Abstract
Background
Although awareness of hypertension in Black patients has increased, blood pressure (BP) is frequently inadequately controlled.
Objective
This prespecified subgroup analysis of the TRINITY study evaluated the efficacy and safety of olmesartan medoxomil (OM) 40 mg, amlodipine besylate (AML) 10 mg, and hydrochlorothiazide (HCTZ) 25 mg triple-combination treatment compared with the component dual-combination treatments in Black and non-Black study participants.
Study Design
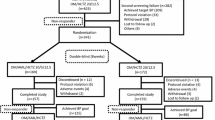
TRINITY was a 12-week, randomized, double-blind, parallel-group evaluation. The first patient was enrolled in May 2008 and the last patient completed the study in February 2009. The study consisted of a 3-week washout period for participants receiving antihypertensive therapy and a 12-week double-blind treatment period. For the treatment phase, all study participants were stratified by age, race, and diabetes mellitus status and randomized to a treatment sequence that led to their final treatment assignment, which they received from weeks 4 to 12 (OM 40 mg/AML 10 mg/HCTZ 25 mg, OM 40 mg/AML 10 mg, OM 40 mg/HCTZ 25 mg, or AML 10 mg/HCTZ 25 mg). In the first 2 weeks of the double-blind treatment period, all participants received either dual-combination treatment or placebo. Participants assigned to dual-combination treatment continued treatment until week 4, and participants receiving placebo were switched at week 2 to receive one of the dual-combination treatments until week 4. At week 4, participants either continued dual-combination treatment or randomly received triple-combination treatment until week 12.
Setting
317 clinical sites in the USA and Puerto Rico were included in the study.
Patients
Study participants eligible for randomization (N = 2492) were ≥18 years of age with mean seated blood pressure (SeBP) ≥140/100 mmHg or ≥160/90 mmHg (off antihypertensive medication).
Intervention
The intervention was with dual- or triple-combination antihypertensive treatment: OM 40 mg/AML 10 mg/HCTZ 25 mg, OM 40 mg/AML 10 mg, OM 40 mg/HCTZ 25 mg, or AML 10 mg/HCTZ 25 mg.
Main Outcome Measure
The primary efficacy variable was the change in least squares (LS) mean seated diastolic BP (SeDBP) from baseline to week 12. Secondary efficacy variables included the LS mean change in seated systolic BP (SeSBP), percentage of study participants reaching BP goal, and safety parameters.
>Results
In both Black and non-Black participants, triple-combination treatment resulted in significant and similar mean reductions in SeDBP and SeSBP (p≤0.0001 vs each dual-combination treatment) with a greater proportion of participants reaching BP goal compared with dual-combination treatments, regardless of race. Most treatment-emergent adverse events were mild or moderate in severity and no new safety concerns were identified.
Conclusion
Triple-combination treatment provided greater BP reductions than dual-combination treatments regardless of race.
Clinical Trial Registration
Registered at ClinicalTrials.gov as NCT00649389.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension control (blood pressure [BP] <140/90 mmHg) in the USA has improved from 27.3% in 1988–1994 to 50.1% in 2007–2008.[1] However, this improvement has not occurred uniformly across demographic groups, and important racial disparities in hypertension awareness, treatment, and control rates continue to exist.[1] Black patients have a greater prevalence of hypertension and develop hypertension earlier in life compared with non-Blacks.[1–8] Black patients also have higher average BP, a disproportionate prevalence of severe hypertension, and significantly more co-morbid conditions, including diabetes mellitus, albuminuria, and chronic kidney disease.[3–7,9] Although awareness of hypertension in Black patients has increased,[2] BP is frequently inadequately controlled over the long term.[10]
In Black patients, the combination of earlier onset, more severe disease, greater co-morbidities, and inadequate treatment leads to excessive target organ damage and mortality risk.[3–5,10–13] In 2005, the death rate from hypertension was 3.4-fold greater in Black men and 2.7-fold greater in Black women compared with White men and women.[5,14] Furthermore, approximately 30% of deaths in Black men and 20% of deaths in Black women with hypertension may be directly attributable to hypertension.[5]
Monotherapy is inadequate to control BP in most patients with hypertension, regardless of race, and combination therapy with two or more antihypertensive agents is required.[12] Thus, the recent International Society on Hypertension in Blacks (ISHIB) consensus statement places major emphasis on the use of effective combination therapy.[5]
TRINITY (Triple Therapy with Olmesartan Medoxomil, Amlodipine, and Hydrochlorothiazide in Hypertensive Patients Study) [registered at ClinicalTrials.gov as NCT00649389] demonstrated that the combination of olmesartan medoxomil (OM) 40 mg/amlodipine besylate (AML) 10 mg/hydrochlorothiazide (HCTZ) 25 mg reduced both mean seated diastolic BP (SeDBP) and mean seated systolic BP (SeSBP) to a significantly greater extent than the component dual-combination treatments (OM 40 mg/AML 10 mg, OM 40 mg/HCTZ 25 mg, or AML 10 mg/HCTZ 25 mg).[15] The objective of this prespecified subgroup analysis from the TRINITY study was to evaluate the efficacy and safety of the triple-combination treatment compared with the component dual-combination treatments in Black and non-Black study participants.
Methods
Study Design and Participants
The methods for the TRINITY study have previously been described in detail.[15] TRINITY was a 12-week, randomized, double-blind, parallel-group evaluation conducted at 317 clinical sites in the USA and Puerto Rico. Study participants eligible for randomization were ≥18 years of age with mean seated blood pressure (SeBP) ≥140/100 mmHg or ≥160/90 mmHg (off antihypertensive medication). Individuals with a history of recent (≤6 months) cardiovascular or cerebrovascular events (including coronary artery disease, stroke, and myocardial infarction), New York Heart Association class III or IV congestive heart failure, secondary hypertension, symptomatic resting bradycardia, heart block greater than first-degree atrioventricular block, and chronic atrial fibrillation or flutter, severe renal insufficiency (defined as creatinine clearance <30 mL/min), or uncontrolled diabetes (defined as glycosylated hemoglobin [HbA1c] >9%) were excluded. However, participants with stable type 1 or type 2 diabetes controlled with insulin, diet, or oral hypoglycemic agents for at least 30 days prior to screening were eligible to participate.
The study protocol, amendment, and informed consent form were approved by the appropriate and applicable institutional review boards. The study was conducted in compliance with ethical principles that have their origin in the Declarations of Helsinki and in accordance with the International Conference on Harmonization E6 Guideline for Good Clinical Practice (GCP), United States Food and Drug Administration (FDA) GCP guidelines, and US Code of Federal Regulations Title 21, Parts 50, 54, 56, and 312. All study participants provided written informed consent prior to participation in any study procedures.
TRINITY consisted of a 3-week washout phase for study participants receiving antihypertensive therapy (period I) followed by a 12-week double-blind treatment phase (period II).[15] For the treatment phase, all study participants were stratified by age, race, and diabetes status and randomized to a treatment sequence that led to their final treatment assignment, which they received from weeks 4 to 12 (OM 40 mg/AML 10 mg/HCTZ 25 mg [given as OM 40 mg/HCTZ 25 mg fixed-dose combination plus AML 10 mg given separately], OM 40 mg/AML 10 mg [fixed-dose combination], OM 40 mg/HCTZ 25 mg [fixed-dose combination], or AML 10 mg/HCTZ 25 mg [given separately]). In the first 2 weeks of the double-blind treatment period, all participants were randomized to receive dual-combination treatment or placebo. All participants assigned to dual-combination treatment continued that treatment until week 4, and those participants receiving placebo were switched at week 2 to receive one of the dual-combination treatments until week 4. At week 4, participants either continued dual-combination treatment or were switched to triple-combination treatment (OM 40 mg/AML 10 mg/HCTZ 25 mg) until week 12.[15] The distribution of patients to the 12 assigned treatment sequences at randomization was managed by an interactive voice response system (IVRS) to provide the four final treatment groups. Study participants were also instructed to take their medications at the same time each day (±2 hours). Participants were instructed to schedule their study visits as such to allow for a trough BP measurement to be taken 24 (±2) hours after the normal dosing time. Participants were instructed to refrain from taking their daily dose of study medication on the morning of clinic visits until after their clinic visit was completed. Participants who inadvertently took study medication on the morning of the clinic visit were rescheduled to return for that clinic visit within 3 days. Investigators and study participants were blinded as to which medication was being administered at any given time.
Outcomes of Interest
For the current prespecified subgroup analysis, data from study participants were grouped and analyzed based on the participant’s self-classified race. Participants selecting multiple races including Black on the enrollment form were classified as Black for this evaluation. The primary efficacy variable was the least squares (LS) mean change from baseline in SeDBP at week 12. Secondary efficacy variables included: LS mean change from baseline in SeSBP at week 12; mean changes from baseline in SeDBP and SeSBP at weeks 6, 8, and 10; proportion of study participants reaching BP goal (<140/90 mmHg or <130/80 mmHg in study participants with diabetes, chronic kidney disease, or chronic cardiovascular disease) at week 12; and mean change from baseline in SeDBP and SeSBP in study participants with severe hypertension (defined as SeSBP ≥180 mmHg or SeDBP ≥110 mmHg at baseline; post hoc analysis). Safety assessments included adverse events, physical examinations, clinical laboratory assessments, and 12-lead electrocardiograms. The severity of adverse events (AEs) (mild, moderate, or severe) and their causality were assessed based on the judgment of the study investigators.
Statistical Analysis
The primary efficacy analysis included all randomized study participants who received at least one dose of study medication and who had both a baseline and at least one post-dose assessment of SeDBP. The primary safety population for the assessment of adverse events was defined as study participants who received at least one dose of study medication at or beyond the week 4 visit, which was the first time study participants randomized to the triple-combination treatment group received this treatment.
Two-sided p-values for testing the significance of the triple-combination treatment against each dual-combination treatment were derived from an analysis of covariance (ANCOVA) model with baseline BP as a covariate as well as final randomized treatment, subgroup (e.g. race subgroup), and final randomized treatment by subgroup interaction as fixed effects. The LS mean difference and standard error (SE), derived from the ANCOVA model, were used to calculate the change from baseline to week 12 in SeBP. With the exception of change from baseline in SeBP at weeks 6, 8, and 10, the last observation carried forward (LOCF) approach was used for missing efficacy measurements during double-blind treatment. Comparisons between triple-combination treatment and each dual-combination treatment were performed using Fisher’s exact test at a 0.05 significance level.
The proportion of study participants reaching BP goal at week 12 (LOCF) in the two race subgroups was summarized and analyzed using chi-squared (χ2) and Fisher’s exact tests with a p-value <0.05 considered significant. Comparison of the efficacy of the various treatments between the two race subgroups (Black vs non-Black) was not carried out, as the study was not designed for this evaluation. The TRINITY study was powered to assess treatment efficacy in the overall study population. Sample size was determined assuming 97% power for each of the three pairwise comparisons of interest so that the desired overall power of 90% could be achieved.
Results
Study Participant Characteristics
Of the 2492 study participants randomized in the TRINITY study, 757 (30.4%) were Black, with a larger proportion of Blacks having severe hypertension compared with non-Blacks. Table I summarizes demographic and clinical characteristics of Black and non-Black participants by treatment group.
Efficacy
The triple-combination treatment resulted in significantly greater reductions at week 12 in LS mean SeDBP (p ≤ 0.0001) and LS mean SeSBP (p < 0.0001) compared with each of the component dual-combination treatments in both race subgroups (prespecified subgroup analysis) [figure 1]. Overall, the triple-combination treatment reduced LS mean SeBP by −37.1/20.8 mmHg in Black and −38.9/21.8 mmHg in non-Black study participants at week 12 compared with LS mean SeBP reductions of −30.7/17.0, −28.9/14.9, and −29.0/14.5 mmHg in Black and −31.5/18.1, −31.9/17.4, and −28.6/14.8 mmHg in non-Black participants receiving OM 40 mg/AML 10 mg, OM 40 mg/HCTZ 25 mg, and AML 10 mg/HCTZ 25 mg, respectively. All four treatment groups significantly reduced SeDBP and SeSBP at week 12 relative to baseline for both race subgroups (p < 0.0001 for all treatment groups). Furthermore, triple-combination treatment resulted in greater mean reductions in SeBP in both race subgroups at weeks 6, 8, 10, and 12 compared with each of the dual-combination treatments. Mean SeSBP and SeDBP for all treatment groups at these time points are provided in figure 2.
Least squares (LS) mean reductions in seated diastolic BP (SeDBP; primary efficacy variable) and seated systolic BP (SeSBP) at week 12 (last observation carried forward) by treatment and race. Error bars depict standard error (SE) of BP change. = amlodipine besylate; BP = blood pressure; HCTZ = hydrochlorothiazide; OM = olmesartan medoxomil. * p < 0.0001 vs baseline; † p ≤ 0.0001 vs each dual-combination treatment for each race subgroup.
Mean () seated systolic BP (SeSBP) and (b) seated diastolic BP (SeDBP) from baseline to week 12 by treatment and race. Week 4 is the first week participants received triple-combination treatment; prior to week 4, study participants randomized to triple-combination treatment were taking one of the three dual-combination treatments. AML = amlodipine besylate; BP = blood pressure; HCTZ = hydrochlorothiazide; OM = olmesartan medoxomil.
A significantly greater percentage of both Black and non-Black study participants (61.5% and 65.5%, respectively) randomized to triple-combination treatment reached BP goal (<140/90 mmHg or <130/80 mmHg in those with diabetes, chronic kidney disease, or chronic cardiovascular disease) at week 12 compared with participants randomized to each dual-combination treatment (prespecified subgroup analysis) [figure 3].
Proportion of study participants achieving seated BP (SeBP) goal at week 12 (last observation carried forward) by treatment and race. SeBP goal was defined as <140/90 mmHg (<130/80 mmHg in study participants with diabetes mellitus, chronic kidney disease, or chronic cardiovascular disease). = amlodipine besylate; BP = blood pressure; HCTZ = hydrochlorothiazide; OM = olmesartan medoxomil. * p ≤ 0.0009 vs each dual-combination treatment for each race subgroup.
The efficacy of triple-combination treatment was maintained in Black and non-Black study participants with severe hypertension (SeSBP ≥180 mmHg or SeDBP ≥110 mmHg; post hoc analysis). Compared with the dual-combination treatments, triple-combination treatment resulted in greater mean reductions in SeBP from baseline to week 12 in Black and non-Black participants with severe hypertension (figure 4). Furthermore, the BP-lowering effect of all treatments in both race subgroups was proportional to the severity of hypertension. In this subset of participants with severe hypertension, 39.6% of Black and 45.5% of non-Black participants receiving triple-combination treatment achieved BP goal by week 12 compared with 22.8–27.8% of Black and 13.5–27.6% of non-Black participants randomized to dual-combination treatment (data not shown). Due to a higher baseline BP, a lower proportion of these participants reached BP goal compared with Black and non-Black participants without severe hypertension.
Mean reductions in seated diastolic BP (SeDBP) and seated systolic BP (SeSBP) at week 12 (last observation carried forward) by treatment and race in study participants with severe hypertension at baseline ( analysis) [severe hypertension was defined as SeSBP ≥180 mmHg or SeDBP ≥110 mmHg]. AML = amlodipine besylate; BP = blood pressure; HCTZ = hydrochlorothiazide; OM = olmesartan medoxomil.
Safety
No new safety concerns were identified for either the triple- or dual-combination treatments that were not already known to occur with the individual component therapies. Treatment-emergent adverse events (TEAEs) occurred in 366 (52.0%) Black and 921 (57.6%) non-Black study participants (table II). Most TEAEs were mild or moderate in severity. In total, 10 (1.4%) Black and 25 (1.6%) non-Black participants had a serious adverse event. The severity of adverse events did not appear to differ across treatment groups. Overall, 15 (2.1%) Black and 37 (2.3%) non-Black participants discontinued the study due to a TEAE.
Across all treatment groups, the most common TEAEs (≥5.0%) experienced by Black study participants were headache (7.7%) and dizziness (5.8%) and the most common TEAEs experienced by non-Black study participants were dizziness (7.5%), peripheral edema (7.2%), fatigue (6.1%), and headache (6.0%). For those receiving triple-combination treatment, the most common TEAEs were dizziness (Black: 6.6%; non-Black: 11.3%), headache (Black: 5.4%; non-Black: 6.9%), nasopharyngitis (Black: 4.2%; non-Black: 3.2%), fatigue (Black: 3.6%; non-Black: 4.4%), and peripheral edema (Black: 3.0%; non-Black: 9.6%).
Discussion
This prespecified subgroup analysis of the TRINITY study demonstrates the effectiveness and safety of OM 40 mg/AML 10 mg/HCTZ 25 mg triple-combination treatment in Black and non-Black study participants with hypertension. Compared with the component dual-combination treatments, triple-combination treatment resulted in significantly greater reductions in mean SeDBP and SeSBP in both race subgroups. This efficacy was observed within 2 weeks of switching from dual- to triple-combination treatment and was similar in both race subgroups. This effect was greatest in participants with severe hypertension, regardless of treatment or race subgroup.
Triple-combination treatment reduced mean SeBP by approximately −44/22 mmHg and −50/24 mmHg in Black and non-Black participants with severe hypertension, respectively. As a result, significantly more Black and non-Black participants reached BP goal by week 12 with triple-combination treatment compared with any of the dual-combination treatments.
Triple-combination treatment was well tolerated in both race subgroups. The incidence of TEAEs was similar in Black (52%) and non-Black (58%) participants and study discontinuation was low in both race subgroups.
Black patients bear an excess burden of cardiovascular disease and overall, rates of non-fatal and fatal stroke, death due to heart disease, and end-stage kidney disease are higher by 1.3-fold, 1.8-fold, 1.5-fold, and 4.2-fold, respectively, in Blacks compared with Whites.[7] A recent analysis of data from the National Health and Nutrition Examination Survey (NHANES) demonstrates that clinical cardiovascular disease, including heart failure, stroke, and myocardial infarction, develops at a much earlier age (30s, 40s, and 50s) in Blacks compared with Whites.[16] As a result, 28% of cardiovascular deaths in Blacks occur in individuals <65 years of age compared with only 13% of cardiovascular deaths in Whites.[16] This excess early cardiovascular mortality risk in Blacks is greater than previously reported.[16,17]
Current guidelines from the JNC 7 report recommend a BP goal of <140/90 mmHg or <130/80 mmHg in patients with co-morbidities that increase cardiovascular risk such as chronic kidney disease and diabetes.[18] A scientific statement from the American Heart Association recommends a BP goal of <130/80 mmHg in patients with high coronary artery disease risk.[19] In addition, the JNC 7 guidelines recommend that initial antihypertensive treatment with two drugs should be considered when BP is >20/10 mmHg above the desired goal.[18] Given the increased risk of hypertension in Blacks, the ISHIB recently published a consensus statement proposing even lower thresholds in Black patients.[5] Specifically, ISHIB recommends a BP goal of <135/85 mmHg for primary prevention and a BP goal of <130/80 mmHg for secondary prevention (preexisting end-organ damage, preclinical cardiovascular disease, or cardiovascular disease) in Black patients with hypertension, as well as the use of combination therapy when SBP is >15 mmHg and/or DBP is >10 mmHg above these goals. Based on these recommendations, most Black patients with hypertension will require combination therapy to achieve BP goal.[20]
Clinical trial data demonstrate that most Black patients can attain their BP goal if properly treated. Blacks comprised 35% of the 33 357 study participants in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial), in which 63.6% of Black men and 58.8% of Black women achieved a BP goal of <140/90 mmHg at year 3, with 22.4% of Black men and 17.3% of Black women requiring ≥3 agents.[21,22] Moreover, most of the participants who reached this goal did so within the first 6 months of the study, with 52.6% and 47.1% of Black men and women having controlled BP, respectively, at 6 months.[22]
Poor adherence with antihypertensive treatment, either due to the complexity of the treatment regimen or adverse effects, is an important barrier to effective BP control and may have a greater impact on Black compared with White patients.[11,23] Increased adherence to antihypertensive agents has been shown to significantly decrease the risk of cardiovascular events in patients with hypertension, with resultant reductions in both hospitalizations and total health care costs.[24] Non-adherence to prescribed therapy is common, especially in Black patients,[23–25] and improving adherence should help patients achieve BP goals and diminish cardiovascular risk. Using fixed-dose combinations as opposed to prescribing the same drugs separately (free-drug combinations) decreases the complexity of the therapeutic regimen, potentially improving adherence. In separate meta-analyses of hypertension trials, patients receiving fixed-dose combinations had 24–29% greater adherence or persistence with their therapeutic regimen compared with patients receiving free-drug combinations.[26,27]
Combination therapy increases the probability of achieving BP goals in high-risk patients who have complicated or severe hypertension.[28,29] Although most study participants, including Blacks, in large clinical trials achieve BP control, attaining and maintaining BP within the target range in these trials typically requires the use of ≥2 antihypertensive agents.[5,22,30–33]
Using a combination of antihypertensive agents with complementary mechanisms of action can improve BP control and decrease the incidence of adverse events.[34–38] For example, a recent meta-analysis of data from 147 randomized trials found that a combination of three drugs at half the standard dose produced approximately 2-fold greater reductions in the risk of coronary heart disease and stroke than single-drug treatment at the standard dose.[39] Prompt control of BP within 1–3 months has a beneficial effect on clinical outcomes, particularly in patients with more severe hypertension or high cardiovascular risk;[30,31,40,41] thus, early intervention resulting in effective BP control may delay or prevent the long-term complications of hypertension.[23,31,42,43]
There are certain limitations to this study. While the evaluation of the Black and non-Black subgroups was prespecified, a statistical evaluation between these subgroups was not intended because of the unequal proportion of participants in the subgroups and treatment arms (approximately 30% of the population was Black). However, the number of Black participants in this study exceeded the demographic proportion representative of the general population. Also, participants with symptomatic heart disease and poorly controlled diabetes were excluded from participation; therefore, caution must be exercised regarding the generalizability of these data to the overall population.
Conclusion
In this prespecified subgroup analysis from the TRINITY study, triple-combination treatment with OM 40 mg/AML 10 mg/HCTZ 25 mg resulted in a significantly greater reduction in SeBP and significantly better attainment of BP goals in both Black and non-Black study participants compared with the component dual-combination treatments. Mean reductions in SeBP were similar in both race subgroups and were greatest in the subset of Black and non-Black participants with severe hypertension. The efficacy of triple- compared with dual-combination treatment was maintained regardless of race, and triple-combination treatment was well tolerated by all participants.
References
Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303 (20): 2043–50
Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008; 52 (5): 818–27
Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol 2007; 6 (2): 67–71
Ferdinand KC. Management of high blood pressure in African Americans and the 2010 ISHIB consensus statement: meeting an unmet need. J Clin Hypertens (Greenwich) 2010; 12 (4): 237–9
Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56 (5): 780–800
Kola LD, Sumaili EK, Krzesinski JM. How to treat hypertension in blacks: review of the evidence. Acta Clin Belg 2009; 64 (6): 466–76
Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics —2012 update: a report from the American Heart Association. Circulation 2012; 125 (1): 188–97
Yoon SS, Ostchega Y, Louis T. Recent trends in the prevalence of high blood pressure and its treatment and control, 1999–2008 [NCHS data brief, no. 48]. National Center for Health Statistics [online]. Available from URL: http://www.cdc.gov/nchs/data/databriefs/db48.pdf [Accessed 2011 Apr 25]
Ferdinand KC. Coronary artery disease in minority racial and ethnic groups in the United States. Am J Cardiol 2006; 97 (2A): 12A–9A
Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics: 2010 update. A report from the American Heart Association. Circulation 2010; 121 (7): 948–54
Douglas JG, Ferdinand KC, Bakris GL, et al. Barriers to blood pressure control in African Americans: overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112 (4): 51–62
Jamerson KA, Basile J. Prompt, aggressive BP lowering in high-risk patients. J Clin Hypertens (Greenwich) 2008; 10 (1 Suppl. 1): 40–8
Levine RS, Foster JE, Fullilove RE, et al. Black-white inequalities in mortality and life expectancy, 1933–1999: implications for healthy people 2010. Public Health Rep 2001; 116 (5): 474–83
Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290 (21): 2805–16
Oparil S, Melino M, Lee J, et al. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther 2010; 32 (7): 1252–69
Jolly S, Vittinghoff E, Chattopadhyay A, et al. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am J Med 2010; 123 (9): 811–8
Mohtashemi M, Levins R. Qualitative analysis of the all-cause black-white mortality crossover. Bull Math Biol 2002; 64 (1): 147–73
Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289 (19): 2560–72
Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007; 115 (21): 2761–88
Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med 2003; 163 (5): 525–41
Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288 (23): 2981–97
Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4 (6): 393–404
Moulton SA. Hypertension in African Americans and its related chronic diseases. J Cult Divers 2009; 16 (4): 165–70
Dragomir A, Cote R, Roy L, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care 2010; 48 (5): 418–25
Gerber BS, Cho YI, Arozullah AM, et al. Racial differences in medication adherence: A cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother 2010; 8 (2): 136–45
Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007; 120 (8): 713–9
Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension 2010; 55 (2): 399–407
Ferdinand KC, Ferdinand DP. Race-based therapy for hypertension: possible benefits and potential pitfalls. Expert Rev Cardiovasc Ther 2008; 6 (10): 1357–66
Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27 (11): 2121–58
Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366 (9489): 895–906
Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363 (9426): 2022–31
Ferdinand KC, Saunders E. Hypertension-related morbidity and mortality in African Americans: why we need to do better. J Clin Hypertens (Greenwich) 2006; 8 (1 Suppl. 1): 21–30
Wright Jr JT, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002; 288 (19): 2421–31
Bakris GL, Weir MR. Achieving goal blood pressure in patients with type 2 diabetes: conventional versus fixed-dose combination approaches. J Clin Hypertens (Greenwich) 2003; 5 (3): 202–9
Chalmers J, Joshi R, Kengne AP, et al. Efficacy and safety of fixed combination of perindopril and indapamide in type 2 diabetes: results from ADVANCE in context of available evidence. J Hypertens 2008; 26 (3 Suppl.): S21–7
Fogari R, Preti P, Zoppi A, et al. Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients. Am J Hypertens 2002; 15 (12): 1042–9
Oparil S, Pimenta E. Efficacy of an olmesartan medoxomil-based treatment algorithm in patients stratified by age, race, or sex. J Clin Hypertens (Greenwich) 2010; 12 (1): 3–13
Tobe S, Kawecka-Jaszcz K, Zannad F, et al. Amlodipine added to quinapril vs quinapril alone for the treatment of hypertension in diabetes: the Amlodipine in Diabetes (ANDI) trial. J Clin Hypertens (Greenwich) 2007; 9 (2): 120–7
Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338: b1665
Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21 (5): 875–86
Weir MR, Zappe D, Orloski LA, et al. How early should blood pressure control be achieved for optimal cardiovascular outcomes? J Hum Hypertens 2011; 25 (4): 211–7
Staessen JA, Thijs L, Fagard R, et al. Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens 2004; 22 (4): 847–57
Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet 2004; 363 (9426): 2049–51
Acknowledgments and Disclosures
Research funds for this study were provided by Daiichi Sankyo, Inc., Parsippany, NJ, USA. The trial was designed by Daiichi Sankyo, Inc. in conjunction with the investigators. All authors contributed to the study design; data analysis/interpretation; drafting, critical revision, and approval of the manuscript. Medpace, Inc. (Cincinnati, OH, USA), a contract research organization, performed project management, data management, clinical and safety monitoring, and statistical analysis in conjunction with Daiichi Sankyo, Inc. Editorial support for this article was provided by Vrinda Mahajan, PharmD, of Peloton Advantage, LLC, Parsippany, NJ, funded by Daiichi Sankyo, Inc. The opinions expressed in the current article are those of the authors. The authors received no honoraria/fees for service or other form of financial support related to the development of this article.
Steven G. Chrysant, MD, has served as a consultant and speakers bureau member for and received grant/research support and honoraria from Daiichi Sankyo, Inc. Joseph L. Izzo, MD, Jr, has served as a consultant or investigator for Daiichi Sankyo, Inc., Boehringer-Ingelheim, Novartis Pharmaceuticals, GlaxoSmithKline, Takeda Pharmaceuticals, and Forest Laboratories. Dean J. Kereiakes, MD, reports no disclosure information. Suzanne Oparil, MD, has received grant/research support from Merck & Co., Medtronic, Novartis, and Takeda, and has served as a consultant for Bayer, Daiichi Sankyo, Inc, Medtronic, Novartis, and Pfizer Inc. Michael Melino, PhD, James Lee, PhD, and Victor Fernandez, BS, are employees of Daiichi Sankyo, Inc. Reinilde Heyrman, MD, is a former employee of Daiichi Sankyo, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are saddened to report the passing in March 2011 of Thomas Littlejohn, III, MD, esteemed physician, investigator, colleague, and co-author of posters and publications from the TRINITY study. His contributions to this manuscript were invaluable.
Rights and permissions
About this article
Cite this article
Chrysant, S.G., Littlejohn, T., Izzo, J.L. et al. Triple-Combination Therapy with Olmesartan, Amlodipine, and Hydrochlorothiazide in Black and Non-Black Study Participants with Hypertension. Am J Cardiovasc Drugs 12, 233–243 (2012). https://doi.org/10.1007/BF03261832
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261832