Abstract
Objectives: Angiotensin II type 1 receptor antagonists (angiotensin receptor blockers [ARBs]) have been shown to be effective and well tolerated in hypertensive patients. Olmesartan is the seventh angiotensin receptor blocker licensed by the US Food and Drug Administration. The aim of this meta-analysis was to determine the efficacy and tolerability of olmesartan medoxomil in comparison with other ARBs.
Data Sources: Reports of randomized controlled trials (RCTs) of olmesartan versus other ARBs were identified through a systematic search of PubMed (up to July 2010), EMBASE (1980 to July 2010), SinoMed (up to July 2010), and the Cochrane Central Register of Controlled Trials (Cochrane Library Issue 7,2010).
Review Methods: Pertinent studies were selected through extensive searches of PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and SinoMed. Two of the authors abstracted data from the identified studies independently. Criteria for inclusion in our meta-analyses were randomized clinical trials in which patients were receiving an ARB and outcome data for blood pressure reduction or the incidence of adverse events were available. Quantitative and qualitative analyses of data from all RCTs meeting the criteria were performed. Our meta-analysis was undertaken according to the Quality of Reporting Meta-analyses (QUOROM) statement.
Results: Twenty-two studies with data from 4892 patients were considered for analyses. Olmesartan provided greater diastolic blood pressure (DBP) and systolic blood pressure (SBP) reductions compared with losartan (DBP: 95% confidence interval [CI] 0.59, 2.62; SBP: 95% CI 0.46, 5.92). Olmesartan provided greater SBP reductions compared with valsartan (95% CI 0.29, 3.16). Similar blood pressure response rates and incidence of adverse events were found with losartan, valsartan, candesartan, and irbesartan.
Conclusion: Olmesartan provides better antihypertensive efficacy than losartan and valsartan and has no association with an increased risk of adverse events in comparison with losartan, valsartan, candesartan, and irbesartan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is considered to be the leading risk factor for death in the world, causing an estimated 7.5 million deaths per year (13% of all deaths).[1] Relationships between elevated blood pressure (BP) levels and cardiovascular disease, stroke, and renal failure have consistently been found.[2] Therefore, lowering BP is crucial in terms of prevention of end-organ damage for hypertensive patients.[3]
The renin-angiotensin-aldosterone system (RAAS) is a major modulator of BP and an important therapeutic target in the treatment of hypertension.[4] By binding to the angiotensin type 1 (AT1) receptor, angiotensin II type 1 receptor antagonists (angiotensin receptor blockers [ARBs]) that inhibit RAAS have been shown to be effective and well tolerated among the different therapeutic classes of antihypertensive agents.[5]
Olmesartan medoxomil is the seventh ARB licensed for the treatment of hypertension by the US Food and Drug Administration (FDA), in 2002.[6,7] Extensive clinical evidence has confirmed the antihypertensive efficacy and good tolerability profile of olmesartan.[5] However, the efficacy and safety of olmesartan in comparison with other sartans (e.g. losartan and valsartan) are controversial. For example, Giles et al.[8] reported that olmesartan was superior to valsartan in BP control. However, Fogari et al.[9] reported that valsartan was more effective for BP reduction than olmesartan. No systematic reviews have been undertaken to evaluate the benefit/risk profiles of olmesartan in comparison with other ARBs.
In order to determine whether olmesartan provides better efficacy for BP control and fewer adverse events (AEs) over other ARBs, we undertook this meta-analysis of all relevant randomized controlled trials (RCTs).
Methods
Searching
Reports of RCTs of olmesartan versus other ARBs were identified through a systematic search of PubMed (up to July 2010), EMBASE (1980 to July 2010), SinoMed (up to July 2010), and the Cochrane Central Register of Controlled Trials (Cochrane Library Issue 7, 2010). The terms used for keywords and text word searching included olmesartan, ARB, losartan, valsartan, irbesartan, candesartan, eprosartan, telmisartan, atacand, teveten, avapro, cozaar, benicar, micardis, and diovan, using Boolean operators and database-specific syntax. The reference lists of original researches, reviews, letters to the editor, and case reports were also scanned to identify those not yet included in the computerized databases. The search was performed without any language restriction.
Selection
Studies meeting the following selection criteria were included in this meta-analysis: (i) study design: prospective RCTs; (ii) population: patients with hypertension, with or without other diseases such as metabolic syndrome and chronic kidney disease; (iii) interventions: olmesartan versus other ARBs, used as monotherapy; (iv) dosing regimens: titration as needed from a starting dose of monotherapy; forced titration of the therapy; parallel-group comparisons of various doses as monotherapy; (v) outcome variables: at least one of either mean seated systolic BP (SBP) or diastolic BP (DBP) reduction; mean BP reduction over 24 hours; therapeutic BP response rates and adverse events including total adverse event rate, drug-related adverse event rates, and incidence of headache, dizziness and diarrhea. The eligibility of a trial to be included in the meta-analysis was determined by two authors (WL, ZJW) independently. All work was reviewed by another author (ZZ). Only the data from the primary series were included if the same group of patients were involved in different reports in order to avoid duplication.
Validity Assessment
Two authors (WL, ZJW) worked independently, using standard criteria (the adequacy of randomization, allocation concealment, blinding method, drop-out reports and follow-ups) to appraise each included article according to an adjusted quality scoring system based on the Jadad scale.[10] The quality scoring system followed was: (i) adequacy of randomization, coded as proper with detailed description of randomization (score 2), randomized but details not reported (score 1), or inappropriate randomization (score 0); (ii) allocation concealment, coded as properly used (score 2), unclear (score 1), or not used (score 0); (iii) blinding method, coded as double-blind (score 2), single-blind (score 1), or open-label or unclear (score 0); (iv) drop-outs and follow-ups, coded as data given (score 1) or data not given (score 0).[11]
Data Extraction
Two of the authors (WL, ZJW) abstracted data from the identified studies independently. Disagreements were resolved by discussion. The data were extracted from each study with a predesigned review form including: the authors of the selected study; the year of publication; the location of the trial; the design of the study (whether double-blind or single-blind, parallel or crossover); the duration of the study; the number of subjects; the patients’ age, sex, baseline SBP and DBP values, end-point SBP and DBP values, change from baseline in SBP and DBP, and therapeutic response rate of SBP and DBP. In addition, we retrieved the number or proportion of adverse events and withdrawals. Only the data associated with monotherapy were extracted if the patients received monotherapy followed by combination therapy if they were reported separately.
Study Characteristics
We attempted to identify and include all RCTs carried out to assess the effects and tolerance associated with the use of olmesartan as compared with other ARBs in hypertensive patients.
The primary antihypertensive efficacy variables were the reduction from baseline to end of treatment in clinic DBP and SBP. A secondary efficacy variable was the therapeutic response rate of DBP (DBP <90 mmHg and/or a reduction of ≥10 mmHg). We assessed the tolerability of olmesartan by considering the overall incidence of AEs and the drug-related incidence of AEs. The incidence of three specific AEs including headache, dizziness, and diarrhea was also evaluated.
We undertook a sensitivity analysis according to the scores of quality assessment based on the Jadad scale. We reanalyzed the results after excluding the studies that scored less than 4. In addition, we conducted additional analyses including only studies published in English.
Quantitative Data Synthesis
Our meta-analysis was undertaken according to the Quality of Reporting Meta-analyses (QUOROM) statement.[12] Not all of the trials reported all the outcomes of interest for our analysis. Separate meta-analyses including DBP reduction, SBP reduction, BP response rate and the incidence of total, drug-related or three specific adverse events were undertaken for each comparison and outcome. We undertook a chi-squared (χ2) test of heterogeneity and the I2 measure of inconsistency to assess the heterogeneity between trials. The indicators were calculated with a fixed-effect mode when I2 <50%, indicating no significant heterogeneity. If the test for heterogeneity showed I2 ≥50%, the analysis was redone with a random effects model. For continuous data, we used weighted mean difference (WMD) as effect size. For dichotomous data, we calculated the relative risk (RR) for each clinical event.
The data analysis was performed using the meta-analysis software Review Manager (Revman 5.0.2, Cochrane Collaboration, Oxford, England). When mean BP reduction and its standard deviation (SD) were not available, we computed them by using the methods given by the Cochrane handbook.[13] In addition, we created L’Abbé plots to analyze the degree of BP reduction with olmesartan in comparison with other ARBs. The scatter of the individual trials lay predominantly between the line of equality and the control axis, which suggests efficacy of olmesartan and other ARBs in BP reduction.
Meta-Regression
Meta-regression using STATA version 10.0 (Stata Corporation, College Station, TX, USA) was performed to determine whether specific characteristics could explain the heterogeneity among studies. We made a meta-regression to examine and test for between-group differences if the heterogeneity (I2) was more than 75% and more than ten trials were included. Co-variables included baseline age, sex, duration, study publication year, and DBP and SBP baseline levels. The p-value for explanatory variables being statistically significant was set as 0.05.[14]
Results
Trial Flow
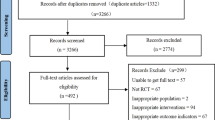
The search strategy generated 171 studies from PubMed, EMBASE, the Cochrane Library, and SinoMed. From all these studies, we identified 22 studies that met the inclusion criteria. The reasons for including the remaining 22 RCTs are listed in figure 1.
Study Characteristics
One hundred and fifty-one studies were excluded due to one of the following: inappropriate control group (n = 42), inappropriate outcomes (n = 50), duplicates (n = 16), not RCTs (n = 32), not monotherapy (n = 3), self-control design (n = 6), studies with no data on outcome variables (n = 2). We obtained the full text of all the 33 included relevant studies and evaluated them in detail. Twenty-two studies met the criteria for inclusion in this analysis. All the included studies used parallel designs. There were 14 double-blind trials,[6–8,15–25] two single-blind trials,[26,27] two open-label trials,[9,28] and four trials not mentioning the blinding methods.[29–32] Seven studies were multicenter RCTs,[7,8,15,17,20,24,25] one was not clear,[27] while the others were single-centered.[6,9,16,18,19,21–23,26,28–32] All the trials were described as randomized and only one trial[27] reported the generation of randomization. None mentioned allocation concealment. Ten trials[6,7,15,19,20,23,26,30–32] used elective titration of the dose; one[18] used forced titration dose and 11 trials[8,9,16,17,21,24,25,27–30] used no titration dose. All the trials reported the follow-up time, ranging from 2 weeks to 24 weeks, but one trial lasted 12 months.[27] Of all the studies, olmesartan was compared with losartan in 13 trials and with valsartan in nine trials. Both losartan and valsartan appeared in three studies.[8,17,18] We carried out a descriptive analysis of candesartan (three trials) or irbesartan (one trial) as there were insufficient data to undertake a meta-analysis. Both candesartan and irbesartan appeared in one trial.[17] Specific characteristics of each included article are listed in table I.
Dose Regimen
In our meta-analysis, the following doses were included: olmesartan 20 and and 20–40 mg, losartan 50 and 50–100 mg, valsartan 80 and 160 mg, candesartan 8 mg, and irbesartan 150 mg. Evaluated doses were all recommended in US, Japanese, and European product labels. Conlin et al.[33] compared the antihypertensive efficacy of losartan, valsartan, irbesartan, and candesartan in 2000. In that meta-analysis, the authors concluded that at recommended doses the four ARBs showed a near-flat dose response curve that suggested that monotherapy dose titration offered limited benefit.[33] In our meta-analysis, we combined fixed dose and dose titration into the same group.
Olmesartan versus Losartan
Efficacy
Twelve trials involving 2133 patients compared olmesartan with losartan in clinic BP reduction. There was a significant difference that favored olmesartan in DBP and SBP (DBP: WMD 1.61; 95% confidence interval [CI] 0.59, 2.62; random effects model, figure 2; SBP: WMD 3.19; 95% CI 0.46, 5.92; random effects model, figure 3). There were seven trials[6,7,15,19,22,26,31] involving 860 patients in response rate, and no significant difference was found between the two arms (RR 1.01; 95% CI 0.95, 1.07).
Tolerability
Ten trials were evaluated for the total incidence of adverse events with olmesartan compared with losartan. There was no significant difference between the two groups (RR 0.98; 95% CI 0.83, 1.15; figure 4). There was also no significant difference in the incidence of drug-related AEs, headache, dizziness, and diarrhea between the two groups (table II).
Meta-Regression
Twelve trials were involved and the heterogeneity (I2) was more than 75% in the meta-analysis of SBP reduction between olmesartan and losartan. We undertook a meta-regression according to study design to determine whether specific characteristics could explain the heterogeneity. The relative meta-regression analysis showed that the patients’ age and sex, duration of the study, number of patients included, publication year of the studies (table III), and BP baseline (figure 5) did not contribute to the heterogeneity.
Meta-regression plots of systolic blood pressure (SBP) reduction differences vs baseline blood pressure (BP) level. Circles represent the estimate from each study, sized according to the precision of each estimate. Fitted dashed lines represent the summary meta-regressions for SBP reduction outcome. The relationship showed no statistical significance for either SBP (p = 0.571) or diastolic BP (DBP) [p = 0.444]. See table I for reference citations. WMD = weighted mean difference.
Sensitivity Analysis
We reanalyzed the results after excluding the studies that scored less than 4 (table I). A significant difference that favored olmesartan still existed in DBP reduction (WMD 1.73; 95% CI 0.58, 2.88; random effects model). However, no significant difference was found in SBP reduction between the two arms (WMD 2.83; 95% CI −0.23, 5.89; random effects model). We also reanalyzed the results after the exclusion of non-English studies. A significant difference that favored olmesartan was found in both DBP and SBP reduction (DBP: WMD 3.06; 95% CI 2.39, 3.74; SBP: WMD 3.66; 95% CI 2.22, 5.09; fixed-effect model).
Olmesartan versus Valsartan
Efficacy
Nine trials involving 1595 patients compared olmesartan with valsartan in clinic BP reduction. There was a significant difference that favored olmesartan in SBP (WMD 1.72; 95% CI 0.29, 3.16; random effects model, figure 3). However, no significant difference was found in DBP reduction between the two arms (WMD 0.65; 95% CI −0.93, 2.22; random effects model, figure 2). There were three trials[6,29,30] involving 228 patients in response rate, and no significant difference was found between the two arms (RR 1.02; 95% CI 0.92, 1.13).
Tolerability
Six trials evaluated the total incidence of adverse events of olmesartan compared with valsartan. There was no significant difference between the two groups (RR 0.86; 95% CI 0.74, 1.01; figure 4). There was also no significant difference in the incidence of drug-related AEs, headache, dizziness, and diarrhea between the two groups (table II).
Sensitivity Analysis
We reanalyzed the results after excluding the studies with low scores (<4) according to table I. A significant difference that favored olmesartan was found in SBP reduction (WMD 2.53; 95% CI 1.15, 3.90; fixed-effect model). We also found a significant difference that favored olmesartan in DBP reduction (WMD 1.92; 95% CI 0.24, 3.59; random effects model). In addition, we reanalyzed the results after the exclusion of non-English studies. There was still a significant difference that favored olmesartan in SBP reduction (WMD 2.37; 95% CI 1.02, 3.73; fixed-effect model). No significant difference was found in DBP reduction (WMD 1.01; 95% CI −1.14, 3.16; random effects model).
Olmesartan versus Candesartan or Irbesartan
There was no significant difference in BP change between olmesartan and candesartan.[27] Olmesartan was superior to candesartan in mean BP reduction over 24 hours.[24] In addition, olmesartan demonstrated greater reductions in both DBP and SBP during the last 4 and 2 hours of the dosing interval.[24] Olmesartan produced a greater reduction in DBP, and a numerically greater but not statistically different SBP change was found in comparison with irbesartan.[17] Moreover, the magnitude of BP lowering with olmesartan was numerically greater than that with irbesartan over 24 hours without statistical significance.[17,25] No significant difference in total adverse events was found between olmesartan and candesartan[24] or irbesartan.[17,25]
L’Abbé Plots
Of all the studies, the detailed efficacy of DBP and SBP reduction between olmesartan and other ARBs (losartan, valsartan, candesartan, irbesartan) was presented in L’Abbé plots (figure 6). Seventy-seven percent of trials (17 of 22) lay under the line of equality in DBP reduction analysis. Seventy-three percent of trials (11 of 15) lay under the line of equality in SBP reduction analysis. Therefore, olmesartan was shown to provide better efficacy compared with other ARBs.
Discussion
Our meta-analysis examined the efficacy and tolerability of olmesartan, losartan, valsartan, candesartan, and irbesartan when administered at their recommended doses. Findings from this meta-analysis of 22 RCTs revealed that olmesartan provided superior BP-lowering efficacy compared with other ARBs. The evidence was sufficient to determine the better efficacy in SBP reduction when olmesartan was compared with losartan or valsartan. Available data indicated that olmesartan was more effective than losartan but as effective as valsartan in DBP reduction. ARBs are well known for having a placebo-like tolerability profile at all recommended dosages.[34] Olmesartan did not differ from losartan or valsartan with regard to the total incidence of adverse events. As indicated by the L’Abbé plots, most trials showed that olmesartan provided better efficacy compared with other ARBs (losartan, valsartan, candesartan, and irbesartan) in BP reduction. These effects can be partially explained by the substantially longer half-life of olmesartan than that of losartan or valsartan, since a longer half-life is associated with a longer duration of action.[17]
RAAS is an important mediator in the pathophysiology of hypertension. The excessive activity in the RAAS plays a key role in target end-organ damage, such as congestive heart failure, myocardial infarction, coronary artery disease, and end-stage renal disease.[5] Although there are other angiotensin peptides with biologic effects, angiotensin II is the major end-product of the system.[35] ARBs including olmesartan block the RAAS through the angiotensin (AT)1 receptor, effectively inhibiting the vasoconstrictor and aldosterone-secreting effects of angiotensin II.[5] Nevertheless, it is still unclear which ARB is preferred in clinical use.[36] It is necessary to determine which ARB is the best agent with a better efficacy for BP control and smaller incidence of adverse events. We undertook this meta-analysis to evaluate the efficacy and tolerability of olmesartan compared with other ARBs. However, we did not include eprosartan or telmisartan in the analyses due to a lack of RCTs with appropriate outcomes.
Previously, one review had evaluated the available literature qualitatively to determine whether all ARBs have equivalent efficacy and tolerability in the treatment of hypertensive patients.[5] Olmesartan provided better antihypertensive efficacy than losartan, valsartan, candesartan, and irbesartan in that review. Several RCTs have also evaluated the antihypertensive efficacy of olmesartan compared with other ARBs. However, our meta-analysis, which combines data across studies to make a quantitative evaluation, had more robust evidence for supporting that conclusion. A previous meta-analysis that compared valsartan with other ARBs in the treatment of hypertension[37] reported that valsartan and olmesartan demonstrated comparable efficacy across dosing ranges. However, five more RCTs were included in our meta-analysis for a total of 803 patients. Our results showed that olmesartan provided better antihypertensive efficacy in SBP reduction.
Our meta-analysis is the first to focus on the superiority of olmesartan over other ARBs with regard to efficacy and tolerability. We used a wide range of clinically relevant variables to evaluate the efficacy and tolerability of its BP reduction. In addition, we undertook L’Abbé plots to evaluate the efficacy visually. For the factors that could potentially influence the results and generate heterogeneity such as patients’ age and sex, duration of study, number of patients, and baseline BP, we performed a meta-regression to examine whether these specific baseline characteristics could explain the heterogeneity among studies.
Our meta-analysis also has some limitations. Due to the lack of head-to-head RCTs, it was difficult to perform a meta-analysis to evaluate the efficacy and tolerability of olmesartan compared with irbesartan or candesartan. Only a small number of RCTs had investigated the ability of ARBs to control 24-hour BP, therefore we could not undertake a meta-analysis of 24-hour BP lowering, especially early morning change, which has been shown to be associated with increased rates of cardiovascular events.[25] The quality of the studies also varied. Some of the included studies were poorly reported, with seven trials scoring less than 4 on the adjusted Jadad score system. Dose regimens also varied.
Pragmatic well-designed RCTs for future research are required. Specifically, further well-designed RCTs should include larger sample sizes and focus on more secondary endpoints such as 24-hour BP control, cardiac-cerebrovascular events, and adverse events. In particular, it seems important to determine how well olmesartan works in patients who fail to respond adequately to other sartans, and whether olmesartan is associated with the long-term reduction of cardiovascular disease morbidity and mortality. More studies should be conducted to determine the differences between olmesartan and other ARBs besides losartan or valsartan.
Conclusion
Olmesartan provides better antihypertensive efficacy in comparison with losartan and valsartan. With regard to the incidence of adverse events, olmesartan shows similar tolerability compared with other ARBs (losartan, valsartan, candesartan, and irbesartan). Therefore, olmesartan is a suitable treatment choice for controlling high BP.
References
Wilkins Campbell NR, Joffres MR, et al. Blood pressure in Canadian adults. Health Rep 2010 Mar; 21 (1): 37–46
Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 2007 Aug 18; 370 (9587): 591–603
Theodoratou D, Maniadakis N, Fragoulakis V, et al. Analysis of published economic evaluations of angiotensin receptor blockers. Hellenic J Cardiol 2009 Mar–Apr; 50 (2): 105–18
Catanzaro DF, Frishman WH. Angiotensin receptor blockers for management of hypertension. South Med J 2010 Jul; 103 (7): 669–73
Scott LJ, McCormack PL. Olmesartan medoxomil: a review of its use in the management of hypertension. Drugs 2008; 68 (9): 1239–72
Zhang YW, Ding R, Wu ZG. The effects and safety of mild and moderate primary hypertension treated with olmesartan medoxomil. World Clinical Drugs 2006; 27 (10): 585–8
Jing S, Sun NL, Ke YN. Effects and safety of mild and moderate primary hypertension treated with olmesartan medoxomil tablet. Chin J Clin Pharmaco 2006; 22 (1): 3–6
Giles TD, Oparil S, Silfani TN, et al. Comparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertension. J Clin Hypertens (Greenwich) 2007 Mar; 9 (3): 187–95
Fogari R, Zoppi A, Mugellini A, et al. Hydrochlorothiazide added to valsartan is more effective than when added to olmesartan in reducing blood pressure in moderately hypertensive patients inadequately controlled by monotherapy. Adv Ther 2006 Sep–Oct; 23 (5): 680–95
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996 Feb; 17 (1): 1–12
Zou Z, Xu F, Wang L, et al. Antihypertensive and renoprotective effects of trandolapril/verapamil combination: a meta-analysis of randomized controlled trials. J Hum Hypertens 2010; 25 (3): 203–10
Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999 Nov 27; 354 (9193): 1896–900
Higgins JPT Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.0.2. September 2009. The Cochrane Collaboration, 2009
Lv Y, Zou Z, Chen G, et al. Amlodipine and angiotensin-converting enzyme inhibitor combination versus amlodipine monotherapy in hypertension: a meta-analysis of randomized controlled trials. Blood Press Monit 2010; 15 (4): 195–204
Hu YR, Chen SX, Zhang J. Efficacy and safety of domestic olmesartan in treatment of mild to moderate essential hypertension. J Shanghai Jiaotong Univ (Medical Science) 2009; 29 (11): 1359–62
He CZ, Lei XG, Zeng ZY. Efficacy of domestic olmesartan in treatment of mild to moderate essential hypertension. Guangxi Med Univ 2008; 25 (6): 922–3
Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich) 2001 Sep–Oct; 3 (5): 283–91, 318
Giles T, Oparil S. Comparison of ascending doses of olmesartan medoxomil (o), losartan potassium (l) and valsartan (v) in patients (pts) with essential hypertension (htn). Am J Hypertens 2005; 18 (5): A56–60
Kong Y, Chu SL, Du J. Efficacy and safety of olmesartan medoxomil in patients with mild to moderate essential hypertension. J Shanghai Jiaotong Univ (Medical Science) 2008; 28 (2): 180–2
Zhu JR, Cai NS, Fan WH, et al. Efficacy and safety of olmesartan medoxomil versus losartan potassium in Chinese patients with mild to moderate essential hypertension [in Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi 2006 Oct; 34 (10): 877–81
Hao TL, Zhang YZ. Effects of Olmesartan M edoxomil in Patients with Primary Hypertension and Left Ventricular Hypertrophy. Journal of Medical Forum 2010; 31 (1): 12–4, 7
Liu XF. Effects of mild and moderate primary hypertension treated with olmesartan. China modern doctor 2009; 47 (18): 228–31
Xi L, Tian F. Antihypertensive efficacy and safety of olmesartan monotherapy in isolated systolic hypertension. Chinese journal of integrative medicine on cardio-/cerebrovascular disease 2009; 7 (12): 1391–2
Brunner HR, Arakawa K. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil in achieving 24-hour blood pressure reductions and ambulatory blood pressure goals. Clin Drug Investig 2006; 26 (4): 185–93
Smith DH, Dubiel R, Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs 2005; 5 (1): 41–50
He RM, Luo XY, Cao JM, et al. Antihypertensive efficacy and safety of domestic olmesartan monotherapy in mild or moderate essential hypertension. Shanghai Med J 2007; 30 (4): 245–51
Tsutamoto T, Nishiyama K, Yamaji M, et al. Comparison of the long-term effects of candesartan and olmesartan on plasma angiotensin II and left ventricular mass index in patients with hypertension. Hypertens Res 2010 Feb; 33 (2): 118–22
Destro M, Scabrosetti R, Vanasia A, et al. Comparative efficacy of valsartan and olmesartan in mild-to-moderate hypertension: results of 24-hour ambulatory blood pressure monitoring. Adv Ther 2005 Jan–Feb; 22 (1): 32–43
Li W, Chen XL, Guan SM. Clinical efficacy and safety of olmesartan medoxomil in patients with mild to moderate primary hypertension. J Clin Cardiol (China) 2008; 24 (10): 737–9
Li W, Chen XL, Guan SM. Efficacy and safety of olmesartan and valsartan in young and middle-aged patients. Clin Med China 2009; 25 (7): 704–5
Chen ZH, Gu Y, Liu QH, et al. Efficacy and safety of olmesartan medoxomil tablet in patients with mild to moderate primary hypertension. Chin J New Drugs Clin Rem 2007; 26 (6): 440–2
Zhang JH, Chen SC, Zhang SZ. Effect and safety of olmesartan medoxomil on treatment of essential hypertension. China Pharmacist 2008; 11 (11): 1354–5
Conlin Pr Fau-Spence JD, Spence Jd Fau-Williams B, Williams B, Fau-Ribeiro AB, Ribeiro Ab Fau-Saito I, Saito I Fau-Benedict C, Benedict C Fau-Bunt AM, et al. Angiotensin II antagonists for hypertension: are there differences in efficacy? Am J Hypertens 2000; 13 (4 Pt 1): 418–26
Ross S, Akhras K, Zhang S, et al. Discontinuation of antihypertensive drugs due to adverse events: a systematic review and meta-analysis. Pharmacotherapy 2001; 21: 940–53
Burnier M. Angiotensin II type 1 receptor blockers. Circulation 2001 Feb 13; 103 (6): 904–12
Smith DH. Comparison of angiotensin II type 1 receptor antagonists in the treatment of essential hypertension. Drugs 2008; 68 (9): 1207–25
Nixon RM, Muller E, Lowy A, et al. Valsartan vs. other angiotensin II receptor blockers in the treatment of hypertension: a meta-analytical approach. Int J Clin Pract 2009 May; 63 (5): 766–75
Acknowledgments
This study was supported by the National Nature Science Foundation of China (81000525) fund. The study was conducted, analyzed, and interpreted by the authors independently of all sponsors.
Drs Long Wang and Jian-wei Zhao contributed equally to this work.
Conflicts of interest: The authors have no conflicts of interest directly relevant to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Zhao, Jw., Liu, B. et al. Antihypertensive Effects of Olmesartan Compared with Other Angiotensin Receptor Blockers. Am J Cardiovasc Drugs 12, 335–344 (2012). https://doi.org/10.1007/BF03261842
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261842