Abstract
The number of childhood cancer survivors is increasing rapidly. According to American Association for Cancer Research, there are more than 750,000 childhood cancer survivors in the United States and Europe. As the number of childhood cancer survivors increases, so does cancer treatment-related cardiac dysfunction (CTRCD), leading to heart failure (HF). It has been reported that childhood cancer survivors who received anthracyclines are 15 times more likely to have late cancer treatment-related HF and have a 5-fold higher risk of death from cardiovascular (CV) disease than the general population. CV disease is the leading cause of death in childhood cancer survivors. The increasing need to manage cancer survivor patients has led to the rapid creation and adaptation of cardio-oncology. Cardio-oncology is a multidisciplinary science that monitors, treats, and prevents CTRCD. Many guidelines and position statements have been published to help diagnose and manage CTRCD, including those from the American Society of Clinical Oncology, the European Society of Cardiology, the Canadian Cardiovascular Society, the European Society of Medical Oncology, the International Late Effects of Childhood Cancer Guideline Harmonization Group, and many others. However, there remains a gap in identifying high-risk patients likely to develop cardiomyopathy and HF in later life, thus reducing primary and secondary measures being instituted, and when to start treatment when there is echocardiographic evidence of left ventricular (LV) dysfunctions without symptoms of HF. There are no randomized controlled clinical trials for treatment for CTRCD leading to HF in childhood cancer survivors. The treatment of HF due to cancer treatment is similar to the guidelines for general HF. This review describes the latest pharmacologic therapy for preventing and treating LV dysfunction and HF in childhood cancer survivors based on expert consensus guidelines and extrapolating data from adult HF trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiomyopathy and heart failure are leading causes of death in childhood cancer survivors. |
Dexrazoxane is the only drug approved by the US FDA for the secondary prevention of anthracycline-related cardiomyopathy. |
Early detection of left ventricular dysfunction and early initiation of cardioprotective therapy can lead to subsequent recovery of function and delay progression to heart failure. |
Standard heart failure therapies should be implemented. Other causes of left ventricular dysfunction should be excluded in children who develop heart failure during treatment or long term after cancer therapy. |
1 Introduction
Cardiac dysfunction and heart failure (HF) in childhood cancer survivors related to treatment with anthracyclines (doxorubicin, daunorubicin) and their analogs (epirubicin, idarubicin, and mitoxantrone) has been widely studied and published in the literature. Several mechanisms postulated for anthracycline-related cardiotoxicity include topoisomerase-II mediated DNA double-strand breaks, reactive oxygen species (ROS) formation, and oxygen free radicals during oxidative respiratory chain reaction in the mitochondria [1]. The common pathway is persistent mitochondrial damage in cardiomyocytes resulting in cardiac dysfunction, including histologic changes (myocyte vacuolization, myofibrillar loss, and cell death), atrophy, and fibrosis [2]. Alkylating agents (cyclophosphamide, ifosfamide, and melphalan) used to treat various childhood cancers can also cause cardiomyopathy, although at much lower rates than anthracyclines [3, 4]. Other newer chemotherapeutic agents such as targeted cancer therapies (immune checkpoint inhibitors [ICI], tyrosine kinase inhibitors [TKI], human epidermal growth factor receptor 2 [HER2]-targeted therapy), vascular endothelial growth factor (VEGF), and cellular therapy (chimeric antigen receptor T [CAR-T] cells) can cause a variety of cardiotoxicities, including acute myocarditis and cardiomyopathy [5]. As a result of radiation therapy (RT), especially when the heart is involved in radiation such as mediastinal and thoracic spine RT, 10–30% of patients may develop cardiac complications [6]. Most cancers in children, including leukemia, lymphoma, and brain tumors, need a combination of chemotherapy and RT. The cumulative toxicity of various cancer therapies increases the risk of these patients later developing cardiomyopathy and HF [7]. Children can have decreased systolic function of the left ventricle (LV) and have a similar clinical presentation to a dilated cardiomyopathy (DCM) phenotype in early stages after chemotherapy, but some children may develop a restrictive pattern of LV dysfunction (restrictive cardiomyopathy, RCM) later in their life, a long time after cancer therapy. Thus, both types of HF, that is, due to reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF), are encountered as late cancer treatment-related cardiac dysfunction (CTRCD) in childhood cancer survivors [7]. Given this new field and the heterogeneity of this cohort of childhood cancer survivors, there have been no randomized controlled trials in children for the treatment of HF due to cancer therapy. Therefore, CTRCD and HF drug treatment is primarily based on expert consensus and extrapolation of data from adult HF trials. This review compares stages of HF due to cancer therapy and focuses on preventing and treating HF in childhood cancer survivors.
2 Prevention of Cancer Treatment-Related Cardiac Dysfunction (CTRCD) and Heart Failure (HF)
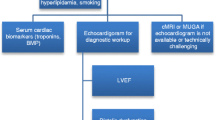
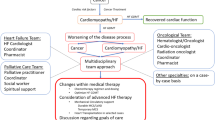
Figure 1 describes three phases of cancer therapy patients: baseline risk, during cancer therapy, and long-term follow-up in cancer survivors. The cardiovascular (CV) risk is minimal at baseline and depends upon pre-existing risk factors (Fig. 2). During cancer therapy, CV outcomes depend upon chemotherapy and combination therapies with RT. The risk of cardiac injury is monitored by echocardiography and cardiac biomarkers (Fig. 3). After cancer therapy, CV risk depends upon CV risk factors (Table 1), and surveillance depends on these factors (Fig. 4). The ultimate goal is to mitigate the CTRCD without decreasing the potency of cancer therapy. Primary prevention is extended to all patients receiving cancer therapy with potential cardiotoxicity (at risk, Stage A HF [8]) and to intervene to optimize their modifiable risk factors (Fig. 2). Secondary prevention in selected high-risk patients showing preclinical signs of cardio-toxicity in the form of elevated cardiac biomarkers (cardiac troponin T (cTnT) and brain-type natriuretic peptides (BNP) or change in echocardiographic parameters such as a decrease in left ventricular ejection fraction (LVEF), decrease in strain and strain rate, and evidence of cardiac modeling such as dilated LV, reduce wall thickness, etc., but clinically asymptomatic (Stage B HF) [9]. Secondary prevention also includes screening for cardiomyopathy in high-risk patients who received either cumulative anthracyclines dose ≥ 250 mg/m2 or RT ≥ 35 Gy) over the long term after completion of cancer therapy (Fig. 4). Early identification of cardiac dysfunction and preemptive intervention is proven to help decrease cardiotoxicity, and early treatment in symptomatic HF (Stage C) will help the recovery of the myocardium by reverse remodeling [10]. HF medications used in childhood cancer survivors are similar to those used in the general population. The choice of medications depends on the individual patient's clinical condition, underlying cause of HF, and other factors [11].
Baseline cardiovascular (CV) risk assessment. BNP brain natriuretic peptide, CHD congenital heart disease, CMR cardiac magnetic resonance, CVD cardiovascular disease, ECG echocardiography, GLS global longitudinal strain, H/O history of, LVEF left ventricular ejection fraction, TTE transthoracic echocardiography
Surveillance for cardiac dysfunction during cancer therapy and management of heart failure (HF) during and after chemotherapy. ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, ARNi angiotensin-receptor-neprilysin inhibitor, BB β-blocker, CV cardiovascular, GDMT guideline-directed medical therapy, HT heart transplantation, LV left ventricle, LVEF left ventricular ejection fraction, MCS mechanical circulatory support, NYHA New York Heart Association, RCT resynchronization therapy, SGLT-2i sodium-glucose transporter-2 inhibitor, Tn troponin
Surveillance and management of CTRCD in childhood cancer survivors. BNP brain natriuretic peptide, CTRCD chemotherapy-related cardiac disease, CV cardiovascular, ECG echocardiography, GLS global longitudinal strain, LVEF left ventricular ejection fraction, MRI magnetic resonance imaging, Tn troponin, TTE transthoracic echocardiography, 2-D two-dimensional, 3-D three-dimensional
2.1 Primary Prevention
Primary prevention applies to all patients receiving cardiotoxic chemotherapy. As per the American College of Cardiology (ACC)/American Heart Association (AHA), all patients requiring cardiotoxic chemotherapy belong to stage A (at risk) HF [8]. For anthracyclines, cardiac damage is dose-dependent. Thus, the goal is to limit the total doses of anthracyclines to < 250 mg/m2 [9]. Recent evidence suggests that patients treated with < 100 mg/m2 total cumulative anthracyclines have not shown a significantly increased risk of HF [11]. However, there is no safe dose of anthracyclines. Factors that can increase the risk of developing cardiac toxicity include patients < 5 years of age, minority race, female gender, Down syndrome, cumulative anthracycline dose > 250 mg/m2, combined with radiation exposure, sedentary lifestyle, poor diet, and comorbidities such as prior LV dysfunction, hypertension, obesity, diabetes, and dyslipidemia [6, 12]. Primary prevention aims to optimize pre-existing modifiable CV risk factors, normalize blood pressure, lower cholesterol, and consume a balanced nutritious diet. Anthracyclines should be avoided in patients with LVEF < 40% unless there is no practical alternative cancer regimen. In patients with LVEF < 50% but ≥ 40% and those exposed to cancer chemotherapy with pre-existing cardiovascular risk factors, a preemptive cardioprotective therapy with angiotensin‐converting enzyme inhibitors (ACEis) (or angiotensin receptor blockers [ARBs]) and/or β‐blockers (BBs) is recommended [13]. Patients with cardiometabolic syndrome undergoing chemotherapy should receive close monitoring of cardiovascular parameters, such as blood pressure, heart rate, and cardiac function, to promptly detect any changes or complications. A 25% increased weight gain in leukemia survivors observed after chemotherapy and RT is associated with an imbalance between energy intake and expenditure [14]. Lifestyle changes include promoting a healthy diet, regular exercise, weight management, and avoiding tobacco and excessive alcohol use, play an important role in the overall management of HF in cancer survivors. However, exercise and dietary modifications should be implemented in consultation with oncologist to ensure safety and appropriateness during cancer treatment [15].
2.1.1 Statins
Statins reduce cholesterol synthesis by inhibiting the enzyme HMG CoA reductase and they are known to exhibit pleiotropic properties and decrease oxidative stress and inflammation. Atorvastatin is approved by the US Food and Drug Administration (FDA) for lowering cholesterol and the risk of heart attack and stroke but is not approved to reduce CTRCD after doxorubicin. In adults, chronic statin treatment concomitantly with anthracyclines was associated with a lower risk for incident HF and less deterioration of LVEF [16, 17]. Recently, it has been found that the efficacy of prophylactic statin therapy in patients with breast cancer and lymphomas with no indication for statin therapy did not affect LV function decline after 2 years [18]. In contrast to the prior study, in the STOP-CA clinical trial, patients who took atorvastatin with chemotherapy before the first dose of anthracycline for 1 year showed less deterioration of LV function compared with those who took a placebo [19]. The study's author has supported the use of atorvastatin when anthracycline group drugs are used for treating lymphoma in adults. A high dose of atorvastatin (i.e., 40 mg) is used in both trials. However, further studies are needed on whether statin use is a cardioprotective therapy in children receiving anthracyclines in the future.
2.1.2 Exercise
Exercise may decrease the CV risk factors for the development of CTRCD and complement pharmacologic risk factor modification. Physical exercise may change the mitochondrial phenotypes that resist ROS-mediated damage and could be beneficial against anthracycline-induced cardiac toxicity [20]. However, exercise may harm children with restrictive LV physiology by precipitating pulmonary edema. Exercise should be individualized over time as childhood cancer survivors’ health changes. Appropriate and safe exercise rehabilitation will decrease the survivor’s CV risks, improve mental health, and decrease adverse cardiometabolic effects. Cardiopulmonary exercise testing (CPET) is beneficial to identify CV versus non-CV factors for decreased exercise capacity in childhood cancer survivors. Cardiac rehabilitation is beneficial in the improvement of cardio-respiratory fitness. In adults, an increase in 1 metabolic equivalent (3.5 mL O2/kg/min) in exercise capacity decreases CV-related mortality [21].
2.1.3 Role of Neurohormonal Antagonists as Primary Prevention of HF
Preclinical studies have shown that angiotensin II [22] and endothelin receptors [23] can mediate anthracycline cardiotoxicity. Furthermore, in animals exposed to anthracyclines, β-1 receptor activation appears to be cardiotoxic, while β-2 receptor activation seems to be cardioprotective [24]. A prophylactic cardioprotective therapy with bisoprolol, perindopril, and eplerenone attenuated doxorubicin-induced adverse myocardial effects in a rodent model compared with treatment initiated after the development of cardiac injury [25]. These observations form the rationale for considering neurohormonal antagonists for prophylactic treatment HF with BBs, ACEis, ARBs, and mineralocorticoid receptor antagonists (MRAs) for chemotherapy-induced cardiomyopathy and HF. The results from preventing LV dysfunction with enalapril and carvedilol showed a minor reduction in LVEF compared with placebo in patients. Pre-treatment with both bisoprolol and perindopril attenuated a decline in LVEF in a group of HER2+ breast cancer patients undergoing treatment with trastuzumab [27]. Candesartan, an ARB, also attenuates the decrease in LVEF in HER2-positive breast cancer patients undergoing anthracycline-containing chemotherapy. In contrast, metoprolol did not prevent a reduction in LVEF [28]. There are conflicting findings from multiple small trials on the role of neurohormonal antagonists as primary prevention in patients receiving anthracyclines [29,30,31,32,33]. Carvedilol has a beneficial effect in decreasing anthracycline-induced decline in LVEF [29,30,31]. However, other studies did not show the benefit of BB therapy with or without ACEi [32, 33]. In the CECCY trial, synchronous use of carvedilol with anthracyclines did not protect against LV systolic dysfunction in HER2-negative breast cancer patients. However, a benefit of β-blockade was noted in the development of diastolic dysfunction [34]. Empagliflozin, a sodium-glucose cotransporter-2 inhibitor (SGLT-2i), was also found to attenuate cardiotoxic effects exerted by doxorubicin on LV function and remodeling in a nondiabetic mice model [35]. Current evidence does not support ACEi and BBs as primary preventive strategies in children receiving anthracycline chemotherapy without any changes in cardiac or imaging biomarkers.
2.1.4 Genetics
Evidence of increasing the burden of rare variants among patients who develop chemotherapy-induced cardiomyopathy suggests that genetic factors are significant determinants of CTRCD susceptibility and outcomes [36,37,38,39,40,41,42,43,44,45,46,47]. Garcia-Pavia and colleagues have reported an increased frequency of rare, truncated variants (TTN) and missense variants (MYH7) of genes in patients who developed cardiomyopathy after cancer therapy [37]. Lipshultz et al. found that 30% of cancer survivors with cardiomyopathy have one or more mutations of mitochondrial DNA compared with healthy controls. Also, they found that cancer survivors have higher mitochondrial DNA copies/cardiomyocytes as a part of the compensation for abnormal functioning mitochondrial DNA in cardiomyocytes [38]. Besides TTN and MYH7 genes, single nucleotide polymorphisms of RARG rs2229774 (G>A) [39, 40], SLC28A3 rs7853758 (G>A) [7], UTG1A6*4 rs17863783 (G>T) [36], and CELF4 rs1786814 [41] genes are also associated with a higher risk of CTRCD. Numerous retrospective studies [42,43,44,45,46] suggest pharmacogenetic testing may be beneficial before chemotherapy. More data should guide clinical decision making in children based on genomics. In the future, identifying genetic risk factors with artificial intelligence (machine learning) for anthracycline cardiotoxicity using a combination of genetic and clinical factors provides new opportunities to identify cancer patients at high risk of CTRCD.
2.2 Secondary Prevention
Secondary prevention could be defined as the appropriate management strategies for preventing symptoms, HF, and cardiovascular events in asymptomatic anthracycline cardiotoxicity. The secondary prevention goal is identifying high-risk patients for the development of HF earlier and and initiating cardioprotective therapy. Although there are no evidence-based guidelines for monitoring cardiotoxicity during and after chemotherapy, there are serum and imaging biomarkers such as elevated high-sensitive (hs) C-reactive protein, cTnT, and BNP or NT-pro-BNP and LVEF in order to detect subclinical cardiotoxicity prior to the development of overt cardiac dysfunction [48,49,50,51,52]. Thus, close monitoring of these patients will help to manage LV dysfunction and to prevent progression to HF. Early impaired LV function with LVEF 40%, but asymptomatic could be treated with ACEi in combination with BBs [48]. New medications used in HF including SGLT-2 inhibitors, angiotensin receptor-neprilysin inhibitor (ARNi), non-steroid MRAs, ivabradine, and ATPase activators such as omecamtiv mecarbil, could be used as potential secondary prevention strategies with promising results in the future [35]. However, more randomized control trials are required.
2.2.1 Early Identification of Left Ventricular Dysfunction by Echocardiography
Echocardiography is the primary modality of screening to determine cardiac remodeling, LVEF, Strain, and Strain rate. LVEF < 50% or a 10% relative reduction is the most commonly used echocardiographic parameter in adults [53]. However, specific guidelines to identify cutoff values do not exist for the pediatric population. LVEF has established predictive capability for HF but depends on preload and afterload, and reproducibility is limited [54]. Furthermore, LVEF is not sensitive to detect regional wall motion abnormalities. Many studies suggest the role of Strain, primarily global longitudinal Strain (GLS), for segmental assessment of myocardial function after starting cancer therapies in children [55, 56]. In adult cancer survivors, LV GLS < − 15% suggests clinically significant cardiotoxicity [57]. GLS appears to have higher sensitivity, but specificity as a marker for long-term cardiotoxicity is not apparent. The SUCCOUR (Strain Surveillance During Chemotherapy for Improving Cardiovascular Outcomes) trial recently showed that echocardiography GLS-guided cardioprotection therapies provide less cardiac dysfunction in survivors of potentially cardiotoxic chemotherapy compared with usual care at 3 years [58]. There are several newer imaging studies such as cardiac magnetic resonance (CMR) imaging, computerized tomography angiography, radionuclide scanning to determine the function and myocardial perfusion (positron emission tomography, PET), and other echocardiographic indices such as 3-D LVEF, 3-D Strain, right ventricular Strain and left atrial Strain for detection of diastolic dysfunction in childhood cancer survivors [59]. At this time, the utility of this advanced imaging in predicting cardiac outcomes after chemotherapy has yet to be satisfactorily established in pediatric patients. The surveillance imaging details are beyond this review's scope; readers can refer to European Society of Cardiology guidelines on cardio-oncology [13].
2.2.2 Cardioprotective Therapy: Dexrazoxane
Dexrazoxane is the only FDA-approved cardiacprotectant against anthracycline-induced cardiotoxicity and is recommended for high-risk patients prone to CTRCD. The drug's administration is usually via intravenous infusion over 15 min. The suggested dosage ratio of dexrazoxane to doxorubicin is 10 to 1. The dose of dexrazoxane requires a reduction in patients who have moderate to significant renal impairment (e.g., creatinine clearance lower than 40 mL/min) by 50%. Administration of doxorubicin within half an hour after the completion of dexrazoxane infusion is recommended. Although the mechanism of cardioprotection by dexrazoxane was thought to be mediated by iron chelation, more recent evidence suggests that inhibition of 2-β-topoisomerase and inhibition of mitochondrial DNA breaks are also possible mechanisms for cardioprotection [60]. Despite compelling evidence of cardioprotective efficacy, dexrazoxane is not routinely administered to patients receiving cancer therapies. The International Late Effects of Childhood Cancer Guideline Harmonization Group reviewed the existing literature and used evidence-based methodology to develop a guideline for dexrazoxane administration in children with cancer who are expected to receive anthracyclines [8]. It is recommended to use dexrazoxane in all patients who receive a total anthracycline dose of ≥ 250 mg/m2 or RT dose ≥ 35 Gy (moderate recommendation) [8]. No recommendation could be formulated for cumulative doxorubicin or equivalent doses of < 250 mg/m2 due to insufficient evidence to determine whether the risk of cardiotoxicity outweighs the possible risk of subsequent neoplasms [8]. Further research is needed to determine the long-term efficacy and safety of dexrazoxane in children with cancer. Lue et al. demonstrated that a synthetic human analog, humanin, increases the protective effect of dexrazoxane in vivo by inhibiting the doxorubicin-induced decrease in LVEF and cardiac fibrosis while protecting mitochondrial function [61]. Another highly conserved and stress-inducible protein, SESN2, maintains mitochondrial function, thus providing a potential future therapeutic approach to doxorubicin-induced cardiomyopathy [62].
2.2.3 Cardioprotective Role of Neurohormonal Agents
The diagnosis of cancer treatment-related cardiomyopathy is a diagnosis of exclusion, and in a comprehensive search for alternatives, etiologies—particularly genetic and infectious etiologies—should be ruled out. The role of neurohormonal antagonists is controversial in children with asymptomatic LV dysfunction. However, they may be considered in some cases. According to the ACC/AHA Task Force on Clinical Practice Guidelines and the Heart Failure Society of America, patients who develop LV dysfunction during anthracycline treatment, even asymptomatic (Stage B) HF patients, should be treated with ACEis and BBs [8]. Enalapril is helpful in children with CTRCD due to anthracycline therapy [63, 64]. Although enalapril seems to decrease the cardiotoxic effects of cancer therapy, the long-term impacts on preventing HF have been disappointing [65, 66]. Multiple studies have shown that early initiation of BBs improves LV function in patients receiving anthracycline therapy over a longer-term follow-up [67,68,69]. The role of BBs in preventing cardiotoxicity is currently undergoing evaluation in a study of the long-term benefits of carvedilol in preventing cardiomyopathy and/or HF in high-risk childhood cancer survivors exposed to high-dose anthracyclines [70]. The results will be helpful to determine if carvedilol can mitigate anthracycline-related HF.
3 Treatment of Cancer Therapy-Related Stage C Heart Failure
Cancer therapy-related HF can be due to reduced LVEF (HFrEF) or preserved LVEF (HFpEF) [71]. There is a lack of randomized controlled trials for chemotherapy-induced HF, either HFrEF or HFpEF. Cancer patients diagnosed with clinical HF either during or after their cancer therapies are currently being treated according to ACC/AHA guidelines for managing HF with ACEis/ARBs/ARNi, BBs, and MRAs [8].
3.1 Treatment of Stage C HF with Reduced Ejection Fraction (HFrEF)
A combination of BB, ACEi, ARB, and MRA is commonly used in guideline-directed medical therapy (GDMT) for Stage C HFrEF [72, 73]. Unfortunately, enalapril improved LV systolic function in the short-term period, but over the long term, there was no effect on the progression of HF in patients who received anthracyclines, as many childhood cancer survivors have developed restrictive cardiac physiology [66, 74]. Newer contemporary drugs for HFrEF include (i) ARNi, a combination of valsartan and sacubitril, (ii) ivabradine, (iii) SGLTi (empagliflozin, dapagliflozin, and canagliflozin), and (iv) soluble guanylate cyclase (sGC) stimulator (vericiguat) that targets the cGMP pathway [75, 76]. Recent meta-analyses have shown that polytherapy with neurohormonal antagonists should be started simultaneously rather than sequentially in subjects with HFrEF [77]. Recently, FDA-approved therapy, ARNi, has improved LV function and decreased BNP in adults with long-standing chemotherapy-induced HF [78,79,80]. However, whether ARNi is helpful for the primary prevention of anthracycline-induced systolic dysfunction is unknown. A recent ongoing trial of ARNi in breast cancer patients will provide more information in the future [81]. Recently, SGLT2i has improved HF outcomes in adults with anthracycline-related cardiomyopathies [83, 84]. Based on adult HF studies, these newer HF agents should be used in children with cancer therapy-induced refractory HF [72]. Lately, Dapagliflozin has been relatively safe and efficacious when added to GDMT in children aged < 21 years with a diagnosis of HF [85].
Soluble guanylate cyclase (sGC) is intimately involved in regulating cardiovascular tone, platelet aggregation, and titin phosphorylation, thus facilitating both the contraction and relaxation of cardiomyocytes [86]. Vericiguat, an sGC stimulator that restores the NO-sGC-cGMP pathway by stimulating sGC levels and enhancing endogenous NO sensitivity, is helpful in HF [87]. The studies suggest an opportunity for cGMP manipulation to prevent cardiac dysfunction in a juvenile mouse model of anthracycline cardiotoxicity [88]. Other preclinical data indicate that cGMP manipulations might be helpful for the primary prevention of cardiac dysfunction [89]. Recent clinical trials on the efficacy, safety, and pharmacodynamics of vericiguat in pediatric participants with heart failure due to LV systolic dysfunction may find a role for vericiguat in children in the future [90]. However, SGLT2i, vericiguat, ivabradine, ATPase activators such as omecamtiv mecarbil, and cGMP manipulators such as vericiguat are not the first lines of therapy, and they are still considered experimental in children.
3.2 Treatment of Stage C HF with Preserved Ejection Fraction (HFpEF)
Indications from the general population have shown that diastolic dysfunction usually progresses to Stage C HF in patients with concomitant CV risk factors [71, 91]. In the St. Jude Lifetime cohort study, diastolic dysfunction after cancer therapy has been described in 11% of all cancer survivors, and 8.7% of them had isolated diastolic dysfunction [92]. Childhood cancer survivors sometimes experience a restrictive cardiomyopathy phenotype due to decreased LV mass index and LV chamber size [93]. There is limited evidence that the conventional GDMT for HFrEF is effective for HFpEF [94, 95]. Although ARNi has a neutral effect on significant cardiovascular adverse outcomes in the PARAMOUNT-HF trial, it has been approved for use in HFrEF and HFpEF because of its improved quality of life and New York Heart Association (NYHA) functional class in adults [96]. In the recently completed EMPEROR-Preserved trial, empagliflozin produced an early and long-lasting reduction in the risk and severity of a broad range of worsening HF events in patients with NYHA class II–IV HFpEF, defined as LVEF between 40 and 49% [97]. Little is known about SGLT2i relieving diastolic dysfunction in cardio-oncologic settings. In limited preclinical in-vivo studies, empagliflozin prevented a doxorubicin-related deterioration of LV function [98]. The protective effect of empagliflozin is due to its cardioprotective property independent of its impact on increasing diuresis and decreasing afterload.
4 Treatment of Cancer-Therapy-Related Advanced HF (Stage D)
When a patient’s HF worsens despite maximizing oral GDMT, referral to an advanced HF specialist to consider a continuous inotropic infusion, cardiac resynchronization therapy (CRT), mechanical circulatory support, and heart transplant is appropriate. Decisions surrounding advanced HF therapies must involve a multidisciplinary team and include shared decision making with the patient and their family.
4.1 CRT for Chemotherapy-Induced Cardiotoxicity
Atrioventricular conduction disturbances are often seen in the setting of cancer therapy. These conduction disturbances produce suboptimal LV filling as well as lower stroke volume. Cardiac resynchronization therapy (CRT) improves symptoms and reduces mortality in HFrEF and HFpEF patients with electrical or mechanical dyssynchronies. Despite meeting clinical indications for this therapy, CRT is underutilized in HF among cancer survivors. It is postulated that this could be related to understanding HF and comorbidities during clinical presentation [99,100,101]. However, utilization of CRT has shown improvement in LV function in cancer therapy-induced cardiomyopathy [102, 103]. A retrospective multicenter study in adults with anthracycline-induced cardiomyopathy derived a significant echocardiographic and symptomatic benefit from CRT, similar to other forms of non-ischemic cardiomyopathy [104, 105]. The pediatric experience with CRT for chemotherapy-induced cardiomyopathy is limited to case reports [106].
4.2 Mechanical Circulatory Support and Heart Transplantation
Specialized interventional procedures such as temporary mechanical circulatory support (MCS) are sometimes necessary for children with acute hemodynamic deterioration during chemotherapy [107]. These temporary percutaneous MCS are reasonable as a ‘bridge to recovery,’ ‘bridge to a decision,’ or ‘bridge to transplantation.’ Durable MCS such as a ventricular assist device (VAD) is beneficial in carefully selected patients with advanced HF as a bridge to cardiac transplantation, transplant candidacy, or destination therapy [108, 109]. The transplant outcome in this population is similar to other non-ischemic cardiomyopathies [110, 111].
4.3 Pluripotent Stem Cells
Cancer treatment has evolved in the last decade with the introduction of new therapies. Despite these successes, chemotherapy's lingering cardiotoxic side effects remain a significant cause of morbidity and mortality in cancer survivors. With the advent of human induced pluripotent stem cell (iPSC) technology, researchers can model cardiac toxicity and discover drugs to protect against chemotherapy-induced cardiotoxicities [112]. In the mice model, mouse embryonic stem cells were transplanted in mice with doxorubicin cardiomyopathy, and improvement in ventricular function was noted [113].
5 Conclusions
The primary purpose of this review is to describe commonly used and future pharmacologic treatment of pediatric cancer patients and survivors at risk for CV toxicities. Cardiotoxicity associated with cancer therapies can be persistent and progressive and can be missed clinically. Cardiomyopathy and HF are leading causes of death in childhood cancer survivors, given the anticipated long post-cancer lifespan. The only FDA-approved cardioprotective therapy is dexrazoxane; which works by reducing anthracycline-induced dose-limiting cardiotoxicities. Multiple therapies, including neurohormonal agents and newer drugs such as ARNi or SGLT2i, should be used simultaneously as combination therapy with ACEi and BB in patients with Stage C and D HF. Early initiation of HF therapies is proven more valuable than at a later stage with advanced HF. There is a need for both clinical researchers and pharmaceutical companies to include cancer-related HF patients in drug trials to accelerate the transition of potentially effective HF drugs from the general population to cardio-oncology patients.
References
Wallace KB, Sardao VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126(7):926–41.
Kitakata H, Endo J, Ikura H, et al. Therapeutic targets for DOX-induced cardiomyopathy: role of apoptosis vs. ferroptosis. Int J Mol Sci. 2022;23(3):1414. https://doi.org/10.3390/ijms23031414.
Kamphius JAM, Linschoten M, Cramer M, et al. Cancer therapy-related cardiac dysfunction of nonanthracycline chemotherapeutics: what is the evidence? JACC CardioOncol. 2019;1(2):280–90.
Zhang X, Zhu Y, Dong S, et al. Role of oxidative stress in cardiotoxicity of antineoplastic drugs. Life Sci. 2019;232: 116526. https://doi.org/10.1016/j.lfs.2019.06.001.
Rhea IB, Oliveira GH. Cardiotoxicity of novel targeted chemotherapeutic agents. Curr Treat Options Cardiovasc Med. 2018;20(7):53.
Mitchell JD, Cehic DA, Morgia M, et al. Cardiovascular manifestations from therapeutic radiation: a multidisciplinary expert consensus statement from the international cardio-oncology society. JACC Cardio Oncol. 2021;3:360–80.
Bansal N, Blanco JG, Sharma U, Pokharel S, Shisler S, Lipshultz SE. Cardiovascular diseases in survivors of childhood cancer. Cancer Metastases Rev. 2020;39(1):55–68.
de Baat EC, van Dalen EC, Mulder RL, et al. Primary cardioprotection with Dexrazoxane in patients with childhood cancer who are expected to receive anthracyclines: recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child Adolesc Health. 2022;6(12):885–94.
Feijen EAM, Font-Gonzalez A, Van der Pal HJH, et al. Risk and temporal changes of heart failure among 5-year childhood cancer survivors: a DCOG-LATER study. JAHA. 2019;8:e009122.
de Baat EC, Feijen EAM, Reulen RC, et al. risk factors for heart failure among Pan-European childhood cancer survivors: a PanCareSurFup and ProCardio cohort and nested case–control study. J Clin Oncol. 2023;41(1):96–106.
Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012;8(4):647–70.
Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80.
Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–60.
Srivastava R, Batra A, Dhawan D, Bakhshi S. Association of energy intake and expenditure with obesity: a cross-sectional study of 150 pediatric patients following treatment for leukemia. Pediatr Hematol Oncol. 2017;34:29–35.
Iughetti L, Bruzzi P, Predieri B, Paolucci P. Obesity in patients with acute lymphoblastic leukemia in childhood. Ital J Pediatr. 2012;38:4.
Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll of Cardiol. 2011;58(9):988–9.
Chotenimitkhun R, D’Agostino R Jr, Lawrence JA, et al. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can J Cardiol. 2015;31(3):302–7.
Hundley WG, D’Agostino R, Crots T. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2022;1(9):EVIDoa2200097.
Nelian TG et al. STOP-CA clinical trial finds statins lower rate of heart decline in lymphoma patients, Late Breaking ACC23 Clinical Trial Reports. ACC.23/WCC Meeting Newspaper. Published on March 4, 2023, in JACC.
Marques-Aleixo I, Santos-Alves E, Oliveira PJ, Moreira PI, Magalhães J, Ascensão A. The beneficial role of exercise in mitigating doxorubicin-induced Mitochondrionopathy. Biochim Biophys Acta Rev Cancer. 2018;1869(2):189–99.
Smarz K, Jaxa-Chamiec T, Chwyczko T, et al. Cardiopulmonary exercise testing in adult cardiology: expert opinion of the Working Group of Cardiac Rehabilitation and Exercise Physiology of the Polish Cardiac Society. Kardiol Pol. 2019;77:730–56.
Toko H, Oka T, Zou Y, et al. Angiotensin II type 1a receptor mediates doxorubicin-induced cardiomyopathy. Hypertens Res. 2002;25:597–603.
Bien S, Riad A, Ritter CA, et al. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res. 2007;67:10428–35.
Bernstein D, Fajardo G, Zhao M, et al. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–9.
Lódi M, Priksz D, Fülöp GÁ, et al. Advantages of prophylactic versus conventionally scheduled heart failure therapy in an experimental model of doxorubicin-induced cardiomyopathy. J Transl Med. 2019;17(1):229. https://doi.org/10.1186/s12967-019-1978-0.
Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (Prevention of left ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive chemotherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355–62.
Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101–breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. 2016;35:870–7.
Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of Candesartan and Metoprolol. Eur Heart J. 2016;37:1671–80.
Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48(11):2258–62.
Jhorawat R, Kumari S, Varma SC, et al. Preventive role of carvedilol in adriamycin-induced cardiomyopathy. Indian J Med Res. 2016;144(5):725–9.
Nabati M, Janbabai G, Baghyari S, Esmaili K, Yazdani J. Cardioprotective effects of carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. 2017;69(5):279–85.
Salehi R, Zamani B, Esfehani A, Ghafari S, Abasnezhad M, Goldust M. Protective effect of carvedilol in cardiomyopathy caused by anthracyclines in patients suffering from breast cancer and lymphoma. Am Heart Hosp J. 2011;9(2):95–8.
Georgakopoulos P, Roussou P, Matsakas E, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol. 2010;85(11):894–6.
Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71(20):2281–90.
Barış VÖ, Dinçsoy AB, Gedikli E, Zırh S, Müftüoğlu S, Erdem A. Empagliflozin significantly prevents the doxorubicin-induced acute cardiotoxicity via non-antioxidant pathways. Cardiovasc Toxicol. 2021;21(9):747–58.
Hitawala G, Jain E, Castellanos L, et al. Pediatric chemotherapy drugs associated with cardiotoxicity. Cureus. 2021;13(11): e19658. https://doi.org/10.7759/cureus.19658.
Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, et al. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation. 2019;140:31–4.
Lipshultz SE, Anderson LM, Miller TL, et al. Impaired mitochondrial function is abrogated by Dexrazoxane in doxorubicin-treated childhood acute lymphoblastic leukemia survivors. Cancer. 2016;122(6):946–53.
Aminkeng F, Bhavsar AP, Visscher H, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47(9):1079–84.
Magdy T, Jiang Z, Jouni M, et al. RARG variant predictive of doxorubicin-induced cardiotoxicity identifies a cardioprotective therapy. Cell Stem Cell. 2021;28(12):2076-2089.e7. https://doi.org/10.1016/j.stem.2021.08.006.
Wang X, Sun CL, Quinones-Lombrana A, et al. CELF4 variant and anthracycline-related cardiomyopathy: a Children’s Oncology Group genome-wide association study. J Clin Oncol. 2016;34(8):863–70.
Ohiri JC, McNally EM. Gene editing and gene-based therapeutics for cardiomyopathies. Heart Fail Clin. 2018;14(2):179–88.
Messinis DE, Melas IN, Hur J, Varshney N, Alexopoulos LG, Bai JPF. Translational systems pharmacology-based predictive assessment of drug-induced cardiomyopathy. CPT Pharmacometrics Syst Pharmacol. 2018;7(3):166–74.
Sapkota Y, Qin N, Ehrhardt MJ, et al. Genetic variants associated with therapy-related cardiomyopathy among childhood cancer survivors of African ancestry. Cancer Res. 2021;81(9):2556–65.
Vinodhini MT, Sneha S, Nagare RP, et al. Evaluation of a polymorphism in MYBPC3 in patients with anthracycline-induced cardiotoxicity. Indian Heart J. 2018;70(2):319–22.
Li L-P, Zhong J, Li M-H, et al. Disruption of MAP7D1 gene function increases the risk of doxorubicin-induced cardiomyopathy and heart failure. Biomed Res Int. 2021. https://doi.org/10.1155/2021/8569921.
Singh P, Wang X, Hageman L, et al. Association of GSTM1 null variant with anthracycline-related cardiomyopathy after childhood cancer—a Children’s Oncology Group ALTE03N1 report. Cancer. 2020;126(17):4051–8.
Anderson BS, Eksborg S, Vidal RF, Sundberg M, Carlberg M. Anthraquinone-induced cell injury: acute toxicity of actinomycin, epirubicin, idarubicin and mitoxantrone in isolated cardiomyocytes. Toxicology. 1999;135(1):11–20.
Leerink JM, Feijen EAM, Moerland PD, et al. Candidate plasma biomarkers to detect anthracycline-related cardiomyopathy in childhood cancer survivors: a case–control study in the Dutch Childhood Cancer Survivor study. J Am Heart Assoc. 2022;11(14): e025935.
Bisoc A, Ciurescu D, Rădoi M, et al. Natriuretic peptide levels in the serum can predict the development of anthracycline-induced cardiomyopathy. Am J Ther. 2020;27(2):e142–50.
Pudil R, Mueller C, Celutkiene J, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1966–83.
Lipshuntz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30:1050–7.
Zamorano JL, Lancellotti P, Aboyans V, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Eur Heart J. 2016;37(36):2768–801.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovi ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84.
Moon TJ, Miyamoto S, Younosazai AK, Landeck BF. Left ventricular strain and strain rates are decreased in children with normal fractional shortening after exposure to anthracycline chemotherapy. Cardiol Young. 2014;24(5):854–65.
Akam-Venkata J, Kadiu G, Galas J, Lipshultz SE, Aggarwal S. Left ventricle segmental function in childhood cancer survivors using speckle-tracking echocardiography. Cardiol Young. 2019;29(12):1494–500.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68.
Negishi T, Thavendiranathan P, Penicka M, et al. Cardioprotection using strain-guided management of potentially cardiotoxic cancer therapy: 3-year results of the SUCCOUR trial. JACC Cardiovasc Imaging. 2023;16(3):269–78.
Doherty JU, Kort S, Mehran R, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/ SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology appropriate use criteria task force, American association for thoracic surgery, American heart association, American society of echocardiography, American society of nuclear cardiology, heart rhythm society, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, society for cardiovascular magnetic resonance, and the society of thoracic surgeons. J Am Coll Cardiol. 2019;73:488–516.
Deng S, Yan T, Jendrny C, et al. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting topoisomerase 2 isoforms. BMC Cancer. 2014;14:842. https://doi.org/10.1186/1471-2407-14-842.
Lue Y, Gao C, Swerdloff R, et al. Humanin analog enhances the protective effect of dexrazoxane against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2018;315:H634–43.
Wnag P, Wang L, Lu J, et al. SESN2 protects against doxorubicin-induced cardiomyopathy by rescuing mitophagy and improving mitochondrial function. J Mol Cell Cardiol. 2019;133:125–37.
Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8.
Gupta V, Singh SK, Agrawal V, Singh TB. Role of ACE inhibitors in anthracycline-induced cardiotoxicity: a randomized, double-blind, placebo-controlled trial. Pediatr Blood Cancer. 2018;65: e27308.
Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–8.
Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–22.
Armenian S, Bhatia S. Predicting and preventing anthracycline-related cardiotoxicity. Am Soc Clin Oncol Educ Book. 2018;38:3–12.
Huang S, Zhao Q, Yang ZG, et al. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity—a systematic review and meta-analysis of carvedilol. Heart Fail Rev. 2019;24(3):325–33.
El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail. 2012;18:607–13.
Armenian SH, Hudson MM, Chen MH, et al. Rationale and design of the Children’s Oncology Group (COG) study ALTE1621: a randomized, placebo-controlled trial to determine if low-dose carvedilol can prevent anthracycline-related left ventricular remodeling in childhood cancer survivors at high risk for developing heart failure. BMC Cardiovasc Disord. 2016;16(1):187. https://doi.org/10.1186/s12872-016-0364-6.
Das B, Deshpande S, Venkata JA, Shakti D, Moskowitz W, Lipshulz SE. Heart failure with preserved ejection fraction in children. Pediatr Cardiol. 2022. https://doi.org/10.1007/s00246-022-02960-7.
Das B, Moskowitz W, Butler J. Current and future drug and device therapies for pediatric heart failure patients: potential lessons from adult trials. Children. 2021;8(5):322. https://doi.org/10.3390/children8050322
Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society for Heart and Lung Transplantation guidelines for the management of pediatric heart failure executive summary. J Heart Lung Transplant. 2014;33:888–909.
Franco VI, Lipshultz SE. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol Young. 2015;25(Suppl 2):107–16.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145:e895–1032.
De Marzo V, Savarese G, Tricarcio L, et al. Network meta-analysis of medical therapy efficacy in more than 90,000 patients with heart failure and reduced ejection fraction. J Intern Med. 2022;292:333–49.
Frey MK, Arfsten H, Pavo N, et al. Sacubitril/valsartan is well tolerated in patients with long-standing heart failure and history of cancer and improves ventricular function: real-world data. Cardio-Oncol. 2021;7:1–6.
Gregorietti V, Fernandez TL, Costa D, Chahla EO, Daniele AJ. Use of Sacubitril/valsartan in patients with cardiotoxicity and heart failure due to chemotherapy. Cardio-Oncology. 2020;6:1–6.
Sheppard CE, Anwar M. The use of sacubitril/valsartan in anthracycline-induced cardiomyopathy: a mini case series. J Oncol Pharm Pract. 2019;25(5):1231–4.
Martín-García A, Díaz-Peláez E, Martín-García AC, et al. Myocardial function and structure improvement with sacubitril/valsartan in cancer therapy-induced cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2020;73(3):268–9.
Mecinaj A, Gulati G, Heck SL, et al. Rationale and design of the prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA II) trial: a randomized, placebo-controlled, multicenter trial. Cardiooncology. 2021;2021(7):33. https://doi.org/10.1186/s40959-021-00115-w.
Chiang CH, Chiang CH, Chiang CH, et al. Impact of sodium–glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer. Heart. 2023;109:470–7.
Gongora CA, Drobni ZD, Costa Silva TQA, et al. Sodium–glucose co-3transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. JACC Heart Fail. 2022;10(8):559–67.
Khouri MG, Greene SJ. Sodium–glucose co-transporter-2 inhibitor therapy [y during anthracycline treatment: is there a role of cardioprotection? JACC Heart Fail. 2022;10:568–70.
Newland DM, Law YM, Albers EL, et al. Early clinical experience with dapagliflozin in children with heart failure. Ped Cardiol. 2023;44:146–52.
Azer J, Hua R, Krishnaswamy PS, Rose RA. Effects of natriuretic peptides on electrical conduction in the sinoatrial node and atrial myocardium of the heart. J Physiol. 2014;592:1025–45.
Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–93.
Nagiub M, Filippone D, Durrant D, Das A, Kukreja RC. Long-acting PDE5 inhibitor tadalafil prevents early doxorubicin-induced left ventricle diastolic dysfunction in juvenile mice: potential role of cytoskeletal proteins. Can J Physiol Pharmacol. 2017;95:295–304.
Frisk M, Le C, Shen X, et al. Etiology-dependent impairment of diastolic cardiomyocyte calcium homeostasis in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2021;4:405–19.
Merck Sharp & Dohme LLC (sponsors). Efficacy, safety, and pharmacokinetics of vericiguat in pediatric participants with heart failure due to left ventricular systolic dysfunction (MK-1242-036). ClinicalTrials.gov identifier (NCT number): NCT0571408592.
Kosmala W, Marwick TH. Asymptomatic left ventricular diastolic dysfunction: predicting progression to symptomatic heart failure. JACC Cardiovasc Imaging. 2020;13:215–7.
Yu W, Li SN, Chan GC, et al. Transmural Strain and rotation gradient in survivors of childhood cancers. Eur Heart J Cardiovasc Imaging. 2013;14:175–82.
Lipshultz SE, Scully R, Stevenson KE, et al. Hearts too small for body size after doxorubicin for childhood ALL: Grinch syndrome. J Clin Oncol. 2014;32(suppl):10021A.
Das B. Therapeutic approaches in heart failure with preserved ejection fraction (HFpEF) in children: present and future. Pediatr Drugs. 2022;24(3):235–46.
Minotti G, Menna P, Camilli M, Salvatorelli E, Levi R. Beyond hypertension: diastolic dysfunction associated with cancer treatment in the era of cardio-oncology. Adv Pharmacol. 2022;94:365–409.
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E. Prospective comparison of ARNI with ARB on management of heart failure with preserved ejection fraction (PARAMOUNT) investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind, randomized controlled trial. Lancet. 2012;380:1387–95.
Packer M, Butler J, Zannad F, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and a preserved ejection fraction: the EMPEROR-preserved trial. Circulation. 2021;144:1284–9.
Sabatino J, De Rosa S, Tammè L, et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc Diabetol. 2020;19:66. https://doi.org/10.1186/s12933-020-01040-5].
Bianco C, Al-Kindi SG, Oliveira GH. Advanced heart failure therapies for cancer therapeutics-related cardiac dysfunction. Heart Fail Clin. 2017;13:327–36.
Fadol AP, Mouhavar E, Reyes-Gibby CC. The use of cardiac resynchronization therapy in cancer patients with heart failure. J Clin Exp Res Cardiol. 2017;3(1). https://doi.org/10.15744/2394-6504.3.105.
Oliveira GH, Qattan MY, Al-Kindi S, Park SJ. Advanced heart failure therapies for patients with chemotherapy-induced cardiomyopathy. Circ Heart Fail. 2014;7:1050–8.
Ezzeddine FM, Saliba AN, Jain V, et al. Outcomes of cardiac resynchronization therapy in patients with chemotherapy-induced cardiomyopathy. Pacing Clin Electrophysiol. 2021;44(4):625–32.
Singh JP, Solomon SD, Fradley MG, MADIT-CHIC Investigators, et al. Association of cardiac resynchronization therapy with change in left ventricular ejection fraction in patients with chemotherapy-induced cardiomyopathy. JAMA. 2019;322(18):1799–805.
Rickard J, Kumbhani DJ, Baranowski B, Martin DO, Tang WH, Wilkoff BL. The usefulness of cardiac resynchronization therapy in patients with adriamycin-induced cardiomyopathy. Am J Cardiol. 2010;105(4):522–6.
Patel D, Kumar A, Moennich LA, et al. Cardiac resynchronization therapy in anthracycline-induced cardiomyopathy. Heart. 2022;108(4):274–8.
Jones BO, Davis A, Alison J, Weintraub RG, Butt W, Cheung MM. Cardiac resynchronization therapy in a child with severe anthracycline-induced congestive heart failure and normal QRS duration. J Heart Lung Transplant. 2007;26:1333–5.
Schlam I, Lee AY, Li S, et al. LeftVentricular assist devices in patients with active malignancies. JACC Cardio Oncol. 2021;3:305–15.
Oliveira GH, Dupont M, Naftel D, et al. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2014;63:240–8.
Puri K, Denfield SW, Adachi I, et al. Ventricular assist device support for children with chemotherapy-induced cardiomyopathy and advanced heart failure: perspectives gained from a single-center experience. Pediatr Transplant. 2022;26: e14286.
Oliveira GH, Hardaway BW, Kucheryavaya AY, Stehlik J, Edwards LB, Taylor DO. Characteristics and survival of patients with chemotherapy-induced cardiomyopathy undergoing heart transplantation. J Heart Lung Transplant. 2012;31:805–10.
Shugh SB, Ryan TD. Heart transplantation in survivors of childhood cancer. Transl Pediatr. 2019;8(4):314–21.
Sayed N, Ameen M, Wu JC. Personalized medicine in cardio-oncology: the role of induced pluripotent stem cell. Cardiovasc Res. 2019;115:949–59.
Santos DSD, Brasil GV, Ramos IPR, et al. Embryonic stem cell-derived cardiomyocytes for the treatment of doxorubicin-induced cardiomyopathy. Stem Cell Res Ther. 2018;9(1):30. https://doi.org/10.1186/s13287-018-0788-2.
Acknowledgements
Ms Sabrina M. Freeman, BSN, RN, has edited the manuscript for English and grammar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Bibhuti Das has no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
BD: Conceptualized, wrote, and revised this manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, B. Pharmacotherapy for Cancer Treatment-Related Cardiac Dysfunction and Heart Failure in Childhood Cancer Survivors. Pediatr Drugs 25, 695–707 (2023). https://doi.org/10.1007/s40272-023-00585-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00585-8