Abstract
Myelodysplastic syndromes (MDS) are typical diseases of the elderly, with a median age of 68–75 years at initial diagnosis. Demographic changes producing an increased proportion of elderly in our societies mean the incidence of MDS will rise dramatically. Considering the increasing number of treatment options, ranging from best supportive care to hematopoietic stem cell transplantation (HSCT), decision making is rather complex in this cohort of patients. Moreover, aspects of the aging process also have to be considered in therapy planning. Treatment of elderly MDS patients is dependent on the patient’s individual risk and prognosis. Comorbidities play an essential role as predictors of survival and therapy tolerance. Age-adjusted models and the use of geriatric assessment scores are described as a basis for individualized treatment algorithms. Specific treatment recommendations for the different groups of patients are given. Currently available therapeutic agents, including supportive care, erythropoiesis-stimulating agents (ESAs), immune-modulating agents, hypomethylating agents, and HSCT are described in detail and discussed with a special focus on elderly MDS patients. The inclusion of elderly patients in clinical trials is of utmost importance to obtain data on efficacy and safety in this particular group of patients. Endpoints relevant for the elderly should be integrated, including maintenance of quality of life and functional activities as well as evaluation of use of healthcare resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Myelodysplastic syndromes (MDS) are typical diseases of elderly people. |
Selecting the appropriate treatment for senior MDS patients is complex because of the heterogeneity of elderly individuals and the increasing number of available treatment options. |
Age-adjusted models and geriatric assessment may be helpful in the design of individualized treatment algorithms. |
1 Epidemiology of Myelodysplastic Syndromes (MDS) in Senior Patients
Myelodysplastic syndromes (MDS) are hematopoietic stem cell disorders characterized by ineffective hematopoiesis resulting in cytopenia and the symptoms thereof. Moreover, MDS carry an inherent risk of transformation to acute myeloid leukemia (AML) [1]. MDS are a typical disease of the elderly, displaying a median age of 68–75 years at diagnosis [2–4]. Whereas the total annual incidence of MDS is about four cases per 100,000 per year, this rate increases dramatically with advancing age to approximately 40 at the age of ≥70 years [5] and to 50 at ≥80 years [4]. Recent studies suggest a continuously increasing incidence of MDS over the last decades, which might be based on raised awareness as well as easier access to referral centers [6]. Importantly, with the aging of societies, the incidence of MDS will increase over the coming decades. In addition, the number of therapy-related MDS (t-MDS) following chemo- or radiotherapy of a primary tumor will increase in the future with the growing number of cancer survivors.
2 The Prevalence and Clinical Relevance of Cytopenias in Elderly
2.1 Anemia
Various cytopenias are a common finding in older individuals. Anemia is the most frequent condition, displaying a mean prevalence of 17 % in individuals aged ≥65 years, as defined by the World Health Organization (WHO) (hemoglobin [Hb] <120 g/L in women and <130 g/L in men [7]). Prevalence of anemia ranges from 12 % in community-dwelling individuals to 47 % in those living in nursing homes [8]. The prevalence rate increases with advanced age, reaching 31 % in individuals aged ≥80 years and 37 % in those aged ≥90 years [9], with men being more frequently affected [10]. Anemia is significantly correlated with increased mortality and hospitalization [11, 12] as well as impaired cognition and limited performance in activities of daily living (ADL) [13, 14]. An estimated 5.0–8.5 % of anemic patients [9, 15, 16] and 17 % of those with unexplained anemia [10] are suspected to suffer from as yet undiagnosed MDS. In light of the profound clinical impact of anemia in the elderly, a thorough diagnostic workup is essential to detect treatable nutritional deficiencies and MDS.
2.2 Thrombocytopenia
Data on the prevalence of thrombocytopenia in the general population are rare. A systematic review by Hui et al. [17] reported prevalence rates between 8 and 68 %. The wide discrepancy most likely resulted from the different thresholds applied in different studies. About 43 % of MDS patients have a platelet count <100 × 109/L, and 7 % of such patients have a count of <20 × 109/L. Importantly, low platelet counts are significantly associated with shortened survival [18].
2.3 Neutropenia
Neutropenia, defined as a neutrophil count <1.5 × 109/L, has a mean prevalence of 1.2 % in a US general population [19]. In lower-risk MDS patients, neutropenia was observed in 7 % [20], and the prevalence of severe infection, defined as sepsis, was increased to 22.5 versus 6.1 % in a reference population [21].
3 Diagnosis of MDS
The diagnosis of MDS is mainly based on the morphologic examination of cells from peripheral blood and bone marrow. A bone marrow biopsy is strongly recommended, as it gives useful information about cellularity, the percentage and localization of CD34+ cells, and the degree of fibrosis [22]. Recommendations on the diagnostic workup and the relevance of laboratory parameters in MDS diagnosis have been summarized recently [22]. Chromosomal abnormalities as assessed by banding analysis are crucial for diagnosis and risk classification. Clonal cytogenetic abnormalities are observed in 50–80 % of MDS patients [23] and are the most relevant prognostic factor defined so far, both in the revised International Prognostic Scoring System (IPSS) IPSS-R [24] and the revised WHO classification-based prognostic scoring system (WPSS-R) [25]. In cases of insufficient bone marrow aspirate or a lack of metaphases, fluorescence in situ hybridization (FISH) can provide cytogenetic information [26]. Other additional tests, like flow cytometry analysis [27] or detection of acquired mutations by single nucleotide polymorphism (SNP) array karyotyping or whole genome sequencing, may provide additional information if MDS is the suspected diagnosis. The routine use of these techniques is not recommended so far.
The term ‘idiopathic cytopenia of unknown significance (ICUS) has been introduced to classify cases that do not completely fulfill the criteria for diagnosis of MDS but reveal unexplained cytopenia (Hb < 110 g/L, granulocytes <1.5 × 109/L, platelets <100 × 109/L) [28]. The category ‘idiopathic dysplasia of unknown significance’ (IDUS) has been introduced for patients who are characterized by pronounced cellular dysplasia but lack marked cytopenia. Despite ICUS and IDUS being useful categories in the classification of cytopenias and dysplasias in the elderly in daily practice, these terms have not been used in the WHO classification [29] so far.
4 Classification
Classification of MDS is based on the WHO classification that was updated in 2008 [29] (Table 1). WHO subtypes characterized by multi-lineage dysplasia or high blast counts are characterized by an unfavorable outcome [22]. Therapy-related myeloid neoplasm, including MDS (t-MDS), AML (t-AML), and myelodysplastic/myeloproliferative neoplasms (t-MDS/MPN) are summarized in a distinct subgroup as they represent a unique clinical syndrome. Another subcategory of MDS/MPN includes chronic myelomonocytic leukemia (CMML) and MDS/MPN unclassifiable.
5 Risk Scoring
Prognostic scores provide information on estimated survival times and the risk of progression to AML and thus form the basis for individualized treatment algorithms. The IPSS was developed in 1997 and divides patients into four risk groups with significant differences in overall and progression-free survival [30]. The revised IPSS (IPSS-R) is currently the gold standard in prognostication (Fig. 1) [24]. One of the major achievements of IPSS-R is the refined classification of cytogenetic risk groups and the fact that cytogenetics now play a more relevant role than the other parameters [31]. In addition, thresholds for bone marrow blasts and the classification of cytopenia have been improved, resulting in five prognostic risk groups. An online tool for calculating the score is available at http://www.ipss-r.com. IPSS-R maintains its prognostic significance even at advanced age. Age has a major impact on survival, and this is more pronounced in lower-risk than in higher-risk patients (Fig. 1) [24]. Thus, age has been incorporated as a prognostic parameter, resulting in IPSS-RA (IPSS-R age). In daily practice and in clinical studies, IPSS-low, IPSS-int-1, IPSS-R very low, IPSS-R low, and IPSS-R intermediate are summarized as ‘lower-risk MDS’, whereas IPSS int-2, IPSS high, IPSS-R high, and IPSS-R very high constitute the ‘higher-risk MDS’ group.
The revised International Prognostic Scoring System [24]. BM bone marrow, Hb hemoglobin, IPSS-R Revised International Prognostic Scoring System
The WPSS is another well-established predictive score [32]. Included predictive parameters are the WHO subtypes, cytogenetic abnormalities according to IPSS, and red blood cell (RBC) transfusion requirements or hemoglobin levels [33]. The advantage of the WPSS is that it was developed for dynamic use at any time during the course of the disease. An improved version of the WPSS was recently published that incorporates the IPSS-R cytogenetic risk groups [25].
6 Age-Adjusted Models and Geriatric Assessment in MDS Patients
Age-adjusted life expectancy forms the basis for decision making in a given patient. Using survival data from local registries, the remaining life expectancy can be estimated and integrated in decision models [34]. Thus, life expectancy in MDS patients can be predicted by means of well-established prognostic scores like IPSS-R and compared with age- and sex-matched reference populations. In this way, it has been unequivocally shown that a majority of elderly MDS patients lose a considerable number of life-years [2]. However, patient subgroups with an excellent prognosis may be defined and reveal an outcome that is not inferior to that of reference populations [32]. Thus, comparisons of MDS- and age-related life expectancies are useful in the design of patient-orientated therapeutic decisions, ranging from a watch-and-wait strategy to a more aggressive disease-modifying therapy.
To date, prognostication in MDS has relied mainly on disease-related factors like cytogenetics or the percentage of blasts [24, 25, 30, 32]. The integration of patient-related parameters, including comorbidities, physical activities, or nutritional status has just begun. Several studies have analyzed the impact of comorbidities in MDS. Structured comorbidity scores have been applied in most studies, with the Charlson Comorbidity Index (CCI) and the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) being the most frequently used. Overall, comorbidities have been shown to display a high prevalence, with one comorbidity in 25 % and two or more comorbidities in 24 % of cases. The highest frequencies were detected for cardiovascular disease (28 %), diabetes (12 %), and prior tumors (10 %) [35]. Importantly, comorbidities have been shown to negatively impact the overall survival of MDS patients [35–42] and have thus been integrated in combined prognostic scores [38, 39]. Comorbidities have been found to increase the risk of non-leukemic death in lower-risk MDS patients, whereas the prognostic impact of the biology of the disease is of higher impact in higher-risk MDS patients. Comorbidities are relevant in higher-risk patients, as they impact clinical outcome by decreasing eligibility for stem cell transplantation (SCT) and tolerance of chemotherapy regimens [22]. Assessment of comorbidities using a validated score should thus be part of therapy planning and management of MDS patients.
The integration of geriatric assessment (GA) scores as a means of defining biological age and prognosis and predicting vulnerability and tolerance to therapy in MDS has only just begun. GA is a systematic procedure that employs validated tools to evaluate distinct dimensions like functional capacities (ADL [43], Instrumental Activities of Daily Living [44]), objective physical activities (Timed Up and Go [45]), mood (Mini-Mental State Examination [46]), or nutritional status (Mini Nutritional Assessment [47] and G8 [48, 49]). However, data on GA in MDS are rare to date [50]. Promising results concerning the prognostic impact of distinct parameters, namely function, mood, and cognition in MDS, on clinical outcome have been published by Deschler et al. [51]. Furthermore, evaluation by GA may be helpful in predicting toxicity and completion of chemotherapy [52] and in identifying targets for improving treatment tolerance [53]. Using the available evidence, an algorithm was proposed to assign patients to one of the three GA risk categories (fit, vulnerable, frail) [54, 55]. This concept enables clinicians to individually tailor treatment approaches (Table 2).
The individual needs and wishes of the elderly patient population should be integrated in future studies, as expectations and treatment goals may differ. Patient-reported outcomes (PROs), including health-related quality of life (HR-QOL) [56], the improvement and preservation of autonomy and functional capacities [57], and the use of healthcare resources, should be integrated as innovative and relevant clinical endpoints to improve personalized decision making in MDS in the future.
7 Treatment Options in Lower-Risk MDS Patients
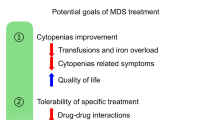
The goal of therapy in lower-risk MDS is to treat cytopenias and improve quality of life (Fig. 2). Although the choice of available drugs has expanded considerably, the number of drugs approved for MDS is limited thus far (Table 3).
Treatment options in senior lower-risk myelodysplastic syndrome patients (low to intermediate-1 IPSS; very low, low to intermediate IPSS-R). ATG anti-thymocyte globulin, CyA cyclosporin A, EMA European Medicines Agency, EPO erythropoietin, ESA erythropoiesis-stimulating agent, G-CSF granulocyte-colony stimulating factor, HLA human leukocyte antigen, HMA hypomethylating agent, IPSS-R revised International Prognostic Scoring System, MDS myelodysplastic syndrome, RBC red blood cell, SCT stem-cell transplant. Number 1 indicates no EMA approval in this indication, number 2 indicates no FDA or EMA approval in this indication
7.1 Red Blood Cell Transfusions and Iron Chelation
Anemia treatment in MDS is of major relevance, as some 90 % of all MDS patients suffer from anemia [58] and about half of them need RBC transfusions at initial diagnosis [4]. Increased RBC transfusion requirements are associated with dismal prognosis [22]. A recent retrospective study showed that lower hemoglobin levels were associated with reduced survival [33]. The main goal of RBC transfusions is to improve quality of life and avoid anemia-related symptoms. Therefore, specific thresholds for RBC transfusions cannot be recommended. An individual decision must be made for each patient. Hemoglobin levels of 80 g/L can be used as a benchmark. In the presence of comorbidities worsened by anemia, poor functional tolerance, or poor quality of life in still very active elderly patients, a threshold of even 90 g/L or 100 g/L can be used. The goal is to increase the hemoglobin level to >100–110 g/L [59]. RBC transfusions should be kept to a minimum to avoid possible deleterious effects, including iron overload [60].
Iron overload may occur as a consequence of regular RBC transfusions. It has been shown that patients who received at least 70 RBC concentrates had a higher heart iron overload in magnetic resonance imaging (MRI) and an increased risk for heart failure [61]. Retrospective studies suggest that iron chelation therapy can improve survival [62] and even achieve hematologic responses [63]. However, no prospective data are available to date. Recommendations suggest that chelation therapy should be started in transfusion-dependent patients who need more than two RBC concentrates per month or who have stable serum ferritin levels of >1000–2000 ng/ml and a life expectancy of more than 2 years. Chelation is also proposed in patients already suffering from organopathy resulting from iron overload, which further reduces life expectancy [64]. Patients who are possible candidates for SCT should be chelated early, as iron overload before SCT is correlated with increased transplant-related mortality [65]. Administration of iron chelation is facilitated by the availability of oral therapy with deferasirox. However, caution is needed, since deferasirox therapy has been associated with gastrointestinal side effects in about 20 % of patients and with a possible increase in serum creatinine levels [66].
7.2 Erythropoiesis-Stimulating Agents and Other Growth Factors
Erythropoiesis-stimulating agents (ESAs) have demonstrated activity in numerous clinical trials and thus represent the first-line therapy in anemic patients without del(5q). Weekly doses of 30,000–80,000 units of recombinant human erythropoietin or 150–300 µg of darbepoetin alpha achieved erythroid responses in 27–58 % of patients with no evidence of a negative impact on AML transformation and are the treatment of choice [67–69] (Fig. 2). Higher dosages of ESAs (60,000–80,000 units of erythropoietin or 300 µg of darbepoetin alpha per week) showed even higher response rates. In clinical practice, higher doses should be administered in patients not responding to standard doses. An iron, B12, or folate deficiency should be corrected before ESA therapy. Benefits of ESA therapy should be carefully weighed along with safety concerns, including thromboembolic events and arterial hypertension. ESA therapy should continue for 8–12 weeks to evaluate response [70]. The median duration of response is about 2 years. Patients with a transfusion requirement of less than two RBC concentrates per month and an endogenous serum erythropoietin level <500 U/L are more likely to respond to ESAs [71, 72]. Likewise, the parameters <5 % bone marrow blasts and lack of multi-lineage dysplasia are predictive for higher response rates [59].
Addition of granulocyte colony-stimulating factor (G-CSF) can further improve the efficacy of ESAs [72]. A dose of G-CSF 300 µg per week divided into two or three doses should be added if no response is observed after a minimum of 8 weeks of ESA treatment. MDS with ring sideroblasts (RARS) respond exceptionally well to ESAs plus G-CSF and should thus be treated with the combination up front [71].
In MDS patients, neutropenia may occur as a manifestation of the disease per se or as a side effect of disease-modifying treatment modalities. In neutropenic infections, the use of G-CSF is recommended on an interventional basis. In addition, broad spectrum antibiotics should be used immediately when fever or other signs of infection occur.
7.3 Thrombopoiesis-Stimulating Agents
Severe thrombocytopenia is correlated with poor prognosis and shortened survival and limits the administration of effective drugs like lenalidomide or azacitidine [73]. Platelet transfusions are a relevant treatment option. Prophylactic administration is recommended in patients with transient platelet counts <10 × 109/L or in patients with platelets <20 × 109/L and any other risk factor for bleeding such as fever, infection, or invasive procedure. As platelet transfusions result in allo-immunization with subsequent loss of response, their administration should be limited [74]. Long-lasting and well-tolerated thrombocytopenia does not need prophylactic platelet transfusions [22].
As high immunogenicity limits the clinical use of recombinant thrombopoietin in thrombocytopenic patients, thrombopoiesis-stimulating agents (TSAs) have been developed [75]. Romiplostim and eltrombopag are the most frequently administered drugs in MDS trials. TSAs are promising in MDS as monotherapy and as a concomitant therapy to facilitate the use of disease-modifying therapies. TSAs are effective in improving platelet counts and in reducing bleeding and transfusion requirements [76–79]. Interpretation of available data is currently complex, as the cohorts have been relatively small. Rising blast counts and the potential for AML transformation remain a concern and should be evaluated in clinical studies [75, 79]. Thus, the available evidence does not yet allow a general recommendation to be made on the use of TSAs in MDS. TSAs may be considered in patients no longer responsive to platelet transfusions who suffer from the clinical consequences of severe thrombocytopenia, so that a transient rise in blasts may be acceptable [75]. However, in vitro data suggest a dose-dependent anti-leukemic effect of eltrombopag. The first phase I trials using eltrombopag in AML patients are ongoing; further clinical studies are needed [80].
7.4 Lenalidomide
Lenalidomide is the treatment of choice in anemic lower-risk MDS patients with del(5q), as shown by better and longer-lasting responses than ESAs plus G-CSF [81]. An initial dose of lenalidomide 10 mg daily for 3 of every 4 weeks is recommended. Lenalidomide brought about RBC transfusion independence in 65 % of patients, with a median duration of 2.2 years. Cytogenetic response was observed in 72 % of patients, including 45 % complete cytogenetic responses. Positive predictors of survival and decreased risk for progression to AML include Del(5q) as the only cytogenetic abnormality, cytogenetic and/or hematological response, blasts <5 %, and a platelet count above 100 × 109/L [82]. Presence of a TP53 gene mutation, which occurs in approximately 29 % of patients, is also associated with a higher risk for leukemic evolution as well as resistance to lenalidomide [83]. Grade 3 or 4 neutropenia and thrombocytopenia are the most frequent adverse events in the first weeks of treatment, with less than 20 % of patients discontinuing treatment as a result [84]. The number of adverse events subsequently decreased after the first two cycles [85]. During this time, close monitoring of blood counts with dose reduction and/or addition of G-CSF is strongly recommended.
Lenalidomide achieves RBC transfusion independence in about 25 % of ESA-refractory MDS patients without del(5q) [86, 87]. These response rates have been confirmed in a randomized phase III study [88] and will form the basis for registration for this indication. A higher percentage and a longer duration of response were observed in patients treated with lenalidomide plus ESAs [89, 90]. Prospective trials are needed to confirm these results. In all, lenalidomide is a valuable therapeutic option in anemic ESA-refractory non-del(5q) patients, although it is not yet registered for this indication.
7.5 Immunosuppressive Drugs
Treatment of patients refractory to ESAs or lenalidomide poses a challenge. Anti-thymocyte globulin (ATG) with or without cyclosporine can yield erythroid responses in about 25–45 % of patients [91, 92]. The rate of responses was higher with ATG plus cyclosporine than with placebo or ATG alone [92]. Factors predictive of a better response to ATG were found to be age <60 years, short duration of RBC transfusion requirement, human leukocyte antigen (HLA)-DR15 phenotype, normal karyotype, <5 % marrow blasts, and hypo-cellular bone marrow [92, 93]. The relevance of ATG in elderly MDS patients is highly limited, because the prerequisites for the therapy are such that elderly patients hardly ever meet them.
7.6 Hypomethylating Agents
Azacitidine yields overall response rates in about 45 % and transfusion independence in 46–61 % of lower-risk MDS patients. It may thus be considered in selected cases of low-risk MDS [94]. However, for low-risk MDS, azacitidine has been registered to date exclusively by the US FDA (Table 3).
7.7 Treatment Options in Higher-Risk MDS Patients
Higher-risk MDS patients exhibit a dismal prognosis, with median survival between 0.4 and 1.5 years and a high risk for AML transformation [24, 30]. Therapeutic options (Fig. 3) should thus aim to modify the natural course of the disease.
Treatment options in senior higher-risk MDS patients (intermediate-2 to high IPSS; high to very high IPSS-R). allo HSCT allo hematopoietic stem-cell transplantation, AML acute myeloid leukemia. Reproduced and modified from Stauder [55]
7.8 Allogeneic Hematopoietic Stem-Cell Transplantation
Allogeneic hematopoietic stem-cell transplantation (allo-HSCT) is the only procedure that can possibly cure the disease. Higher-risk disease with increased percentage of marrow blasts and high-risk cytogenetics is a clear indication for allo-HSCT. Low- and intermediate-risk disease with life-threatening cytopenia or transfusion dependency associated with the risk of iron overload can also prompt us to think about moving forward to allo-HSCT. But is allo-HSCT a reasonable therapeutic option for elderly MDS patients? Age per se should not serve as an exclusion factor for allo-HSCT, as new reduced-intensity conditioning regimens allow elderly patients to also be transplanted. More and more older patients—a few up to the age of 70 years—are being included in transplant studies [95]. These studies have shown no statistically significant difference between patients aged below or above 65 years, independent of conditioning regimen [96–98]. Nevertheless, only a very small proportion (<10 %) of patients aged >65 years are definitely going to be transplanted [99].
Outcome of allo-HSCT is strongly influenced by comorbidities [99, 100]. Comorbidities may be defined from assessment scores such as CCI or HCT-CI, although HCT-CI was found to be superior [101–103]. HCT-CI covers different clinical and laboratory-based definitions for a large number of comorbidities, and was found to discriminate different risk groups for prediction of non-relapse mortality, overall survival, and acute graft-versus-host disease (GvHD) and post-GvHD mortality [103, 104]. Co-morbid conditions as important predictors of outcome should be evaluated carefully prior to transplant, especially in elderly MDS patients. The use of performance scales is simple but recommended only in combination with HCT-CI during pre-transplant assessment [105]. Geriatric assessment and frailty measures appear to be of additional importance in determining whether or not a patient should be transplanted, although their prognostic significance has not been adequately tested in the transplant setting. The prospective use of geriatric and quality-of-life assessment in elderly MDS patients has been shown to be predictive of outcome. The combination of functional and symptom parameters measured by Karnofsky, ADL and ‘fatigue’, was associated with survival [51]. Prospective studies are needed, particularly in the transplant setting.
7.9 Remission-Induction Chemotherapy
Conventional chemotherapy regimens for AML are able to induce complete remissions in only about 35 % of patients with MDS [106, 107]. Long post-chemotherapy neutropenia causes lower remission rates in MDS patients compared with patients with primary AML due to increased rates of infection-related deaths [108]. Induction chemotherapy is thought to be a valid treatment option for patients aged <65 years who may not proceed to allogeneic stem-cell transplantation because of lack of a suitable donor. Additionally, patients should have more than 10 % bone marrow blasts and no adverse cytogenetic risk factors [22].
7.10 Low-Dose Chemotherapy
Low-dose cytarabine (ara-C [LDAC]) and low-dose oral melphalan achieve overall response rates of 30 and 40 %, respectively. However, existing evidence does not allow for recommendation of routine use of these agents in MDS patients [109, 110].
7.11 Hypomethylating Agents
Hypomethylating therapy with azacitidine or decitabine (only FDA approved as yet) is recommended for all higher-risk MDS patients not eligible for allo-HSCT. Azacitidine is the standard of care in higher-risk elderly MDS patients.
Azacitidine was able to produce an overall survival benefit as compared with conventional care regimens (median survival 24.5 vs. 15 months) [111]. A subgroup analysis of elderly patients (>75 years) confirmed efficacy in this particular group. Tolerability of azacitidine is good [112]. Most common hematological grade 3/4 toxicities were cytopenias. Non-hematological toxicities were mainly nausea, vomiting, fatigue, and diarrhea, but these were generally easy to manage. Patients aged ≥80 years can be treated with azacitidine because of its favorable toxicity profile. Response is often achieved after several cycles of treatment, which means administration of at least four to six cycles of azacitidine is recommended before response evaluation. Not only complete or partial remission but also hematologic improvement and stable disease were shown to be associated with prolonged survival [113]. Azacitidine treatment beyond the first response may improve responses. It is therefore recommended that azacitidine be continued in responding patients. Azacitidine 75 mg/m2 days is administered on days 1–7 of a 28-day cycle. Administration of azacitidine on weekends is often associated with logistic difficulties. Therefore, a regimen of 7 days with a 2-day break (5-2-2) is used. However, recently published data on high-risk patients compared the administration of azacitidine for 5 days (AZA 5) versus AZA 5-2-2 and azacitidine for 7 days (AZA 7). Median response rates and median overall survival seemed to be best in the AZA 7 arm [114]. An oral formulation of azacitidine has recently been developed and showed an overall response rate of 35 % in previously treated patients and 75 % in previously untreated patients [115]. Oral azacitidine is currently being tested in a phase III study.
Only 40–50 % of patients treated with hypomethylating agents achieve hematologic improvement; complete responses are seen in as few as 10–15 % of patients [103]. Therefore, effective methods for identifying those patients who are most likely to benefit from treatment with hypomethylating agents would be of great clinical and economic interest. Platelet count doubling after the first cycle of azacitidine was an early predictor of achieving objective response and overall survival [116, 117]. However, patients without platelet doubling after the first cycle (about 46 %) were also able to later achieve a partial or complete response. Therefore, early discontinuation of azacitidine cannot be recommended at that time. The association of TET2 mutations with an increased response to hypomethylating agents was shown by Bejar et al. [118] and Itzykson et al. [119]. A prognostic score published by the Groupe Francophone des Myelodysplasies (GFM) showed previous LDAC treatment, bone marrow blasts >15 %, and abnormal karyotype to be associated with lower response rates to azacitidine treatment. Other factors associated with poorer overall survival were complex karyotype, performance status ≥2, intermediate- and poor-risk cytogenetics, presence of circulating blasts, and RBC transfusion dependency ≥4 units/8 weeks. Using these factors, three different subgroups of patients with a distinct prognosis under azacitidine treatment can be discriminated [120].
Nonetheless, patients with high-risk MDS continue to experience high mortality. Overall survival after relapse or failure of azacitidine treatment is still low at about 6 months [121]. The multi-kinase inhibitor rigosertib showed encouraging results in a phase I clinical trial in patients with MDS [122]. The first analysis of phase III data for rigosertib versus best supportive care in MDS patients who have progressed on or failed or relapsed after azacitidine or decitabine treatment seems to show a better median survival for patients in the rigosertib group [123]. No standard treatment is available for patients not responding to azacitidine or with diminishing response to azacitidine. Therefore, treatment within clinical trials is highly recommended. Decitabine achieved a longer median time to AML progression and death in patients with higher-risk MDS but was not able to show a survival benefit [124]. Retrospective comparative analyses demonstrated significantly better survival in patients treated with azacitidine than with decitabine [125, 126]. The use of decitabine is limited, as it is, as yet, not licensed by the European Medicines Agency (EMA) for the treatment of MDS patients (Table 3).
Efforts to improve treatment of higher-risk MDS are ongoing. A combination of multiple classes of medications (hypomethylating agents, histone deacetylase inhibitors, tumor necrosis factor [TNF]-alpha inhibitors, bortezomib, classical chemotherapeutic agents) in individual patients seems to produce promising results [127–131]. Randomized trials comparing standard monotherapy and combination therapies are needed to establish the significance and possible use of combinations, especially in elderly patients.
8 Future Aspects
Demographic changes in Western countries will cause the incidence of MDS to increase in the near future. Allo-HSCT, the only potentially curative treatment option, is available for only a minority of MDS patients. Reduced-intensity regimens and improved management of side effects may increase the proportion of elderly patients successfully transplanted. Instruments to better stratify patients for allo-HSCT versus supportive treatment are urgently needed. Prevention of cytopenia-related morbidity and preservation of quality of life are the main issues in MDS patients not fit for HSCT. Promising new drugs, including hypomethylating agents and growth factors, will improve the treatment options in elderly MDS patients. A new group of antagonists of activin receptor signaling (sotatercept, luspatercept, dalantercept) increases RBCs markedly in murine models as well as in healthy volunteers. First data in patients with MDS seem to be very promising. Phase III studies are currently planned.
References
Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–85.
Nösslinger T, Tüchler H, Germing U, Sperr WR, Krieger O, Haase D, Lübbert M, Stauder R, Giagounidis A, Valent P, Pfeilstöcker M. Prognostic impact of age and gender in 897 untreated patients with primary myelodysplastic syndromes. Ann Oncol. 2010;21(1):120–5.
Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536–42.
de Swart L, Smith A, Johnston T, Haase D, Droste J, Fenaux P, Symeonidis A, Sanz G, Hellström-Lindberg E, Cermak J, Germing U, Stauder R, Georgescu O, MacKenzie M, Malcovati L, Skov-Holm M, Almeida AM, Mądry K, Slama B, Guerci-Bresler A, Sanhes L, Beyne-Rauzy O, Luno E, Bowen D, De Witte T. Validation of the Revised International Prognostic Scoring System (IPSS-R) in patients with lower-risk myelodysplastic syndromes: a report from the prospective European LeukemiaNet MDS (EUMDS) Registry. Br J Haematol. 2015;170(3):372–83.
Neukirchen J, Schoonen WM, Strupp C, Gattermann N, Aul C, Haas R, Germing U. Incidence and prevalence of myelodysplastic syndromes: data from the Düsseldorf MDS-registry. Leuk Res. 2011;35(12):1591–6.
Avgerinou C, Alamanos Y, Zikos P, Lampropoulou P, Melachrinou M, Labropoulou V, Tavernarakis I, Aktypi A, Kaiafas P, Raptis C, Kouraklis A, Karakantza M, Symeonidis A. The incidence of myelodysplastic syndromes in Western Greece is increasing. Ann Hematol. 2013;92(7):877–87.
World Health Organisation. Nutritional anemia: report of a WHO Scientific Group. Geneva: WHO; 1968.
Gaskell H, Derry S, Andrew Moore R, McQuay HJ. Prevalence of anaemia in older persons: systematic review. BMC Geriatr. 2008;8:1.
Bach V, Schruckmayer G, Sam I, Kemmler G, Stauder R. Prevalence and possible causes of anemia in the elderly: a cross-sectional analysis of a large European university hospital cohort. Clin Interv Aging. 2014;9:1187–96.
Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8.
Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PHM, Newman AB, Cushman M. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–20.
Riva E, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Guala A, Fasolo G, Lucca U. Association of mild anemia with hospitalization and mortality in the elderly: the Health and Anemia population-based study. Haematologica. 2009;94:22–8.
Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–34.
Den Elzen WPJ, Willems JM, Westendorp RGJ, De Craen AJM, Assendelft WJJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus Study. CMAJ. 2009;181:151–7.
Joosten E, Pelemans W, Hiele M, Noyen J, Verhaeghe R, Boogaerts MA. Prevalence and causes of anaemia in a geriatric hospitalized population. Gerontology. 1992;38:111–7.
Tettamanti M, Lucca U, Gandini F, Recchia A, Mosconi P, Apolone G, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Savoia L, Fasolo G, Ponchio L, Della Porta MG, Riva E. Prevalence, incidence and types of mild anemia in the elderly: the ‘Health and Anemia’ population-based study. Haematologica. 2010;95:1849–56.
Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139:271–8.
Neukirchen J, Blum S, Kuendgen A, Strupp C, Aivado M, Haas R, Aul C, Gattermann N, Germing U. Platelet counts and haemorrhagic diathesis in patients with myelodysplastic syndromes. Eur J Haematol. 2009;83(5):477–82.
Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the US population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486–92.
Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthakur G, Wierda WG, Pierce S, Estey E, Liu J, Huang X, Kantarjian H. A prognostic score for patients with lower risk myelodysplastic syndrome. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2008;22:538–43.
Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, Laouri M. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28:2847–52.
Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, Della Porta MG, Fenaux P, Gattermann N, Germing U, Jansen JH, Mittelman M, Mufti G, Platzbecker U, Sanz GF, Selleslag D, Skov-Holm M, Stauder R, Symeonidis A, Van De Loosdrecht AA, De Witte T, Cazzola M. Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood. 2013;122:2943–64.
Haase D, Germing U, Schanz J, Pfeilstöcker M, Nösslinger T, Hildebrandt B, Kundgen A, Lübbert M, Kunzmann R, Giagounidis AAN, Aul C, Trümper L, Krieger O, Stauder R, Müller TH, Wimazal F, Valent P, Fonatsch C, Steidl C. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–95.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SMM, Miyazaki Y, Pfeilstöcker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, Van De Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–65.
Porta MGD, Tuechler H, Malcovati L, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SMM, Miyazaki Y, Pfeilstöcker M, Sekeres MA, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D, Greenberg PL, Cazzola M. Validation of WHO classification-based Prognostic Scoring System (WPSS) for myelodysplastic syndromes and comparison with the revised International Prognostic Scoring System (IPSS-R). A study of the International Working Group for Prognosis in Myelodyspla. Leukemia. 2015;29(7):1502–13.
Braulke F, Platzbecker U, Muller-Thomas C, Gotze K, Germing U, Brummendorf TH, Nolte F, Hofmann W-K, Giagounidis AAN, Lubbert M, Greenberg PL, Bennett JM, Sole F, Mallo M, Slovak ML, Ohyashiki K, Le Beau MM, Tuchler H, Pfeilstocker M, Nosslinger T, Hildebrandt B, Shirneshan K, Aul C, Stauder R, Sperr WR, Valent P, Fonatsch C, Trumper L, Haase D, Schanz J. Validation of cytogenetic risk groups according to International Prognostic Scoring Systems by peripheral blood CD34+ FISH: results from a German diagnostic study in comparison with an international control group. Haematologica. 2015;100(2):205–13.
Van De Loosdrecht AA, Westers TM, Westra AH, Dräger AM, Van Der Velden VHJ, Ossenkoppele GJ. Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood. 2008;111(3):1067–77.
Valent P, Horny HP, Bennett JM, Fonatsch C, Germing U, Greenberg P, Haferlach T, Haase D, Kolb HJ, Krieger O, Loken M, van de Loosdrecht A, Ogata K, Orfao A, Pfeilstöcker M, Rüter B, Sperr WR, Stauder R, Wells DA. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: consensus statements and report from a working conference. Leuk Res. 2007;31(6):727–36.
Bruning RD, Orazi A, Germing U, Le Beau MM, Porwit A, Baumann I. Myelodysplastic syndromes. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization classification of tumours of the hematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88.
Schanz J, Steidl C, Fonatsch C, Pfeilstöcker M, Nösslinger T, Tuechler H, Valent P, Hildebrandt B, Giagounidis A, Aul C, Lübbert M, Stauder R, Krieger O, Garcia-Manero G, Kantarjian H, Germing U, Haase D, Estey E. Coalesced multicentric analysis of 2,351 patients with myelodysplastic syndromes indicates an underestimation of poor-risk cytogenetics of myelodysplastic syndromes in the International Prognostic Scoring System. J Clin Oncol. 2011;29(15):1963–70.
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B, Bernasconi P, Knipp S, Strupp C, Lazzarino M, Aul C, Cazzola M. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10.
Malcovati L, DellaPorta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K, Travaglino E, Invernizzi R, Pascutto C, Lazzarino M, Germing U, Cazzola M. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based prognostic scoring system (WPSS). Haematologica. 2011;96(10):1433–40.
Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–6.
Bammer C, Sperr WR, Kemmler G, Wimazal F, Nösslinger T, Schönmetzler A, Krieger O, Pfeilstöcker M, Valent P, Stauder R. Clustering of comorbidities is related to age and sex and impacts clinical outcome in myelodysplastic syndromes. J Geriatr Oncol. 2014;5(3):299–306.
Sperr WR, Wimazal F, Kundi M, Baumgartner C, Nösslinger T, Makrai A, Stauder R, Krieger O, Pfeilstöcker M, Valent P. Comorbidity as prognostic variable in MDS: comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS study group. Ann Oncol. 2009;21(1):114–9.
Stauder R, Nösslinger T, Pfeilstöcker M, Sperr WR, Wimazal F, Krieger O, Valent P. Impact of age and comorbidity in Myelodysplastic syndromes. JNCCN J Natl Compr Cancer Netw. 2008;6(9):927–34.
Naqvi K, Garcia-Manero G, Sardesai S, Oh J, Vigil CE, Pierce S, Lei X, Shan J, Kantarjian HM, Suarez-Almazor ME. Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol. 2011;29(16):2240–6.
Sperr WR, Kundi M, Wimazal F, Nosslinger T, Schonmetzler-Makrai A, Stauder R, Krieger O, Neukirchen J, Germing U, Pfeilstocker M, Valent P. Proposed score for survival of patients with myelodysplastic syndromes. Eur J Clin Invest. 2013;43(11):1120–8.
Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E, Travaglino E, Invernizzi R, Pascutto C, Lazzarino M, Germing U, Cazzola M. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96(3):441–9.
Breccia M, Federico V, Latagliata R, Mercanti C, D’Elia GM, Cannella L, Loglisci G, Salaroli A, Santopietro M, Alimena G. Evaluation of comorbidities at diagnosis predicts outcome in myelodysplastic syndrome patients. Leuk Res. 2011;35(2):159–62.
Wang R, Gross CP, Halene S, Ma X. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res. 2009;33(12):1594–8.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–5.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8.
Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Guigoz Y, Vellas BJ. Malnutrition in the elderly: the Mini Nutritional Assessment (MNA). Ther Umsch. 1997;54(6):345–50.
Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, Soubeyran PL. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–72.
Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol. 2014;93(6):1031–40.
Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy—a systematic review. Leuk Res. 2014;38(3):275–83.
Deschler B, Ihorst G, Platzbecker U, Germing U, März E, De Figuerido M, Fritzsche K, Haas P, Salih HR, Giagounidis A, Selleslag D, Labar B, de Witte T, Wijermans P, Lübbert M. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208–16.
Wildes TM, Ruwe AP, Fournier C, Gao F, Carson KR, Piccirillo JF, Tan B, Colditz GA. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4(3):227–34.
Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014;32(24):2541–52.
Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5(3):224–37.
Stauder R. The challenge of individualised risk assessment and therapy planning in elderly high-risk myelodysplastic syndromes (MDS) patients. Ann Hematol. 2012;91(9):1333–43.
Efficace F, Gaidano G, Breccia M, Criscuolo M, Cottone F, Caocci G, Bowen D, Lübbert M, Angelucci E, Stauder R, Selleslag D, Platzbecker U, Sanpaolo G, Jonasova A, Buccisano F, Specchia G, Palumbo GA, Niscola P, Wan C, Zhang H, Fenu S, Klimek V, Beyne-Rauzy O, Nguyen K, Mandelli F. Prevalence, severity and correlates of fatigue in newly diagnosed patients with myelodysplastic syndromes. Br J Haematol. 2015;168(3):361–70.
Hamaker ME, Stauder R, van Munster BC. On-going clinical trials for elderly patients with a hematological malignancy: are we addressing the right end points? Ann Oncol. 2014;25(3):675–81.
Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383(9936):2239–52.
Fenaux P, Hasse D, Sanz GF, Santini V, Buske C. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii57–69.
Hicks LK, Bering H, Carson KR, Kleinerman J, Kukreti V, Ma A, Mueller BU, O’Brien SH, Pasquini M, Sarode R, Solberg L, Haynes AE, Crowther MA. The ASH Choosing Wisely®campaign: five hematologic tests and treatments to question. Hematol Am Soc Hematol Educ Program. 2013;2013:9–14.
Jensen PD, Jensen FT, Christensen T, Eiskjær H, Baandrup U, Nielsen JL. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood. 2003;101(11):4632–9.
Rose C, Brechignac S, Vassilief D, Pascal L, Stamatoullas A, Guerci A, Larbaa D, Dreyfus F, Beyne-Rauzy O, Chaury MP, Roy L, Cheze S, Morel P, Fenaux P. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myélodysplasies). Leuk Res. 2010;34(7):864–70.
Breccia M, Voso MT, Aloe Spiriti MA, Fenu S, Maurillo L, Buccisano F, Tafuri A, Alimena G. An increase in hemoglobin, platelets and white blood cells levels by iron chelation as single treatment in multitransfused patients with myelodysplastic syndromes: clinical evidences and possible biological mechanisms. Ann Haematol. 2015;94(5):771–7.
Valent P, Krieger O, Stauder R, Wimazal F, Nösslinger T, Sperr WR, Sill H, Bettelheim P, Pfeilstöcker M. Iron overload in myelodysplastic syndromes (MDS)—diagnosis, management, and response criteria: a proposal of the Austrian MDS platform. Eur J Clin Invest. 2008;38(3):143–9.
Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, Soiffer RJ, Antin JH. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–8.
List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez-Lopez N, Paley C, Feigert J, Besa E. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol. 2012;30(17):2134–9.
Ross SD, Allen IE, Probst CA, Sercus B, Crean SM, Ranganathan G. Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis. Oncologist. 2007;12(10):1264–73.
Mundle S, Lefebvre P, Vekeman F, Duh MS, Rastogi R, Moyo V. An assessment of erythroid response to epoetin α as a single agent versus in combination with granulocyte- or granulocyte- macrophage-colony-stimulating factor in myelodysplastic syndromes using a meta-analysis approach. Cancer. 2009;115(4):706–15.
Moyo V, Lefebvre P, Duh MS, Yektashenas B, Mundle S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol. 2008;87(7):527–36.
Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(1):97–108.
Hellström-Lindberg E, Negrin R, Stein R, Krantz S, Lindberg G, Vardiman J, Ost A, Greenberg P. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol. 1997;99(2):344–51.
Hellström-Lindberg E, Gulbrandsen N, Lindberg G, Ahlgren T, Dahl IMS, Dybedal I, Grimfors G, Hesse-Sundin E, Hjorth M, Kanter-Lewensohn L, Linder O, Luthman M, Löfvenberg E, Öberg G, Porwit-MacDonald A, Rådlund A, Samuelsson J, Tangen JM, Winquist I, Wisloff F. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120(6):1037–46.
Gonzalez-Porras JR, Cordoba I, Such E, Nomdedeu B, Vallespi T, Carbonell F, Luño E, Ardanaz M, Ramos F, Pedro C, Gomez V, De Paz R, Sanchez-Barba M, Sanz GF, Del Cañizo C. Prognostic impact of severe thrombocytopenia in low-risk myelodysplastic syndrome. Cancer. 2011;117(24):5529–37.
Stolla M, Refaai MA, Heal JM, Spinelli SL, Garraud O, Phipps RP, Blumberg N. Platelet transfusion—the new immunology of an old therapy. Front Immunol. 2015;6:28.
Brierley CK, Steensma DP. Thrombopoiesis-stimulating agents and myelodysplastic syndromes. Br J Haematol. 2015;169(3):309–23.
Kantarjian HM, Giles FJ, Greenberg PL, Paquette RL, Wang ES, Gabrilove JL, Garcia-Manero G, Hu K, Franklin JL, Berger DP. Phase 2 study of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving azacitidine therapy. Blood. 2010;116(17):3163–70.
Kantarjian H, Fenaux P, Sekeres MA, Becker PS, Boruchov A, Bowen D, Hellstrom-Lindberg E, Larson RA, Lyons RM, Muus P, Shammo J, Siegel R, Hu K, Franklin J, Berger DP. Safety and efficacy of romiplostim in patients with lower-risk myelodysplastic syndrome and thrombocytopenia. J Clin Oncol. 2010;28(3):437–44.
Oliva EN, Santini V, Zini G, Palumbo GA, Poloni A, Cortelezzi A, Voso MT, Molteni A, Sanpaolo G, Marino A, Rodà F, Alati C, Ronco F, Di Raimondo F, Leoni P, Alimena G, Finotto S, Latagliata R, Nobile F. Efficacy and safety of eltrombopag for the treatment of thrombocytopenia of low and intermediate-1 IPSS risk myelodysplastic syndromes: interim analysis of a prospective, randomized, single-blind, placebo-controlled trial (EQoL-MDS). Blood. 2012;120:20.
Giagounidis A, Mufti GJ, Fenaux P, Sekeres MA, Szer J, Platzbecker U, Kuendgen A, Gaidano G, Wiktor-Jedrzejczak W, Hu K, Woodard P, Yang AS, Kantarjian HM. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120(12):1838–46.
Kalota A, Selak MA, Garcia-Cid LA, Carroll M. Eltrombopag modulates reactive oxygen species and decreases acute myeloid leukemia cell survival. PLoS One. 2015;10(4):e0126691.
Kelaidi C, Park S, Brechignac S, Mannone L, Vey N, Dombret H, Aljassem L, Stamatoullas A, Adès L, Giraudier S, de Botton S, Raynaud S, Lepelley P, Picard F, Leroux G, Daniel MT, Bouscary D, Dreyfus F, Fenaux P. Treatment of myelodysplastic syndromes with 5q deletion before the lenalidomide era; the GFM experience with EPO and thalidomide. Leuk Res. 2008;32(7):1049–53.
List AF, Bennett JM, Sekeres MA, Skikne B, Fu T, Shammo JM, Nimer SD, Knight RD, Giagounidis A. Extended survival and reduced risk of AML progression in erythroid-responsive lenalidomide-treated patients with lower-risk del(5q) MDS. Leukemia. 2014;28(5):1033–40.
Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, Hedlund A, Hast R, Schlegelberger B, Porwit A, Hellström-Lindberg E, Mufti GJ. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29(15):1971–9.
Syed YY, Scott LJ. Lenalidomide: a review of its use in patients with transfusion-dependent anaemia due to low- or intermediate-1-risk myelodysplastic syndrome associated with 5q chromosome deletion. Drugs. 2013;73(11):1183–96.
Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M, Muus P, Te Boekhorst P, Sanz G, Del Cañizo C, Guerci-Bresler A, Nilsson L, Platzbecker U, Lübbert M, Quesnel B, Cazzola M, Ganser A, Bowen D, Schlegelberger B, Aul C, Knight R, Francis J, Fu T, Hellström-Lindberg E. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765–76.
Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, Dreisbach L, Schiffer CA, Stone RM, Greenberg PL, Curtin PT, Klimek VM, Shammo JM, Thomas D, Knight RD, Schmidt M, Wride K, Zeldis JB, List AF. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1-risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93.
Abou Zahr A, Saad Aldin E, Komrokji R, Zeidan A. Clinical utility of lenalidomide in the treatment of myelodysplastic syndromes. J Blood Med. 2014;6:1.
Santini V, Almeida AM, Giagounidis A, Gröpper S, Jonasova A, Vey N, Mufti GJ, Buckstein R, Mittelman M, Platzbecker U, Shpilberg O, Ram R, Del Cañizo C, Gattermann N, Ozawa K, Zhong J, Séguy F, Hoenekopp A, Beach CL, Fenaux P. Paper: efficacy and safety of lenalidomide (LEN) versus placebo (PBO) in RBC-transfusion dependent (TD) patients (Pts) with IPSS low/intermediate (int-1)-risk myelodysplastic syndromes (MDS) without Del(5q) and unresponsive or refractory to erythropoiesis. https://ash.confex.com/ash/2014/webprogram/Paper70494.html. Accessed 13 April 2015.
Sibon D, Cannas G, Baracco F, Prebet T, Vey N, Banos A, Besson C, Corm S, Blanc M, Slama B, Perrier H, Fenaux P, Wattel E. Lenalidomide in lower-risk myelodysplastic syndromes with karyotypes other than deletion 5q and refractory to erythropoiesis-stimulating agents. Br J Haematol. 2012;156(5):619–25.
Komrokji RS, Lancet JE, Swern AS, Chen N, Paleveda J, Lush R, Saba HI, List AF. Combined treatment with lenalidomide and epoetin alfa in lower-risk patients with myelodysplastic syndrome. Blood. 2012;120(17):3419–24.
Passweg JR, Giagounidis AAN, Simcock M, Aul C, Dobbelstein C, Stadler M, Ossenkoppele G, Hofmann W-K, Schilling K, Tichelli A, Ganser A. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care—SAKK 33/99. J Clin Oncol. 2011;29(3):303–9.
Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505–11.
Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102(8):3025–7.
Lyons RM, Cosgriff TM, Modi SS, Gersh RH, Hainsworth JD, Cohn AL, McIntyre HJ, Fernando IJ, Backstrom JT, Beach CL. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850–6.
Hahn T, McCarthy PL, Hassebroek A, Bredeson C, Gajewski JL, Hale GA, Isola LM, Lazarus HM, Lee SJ, Lemaistre CF, Loberiza F, Maziarz RT, Rizzo JD, Joffe S, Parsons S, Majhail NS. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437–49.
Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, Beelen D, Devergie A, Alessandrino E, Willemze R, Ruutu T, Boogaerts M, Falda M, Jouet J-P, Niederwieser D, Kroger N, Mufti GJ, De Witte TM. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405–11.
McClune BL, Weisdorf DJ, Pedersen TL, Da Silva GT, Tallman MS, Sierra J, DiPersio J, Keating A, Gale RP, George B, Gupta V, Hahn T, Isola L, Jagasia M, Lazarus H, Marks D, Maziarz R, Waller EK, Bredeson C, Giralt S. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–87.
Tamari R, Castro-Malaspina H. Transplant for MDS: Challenges and emerging strategies. Best Pract Res Clin Haematol. 2015;28(1):43–54.
Atallah E, Bylow K, Troy J, Saber W. Treatment of older patients with high-risk myelodysplastic syndromes (MDS): the emerging role of allogeneic hematopoietic stem cell transplantation (allo HSCT). Curr Hematol Malig Rep. 2014;9(1):57–65.
Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, Maloney DG, Storb R. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961–8.
Zipperer E, Pelz D, Nachtkamp K, Kuendgen A, Strupp C, Gattermann N, Haas R, Germing U. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica. 2009;94(5):729–32.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9.
Sorror ML, Martin PJ, Storb RF, Bhatia S, Maziarz RT, Pulsipher MA, Maris MB, Davis C, Deeg HJ, Lee SJ, Maloney DG, Sandmaier BM, Appelbaum FR, Gooley TA. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124(2):287–95.
Sorror ML, Estey E. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia in older adults. Hematol Am Soc Hematol Educ Program. 2014;2014(1):21–33.
Hoyle CF, de Bastos M, Wheatley K, Sherrington PD, Fischer PJ, Rees JK, Gray R, Hayhoe FG. AML associated with previous cytotoxic therapy, MDS or myeloproliferative disorders: results from the MRC’s 9th AML trial. Br J Haematol. 1989;72(1):45–53.
Kantarjian HM, Estey EH, Keating MJ. Treatment of therapy-related leukemia and myelodysplastic syndrome. Hematol Oncol Clin N Am. 1993;7(1):81–107.
Estey EH, Keating MJ, McCredie KB, Bodey GP, Freireich EJ. Causes of initial remission induction failure in acute myelogenous leukemia. Blood. 1982;60(2):309–15.
Cheson BD, Jasperse DM, Simon R, Friedman MA. A critical appraisal of low-dose cytosine arabinoside in patients with acute non-lymphocytic leukemia and myelodysplastic syndromes. J Clin Oncol. 1986;4(12):1857–64.
Cheson BD, Simon R. Low-dose ara-C in acute nonlymphocytic leukemia and myelodysplastic syndromes: a review of 20 years’ experience. Semin Oncol. 1987;14(2 Suppl 1):126–33.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32.
Seymour JF, Fenaux P, Silverman LR, Mufti GJ, Hellström-Lindberg E, Santini V, List AF, Gore SD, Backstrom J, McKenzie D, Beach CL. Effects of azacitidine compared with conventional care regimens in elderly (≥75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol/Hematol. 2010;76(3):218–27.
Gore SD, Fenaux P, Santini V, Bennett JM, Silverman LR, Seymour JF, Hellström-Lindberg E, Swern AS, Beach CL, List AF. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica. 2013;98(7):1067–72.
García-Delgado R, de Miguel D, Bailén A, González JR, Bargay J, Falantes JF, Andreu R, Ramos F, Tormo M, Brunet S, Figueredo A, Casaño J, Medina Á, Badiella L, Jurado AF, Sanz G. Effectiveness and safety of different azacitidine dosage regimens in patients with myelodysplastic syndromes or acute myeloid leukemia. Leuk Res. 2014;38(7):744–50.
Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, Macbeth KJ, Laille E, Giordano H, Sakoian S, Jabbour E, Kantarjian H, Skikne B. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29(18):2521–7.
Zeidan AM, Lee J-W, Prebet T, Greenberg P, Sun Z, Juckett M, Smith MR, Paietta E, Gabrilove J, Erba HP, Katterling RP, Tallman MS, Gore SD. Platelet count doubling after the first cycle of azacitidine therapy predicts eventual response and survival in patients with myelodysplastic syndromes and oligoblastic acute myeloid leukaemia but does not add to prognostic utility of the revised IPSS. Br J Haematol. 2014;167:62–8.
van der Helm LH, Alhan C, Wijermans PW, van Marwijk Kooy M, Schaafsma R, Biemond BJ, Beeker A, Hoogendoorn M, van Rees BP, de Weerdt O, Wegman J, Libourel WJ, Luykx-de Bakker SA, Minnema MC, Brouwer RE, Croon-de Boer F, Eefting M, Jie KSG, van de Loosdrecht AA, Koedam J, Veeger GM, Vellenga E, Huls G. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named p”. Br. J. Haematol. 2011;155(5):599–606.
Bejar R, Lord A, Stevenson K, Bar-Natan M, Pérez-Ladaga A, Zaneveld J, Wang H, Caughey B, Stojanov P, Getz G, Garcia-Manero G, Kantarjian H, Chen R, Stone RM, Neuberg D, Steensma DP, Ebert BL. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705–12.
Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, Quesnel B, Vey N, Gelsi-Boyer V, Raynaud S, Preudhomme C, Adès L, Fenaux P, Fontenay M. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2011;25(7):1147–52.
Itzykson R, Thépot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, De Botton S, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Adès L, Fenaux P. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403–11.
Prébet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, Dreyfus F, Rauzy OB, Recher C, Adès L, Quesnel B, Beach CL, Fenaux P, Vey N. Outcome of high-risk myelodysplastic syndrome after azacitidine Treatment failure. J Clin Oncol. 2011;29(24):3322–7.
Komrokji RS, Raza A, Lancet JE, Ren C, Taft D, Maniar M, Wilhelm F, List AF. Phase I clinical trial of oral rigosertib in patients with myelodysplastic syndromes. Br J Haematol. 2013;162(4):517–24.
Garcia-Manero G, Fenaux P, Al-Kali A, Baer MR, Sekeres MA, Roboz G, Gaidano G, Scott BL, Greenberg PL, Platzbecker U, Steensma DP, Kambhampati S, Kreuzer K-A, Godley LA, Collins R, Atallah E, Wilhelm F, Wilhelm I, Azarnia N, Maniar M, Silverman LR. Overall survival and subgroup analysis from a randomized phase III study of intravenous rigosertib versus best supportive care (BSC) in patients (pts) with higher-risk myelodysplastic syndrome (HR-MDS) after failure of hypomethylating agents (HMAs). Blood. 2014;124:163.
Kantarjian H, Issa J-PJ, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803.
Lee YG, Kim I, Yoon SS, Park S, Cheong JW, Min YH, Lee JO, Bang SM, Yi HG, Kim CS, Park Y, Kim BS, Mun YC, Seong CM, Park J, Lee JH, Kim SY, Lee HG, Kim YK, Kim HJ. Comparative analysis between azacitidine and decitabine for the treatment of myelodysplastic syndromes. Br J Haematol. 2013;161(3):339–47.
Lee JH, Choi Y, Kim SD, Kim DY, Lee JH, Lee KH, Lee SM, Cho SH, Lee WS, Joo YD. Comparison of 7-day azacitidine and 5-day decitabine for treating myelodysplastic syndrome. Ann Hematol. 2013;92(7):889–97.
Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, Cortes J, Wierda WG, Ouzounian S, Quezada A, Pierce S, Estey EH, Issa JPJ, Kantarjian HM, Garcia-Manero G. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110(7):2302–8.
Kuendgen A, Bug G, Ottmann OG, Haase D, Schanz J, Hildebrandt B, Nachtkamp K, Neukirchen J, Dienst A, Haas R, Germing U, Gattermann N. Treatment of poor-risk myelodysplastic syndromes and acute myeloid leukemia with a combination of 5-azacytidine and valproic acid. Clin Epigenet. 2011;2(2):389–99.
Silverman LR, Amit V, Odchimar-Reissig R, Feldman EJ, Navada SC, Demakos EP, Baer MR, Najfeld V, Sparano J, Piekarz R. A phase II trial of epigenetic modulators vorinostat in combination with azacitidine (azaC) in patients with the myelodysplastic syndrome: initial results of study 6898 of the New Yorker Cancer Consortium. Blood. 2013;122 (21):386.
Scott BL, Ramakrishnan A, Storer B, Becker PS, Petersdorf S, Estey EH, Deeg HJ. Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept: short report. Br J Haematol. 2010;148(6):944–7.
Liesveld JL, Rosell KE, Bechelli J, Lu C, Messina P, Mulford D, Ifthikharuddin JJ, Jordan CT, Phillips Ii GL. Proteasome inhibition in myelodysplastic syndromes and acute myelogenous leukemia cell lines. Cancer Invest. 2011;29(7):439–50.
Acknowledgments
Mary Heaney Margreiter proofread the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by ‘Austrian Science Fund (FWF): Projekt I 1576-B19’ and by Verein Seniorenkrebshilfe.
Conflicts of interest
SB has received research support from Celgene and honoraria from Celgene, Novartis, and AOP. RS has received research support from Celgene and TEVA, honoraria from Celgene, Teva, and Novartis, and is a scientific advisory board member of Celgene. PW declares no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Burgstaller, S., Wiesinger, P. & Stauder, R. Myelodysplastic Syndromes in the Elderly: Treatment Options and Personalized Management. Drugs Aging 32, 891–905 (2015). https://doi.org/10.1007/s40266-015-0312-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-015-0312-7