical activity during late pregnancy. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, IG intervention group, RR relative risk, SGA small for gestational age
Meta-analysis of the effect of physical activity on being born large for gestational age (defined as birth weight above the 90th centile or 4000 g): relative risk ± 95 % confidence interval in randomized controlled trials. De Oliveria Melo et al. [31] (a) = exercise initiated at 13 weeks; De Oliveria Melo et al. [31] (b) = exercise initiated at 20 weeks. The point estimate drawn represents graphically the weight of the study in the random-effects analysis. CG control group, CI confidence interval, ES effect size, IG intervention group, RR relative risk
For cohort studies (Fig. 2b), most studies dichotomized the outcome as exceeding or not exceeding the GWG guidelines from the IOM [19]. Active women during pregnancy had an 18 % lower risk of GWG that exceeded the IOM recommendations as compared with inactive women (OR 0.82; 95 % CI 0.68–0.99). The sample of cohort studies included 9795 women, and the studies exhibited moderate heterogeneity (I 2 = 60.2 %; p = 0.005). The article by Schlaff et al. [63] included separate findings for moderate- and vigorous-intensity physical activity, and both were included in the meta-analysis. The papers by Chasan-Taber et al. [64] and by Jiang et al. [65] included measures of early, mid, and late pregnancy, and all results were included in the meta-analysis.
3.4 Meta-Analysis: Gestational Diabetes
In terms of GDM, ten trials were included, with 1907 women in the control groups and 1883 in the exercise groups. Barakat et al. [43] included analysis with two criteria for GDM diagnostics: World Health Organization criteria and the International Association for Diabetes in Pregnancy Study Group criteria; both findings were included in the meta-analysis. The meta-analysis suggested a protective role of exercise interventions on the development of GDM (relative risk [RR] 0.67; 95 % CI 0.49–0.92). The studies showed low heterogeneity (I 2 = 33 %; p = 0.135).
When taking cohort studies into account, the total sample size was 6754. The summary OR for GDM comparing high/moderate and low/no LTPA was 0.75 (95 % CI 0.55–1.01), with no evidence of heterogeneity across studies (I 2 = 0 %; p = 0.615). The paper by Chasan-Taber et al. [66] included measures of early- and mid-pregnancy physical activity; both findings were included in the meta-analysis (Fig. 3).
3.5 Meta-Analysis: Preeclampsia
Data from three trials were included in the meta-analysis of preeclampsia, with 708 women in the control groups and 709 in the exercise groups. No evidence of an association between exercise interventions in pregnancy and risk of preeclampsia was observed (RR 0.93; 95 % CI 0.55–1.57). There was no evidence of heterogeneity across the trials (I 2 = 0 %; p = 0.872).
In the cohort analysis, eight studies were included, with a sample size of 155,414 women. Similar to the findings of the randomized trials, there was no evidence of an association between LTPA in pregnancy and preeclampsia. There was low heterogeneity across cohort studies (I 2 = 19.4 %; p = 0.270). The paper by Vollebregt et a l. [67] included effect measures for moderate-intensity and vigorous-intensity physical activity, and both were included in the meta-analysis (Fig. 4).
3.6 Meta-Analysis: Birthweight
A total of 22 randomized trials (2431 women in exercise groups and 2478 in control groups) evaluated the effect of exercise interventions on birthweight. There was no evidence of an effect of LTPA on average birthweight (MD −31.09 g; DSE −69.91; 7.73). However, high heterogeneity was detected across randomized trials (I 2 = 98.8 %; p < 0.001).
The analysis of the six cohort studies (n = 62,127 women) showed a small effect of LTPA on mean birthweight (β −1.05 g; 95 % CI −1.49; −0.62) and no heterogeneity (I 2 = 0 %; p = 0.445). Sternfeld et al. [68] and Hatch et al. [69] included measures of early, mid, and late pregnancy physical activity; again, all estimates were included in the meta-analysis. Hegaard et al. [70] and Fleten et al. [71] included measures of early and mid/late pregnancy physical activity; both were included in the meta-analysis (Fig. 5).
3.7 Meta-Analysis: Gestational Age
Data from 17 trials were included in the meta-analysis of gestational age, comprising 2169 women in the control groups and 2109 in the exercise groups. The meta-analysis showed no difference between the groups in gestational age at delivery (MD −0.07 weeks, DSE −0.29; 0.16). There was no heterogeneity across the trials (I 2 = 0 %; p < 0.001).
The 11 cohort studies (n = 81,595) showed an inverse association between LTPA and the risk of preterm birth (OR 0.80; 95 % CI 0.70–0.91). A low heterogeneity across studies was found (I 2 = 13.4 %; p = 0.310). Owe et al. [72] and Evenson et al. [73] included more than one measure of LTPA in pregnancy, and all findings were included in the meta-analysis (Fig. 6).
3.8 Meta-Analysis: Fetal Growth
Four trials (754 women in exercise groups and 745 in control groups) evaluated the effect of exercise interventions on the risk of being born SGA, and three trials (302 women in exercise groups and 301 in control groups) evaluated the effect of exercise interventions on the risk of being born LGA. No association was observed between exercise and SGA (Fig. 7), but women undergoing exercise interventions during pregnancy had a lower risk of having an LGA baby (RR 0.51; 95 % CI 0.30–0.87) (Fig. 8). There was no heterogeneity across the trials (I 2 = 0 %; p < 0.001) in both analyses, LGA and SGA.
In cohort studies, it was only possible to analyze the outcome ‘SGA’ given the low number of studies including the outcome ‘LGa’. Data from three studies were included in the meta-analysis for SGA. There was no association between LTPA and SGA (OR 1.03; 95 % CI 0.81–1.30), and low heterogeneity across studies (I 2 = 25.2 %; p = 0.245). Harrod et al. [74] and Gollenberg et al. [75] included more than one measure of physical activity during pregnancy; all estimates were included in the meta-analysis (Fig. 7).
4 Discussion
To the best of our knowledge, this is the first meta-analysis comparing associations between LTPA during pregnancy and maternal and child health between experimental and cohort studies. Previous reviews on the associations of LTPA on maternal–child outcomes were limited to specific groups of women of specific study designs [76, 77].
Consistent associations for LTPA in pregnancy were observed for weight gain (active women gained less weight during pregnancy), GDM (active women were less likely to develop GDM), preterm birth (active women were less likely to deliver a preterm infant), and fetal growth (active women were less likely to deliver an LGA baby). In methodological terms, the comparisons presented here are particularly relevant because of the different features of experimental and cohort studies.
The first difference between these two study designs relates to the nature of the exposure variable (i.e., physical activity). Cohort studies relied on self-reported LTPA and moderate–vigorous physical activity from accelerometry, whereas most experimental studies delivered structured exercise interventions to pregnant women. Observational studies therefore face higher variability in physical activity levels, whereas randomized trials are more prone to homogeneous physical activity levels in the intervention groups.
Another issue is the possibility of confounding in cohort studies, which is not the case for RCTs of sufficient sample size. For example, high socioeconomic status women are more active in leisure time [13] and have better maternal and child health indicators. In unadjusted analysis, it is therefore likely that active women will present better health indicators than inactive women, at least partly because they are from high socioeconomic groups. Adjusting for socioeconomic status could theoretically remove this artefact, but residual confounding is always a possibility.
Sample sizes also varied considerably between cohort and experimental studies. Most trials had fewer than 100 participants per group, whereas most cohort studies had sample sizes of more than 1000 women. Therefore, differences of the same magnitude could have been captured as significant in cohort studies, and not in experimental studies. Furthermore, another important issue when comparing RCTs and cohort studies is the selection of exposure to physical activity during pregnancy. While some cohort studies considered physical activity before pregnancy to be a confounding variable in the analysis, most did not specify whether women were active before pregnancy; in most RCTs, being inactive before pregnancy was an inclusion criterion. The positive effects found in cohort studies, but not in intervention studies could be attributed to long-term physical activity participation and not just to physical activity during pregnancy.
Despite differences between cohort and experimental studies, some of the findings were markedly consistent. In terms of weight gain during pregnancy, findings were similar when comparing cohort (n = 11) and experimental (n = 18) analyses; regardless of the study design, active women had lower weight gain during pregnancy. These results are consistent with the meta-analysis of 12 studies conducted by Streuling et al. [2], which showed an average weight gain significantly lower in the intervention groups (−0.61; 95 % CI −1.17 to 0.06) compared with controls. On the other hand, our findings differ from those of a Cochrane review of four articles on aerobic exercise during pregnancy, in which Kramer and McDonald [78] concluded that exercise showed no significant effect on weight gain during pregnancy.
Regarding GDM, our meta-analysis of 11 experimental studies showed a protective effect in women who engaged in physical activity during pregnancy. The same direction of association was observed in seven cohort analyses, although the difference was borderline in terms of statistical significance. In a previous meta-analysis of five cohort and case–control studies, Tobias et al. [3] showed a protective effect of physical activity on the development of GDM, but the same was not observed in the meta-analysis of five RCTs conducted by Yin et al. [18].
A protective effect of physical activity on the incidence of preeclampsia was not confirmed in our meta-analysis of four experimental and nine cohort studies. Aune et al. [6] conducted a systematic review and meta-analysis of seven cohort and four case–control studies, and found an inverse association between physical activity and preeclampsia. However, this association was not observed in a Cochrane review of three trials conducted by Meher and Duley [19].
There were no differences in mean birthweight of newborns according to maternal LTPA in pregnancy, consistent with two previous meta-analyses [20, 79]. A 2015 meta-analysis of 15 RCTs found that active women delivered lighter babies [4]; however, this study did not include articles published in 2014 and 2015, which were the studies tending to show results in the opposite direction. All weights were similarly distributed, with the exception of the meta-analysis of mean birth weight in cohort studies, where two studies carried almost 100 % of the weighted effect. The observed difference was because Fleten et al. [71] had a sample of 43,705 individuals, whereas other studies had fewer participants. However, we used a model that takes into consideration the size of the sample in the meta-analysis to distribute the weights evenly considering the contribution of studies with small sample size as well as heterogeneity between studies. In addition, we performed an additional analysis to observe a possible change in the effect estimation with the withdrawal of the Fleten et al. [71] study. No significant changes in the pooled effect were observed.
In terms of preterm births, experimental evidence does not support an association with physical activity in pregnancy, similar to the findings by Sanabria-Martinez et al. [4]. In the past, there was a concern in the literature as to whether exercise during pregnancy could cause adverse effects on maternal and fetal growth and increase the risk of premature births [80]. According to Goldenberg et al. [81] working long hours and undertaking hard physical labor under stressful conditions are probably associated with an increase in preterm birth, especially in relation to occupational physical activity. However, several studies that focused on vigorous sports training several times a week during pregnancy found it was associated with a decreased or unchanged risk of preterm delivery [69, 73, 82]. Observational studies have shown that regular LTPA during pregnancy can even reduce the incidence of preterm births [83, 84], an association that was confirmed in our meta-analysis of 13 cohort studies.
Our meta-analysis of RCTs suggests that exercise during pregnancy leads to decreased odds of delivering an LGA newborn. Similar results were found in the meta-analysis conducted by Wiebe et al. [5]. It is important to highlight that the available literature focusing on fetal growth is still limited compared with other outcomes.
Most physical activity interventions designed to prevent prenatal complications have focused on LTPA of moderate intensity. Less attention has been paid to sedentary behavior or light-intensity activity during pregnancy [85]. Increasing time spent in light-intensity activity could have important health implications during pregnancy by directly reducing time spent in sedentary behavior. Future studies might help address this literature gap. Another important research question is the impact of exercise intensity on maternal and child health outcomes. In our meta-analysis, it was not possible to evaluate the separate effects of moderate- and vigorous-intensity activities due to the small number of studies focusing on vigorous-intensity activities.
Our systematic review and meta-analysis differs from others published in the literature in some respects because we adopted methodological criteria such as the inclusion of studies in three languages (English, Portuguese, and Spanish), allowing a greater number of studies to be included, whereas most previously published reviews included only studies in English. Furthermore, we performed a meta-analysis only with LTPA from observational studies to enable a better comparison with intervention studies, whereas other meta-analyses included all domains of physical activity (occupational, leisure-time, domestic, and active commuting) in the same analysis [6].
5 Conclusion
The available evidence supports associations between LTPA during pregnancy and the following outcomes: weight gain in pregnancy, GDM, preterm births, and LGA. No evidence of an association with preeclampsia was detected, but only three trials have been conducted on this topic so far. Collectively, our findings support the promotion of LTPA in pregnancy as a strategy to improve maternal and child health.
References
Hopkins SA, Baldi JC, Cutfield WS, et al. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab. 2010;95(5):2080–8. doi:10.1210/jc.2009-2255.
Streuling I, Beyerlein A, Rosenfeld E, et al. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118(3):278–84. doi:10.1111/j.1471-0528.2010.02801.x.
Tobias DK, Zhang C, Van Dam RM, et al. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care. 2011;34:223–9.
Sanabria-Martiınez G, Garcia-Hermoso A, Poyatos-Leon R, et al. Effects of exercise-based interventions on neonatal outcomes: A meta-analysis of randomized controlled trials. Am J Health Promot. 2015;. doi:10.4278/ajhp.140718-LIT-351 (Epub ahead of print).
Wiebe HW, Boulé NG, Chari R, et al. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol. 2015;125(5):1185–1194.
Aune D, Saugstad OD, Henriksen T, et al. Physical activity and the risk of pre- eclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25:331–43. doi:10.1097/EDE.0000000000000036.
Domingues MR, Matijasevich A, Barros AJ. Physical activity and preterm birth: a literature review. Sports Med. 2009;39(11):961–75. doi:10.2165/11317900-000000000-00000.
Tomic V, Sporis G, Tomic J, et al. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat Med J. 2013;54(4):362–8.
Rich-Edwards JW, Fraser A, Lawlor DA, et al. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70. doi:10.1093/epirev/mxt006.
Evenson K, Barakat R, Brown W, et al. Guidelines for physical activity during pregnancy: comparisons from around the world. Am J Lifestyle Med. 2014;8(2):102–21.
Evenson K, Wen F. National trends in self-reported physical activity and sedentary behaviors among pregnant women: NHANES 1999–2006. Prev Med. 2010;50(3):123–8. doi:10.1016/j.ypmed.2009.12.015.
Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18(6):400–7.
Domingues MR, Barros AJ. Leisure-time physical activity during pregnancy in the 2004 Pelotas Birth Cohort Study. Rev Saude Publica. 2007;41(2):173–80.
Coll C, Domingues M, Santos I, et al. Changes in leisure-time physical activity from the prepregnancy to the postpartum period: 2004 Pelotas (Brazil) Birth Cohort Study. J Phys Act Health. 2016;13(4):361–5.
Owe KM, Nystad W, Bø K. Association between regular exercise and excessive newborn birth weight. Obstet Gynecol. 2009;114(4):770–6. doi:10.1097/AOG.0b013e3181b6c105.
Hegaard HK, Petersson K, Hedegaard M, et al. Sports and leisure-time physical activity in pregnancy and birth weight: a population-based study. Scand J Med Sci Sports. 2010;20(1):e96–102. doi:10.1111/j.1600-0838.2009.00918.x.
Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med. 2011;53(1-2):39–43. doi:10.1016/j.ypmed.2011.04.014.
Yin YN, Li XL, Tao TJ, et al. Physical activity during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Br J Sports Med. 2014;48(4):290–5.
Meher S, Duley I. Exercise or other physical activity for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2006;2:CD005942.
Leet T, Flick L. Effect of exercise on birthweight. Clin Obstet Gynecol. 2003;46:423–31.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
Higgins JP, Green, S (editors). Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/. Accessed 31 July 2015.
IOM (Institute of Medicine) and NRC (National Research Council). Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies; 2009.
American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus (position statement. Diabetes Care. 2009;32(1):62–7. doi:10.2337/dc09-S062.
World Health Organization. Recommendations for Prevention and treatment of pre-eclampsia and eclampsia. Geneva: WHO. 2011. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548335/en/. Accessed 31 Sept 2015.
United Nations Children’s Fund and World Health Organization. Low birthweight: country, regional and global estimates. New York: UNICEF; 2004.
World Health Organization. Born too soon: the global action report on preterm birth. Geneva: WHO; 2012.
Carlo WA. The high-risk infant. In: Kliegman RM, Stanton BF, St. Geme JW, et al., editors. Nelson textbook of pediatrics. 20th ed. Philadelphia: Elsevier Saunders; 2015.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa: Department of Epidemiology and Community Medicine, University of Ottawa. 2009. www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 4 Apr 2016.
de Oliveria Melo AS, Silva JL, Tavares JS, et al. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol. 2012;120(2 Pt 1):302–10. doi:10.1097/AOG.0b013e31825de592.
Clapp JF, Kim H, Burciu B, et al. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol. 2000;183(6):1484–8.
Salvesen KA, Stafne SN, Eggebo TM, et al. Does regular exercise in pregnancy influence duration of labor? A secondary analysis of a randomized controlled trial. Acta Obstet Gynecol Scand. 2014;93(1):73–9. doi:10.1111/aogs.12260.
Barakat R, Pelaez M, Montejo R, et al. Exercise throughout pregnancy does not cause preterm delivery: a randomized, controlled trial. J Phys Act Health. 2014;11(5):1012–7. doi:10.1123/jpah.2012-0344.
Murtezani A, Paçarada M, Ibraim Z, et al. The impact of exercise during pregnancy on neonatal outcomes: a randomized controlled trial. J Sports Med Phys Fitness. 2014;54(6):802–8.
Ruiz JR, Perales M, Pelaez M, et al. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc. 2013;88(12):1388–97. doi:10.1016/j.mayocp.2013.07.020.
Kihlstrand M, Stenman B, Nilsson S, et al. Water-gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstet Gynecol Scand. 1999;78(3):180–5.
Hopkins SA, Baldi JC, Cutfield WS, et al. Effects of exercise training on maternal hormonal changes in pregnancy. Clin Endocrinol (Oxf). 2011;74(4):495–500. doi:10.1111/j.1365-2265.2010.03964.x.
Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn’s birth size: a randomised controlled trial. Int J Obes (Lond). 2009;33(9):1048–57. doi:10.1038/ijo.2009.150.
Barakat R, Stirling JR, Lucia A. Does exercise training during pregnancy affect gestational age? A randomised controlled trial. Br J Sports Med. 2008;42(8):674–8. doi:10.1136/bjsm.2008.047837.
Sedaghati P, Ziaee V, Ardjmand A. The effect of an ergometric training program on pregnants’ weight gain and low back pain. Gazz Med Ital. 2007;166:209–13.
Stafne SN, Salvesen KA, Romundstad PR, et al. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol. 2012;119(1):29–36. doi:10.1097/AOG.0b013e3182393f86.
Barakat R, Pelaez M, Lopez C, et al. Exercise during pregnancy and gestational diabetes-related adverse effects: a randomised controlled trial. Br J Sports Med. 2013;47(10):630–6. doi:10.1136/bjsports-2012-091788.
Barakat R, Cordero Y, Coteron J, et al. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: a randomised controlled trial. Br J Sports Med. 2012;46(9):656–61. doi:10.1136/bjsports-2011-090009.
Barakat R, Pelaez M, Montejo R, et al. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol. 2011;204(5):402.e1–7. doi:10.1016/j.ajog.2011.01.043.
Cordero Y, Mottola MF, Vargas J, et al. Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc. 2015;47(7):1328–33. doi:10.1249/MSS.0000000000000547.
Petrov Fieril K, Glantz A, Fagevik OM. The efficacy of moderate-to-vigorous resistance exercise during pregnancy: a randomized controlled trial. Acta Obstet Gynecol Scand. 2015;94(1):35–42. doi:10.1111/aogs.12525.
Barakat R, Perales M, Bacchi M, et al. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot. 2014;29(1):2–8. doi:10.4278/ajhp.130131-QUAN-56.
Garshasbi A, Zadeh SF. The effect of exercise on the intensity of low back pain in pregnant women. Int J Gynaecol Obstet. 2005;88(3):271–5.
Haakstad LA, Bo K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2011;16(2):116–25. doi:10.3109/13625187.2011.560307.
Haakstad LA, Bo K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC Pregnancy Childbirth. 2011;30(11):66. doi:10.1186/1471-2393-11-66.
Ramírez-Vélez R, Aguilar de Plata AC, Mosquera-Escudero M, et al. Efecto del ejercicio físico aeróbico sobre el consumo de oxígeno de mujeres primigestantes saludables: estudio clínico aleatorizado. [The effect of aerobic exercise on oxygen consumption in healthy first-pregnancy females: a randomized clinical trial]. Rev Colomb Obstet Ginecol. 2011;62(1):15–23.
Marquez-Sterling S, Perry AC, Kaplan TA, et al. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med Sci Sports Exerc. 2000;32(1):58–62.
Price BB, Amini SB, Kappeler K. Exercise in Pregnancy: effect on fitness and obstetric outcomes—a randomized trial. Med Sci Sports Exerc. 2012;44(12):2263–9. doi:10.1249/MSS.0b013e318267ad67.
Rodríguez YC, Puente MP, Abad MDM, et al. Puede el ejercicio físico moderado durante el embarazo actuar como un factor de prevención de la diabetes gestacional? [Can moderate physical exercise during pregnancy act as a factor in preventing gestational diabetes?]. Rev Int Cienc Deporte. 2012;8(27):3–19.
Cavalcante SR, Cecatti JG, Pereira RI, et al. Water aerobics II: maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reprod Health. 2009;6(6):1. doi:10.1186/1742-4755-6-1.
Baciuk EP, Pereira RI, Cecatti JG, et al. Water aerobics in pregnancy: cardiovascular response, labor and neonatal outcomes. Reprod Health. 2008;5:10. doi:10.1186/1742-4755-5-10.
Prevedel T, Calderon I, De Conti M, et al. Maternal and perinatal effects of hydrotherapy in pregnancy. Rev Bras Ginecol Obstet. 2003;25:53–9.
Melzer K, Schutz Y, Soehnchen N, et al. Effects of recommended levels of physical activity on pregnancy outcomes. Am J Obstet Gynecol. 2010;202(3):266.e1–6. doi:10.1016/j.ajog.2009.10.876.
Morgan KL, Rahman MA, Hill RA, et al. Physical activity and excess weight in pregnancy have independent and unique effects on delivery and perinatal outcomes. PLoS One. 2014;9(4):e94532. doi:10.1371/journal.pone.0094532.
Morkrid K, Jenum AK, Berntsen S, et al. Objectively recorded physical activity and the association with gestational diabetes. Scand J Med Sci Sports. 2014;24(5):e389–97. doi:10.1111/sms.12183.
Perkins CC, Pivarnik JM, Paneth N, et al. Physical activity and fetal growth during pregnancy. Obstet Gynecol. 2007;109(1):81–7.
Schlaff RA, Holzman C, Maier KS, et al. Associations among gestational weight gain, physical activity, and pre-pregnancy body size with varying estimates of pre-pregnancy weight. Midwifery. 2014;30(11):1124–31. doi:10.1016/j.midw.2014.03.014.
Chasan-Taber L, Silveira M, Lynch KE, et al. Physical activity and gestational weight gain in Hispanic women. Obesity. 2014;22(3):909–18. doi:10.1002/oby.20549.
Jiang H, Qian X, Li M, et al. Can physical activity reduce excessive gestational weight gain? Findings from a Chinese urban pregnant women cohort study. Int J Behav Nutr Phys Act. 2012;9(9):12. doi:10.1186/1479-5868-9-12.
Chasan-Taber L, Schmidt MD, Pekow P, et al. Physical activity and gestational diabetes mellitus among Hispanic women. J Womens Health. 2008;17(6):999–1008. doi:10.1089/jwh.2007.0560.
Vollebregt KC, Wolf H, Boer K, et al. Does physical activity in leisure time early in pregnancy reduce the incidence of preeclampsia or gestational hypertension? Acta Obstet Gynecol Scand. 2010;89(2):261–7. doi:10.3109/00016340903433982.
Sternfeld B, Quesenberry CP, Eskenazi B, et al. Exercise during pregnancy and pregnancy outcome. Med Sci Sports Exerc. 1995;27(5):634–40.
Hatch MC, Shu XO, McLean DE, et al. Maternal exercise during pregnancy, physical fitness, and fetal growth. Am J Epidemiol. 1993;137(10):1105–14.
Hegaard HK, Hedegaard M, Damm P, et al. Leisure time physical activity is associated with a reduced risk of preterm delivery. Am J Obstet Gynecol. 2008;198(2):180.e1–5. doi:10.1016/j.ajog.2007.08.038.
Fleten C, Stigum H, Magnus P, et al. Exercise during pregnancy, maternal prepregnancy body mass index, and birth weight. Obstet Gynecol. 2010;115(2 Pt 1):331–7. doi:10.1097/AOG.0b013e3181ca4414.
Owe KM, Nystad W, Skjaerven R, et al. Exercise during pregnancy and the gestational age distribution: a cohort study. Med Sci Sports Exerc. 2012;44(6):1067–74. doi:10.1249/MSS.0b013e3182442fc9.
Evenson KR, Siega-Riz AM, Savitz DA, et al. Vigorous leisure activity and pregnancy outcome. Epidemiology. 2002;13(6):653–9.
Harrod CS, Chasan-Taber L, Reynolds RM, et al. Physical activity in pregnancy and neonatal body composition: the healthy start study. Obstet Gynecol. 2014;124(2 Pt 1):257–64. doi:10.1097/AOG.0000000000000373.
Gollenberg AL, Pekow P, Bertone-Johnson ER, et al. Physical activity and risk of small-for-gestational-age birth among predominantly Puerto Rican women. Matern Child Health J. 2011;15(1):49–59. doi:10.1007/s10995-009-0563-1.
Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database Syst Rev. 2006;3:CD004225.
Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum: a systematic review and meta-analysis of randomized controlled trials. Prev Med. 2013;56(6):351–64. doi:10.1016/j.ypmed.2013.02.021.
Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;3:CD000180.
Lokey EA, Tran ZV, Wells CL, et al. Effects of physical exercise on pregnancy outcomes: a meta-analytic review. Med Sci Sports Exerc. 1991;23(11):1234–9.
Pivarnik JM, Chambliss HO, Clapp JF, et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38(5):989–1006.
Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi:10.1016/S0140-6736(08)60074-4.
Misra DP, Strobino DM, Stashinko EE, et al. Effects of physical activity on preterm birth. Am J Epidemiol. 1998;147(7):628–35.
Field T. Prenatal exercise research. Infant Behav Dev. 2012;35(3):397–407. doi:10.1016/j.infbeh.2011.10.001.
Domingues MR, Barros AJ, Matijasevich A. Leisure time physical activity during pregnancy and preterm birth in Brazil. Int J Gynaecol Obstet. 2008;103:9–15.
Di Fabio DR, Blomme CK, Smith KM, et al. Adherence to physical activity guidelines in mid-pregnancy does not reduce sedentary time: an observational study. Int J Behav Nutr Phys Act. 2015;24(12):27. doi:10.1186/s12966-015-0191-7.
McCullough LE, Mendez MA, Miller EE, et al. Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics. 2015;10(7):597–606. doi:10.1080/15592294.2015.1045181.
Chasan-Taber L, Silveira M, Pekow P, et al. Physical activity, sedentary behavior and risk of hypertensive disorders of pregnancy in Hispanic women. Hypertens Pregnancy. 2015;34(1):1–16. doi:10.3109/10641955.2014.946616.
Currie L, Woolcott C, Fell D, et al. The association between physical activity and maternal and neonatal outcomes: a prospective cohort. Matern Child Health J. 2014;18(8):1823–30. doi:10.1007/s10995-013-1426-3.
Portela SN, Rocha-de-Souza R, Oppermann-Lisboa K, et al. Maternal physical activity, cervical length and its relation to spontaneous vaginal birth at term. Arch Gynecol Obstet. 2014;290(2):257–62. doi:10.1007/s00404-014-3198-4.
Restall A, Taylor RS, Thompson JM, et al. Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. J Obes. 2014;2014:148391. doi:10.1155/2014/148391.
Sealy-Jefferson S, Hegner K, Misra DP. Linking nontraditional physical activity and preterm delivery in urban African-American women. Womens Health Issues. 2014;24(4):e389–95. doi:10.1016/j.whi.2014.04.007.
Tinloy J, Chuang CH, Zhu J, et al. Exercise during pregnancy and risk of late preterm birth, cesarean delivery, and hospitalizations. Womens Health Issues. 2014;24(1):e99–104. doi:10.1016/j.whi.2013.11.003.
Kraschnewski JL, Chuang CH, Downs DS, et al. Association of prenatal physical activity and gestational weight gain: results from the first baby study. Womens Health Issues. 2013;23(4):e233–8. doi:10.1016/j.whi.2013.04.004.
Mudd LM, Pivarnik J, Holzman CB, et al. Leisure-time physical activity in pregnancy and the birth weight distribution: where is the effect? J Phys Act Health. 2012;9(8):1168–77.
Jukic AMZ, Evenson KR, Daniels JL, et al. Prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Matern Child Health J. 2012;16(5):1031–44. doi:10.1007/s10995-011-0831-8.
Doustan M, Seifourian M, Zarghami M, et al. Relationship between physical activity of mothers before and during pregnancy with the newborn health and pregnancy outcome. J Phys Educ Sport. 2012;12(2):222–9.
Fortner RT, Pekow PS, Whitcomb BW, et al. Physical activity and hypertensive disorders of pregnancy among Hispanic women. Med Sci Sports Exerc. 2011;43(4):639–46. doi:10.1249/MSS.0b013e3181f58d3e.
Juhl M, Kogevinas M, Andersen PK, et al. Is swimming during pregnancy a safe exercise? Epidemiology. 2010;21(2):253–8. doi:10.1097/EDE.0b013e3181cb6267.
Juhl M, Olsen J, Andersen PK, et al. Physical exercise during pregnancy and fetal growth measures: a study within the Danish National Birth Cohort. Am J Obstet Gynecol. 2010;202(1):63.e1–8. doi:10.1016/j.ajog.2009.07.033.
Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201(1):58.e1–8. doi:10.1016/j.ajog.2009.02.025.
Tavares JS, Melo AS, Amorim MM, et al. Association between maternal physical activity, gestational weight gain and birth weight in a cohort of 118 pregnant women in Campina Grande, Northeast of Brazil. Rev Assoc Med Bras. 2009;55(3):335–41.
Osterdal ML, Strom M, Klemmensen AK, et al. Does leisure time physical activity in early pregnancy protect against pre-eclampsia? Prospective cohort in Danish women. BJOG. 2009;116(1):98–107. doi:10.1111/j.1471-0528.2008.02001.x.
Rudra CB, Sorensen TK, Luthy DA, et al. A prospective analysis of recreational physical activity and preeclampsia risk. Med Sci Sports Exerc. 2008;40(9):1581–8. doi:10.1249/MSS.0b013e31817cab1.
Magnus P, Trogstad L, Owe KM, et al. Recreational physical activity and the risk of preeclampsia: a prospective cohort of Norwegian women. Am J Epidemiol. 2008;168(8):952–7. doi:10.1093/aje/kwn189.
Juhl M, Andersen PK, Olsen J, et al. Physical exercise during pregnancy and the risk of preterm birth: a study within the Danish national birth cohort. Am J Epidemiol. 2008;167(7):859–66. doi:10.1093/aje/kwm364.
Dwarkanath P, Muthayya S, Vaz M, et al. The relationship between maternal physical activity during pregnancy and birth weight. Asia Pac J Clin Nutr. 2007;16(4):704–10.
Downs DS, Hausenblas HA. Pregnant women’s third trimester exercise behaviors, body mass index, and pregnancy outcomes. Psychol Health. 2007;22(5):545–59.
Watson PE, McDonald BW. Activity levels in pregnant New Zealand women: relationship with socioeconomic factors, well-being, anthropometric measures, and birth outcome. Appl Physiol Nutr Metab. 2007;32(4):733–42.
Oken E, Ning Y, Rifas-Shiman SL, et al. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108(5):1200–7.
Duncombe D, Skouteris H, Wertheim EH, et al. Vigorous exercise and birth outcomes in a sample of recreational exercisers: a prospective study across pregnancy. Aust N Z J Obstet Gynaecol. 2006;46(4):288–92.
Takito MY, Benicio MH, Latorre MR. Maternal posture and its influence on birthweight. Rev Saude Publica. 2005;39(3):325–32.
Dempsey JC, Sorensen TK, Williams MA, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol. 2004;159(7):663–70.
Magann EF, Evans SF, Weitz B, et al. Antepartum, intrapartum, and neonatal significance of exercise on healthy low-risk pregnant working women. Obstet Gynecol. 2002;99(3):466–72.
Nieuwenhuijsen MJ, Northstone K, Golding J. Swimming and birth weight. Epidemiology. 2002;13(6):725–8.
Hatch M, Levin B. Maternal leisure-time exercise and timely delivery. Am J Public Health. 1998;88(10):1528–33.
Acknowledgments
Shana G. da Silva thanks the National Council of Scientific and Technological Development (CNPq) for her scholarship. Luiza I. Ricardo thanks the Coordination for the improvement of Higher Education Personnel (CAPES) for her scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflicts of interest
Shana G da Silva, Luiza I Ricardo, Kelly R Evenson, and Pedro C Hallal have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
da Silva, S.G., Ricardo, L.I., Evenson, K.R. et al. Leisure-Time Physical Activity in Pregnancy and Maternal-Child Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Cohort Studies. Sports Med 47, 295–317 (2017). https://doi.org/10.1007/s40279-016-0565-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0565-2