Abstract
Olmesartan medoxomil (OLM)-based antihypertensive treatment is a valuable option in the treatment of patients with mild to severe hypertension, including those with difficult-to-treat disease. Once-daily OLM, as monotherapy or in combination with hydrochlorothiazide (HCT) and/or amlopidine (AML), provides blood pressure (BP) control over the entire 24-h dosing interval, reduces systolic and diastolic BP, enables patients to achieve BP goals and is generally well tolerated. In patients who require treatment with two or more antihypertensives, treatment with fixed-dose combinations (FDC) of OLM (an angiotensin II receptor blocker) + AML (a calcium channel blocker) and/or HCT (a diuretic) is a rational choice, as the drugs have complementary mechanisms of action, and the use of FDCs reduces pill burden, which may improve patient adherence and persistence to treatment, and clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adis evaluation of olmesartan medoxomil-based therapy in adults with hypertension
Provides effective and sustained reductions in blood pressure across all stages of hypertension |
At least as, or more effective than, many other antihypertensive mono- or combination-therapy regimens |
Components of fixed-dose combinations have different and complementary mechanism of action |
Fixed-dose combinations reduce pill burden, which may improve compliance with antihypertensive therapy |
Convenient once-daily administration provides efficacy over 24 h (including during the early morning period) |
Generally well tolerated |
What is the rationale for using OLM-based treatment?
When treating hypertension, the primary goal is to reduce blood pressure (BP) to the guideline-recommended target of <140/90 mmHg (or <130/80 mmHg in patients with diabetes mellitus and those with high cardiovascular risk), which, in turn, reduces the risk of cardiovascular events, cerebrovascular disorders, renal failure and premature death [1, 2]. However, suboptimal BP control is common, resulting in a substantial health and economic burden [3, 4].
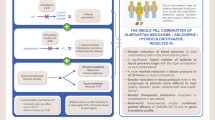
Some patients may be able to achieve BP control with treatment with a single antihypertensive. Among the recommended first-line options are angiotensin II receptor blockers/antagonists (ARBs) [1, 2]. Olmesartan mesylate [OLM; tradenames include Olmetec® (EU) [5] and Benicar® (USA) [6])] is an oral once-daily ARB with well-established efficacy, tolerability and safety profiles in the treatment of hypertension. However, most patients require treatment with two or more antihypertensive agents to achieve their target BP [1, 2, 7]. In patients requiring treatment with more than one antihypertensive, the use of a single-pill fixed-dose combination (FDC) of two or three antihypertensives is convenient. As a result, patient compliance and treatment adherence may be improved, which, in turn, may improve morbidity and mortality rates, and reduce healthcare costs [4, 7, 8]. The potential compliance advantages associated with regimen simplification have resulted in the development of a variety of FDCs of well-established antihypertensive agents, including once-daily FDCs of OLM + hydrochlorothiazide (HCT) [tradenames include Olmetec Plus® (EU) [9] and Benicar HCT® (USA) [10]], OLM + amlodipine (AML) [tradenames include Sevikar® (EU) [11] and Azor® (USA) [12]], and OLM + AML + HCT [tradenames include Sevikar HCT® (EU) [13] and Tribenzor® (USA) [14]].
How do OLM, AML and HCT work?
OLM is an ARB that acts at the angiotensin II type 1 (AT1) receptor to block the effects of angiotensin II (i.e. vasoconstriction and aldosterone secretion) [5, 6]. Following oral administration, the mesylate salt of olmesartan is rapidly metabolized to olmesartan during absorption from the gastrointestinal tract. This active metabolite binds with high selectivity to the AT1 receptor [but not to the type 2 (AT2) receptor], and blocks the binding of angiotensin I [15]. Angiotensin II is the primary effector peptide of the renin–angiotensin–aldosterone system (RAAS), which is an important mediator in the pathophysiology of hypertension. Excessive activity in the RAAS plays a key role in target end-organ damage (e.g. myocardial infarction, congestive heart failure, coronary artery disease and end-stage renal disease) [16]. The cardiovascular effects of angiotensin II rely on activation of the AT1 receptor by angiotensin II, resulting in acute vasoconstriction and increases in salt retention, fluid volume, aldosterone secretion and sympathetic activity. Olmesartan binds to the AT1 receptor with a high degree of insurmountability and with greater affinity than most other ARBs [16].
OLM is available as a FDC with HCT for the treatment of patients whose hypertension is not controlled with monotherapy with OLM [9, 10] or HCT [10]. HCT, a thiazide diuretic, has complementary BP-lowering mechanisms of action to those of OLM. Similar to other drugs in this class, HCT affects the electrolyte reabsorption mechanisms of the kidney tubules, causing sodium and chloride excretion to increase, with a resultant diuretic effect that decreases plasma volume. However, the exact mechanism by which thiazide diuretics reduce BP is not completely understood. The concomitant use of HCT with an ARB, such as OLM, tends to reduce the potassium loss associated with thiazide diuretics [9, 10].
A FDC of OLM and AML is available for the treatment of patients whose BP is inadequately controlled with monotherapy with OLM [11, 12] (or another ARB) [12] or AML [11, 12] (or another CCB) [12], as replacement therapy for its individual components [12], or as initial therapy in adults who are likely to need treatment with multiple antihypertensives to achieve BP control [12]. The mechanisms of action of AML in lowering BP are complementary to those of OLM [11, 12]. AML is a dihydropyridine calcium channel blocker (CCB) that inhibits the transmembrane influx of calcium into vascular smooth muscle and cardiac muscle cells, resulting in peripheral arterial vasodilation and reduced peripheral vascular resistance.
OLM is also available as a FDC that includes both AML and HCT as replacement therapy for its individual components [13, 14], or as add-on/switch therapy if BP is inadequately controlled with OLM [13, 14] (or another ARB) [14], AML [13, 14] (or another CCB) [14] or HCT [13, 14] (or another diuretic) [14]. The three components of this FDC have complementary mechanisms of action, providing BP-lowering effects by blocking the RAAS (OLM) and calcium channels (AML), and providing diuretic activity (HCT) [13, 14].
The rate and extent of absorption of OLM, AML and HCT when administered as components of a FDC is similar to that following administration of the individual components [9–14].
For whom is OLM-based treatment indicated?
OLM-based treatment (i.e. OLM alone and as a component of FDCs with AML and/or HCT) is indicated in the treatment of patients with hypertension in many countries worldwide. Table 1 provides a brief summary of the approved indications and administration of once-daily OLM monotherapy [5, 6] and OLM-based FDC therapy [9–14] in adults in the EU [5, 9, 11, 13] and USA [6, 10, 12, 14], as representative of its use worldwide.
Recommendations for the use of OLM alone or in FDCs in special populations are consistent with those of the individual drug components [5, 6, 9–14]. As precautions, warnings and contraindications vary somewhat between countries, local prescribing information should be consulted for details. In general, OLM-based therapy should not be used during pregnancy or while breastfeeding, and may require caution and/or dosage adjustments in patients with moderate to severe renal or hepatic impairment or other underlying conditions [5, 6, 9–14]. Cautious dose titration and regular monitoring of BP and other parameters (e.g. levels of serum potassium and/or other electrolytes) are recommended in patients who are considered to be at risk for adverse events associated with OLM, AML and/or HCT [5, 6, 9–14]. Of note, the BP-lowering effect of ARBs, including OLM, may be somewhat less in Black patients than in non-Black patients [5, 6, 9–14].
The potential drug interactions with OLM-based therapy are also consistent with those of OLM and the other components of the FDCs [5, 6, 9–14]. The most clinically relevant potential drug interactions with OLM and the other components of OLM-based FDCs include [5, 6, 9–14]:
-
interactions between OLM and NSAIDs, other agents that block the RAAS (concomitant use of OLM and aliskiren in patients with diabetes or renal impairment is contraindicated or should be avoided), colesevalam hydrochloride and lithium;
-
interactions between AML and simvastatin;
-
interactions between HCT and oral antidiabetics and insulin, cholestyramine and colestipol resins, corticosteroids, NSAIDs, and alcohol, barbiturates and narcotics.
Consult local prescribing information for further details on these and other potential drug interactions with OLM and the other components of OLM-based FDCs.
What is the clinical efficacy of OLM-based treatment?
The antihypertensive efficacy of OLM-based treatment has been established in many clinical trials in patients with hypertension. This section focuses on the key results of pivotal, large, well-designed clinical trials in the treatment arms that used clinically relevant once-daily dosages of OLM as monotherapy or FDC therapy. Unless otherwise indicated, adult patients with hypertension were enrolled in the trials, all drug regimens were administered once daily, changes in BP-related parameters are relative to baseline, and systolic BP (SBP) and diastolic BP (DBP) values refer to mean values for seated BP. BP treatment goals were generally based on SBP/DBP values of <140/90 mmHg (<130/80 mmHg in patients with comorbid diabetes).
OLM monotherapy
In adults
OLM monotherapy was more effective than placebo in lowering BP [17, 18]. In a dose-ranging trial in patients with mild to moderate hypertension [17], the optimal daily dosage of OLM was 10–40 mg (i.e. the recommended dosage range), with no further increase in efficacy with 80 mg. The reduction in DBP after 12 weeks’ treatment with OLM 10–40 mg was 12.9–15.5 mmHg greater than that with placebo (all p < 0.05). At 12 weeks, BP response rates were also significantly (p < 0.01) greater with OLM 10–80 mg than with placebo (66–78 vs. 46 %) [17].
In randomized, double-blind trials of up to 12 weeks’ duration, OLM monotherapy provided better antihypertensive efficacy than monotherapy with other ARBs, including candesartan cilexetil [19], losartan [20–23] and irbesartan [21], and was at least as effective as valsartan monotherapy [21, 22]. The between-group difference (BGD) in reductions in trough seated or daytime ambulatory DBP were evident from 1 or 2 weeks onwards, indicating a faster onset of action with OLM than with the comparator ARB [19, 21, 22]. At most timepoints, reductions in SBP, response rates and BP normalization rates also generally favoured OLM over other ARBs [19–22]. Moreover, in a meta-analysis of 22 randomized trials of OLM versus other ARBs, the overall antihypertensive efficacy of OLM was better than that of losartan and valsartan, and comparable to that of candesartan and irbesartan [24].
Compared with monotherapy with drugs in other antihypertensive classes, OLM monotherapy was as effective as AML [25], felodipine and atenolol [26], and generally provided better antihypertensive efficacy than captopril [20] and ramipril [27, 28], in 8- to 12-week trials.
OLM treatment for 24 weeks was also effective in lowering BP in noncomparative trials in Chinese patients with mild to moderate hypertension [29, 30], with significant reductions in morning BP surge being shown in a subgroup of patients with a morning BP surge of ≥23 mmHg [30].
OLM was also effective in the real-world setting [31, 32]. Reductions in DBP and SBP were 14.2 and 28.4 mmHg, respectively, at week 12 in a study in ≈12,000 German patients with hypertension receiving treatment with OLM [31]. In Japanese patients with masked, white-coat, poorly controlled or well-controlled hypertension, OLM decreased high morning BP when measured at home or at a clinic, but the decreases were not excessive relative to normal BP values [32].
In paediatric patients
In the USA, OLM monotherapy is also indicated in the treatment of hypertension in children and adolescents aged 6–16 years (Table 1) [6]. In the 3-week dose-response phase of a trial in this patient population, low-dose (2.5 or 5.0 mg) and high-dose (20 or 40 mg) weight-dependent OLM significantly (p < 0.015) and dose-dependently reduced SBP and DBP [33]. The response was significant for both race-stratified cohorts [cohort A was mixed race (62 % White) and cohort B was 100 % Black]. In the following 2-week phase of the trial in which some OLM-recipients were randomly switched to placebo, relative to placebo, BP control was maintained with OLM in cohort A and the combined A+ B cohorts, but not in cohort B [33].
OLM + HCT dual therapy
OLM/HCT was more effective than placebo in reducing BP in randomized, double-blind trials [34, 35]. At week 8 in a trial with a factorial design, all dosages of OLM/HCT reduced both DBP and SBP in a dose-dependent manner to a significantly greater extent than placebo [36]. Reductions in trough SBP/DBP were 20.1/16.4 and 26.8/21.9 mmHg with OLM/HCT 20 mg/12.5 mg and 40 mg/25 mg, respectively, versus 3.3/8.2 mmHg with placebo [36]. Moreover, the proportion of patients achieving all BP goals was significantly (p < 0.05) greater with OLM/HCT 20 mg/25 mg, 40 mg/12.5 mg and 40 mg/25 mg than with placebo [34].
In a trial in patients with stage 1 or 2 hypertension, an OLM/HCT titration regimen was significantly (p < 0.0001) more effective than placebo with regard to improving SBP/DBP (−22.3/−12.1 vs. −0.1/+0.8 mmHg), and with regard to the proportion of patients who achieved a BP target of <140/90 mmHg (74.1 vs. 30.7 %) [35]. In the subgroup of patients with stage 1 hypertension, significantly more OLM-based therapy than placebo recipients achieved a normalized BP of <120/80 mmHg (44.8 vs. 1.4 %; p < 0.0001) [35]. OLM-based therapy also reduced SPB, with >80 and 44 % of patients with stage 1 or 2 hypertension achieving SBP reductions of ≥15 and 30 mmHg, respectively [37]. The OLM/HCT titration regimen was also effective in reducing BP in an open-label study in hypertensive patients with comorbid diabetes; ambulatory BP targets of <130/80, <125/75, and <120/80 mmHg were achieved by 61.6, 47.1 and 39.0 % of patients [38].
OLM/HCT combination therapy was more effective in reducing BP than OLM monotherapy in randomized trials in patients with harder-to-treat hypertension [39, 40]. In patients with moderate to severe hypertension, OLM/HCT 40 mg/12.5 mg combination therapy was significantly (p < 0.0001) more effective than OLM 40 mg monotherapy, as assessed by changes in SBP and DBP, and the proportion of patients achieving BP goals during the initial 8-week randomized phase [39]. In the subsequent 8-week uptitration phase, the addition of HCT 12.5 mg or uptitration to OLM/HCT 40 mg/25 mg in patients who had not responded to treatment with OLM 40 mg or OLM/HCT 40 mg/12.5 mg, respectively, provided further BP reductions and increased the proportion of patients achieving BP goals [39]. Likewise, the addition of HCT 12.5 or 25 mg to OLM 40 mg monotherapy significantly (p < 0.0001) improved BP reductions and BP goal rates in patients with grade 2 or 3 hypertension in an 8-week trial [40]. However, in a noninferiority trial in patients with mild to moderate hypertension [41], the change in DBP during the randomized treatment period did not establish noninferiority between OLM/HCT and OLM monotherapy, which may reflect that 76 % of patients achieved a response at the end of 8 weeks’ open-label treatment with OLM 20 mg, which resulted in fewer patients than predicted entering the randomized, double-blind phase of the trial.
Combination therapy with OLM/HCT was at least as effective as treatment with other antihypertensive combinations in randomized, double-blind, 12-week trials [20, 42, 43]. OLM/HCT was as effective as losartan/HCT in reducing trough DBP at week 12 in patients with moderate to severe hypertension, with BGDs favouring OLM/HCT being shown at earlier timepoints [42]. BGDs in reductions in trough SBP favoured OLM/HCT at all timepoints from 1 week onwards. At study end, a significantly higher proportion of OLM/HCT than losartan/HCT recipients achieved a BP of <140/90 mmHg [42]. In patients with moderate to severe hypertension, OLM/HCT was noninferior to atenolol/HCT, as assessed by reductions in DBP and SBP at study end, with no significant BGDs in responder rates [20]. In another noninferiority trial, OLM/HCT provided antihypertensive efficacy that was superior to that with benazepril/AML, as assessed by reductions in SBP, with no significant BGD in reductions in DBP [43]. Generally, significantly more OLM/HCT than benazepril/AML recipients achieved BP targets by week 12 [43].
OLM + AML dual therapy
OLM/AML was effective in the treatment of patients with mild to severe hypertension without an adequate response to monotherapy with one of its individual components in randomized, double-blind [44–47] or open-label trials [48]. In clinical trials and/or subgroup analyses, OLM/AML was effective in treating patients regardless of the severity of their baseline hypertension [49, 50], and in patient populations with difficult-to-treat hypertension (i.e. those aged ≥65 years [50–53], with comorbid obesity [51, 54], with type 2 diabetes [51, 52, 55] or who were Black [51, 52, 56]).
In an 8-week trial in adults with mild to severe hypertension, OLM/AML 20 mg/5 mg, 40 mg/5 mg and 40 mg/10 mg were significantly more effective in reducing DBP and SBP than equivalent dosages of OLM or AML monotherapy [44]. In addition, the proportion of patients who met their BP goal was significantly (p < 0.005) greater with OLM/AML than with OLM or AML monotherapy (42.5–51.0 vs. 26.4–36.3 %). Only 8.8 % of placebo recipients achieved BP goals [44]. During a 44-week, open-label extension of this trial [57], target BP goals were attained by 67 % of patients overall, 80 % of those who continued to receive OLM/AML 40 mg/5 mg, 71 % of those uptitrated to OLM/AML 40 mg/10 mg, and 67 and 46 % of those who required the addition of HCT 12.5 or 25 mg to achieve BP goals, respectively [10]. Similar results were shown in two 8-week trials in Chinese patients whose mild to moderate BP was not controlled with OLM 20 mg or AML 5 mg monotherapy [47]. Relative to monotherapy with OLM 40 mg and AML 5 mg, dual OLM/AML 20 mg/5 mg therapy significantly lowered both SBP and DBP at weeks 4 and 8 [47].
OLM/AML also reduced BP in patients with moderate to severe hypertension who had not responded to AML monotherapy [45]. Following 8 weeks of treatment with AML 5 mg monotherapy, patients who had not reached BP goals were randomized to 8 weeks’ treatment with AML 5 mg or OLM/AML 10 mg/5 mg, 20 mg/5 mg or 40 mg/5 mg, and then entered a final 8-week phase that included uptitration of OLM/AML dosages in patients who did not respond to treatment; responders continued the randomized treatment [45]. Compared with continuing AML 5 mg monotherapy, OLM/AML 20 mg/5 mg and 40 mg/5 mg for 8 weeks significantly (p < 0.0001) improved SBP/DBP (BGD 5.7/3.8 and 7.1/3.9 mmHg, respectively) [45], and the proportion of patients achieving BP goals (30 % with AML 5 mg vs. 54 and 51 % with OLM/AML 20 mg/5 mg and 40 mg/5 mg) [8]. During the uptitration phase, additional BP reductions were shown, with total reductions in SBP/DBP ranging from 22.3/13.9 mmHg (AML 5 mg uptitrated to OLM/AML 20 mg/5 mg) to 29.1/17.8 mmHg (OLM/AML 40 mg/5 mg uptitrated to OLM/AML 40 mg/10 mg) [45]. By the end of the trial, BP goals were met by >70 % of patients who received OLM/AML (±uptitration) [45].
OLM/AML reduced BP in patients with moderate to severe hypertension who had not reached BP goals after receiving OLM 20 mg monotherapy for 8 weeks, and were then randomized to receive OLM 20 mg or OLM/AML 20 mg/5 mg or 20 mg/10 mg for 8 weeks [46]. Compared with continuing OLM monotherapy, OLM/AML 20 mg/5 mg led to significantly (p ≤ 0.001) greater reductions in DBP and SBP (BGD 2.7 and 5.9 mmHg, respectively), and a greater proportion of patients who achieved BP control (44.5 vs. 28.5 % with OLM 20 mg) [46].
In an open-label titrate-to-goal trial in patients with uncontrolled BP despite antihypertensive monotherapy, a switch to OLM/AML 20 mg/5 mg was followed by uptitration every 4 weeks to OLM/AML 40 mg/5mg and 40 mg/10 mg, and finally the addition of HCT 12.5 or 25 mg, in patients who had not achieved BP goals. By week 12, 75.8 % of patients receiving OLM/AML (± HCT) had received SBP goals, with 90.3 % of patients achieving SBP/DBP goals by week 20 [48]. In a subgroup analysis of this trial, 72.7 and 76.9 % of patients previously treated with CCB or ARB, respectively, achieved SBP goals at week 12 of the OLM/AML titration regimen. In the groups previously treated with CCB or ARB monotherapy, decreases in SBP/DBP were dose-proportional (ranged from 9.9/5.8 and 13.9/7.6 mmHg, respectively, with OLM/AML 20 mg/5 mg to 21.8/11.6 and 26.2/−15.0 mmHg with OLM/AML 40 mg/10 mg + HCT 25 mg) and significant (all p < 0.0001 vs. baseline) [58].
OLM + AML + HCT triple therapy
Triple combination therapy with OLM/AML/HCT was effective in reducing BP in patients whose BP was not controlled by dual therapy in randomized, double-blind trials [59–62]. OLM/AML/HCT 40 mg/10 mg/25 mg was more effective in reducing BP than dual therapy with OLM/AML 40 mg/10 mg, OLM/HCT 40 mg/25 mg and AML/HCT 10 mg/25 mg in patients with moderate to severe hypertension in the 12-week TRINITY trial [59]. At weeks 6, 8, 10 and 12, significantly (p < 0.001) greater reductions in both DBP and SBP were seen in the triple-therapy group than in any of the three dual-therapy groups [59]. At week 12, significantly (p < 0.001) more patients in the triple therapy group than in any of the dual therapy groups achieved the following BP targets [59]:
-
SBP/DBP of <140/90 mmHg (69.9 vs. 41.1–53.4 %)
-
SBP/DBP of <120/80 mmHg (27.2 vs. 5.4–15.2 %)
-
SBP of <140 mmHg (73.6 vs. 51.3–58.8 %)
-
DBP of <90 mmHg (85.7 vs. 64.2–77.1 %).
According to subgroup analyses, triple therapy was more effective in reducing BP and achieving BP goals than each of the dual therapies, regardless of patients’ baseline hypertension severity [59], age [63], race [64, 65], weight [54, 66] or presence of comorbid diabetes [67], chronic kidney disease [67] or chronic cardiovascular disease [67]. Reductions in BP and increases in BP control were associated with improvements in measures of health-related quality of life [68].
In a large 10-week trial in patients with moderate-to-severe hypertension, the addition of HCT 12.5 or 25 mg to OLM/AML 20 mg/5 mg, 40 mg/5 mg or 40 mg/10 mg improved BP control [60]. DBP and SBP were significantly decreased, and the proportion of patients achieving BP goals was significantly increased, in all groups receiving triple therapy with OLM/AML/HCT compared with those receiving corresponding dual therapy with OLM/AML (all p ≤ 0.05). More than 70 % of patients in the OLM/AML/HCT 40 mg/5 mg/12.5 mg, 40 mg/5 mg/25 mg and 40 mg/10 mg/25 mg groups achieved the target SBP/DBP goal of <140/90 mmHg [60]. Likewise, during the 8-week double-blind phase of a trial in Korean patients with moderate hypertension that was not controlled with dual OLM/HCT 20 mg/12.5 mg treatment [61], switching to triple OLM/AML/HCT 20 mg/5 mg/12.5 mg treatment was associated with significant (p < 0.0001) reductions in BP relative to baseline and dual OLM/HCT therapy. The proportion of patients achieving BP goals was also significantly higher with triple than with dual therapy; for example, 65.3 versus 37.4 % of patients achieved both seating SBP and DBP goals [61].
In a trial in patients whose BP was inadequately controlled with OLM/AML 40mg /10 mg, the addition of HCT 12.5 or 25 mg significantly (p< 0.0001) reduced DBP by 2.8 mmHg, as well as significantly improving most other SBP- and DBP-related outcomes, including BP goal achievement rates [62].
OLM/AML/HCT provides effective long-term treatment for hypertension [69, 70]. In a 44-week open-label extension of an 8-week trial, patients who did not achieve their BP goal with OLM/AML 40 mg/10 mg added HCT 12.5 mg to their regimen, resulting in decreases in SBP and DBP of 7.7 and 4.5 mmHg. In patients whose BP remained uncontrolled, the HCT dose was increased to 25 mg, resulting in additional decreases in SBP and DBP of 9.9 and 6.0 mmHg [69]. After a total of up to 52 weeks of treatment, decreases from baseline in SBP/DBP were 34.8/21.2 mmHg in OLM/AML/HCT 40 mg/10 mg/12.5 mg recipients, and 36.1/19.8 mmHg in OLM/AML/HCT 40 mg/10 mg/25 recipients, and 66.6 and 46.3 % of patients in the respective treatment groups achieved their BP goal [69]. Likewise, at the end of an 40-week open-label extension of TRINITY, 44.5–79.8 % of OLM/AML/HCT recipients reached their BP goal, with SBP decreasing from 168.6 at baseline to 125.0–136.8 mmHg, and DBP decreasing from 100.7 at baseline to 77.8–82.5 mmHg, depending on treatment [70].
24-h control of blood pressure
The BP-lowering effects of antihypertensive treatment should be assessed over the entire dosing interval, in order to ensure adequate assessment of the efficacy of the treatment over the time between doses. As assessed by 24-h ambulatory BP monitoring (ABPM), once-daily OLM-based therapy provided 24 h of antihypertensive activity in trials in patients with hypertension [18, 38, 71–76]. Relative to placebo, OLM 5 or 20 mg for 8 weeks significantly (p < 0.0001) reduced 24-h DBP [BGD 9.6 and 12.2 mmHg, respectively] and 24-h SBP (BGD 14.5 and 16.5 mmHg, respectively) [18]. The 24-h duration of action of OLM on BP was confirmed using the placebo-subtracted trough-to-peak (TTP) ratio for DBP and SBP, as TTP ratios for both OLM regimens exceeded the level that indicates a once-daily drug is considered an effective antihypertensive agent (i.e. ≥50 % of the peak effect remained at the end of the 24-h BP assessment period) [18].
An OLM/HCT-based titration regimen also safely reduced BP throughout the 24-h dosing interval in patients with moderate to severe hypertension [77] and elderly patients (aged ≥65 years) [74], including subgroups of elderly patients with stage 1 or 2 hypertension or isolated systolic hypertension [75], as well as in patients with comorbid type 2 diabetes [38], regardless of age, race, sex or severity of hypertension [76].
In substudies of clinical trials of OLM + AML ± HCT therapy, OLM/AML 20 mg/5 mg, 40 mg/5 mg and 40/10 mg [48, 71], and/or OLM/AML/HCT 40 mg/10 mg/25 mg [48, 72] also effectively reduced BP over the 24-h dose interval in patients with hypertension [71, 72]. Importantly, subgroup analysis of 24-h APBM results in clinical trials also indicated that OLM-based therapy provided BP control throughout the full 24-h dosing period in high-risk patients with difficult-to-treat hypertension (i.e. Blacks, patients with obesity, stage 2 hypertension or type 2 diabetes) [73].
What is the tolerability profile of OLM-based treatment?
OLM-based therapy is generally well tolerated in patients with hypertension, with adverse events usually being of mild or moderate severity [5, 6, 9–14]. In general, the adverse events associated with OLM-based therapy are of a similar clinical nature to those associated with the individual components. The following are the most commonly reported treatment-emergent adverse events (TEAEs) associated with OLM-based therapy:
-
OLM monotherapy Headache, influenza-like symptoms and dizziness (7.7, 4.0 and 3.7 % of patients, respectively), with only dizziness being considered as being unequivocally related to treatment [5];
-
OLM/HCT dual therapy Headache, dizziness and fatigue (2.9, 1.9 and 1.0 % of patients) [9];
-
OLM/AML dual therapy Peripheral oedema, headache and dizziness (11.3, 5.3 and 4.5 % of patients) [11];
-
OLM/AML/HCT triple therapy Peripheral oedema, headache and dizziness (rates not reported) [13].
Table 2 presents a summary of the ‘common’ (i.e. reported in ≥1 to <10 % of patients) TEAEs associated with OLM-based mono-, dual and triple therapy, as reported in current EU summaries of product characteristics [5, 9, 11, 13]. None of the adverse events reported with any of the OLM-based formulations were considered to be ‘very common’ (i.e. reported in ≥10 % of patients).
Of note, although hypotension is ‘rare’ (i.e. reported in ≥0.01 to <0.1 % of patients) in the overall patient population, its incidence may increase to ‘uncommon’ (i.e. reported in ≥0.1 to <1.0 % of patients) in elderly patients receiving OLM [5]. Treatment with HCT may cause or exacerbate volume depletion (which may lead to electrolyte imbalance) [9], and single cases of rhabdomyolysis and extrapyramidal syndrome have been reported with ARBs and AML, respectively [13]. OLM does not prolong the corrected QT interval, according to the results of a thorough study evaluating the results of therapeutic and supratherapeutic doses of OLM on cardiac repolarization [78].
Peripheral oedema
Peripheral oedema (unrelated to congestive heart failure) is a well-recognized adverse event associated with CCBs, including AML [79]. The addition of drugs that cause venous vasodilation, such as the ARBs and ACE inhibitors, have the potential to compensate for increased capillary blood flow and pressure, thereby reducing peripheral oedema [79].
In a clinical trial of OLM/AML in patients with middle to severe hypertension, the incidence of peripheral oedema was specifically assessed in order to obtain a reliable estimate of the reduction in oedema that could be achieved by adding OLM to AML [15]. Peripheral oedema (generally of mild severity) was observed in 13.6 % of patients at baseline and 19.8 % during the trial. As anticipated, the rates of peripheral oedema were significantly (p < 0.05) higher in patients receiving AML 10 mg monotherapy (36.8 %) than in patients receiving OLM 20 or 40 mg + the equivalent dosage of AML (26.5 and 23.5 % in OLM/AML 20 mg/10 mg and 40 mg /10 mg recipients, respectively), The frequency was lowest in patients receiving OLM monotherapy (14.3, 9.9 and 18.5 % of OLM 10, 20 and 40 mg recipients, respectively), AML 5 mg as monotherapy (13.0 %) or in combination with OLM (20.9, 18.0 and 18.5 % of OLM/AML 10 mg/5 mg, 20 mg/5 mg and 40 mg/5 mg recipients) and placebo (12.3 %). Most cases of peripheral oedema were mild or moderate in severity; severe oedema occurred in 1.2 and 0.6 % of AML 10 mg and OLM/AML 40 mg/10 mg recipients, respectively [15].
What is the current clinical positioning of OLM-based treatment?
A key strategy for reducing the burden of hypertension-related cardiovascular diseases is the effective treatment of high BP; however, despite the large number of available antihypertensive agents, BP is often poorly controlled [80, 81]. The choice of antihypertensive regimen should be based on the needs and medical profile of the individuals and the characteristics and mechanism of action of the antihypertensive [1, 2]. The dosages of antihypertensive agents should be optimized and/or the number of antihypertensive treatments increased until the patient achieves his or her BP goal. BP can be reduced to a significantly greater extent when combinations of antihypertensive agents with complementary mechanisms of action are used than when antihypertensive monotherapy is used [1, 7]. Initial treatment with dual antihypertensive therapy is recommended in some patients, such as those with marked BP elevation or high/very high cardiovascular risk, and those with a low target BP goal [1].
OLM-based antihypertensive treatment is a valuable option in the treatment of patients with mild to severe hypertension, including those with hard-to-treat disease. As monotherapy, OLM provides BP control that is similar or better than that shown with monotherapy with other antihypertensives and is well tolerated. In patients who require treatment with two or more antihypertensive from different drug classes to achieved BP goals. the combination of a RAAS blocker (such as OLM) with a CCB (such as AML) and/or a thiazide diuretic (such as HCT) are a rational choice, as the drugs have complementary mechanisms of action, and provide effective and safe lowering of both SBP and DBP [80, 81]. In patients whose BP does not respond adequately to monotherapy with OLM, the addition of HCT improves BP control. Likewise, in patients who do not respond adequately to OLM or AML monotherapy, dual therapy with OLM/AML reduces BP and helps patients with mild to severe hypertension, including those with difficult-to-treat hypertension, to achieve BP goals. Of note, dual treatment with OLM/AML is approved as initial therapy in adults who are likely to need treatment with multiple antihypertensives to achieve BP control in the USA (Table 1) [12]. In patients requiring treatment with three antihypertensive drugs, triple therapy with OLM/AML/HCT effectively reduces BP, improves rates of BP control, and enhances health-related quality of life in patients whose BP was not controlled by dual therapy, regardless of baseline patient characteristics.
OLM-based treatment is generally well-tolerated, with tolerability and safety profiles that are consistent with those of the individual drugs. In particular, the incidence of peripheral oedema, an adverse event associated with CCBs, was significantly lower in patients receiving OLM/AML than in those receiving an equivalent AML dosage as monotherapy.
Importantly, the use of FDCs of two or three antihypertensives with different mechanisms of action may improve patient adherence and persistence to treatment. For example, on a study of single-, dual- or triple-pill antihypertensive regimens in a US clinical practice setting, decreased adherence and persistence was directly and significantly related to greater antihypertensive pill burden [8]. As the use of FDCs reduces pill burden, patients may be more likely to continue treatment, which ultimately improves clinical outcomes. Therefore, switching patients who are receiving separate tablets of OLM together with separate tablets of HCT and/or AML to treatment with FDC of OLM/HCT or OLM/AML will simplify the treatment schedule, which may improve patient compliance and clinical outcomes. Likewise, in patients who are receiving triple OLM-based therapy (i.e. OLM + AML + HCT as individual tablets, or as a FDC of OLM/HCT or OLM/AML + a separate tablet of AML or HCT), switching to the FDC of OLM/AML/HCT will simplify administration and may improve compliance and outcomes.
References
Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87.
Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
Wang YR, Alexander GC, Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167(2):141–7.
Erdine S. How do compliance, convenience, and tolerability affect blood pressure goal rates? Am J Cardiovasc Drugs. 2012;12(5):295–302.
Olmetec (olmesartan medoxomil) film-coated tablets: UK summary of product characteristics. Gerrards Cross; Daiichi Sankyo UK Limited; 2015.
Benicar (olmesartan medoxomil) tablets: US prescribing information. Parsippany (NJ): Daiichi Sankyo, Inc.; 2016.
Neutel JM. Prescribing patterns in hypertension: the emerging role of fixed-dose combinations for attaining BP goals in hypertensive patients. Curr Med Res Opin. 2008;24(8):2389–401.
Xie L, Frech-Tamas F, Marrett E, et al. A medication adherence and persistence comparison of hypertensive patients treated with single-, double- and triple-pill combination therapy. Curr Med Res Opin. 2014;30(12):2415–22.
Olmetec Plus (olmesartan medoxomil + hydrochlorothiazide) film-coated tablets: UK summary of product characteristics. Gerrards Cross; Daiichi Sankyo UK Limited; 2015.
Benicar HCT (olmesartan medoxomil + hydrochlorothiazide) tablets: US prescribing information. Parsippany (NJ): Daiichi Sankyo, Inc.; 2016.
Sevikar (olmesartan medoxomil + amlodipine besilate): UK summary of product characteristics. Gerrards Cross: Daiichi Sankyo UK Limited; 2015.
Azor (olmesartan medoxomil + amlodipine) tablets: US prescribing information. Parsippany (NJ): Daiichi Sankyo, Inc.; 2016.
Sevikar HCT (olmesartan medoxomil + amlodipine besilate + hydrochlorothiazide): UK summary of product characteristics. Gerrards Cross: Daiichi Sankyo UK Limited; 2015.
Tribenzor HCT (olmesartan medoxomil + amlodipine + hydrochlorothiazide) tablets: US prescribing information. Parsippany (NJ): Daiichi Sankyo, Inc.; 2016.
Mizuno M, Sada T, Ikeda M, et al. Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol. 1995;285(2):181–8.
Scott LJ, McCormack PL. Olmesartan medoxomil: a review of its use in the management of hypertension. Drugs. 2008;68(9):1239–72.
Brunner HR, Nussberger J. Relevance of clinical pharmacological models for the evaluation of therapeutic dose range of an AT1-receptor antagonist. J Hypertens. 2001;19(Suppl 1):15–20.
Neutel JM, Elliott WJ, Izzo JL Jr, et al. Antihypertensive efficacy of olmesartan medoxomil, a new angiotensin II receptor antagonist, as assessed by ambulatory blood pressure measurements. J Clin Hypertens. 2002;4(5):325–31.
Brunner HR, Stumpe KO, Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Invest. 2003;23(7):419–30.
Ball KJ, Williams PA, Stumpe KO. Relative efficacy of an angiotensin II antagonist compared with other antihypertensive agents: olmesartan medoxomil versus antihypertensives. J Hypertens. 2001;19(Suppl 1):49–56.
Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan and irbesartan in the control of essential hypertension. J Clin Hypertens. 2001;3(5):283–91.
Giles TD, Oparil S, Silfani TN, et al. Comparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertension. J Clin Hypertens. 2007;9(3):187–95.
Flack JM, Graff A, Li W, et al. Efficacy/safety of olmesartan medoxomil versus losartan potassium in patients by stage 1 or 2 hypertension. Postgrad Med. 2012;124(3):59–70.
Wang L, Jian-wei Zhao, Liu B, et al. Antihypertensive effects of olmesartan compared with other angiotensin receptor blockers. Am J Cardiovasc Drugs. 2012;12(5):335–44.
Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild-to-moderate hypertension. J Hum Hypertens. 2003;17(6):425–32.
Stumpe KO, Ludwig M. Antihypertensive efficacy of olmesartan compared with other antihypertensive drugs. J Hum Hypertens. 2002;16(Suppl 2):24–8.
Omboni S, Malacco E, Mallion JM, et al. Olmesartan vs ramipril in the treatment of hypertension and associated clinical conditions in the elderly: a reanalysis of two large double-blind, randomized studies at the light of the most recent blood pressure targets recommended by guidelines. Clin Interv Aging. 2015;10:1575–86.
Malacco E, Omboni S, Volpe M, et al. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens. 2010;28(11):2342–50.
Wang JG, Sun NL, Ke YN, et al. Long-term efficacy of olmesartan medoxomil in Chinese hypertensive patients as assessed by clinic, ambulatory and home blood pressure measurements. Clin Drug Investig. 2012;32(11):729–34.
Jiao Y, Ke Y, Sun N, et al. Reduction of the morning blood pressure surge treated with olmesartan in Chinese patients with mild to moderate essential hypertension: a multicenter, open-label, single treatment group clinical study. Eur Rev Med Pharmacol Sci. 2012;16(5):653–9.
Brunner HR. Olmesartan medoxomil: current status of its use in monotherapy. Vasc Health Risk Manag. 2006;2(4):327–40.
Kario K, Saito I, Kushiro T, et al. Effects of olmesartan-based treatment on masked, white-coat, poorly controlled, and well-controlled hypertension: HONEST study. J Clin Hypertens (Greenwich). 2014;16(6):442–50.
Hazan L, Hernandez Rodriguez OA, Bhorat AE, et al. A double-blind, dose-response study of the efficacy and safety of olmesartan medoxomil in children and adolescents with hypertension. Hypertension. 2010;55(6):1323–30.
Chrysant SG, Chavanu KJ, Xu J. Combination therapy with olmesartan medoxomil and hydrochlorothiazide: secondary analysis of the proportion of patients achieving recommended blood pressure goals from a randomized, double-blind, factorial study. Am J Cardiovasc Drugs. 2009;9(4):241–51.
Oparil S, Chrysant SG, Kereiakes D, et al. Results of an olmesartan medoxomil-based treatment regimen in hypertensive patients. J Clin Hypertens (Greenwich). 2008;10(12):911–21.
Chrysant SG, Weber MA, Wang AC, et al. Evaulation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens. 2004;17(3):252–9.
Kereiakes DJ, Maa J-F, Shojaee A, et al. Effects of an olmesartan medoxomil-based treatment algorithm of systolic blood pressure in patients with stage 1 or 2 hypertension. Am J Cardiovasc Drugs. 2010;20(4):239–46.
Neutel JM, Kereiakes DJ, Waverczak WF, et al. Effects of an olmesartan medoxomil based treatment algorithm on 24-hour blood pressure control in patients with hypertension and type 2 diabetes. Curr Med Res Opin. 2010;26(3):721–8.
Fogari R, Tadde S, Holm-Bentzen M, et al. Efficacy and safety of olmesartan medoxomil 40 mg/hydrochlorothiazide 12.5 mg combination therapy versus olmesartan medoxomil 40 mg monotherapy in patients with moderate to severe hypertension: a randomized, double-blind, parallel-group, multicentre, multinational, phase III study. Clin Drug Investig. 2010;30(9):581–97.
Rump LC, Girerd X, Sellin L, et al. Effects of high dose olmesartan medoxomil plus hydrochlorothiazide on blood pressure control in patients with grade 2 and grade 3 hypertension. J Hum Hypertens. 2011;25(9):565–74.
Barrios V, Boccanelli A, Ewald S, et al. Efficacy and tolerability of olmesartan medoxomil in patients with mild to moderate essential hypertension: the OLMEBEST study. Clin Drug Investig. 2007;27(8):545–58.
Rump LC, Ambrosioni E, Burnier M, et al. Initial combination therapy with olmesartan/hydrochlorothiazide in moderate-to-severe hypertension. J Hum Hypertens. 2006;20(4):299–301.
Kereiakes DJ, Neutel JM, Punzi HA, et al. Efficacy and safety of olmesartan medoxomil and hydrochlorothiazide compared with benazepril and amlodipine besylate. Am J Cardiovasc Drugs. 2007;7(5):361–72.
Chrysant SG, Melino M, Karki S, et al. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30(4):587–604.
Volpe M, Brommer P, Haag U, et al. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin Drug Invest. 2009;29(1):11–25.
Barrios V, Brommer P, Haag U, et al. Olmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009;29(7):427–39.
Zhu J-R, Zhang S-Y, Gao P-J. Efficacy and safety of olmesartan medoxomil/amlodipine fixed-dose combination for hypertensive patients uncontrolled with monotherapy. Arch Pharm Res. 2014;37(12):1588–98.
Weir MR, Hsueh WA, Nesbitt SD, et al. A titrate-to-goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed-dose combinations of amlodipine and olmesartan medoxomil ± hydrochlorothiazide. J Clin Hypertens (Greenwich). 2011;13(6):404–12.
Oparil S, Lee J, Karki S, et al. Subgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: evaluation by baseline hypertension stage and prior antihypertensive medication use. J Cardiovasc Pharmacol. 2009;54(5):427–36.
Schmieder RE, Böhm M. Efficacy and safety of olmesartan medoxomil plus amlodipine in age, gender and hypertension severity defined subgroups of hypertensive patients. J Hum Hypertens. 2011;25(6):354–63.
Chrysant SG, Lee J, Melino M, et al. Efficacy and tolerability of amlodipine plus olmesartan medoxomil in patients with difficult-to-treat hypertension. J Hum Hypertens. 2010;24(11):730–8.
Oparil S, Chrysant SG, Melino M, et al. Long-term efficacy of a combination of amlodipine and olmesartan medoxomil ± hydrochlorothiazide in patients with hypertension stratified by age, race and diabetes status: a substudy of the COACH trial. J Hum Hypertens. 2010;24(12):831–8.
Ding S, Liu J, Fu Q, et al. Clinical effects of combined olmesartan medoxomil and amlodipine on clinic and ambulatory blood pressure in elderly patients with resistant hypertension. Arch Gerontol Geriatr. 2013;57(3):423–7.
Hsueh WA, Shojaee A, Maa JF, et al. Efficacy of amlodipine/olmesartan medoxomil ± HCTZ in obese patients uncontrolled on antihypertensive monotherapy. Curr Med Res Opin. 2012;28(11):1809–18.
Nesbitt SD, Shojaee A, Maa JF, et al. Efficacy of an amlodipine/olmesartan treatment algorithm in patients with or without type 2 diabetes and hypertension (a secondary analysis of the BP-CRUSH study). J Hum Hypertens. 2013;27(7):445–52.
Nesbitt S, Shojaee A, Maa JF. Efficacy/safety of a fixed-dose amlodipine/olmesartan medoxomil-based treatment regimen in hypertensive blacks and non-blacks with uncontrolled BP on prior antihypertensive monotherapy. J Clin Hypertens (Greenwich). 2013;15(4):247–53.
Chrysant SG, Oparil S, Melino M, et al. Efficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens (Greenwich). 2009;11(9):475–82.
Neutel J, Shojaee A, Maa JF. Efficacy of amlodipine/olmesartan ± hydrochlorothiazide in patients uncontrolled on prior calcium channel blocker or angiotensin II receptor blocker monotherapy. Adv Ther. 2012;29(6):508–23.
Oparil S, Melino M, Lee J, et al. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32(7):1252–69.
Volpe M, Christian Rump L, Ammentorp B, et al. Efficacy and safety of triple antihypertensive therapy with the olmesartan/amlodipine/hydrochlorothiazide combination. Clin Drug Investig. 2012;32(10):649–64.
Sohn IS, Kim C-J, On B-H, et al. Efficacy and safety study of olmesartan medoxomil, amlodipine, and hydrochlorothiazide combination therapy in patients with hypertension not controlled with olmesartan medoxomil and hydrochlorothiazide combination therapy: results of a randomized, double-blind, multicenter trial. Am J Cardiovasc Drugs. 2016;16(2):129–38.
Rump LC, Ammentorp B, Laeis P, et al. Adding hydrochlorothiazide to olmesartan/amlodipine increases efficacy in patients with inadequate blood pressure control on dual-combination therapy. J Clin Hypertens (Greenwich). 2016;18(1):60–9.
Lewin AJ, Izzo JL Jr, Melino M, et al. Combined olmesartan, amlodipine, and hydrochlorothiazide therapy in randomized patients with hypertension: a subgroup analysis of the TRINITY study by age. Drugs Aging. 2013;30(7):549–60.
Chrysant SG, Littlejohn T III, Izzo JL Jr, et al. Triple combination therapy with olmesartan, amlodipine, and hydrochlorothiazide in Black and non-Black study participants with hypertension: the TRINITY randomized, double-blind 12-week, parallel-group study. Am J Cardiovasc Drugs. 2012;12(4):233–43.
Lewin AJ, Kereiakes DJ, Chrysant SG, et al. Triple-combination treatment with olmesartan medoxomil/amlodipine/hydrochlorothiazide in Hispanic/Latino patients with hypertension: the TRINITY study. Ethn Dis. 2014;24(1):41–7.
Roth EM, Oparil S, Melino M, et al. Olmesartan/amlodipine/hydrochlorothiazide in obese participants with hypertension: a TRINITY subanalysis. J Clin Hypertens (Greenwich). 2013;15(8):584–92.
Kereiakes DJ, Chrysant SG, Izzo JL Jr, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double-blind, parallel-group TRINITY study. Cardiovasc Diabetol. 2012;11:134.
da Silva M, Haag U, Guest JF, et al. Health-related quality of life impact of a triple combination of olmesartan medoxomil, amlodipine besylate and hydrochlorotiazide in subjects with hypertension. Health Qual Life Outcomes. 2015;13:24.
Chrysant SG, Oparil S, Melino M, et al. Efficacy and safety of longterm treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertension. 2009;11(9):475–82.
Kereiakes DJ, Chrysant SG, Izzo JL Jr, et al. Long-term efficacy and safety of triple-combination therapy with olmesartan medoxomil and amlodipine besylate and hydrochlorothiazide for hypertension. J Clin Hypertens (Greenwich). 2012;14(3):149–57.
Punzi H, Neutel JM, Kereiakes DJ, et al. Efficacy of amlodipine and olmesartan medoxomil in patients with hypertension: the AZOR Trial Evaluating Blood Pressure Reductions and Control (AZTEC) study. Ther Adv Cardiovasc Dis. 2010;4(4):209–21.
Izzo JL Jr, Chrysant SG, Kereiakes DJ, et al. 24-hour efficacy and safety of triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazide: the TRINITY ambulatory blood pressure substudy. J Clin Hypertens (Greenwich). 2011;13(12):873–80.
Chrysant SG, Germino FW, Neutel JM. Olmesartan medoxomil-based antihypertensive therapy evaluated by ambulatory blood pressure monitoring: efficacy in high-risk patient subgroups. Am J Cardiovasc Drugs. 2012;12(6):375–89.
Kerediakes DJ, Neutal J, Stoakes KA, et al. The effects of an olmesartan medoxomil-based treatment algorithm on 24-hour blood pressure levels in elderly patients aged 65 years and older. J Clin Hypertens. 2009;11(8):411–21.
Germino FW, Neutel JM, Dubiel R, et al. Efficacy of olmesartan medoxomil and hydrochlorothiazide fixed-dose combination therapy in patients aged 65 years and older with stage 1 and 2 hypertension or isolated systolic hypertension. Am J Cardiovasc. 1012;12(5):325–33.
Neutel JM, Kereiakes DJ, BENIFICIARY Investigators. An olmesartan-medoxomil-based treatment algorithm is effective in achieving 24-hour BP control in patients with type 2 diabetes mellitus, regardless of age, race, sex, or severity of hypertension: subgroup analysis of the BENIFICIARY study. Am J Cardiovasc Drugs. 2010;10(5):289–303.
Rosenbaum D, Girerd X. Olmesartan medoxomil combined with hydrochlorothiazide improves 24-hour blood pressure control in moderate-to-severe hypertension. Curr Med Res Opin. 2012;28(2):179–86.
Mendell J, Matsushima N, O’Reilly TE, et al. A thorough QTc study demonstrates that olmesartan medoxomil does not prolong the QTc interval. J Clin Pharmacol. 2016;56(4):484–91.
Epstein BJ, Vogel K, Palmer BF. Dihydropyridine calcium channel antagonists in the management of hypertension. Drugs. 2007;67(9):1309–27.
Volpe M, Tocci G. Rationale for triple fixed-dose combination therapy with an angiotensin II receptor blocker, a calcium channel blocker, and a thiazide diuretic. Vasc Health Risk Manag. 2012;8:371–80.
Chrysant SG. Single-pill triple-combination therapy: an alternative to multiple-drug treatment of hypertension. Postgrad Med. 2011;123(6):21–31.
Acknowledgments
The article was reviewed by: R. B. Shah, GMERS Medical College and Hospital, Gandhinagar, Gujarat, India; N. Yasmeen, Shadan College of Pharmacy, Hyderabad, India. During the peer review process, the manufacturer of OLM/OLM-based FDCs was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
K. A. Lyseng-Williamson is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Olmesartan medoxomil: a guide to its use as monotherapy or in fixed-dose combinations with amlodipine and/or hydrochlorothiazide. Drugs Ther Perspect 32, 369–380 (2016). https://doi.org/10.1007/s40267-016-0335-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0335-0