Abstract
Combination antiretroviral treatment is associated with clear benefits in HIV-positive subjects, and is also effective in the central nervous system (CNS), meaning HIV-associated dementia is now an uncommon event. Nevertheless, a significant number of patients show symptoms of neurocognitive impairment which may negatively affect their quality of life. Although several risk factors for HIV-associated neurocognitive disorders have been identified, there is no clear recommendation for their prevention and management. In this review, the penetration of drugs into the cerebrospinal fluid/CNS is discussed as well as the viral and clinical consequences associated with higher/lower compartmental exposure. We also review the potential interventions according to the currently identified underlying mechanisms, including persistent CNS immune activation, legacy effects, low-level viral replication and escape, co-morbidities, and antiretroviral-associated direct and indirect ‘neurotoxicity’. Adjunctive therapies and interventions (including neuro-rehabilitation) are then briefly discussed. The treatment of HIV infection in the CNS is a complex area of therapeutics requiring multidisciplinary interventions and further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
HIV affects the central nervous system and may cause neurocognitive function impairment (memory, attention, fine motor skills), usually called ‘HAND’ (HIV-associated neurocognitive disorder). |
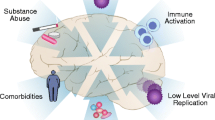
Neurocognitive impairment is associated with HIV persistence, immune system dysregulation, vascular abnormalities and, potentially, with the toxic effects of certain medications. |
Specific interventions including tailoring of antiretroviral treatment, adjunctive therapies and rehabilitation need to be addressed in prospective studies as significant uncertainty still exists regarding the appropriate management of HIV-positive patients with HAND. |
1 Introduction
The evolution of antiretroviral therapy has led to extraordinary success in the treatment of HIV-positive subjects—with the introduction of combination antiretroviral treatment (cART), the prognosis changed from a death sentence to a chronic stable disease. Besides enormous beneficial effects in terms of survival and quality of life, the incidence of opportunistic infections in efficaciously treated HIV-positive subjects reduced to approximately zero; thus, age- and inflammation-associated disorders emerged as leading causes of morbidity in these patients. Before the introduction of antiretroviral drugs (ARVs) the central nervous system (CNS) was often affected by HIV and patients presented a so-called ‘subacute encephalitis’; 20–30% of untreated subjects developed AIDS dementia complex, now termed HIV-associated dementia (HAD) [1]. With appropriate combinations of ARVs, the incidence of HAD has become rather infrequent but less severe forms are still highly prevalent and significantly affect patients’ quality of life [2]. The exact prevalence of non-confounded HIV-associated neurocognitive disorder (HAND), its clinical course and appropriate management are still debated [3, 4]. However, acute neurological symptoms may emerge in cases of selective viral replication in the cerebrospinal fluid (CSF), a rare event termed ‘symptomatic CSF escape’. Several factors may influence the effectiveness of ARVs in the central compartment, thus highlighting the need for a better understanding of HIV infection in the CNS.
2 Untreated HIV Infection of the Central Nervous System (CNS)
Following HIV infection, the virus reaches the CNS and may be detected both in the CSF and brain tissue as early as 8 days post-infection [5]. Besides being associated with acute neurological symptoms similar to viral meningoencephalitis, HIV neuroinvasion leads to a high CSF viral load, local immune activation (including higher CSF pleocytosis and neopterin, a macrophage-derived marker of immune activation), changes in magnetic resonance imaging (MRI) (such as putamen volume) and partially reversible neurocognitive impairment in a few patients [6–8]. In the months following HIV neuroinvasion, these inflammatory-mediated perturbations seem to increase and may pave the way to chronic neuronal damage—several pieces of evidence support the relationship between advanced immune depletion (after years of uncontrolled HIV infection) and the incidence of HAND [9].
Viral particles may enter the CNS directly or through HIV-infected lymphocytes and, potentially, monocytes; once there, they have been shown to infect microglia, perivascular macrophages and, although through a restricted infection, astrocytes [10]. Early CNS entry seems to be dependent on α4 integrin, and natalizumab blocks viral trafficking in macaques; brain tissue analysis shows superior benefits of this approach when used early after infection [11]. Neuronal damage seems to be indirect following the production of neurotoxic products (such as free radicals and reactive oxygen species) by infected cells. HIV proteins influence several functions of the CNS immune system and the permeability of the blood–brain barrier (BBB). HIV trans-activating regulatory protein (TAT) has been extensively studied and it has been associated with several pathogenic pathways that may explain HIV-associated neuronal damage [12]. Years after primary infection, CSF HIV replication persists at lower levels. Although genetically distinct CSF viruses have been described, viral evolution over time does not seem to be relevant, suggesting that continuous virus replication is not the major cause of viral persistence in the CNS [13]. Simultaneously, several markers of neuronal damage increase with advanced immune suppression and HAD—neurofilament and tau protein seem to increase with disease progression [14].
While only 5–10% of astrocytes are infected and mature viral particles are not produced, the involvement of these cells seems relevant as they participate in the neurovascular unit and affect the permeability of the BBB. HIV may alter the stability of tight junctions, thus affecting BBB permeability [15, 16]. An altered BBB was observed in almost 100% of patients with HAD but also in severely immune-suppressed patients without neurocognitive disorders; furthermore, an altered BBB may refuel HIV replication in the CNS by enhancing viral trafficking from and to the systemic circulation [17, 18].
The clinical consequence of these processes is dementia, which is now defined as a marked acquired impairment in cognitive functioning involving at least two ability domains and producing relevant interference with day-to-day functioning (work, home life, social activities) [19]. An alternative diagnosis has to be sought in cases of active delirium, untreated major depression, active substance abuse or in the presence of pre-existing causes of dementia.
3 Antiretrovirals and HIV Infection in the CNS
After the introduction of cART, CSF HIV RNA declines and usually parallels the plasma viral load: CSF replication is undetectable in 90% of adequately treated patients. This compartmental efficacy leads to a decrease in all markers of immune activation and neuronal damage, although it is still higher than that observed in HIV-negative subjects [20]. Dementia is now observed in late presenters and in elderly patients with several co-morbidities. Neuropathological studies suggest significant changes in brain tissue after the introduction of cART; although microglial activation and neuroinflammation were, surprisingly, not reduced, they are now observed in different brain areas. While pre-cART examinations showed basal ganglia involvement, post-cART specimens suggest pronounced inflammation in the hippocampus and adjacent parts of the entorhinal and temporal cortex; in contrast, lymphocyte infiltration is now rarely observed with the exception of patients with immune reconstitution inflammatory syndrome (IRIS) or CD8 encephalitis [21, 22].
However, cART is not completely effective in the CNS: low-level compartmental replication, CSF escape, mild neurocognitive symptoms and, potentially, neurotoxicity have all been described.
3.1 Low-Level Cerebrospinal Fluid (CSF) Replication
CSF HIV RNA is usually 1 Log10 lower than plasma levels and with antiretroviral treatment it rapidly decreases to below the limit of detection of conventional methods (20–50 copies/mL). Using sensitive methods (including the single copy assay) low-level replication has been demonstrated in 17–60% of patients with an undetectable plasma viral load: the lowest prevalence was observed in patients with 10 years of efficacious antiretroviral treatment [23, 24]. In these papers [23, 24] the lowest CSF viral load strata was the one associated with the lowest, and almost normal, concentrations of neopterin. These data suggest that the strictest viral control is associated with the lowest immune activation. The origin of this low-level HIV RNA is still debated: controversy regarding the ‘ongoing replication’ versus ‘dismissal from reservoirs’ origin hypotheses exists. An interesting study showed that intensification with drugs crossing the BBB or remaining in the systemic circulation had no effect on CSF residual HIV RNA [25].
3.2 CSF Escape
CSF escape is defined as a detectable CSF HIV RNA with undetectable plasma HIV RNA or a CSF HIV RNA 1 Log10 (0.5 Log10 in some papers) higher than plasma HIV RNA. CSF escape has been observed in approximately 10% of patients but its clinical relevance is still unknown. Preliminary data suggest that 25% of patients may have detectable CSF HIV RNA without clinical progression, resembling what happens with plasma viral blips [26, 27]. Nightingale et al. [28] observed a significantly higher prevalence of CSF/plasma discordance (18%) in patients with unexplained episodes of low-level HIV RNA in plasma in the previous 12 months.
CSF escape may be asymptomatic, symptomatic or ‘secondary’ (i.e. in association with a concomitant non-HIV infection such as neurosyphilis or herpesvirus as a consequence of the local inflammatory response) [29]. The most relevant cases are those with symptomatic CSF escape, which present acute neurological symptoms varying from headache to coma. True to form, CSF replication with associated resistance-associated mutations has been observed [30, 31]. This event is rare (there are less than 30 published cases) and it is reversible with optimised antiretroviral therapy. Recent unpublished data suggest that it may be far more common in intermediate- and low-income countries: in a case series from India, the incidence of symptomatic CSF escape was 1% and worrisome resistance patterns were described in the CSF of these subjects [32]. The rarity of this event does not allow analysis of the predictors of symptomatic CSF escape; however, a low CD4 nadir and the presence of resistance-associated mutations in plasma (thus limiting the efficacy in both compartments) have been reported. Incomplete penetration of ARVs in the CNS might be an issue and are discussed in Sect. 4. There is no evidence of a higher prevalence of CSF escape or neurocognitive function decay over time in patients on protease inhibitor (PI) monotherapy [33]. However, treatment failures have been observed (both in plasma and CSF) in patients with a CD4 nadir below 200 cells/µL and cases of symptomatic CSF escape have been described, which suggests that the less powerful antiretroviral strategies in patients with large compartmental infection (secondary to severe immune depletion) might be a risk factor [34, 35]. CSF replication in these cases is usually low (typically below 104 Log10 copies/mL) but MRI shows a significant involvement of white matter with acute inflammatory changes; therefore, CSF HIV RNA may be the trigger for immune-mediated changes in brain tissue [31, 36].
3.3 Factors Associated with HIV-Associated Neurocognitive Disorder (HAND)
The exact prevalence of milder forms of neurocognitive impairment is still debated: while dementia is rare (<2%), mild neurocognitive disorders (MNDs) or asymptomatic neurocognitive impairment (ANI) are observed in 15–50% of treated patients [37, 38]. Patients with ANI report normal results in the Independence Activity in Daily Living test as opposed to those diagnosed with MND; however, they score poorly on performance-based tests (rather than self-reported perception of their own disability) and there is a significant risk of progression to more severe forms of impairment over time [39–41]. One of the performance-based tests that patients with ANI scored poorly on (the Medication Management Test-Revised [MMT-R]) was clearly associated with adherence to medication in a large group of HIV-positive subjects [42].
Besides sociodemographic features and alcohol/substance abuse, two groups of factors have been constantly associated with the prevalence of HAND: advanced immune depletion and vascular abnormalities [3]. A recent study prospectively followed 99 subjects on suppressive cART, measuring CSF biomarkers on two occasions: the authors observed that mild HAND (ANI and MND) was associated with increased intrathecal immune activation. Furthermore, the authors reported a correlation between neopterin and neurofilament that supports an association between neurocognitive impairment, CNS inflammation and neuronal damage [43].
Advanced immunosuppression before treatment (as testified by a low CD4+ nadir cell count) is usually associated with persistent immune and glial cell activation, BBB damage and the incidence of HAND. Peripheral blood mononuclear cell (PBMC) HIV DNA, usually considered a marker of the amount of HIV in body reservoirs, is inversely correlated with CD4 nadir and has been associated with HAND prevalence, neurocognitive worsening and cortical atrophy on brain MRI [44–46]. Hepatitis C virus (HCV) infection has been associated with worse neurocognitive performance, although its effect in HIV-positive individuals is controversial [47, 48].
On the other hand, an increasing amount of data have highlighted the higher cardio- and cerebrovascular risk in HIV-positive patients: chronic inflammation, viral replication and the effects of drugs have been shown to interact with age and traditional risk factors [49]. Cardiovascular risk factors, central obesity, insulin resistance, diabetes mellitus and atherosclerosis (as measured by a higher intima media thickness on carotid ultrasound examination or by ophthalmic artery resistance) have been associated with neurocognitive impairment in HIV-positive subjects [50–52]. Standard and perfusion brain MRI confirm the high prevalence of cerebrovascular abnormalities in symptomatic and asymptomatic HIV-positive patients [53]. With the longer life expectancy of cART-treated HIV-positive patients, a differential diagnosis needs to be performed in older subjects to exclude Alzheimer’s and vascular dementias (among others) [54].
3.4 Neurotoxicity
Neurological adverse effects have been demonstrated for several ARVs, such as thymidine analogues (peripheral neuropathy), efavirenz (neuropsychiatric symptoms including dizziness and insomnia) and integrase inhibitors (headache and sleep disturbances), among others. Several in vitro pieces of evidence support the theory of a neuronal toxicity induced by certain ARVs, as demonstrated in cell cultures and in macaques [55]. After 14 days of incubation, foetal rat cortical neuron cultures showed some degree of functional injury with all drugs, with no additive effect, and with efavirenz having the highest toxicity and the lowest toxicity being for emtricitabine, tenofovir, darunavir and maraviroc [56]. Furthermore, ARVs can induce oxidative stress in neuronal cultures, PIs disrupt astrocytic glutamate transporter function (and alter neurobehavioural performance in rats) and β-amyloid metabolism may be impaired by efavirenz and PIs [57, 58].
Efavirenz, besides its well-known adverse effects, has been associated with a higher prevalence of HAND [59, 60]. Although still debated, the adverse drug effects (which seem to be dose dependant and susceptible to dose optimisation) and in vitro data suggest that efavirenz should be avoided in patients with neurocognitive disturbances. A prospective study of patients who elected to discontinue cART showed that there was an improvement in two neuropsychological tests (Trail-Making Test A & B and the Wechsler Adult Intelligence Scale-Revised Digit Symbol subtest) for up to 96 weeks; this effect was higher in efavirenz recipients but was still significant in those who were on different ARVs [61]. Vague neuropsychiatric symptoms (including worsening depression), headache and sleep disturbances have been reported with dolutegravir. US Department of Health and Human Services guidelines suggest that the use of efavirenz and rilpivirine in patients with psychiatric illnesses and efavirenz in those with HAD should be avoided [62].
The last point to be considered is the potential endothelial toxicity caused by ARVs. In a post-mortem brain tissue gene array study, two different pictures of impairment were observed: a rare (<10%) inflammatory pattern with strong immune response and a common (>35%) pattern with upregulated genes of endothelial origin (such as JAG1, PECAM1 and TFRC) [63]. PIs have been associated with severe lipid metabolism abnormalities and lopinavir/ritonavir has been associated with a higher cumulative incidence of cardiovascular disorders. In another autoptic study, cerebral small vessel disease was observed in more than 60% of brains and was associated with PI-based cART and the presence of diabetes [64, 65].
4 Determinants of the Efficacy of Antiretrovirals in the CNS
cART initiation has beneficial effects on CSF HIV RNA, immune activation, compartmental inflammation and neurocognitive function. Nevertheless, none of the CSF biomarkers has been found to normalise despite antiretroviral treatment. The number of ARVs does not seem to affect CNS efficacy since three-drug and intensified regimens do not seem to differ substantially: provided patients are accurately selected, even less drug regimens seem to be comparably efficacious [66]. While the START (Strategic Timing of Antiretroviral Therapy) trial provided no strong evidence for a beneficial effect on neurocognitive function with early initiation of cART (above or below 500 CD4+ T lymphocytes/µL), preliminary data support the use of ARVs in patients with primary HIV infection (PHI). In an as-yet unpublished study, ARV treatment during acute HIV infection was associated with a reduction in CSF neopterin to levels comparable with those observed in HIV-negative subjects [67, 68]. In another study, most individuals had normal neuropsychiatric performance during PHI and early cART improved their psychomotor function; however, approximately 25% had impaired neuropsychiatric performance that did not improve with early cART, possibly indicating limited reversibility of cognitive impairment in a subset of PHI individuals [8].
There is considerable uncertainty regarding the differential neuroefficacy of ARVs—reasons exist both in favour and against such a hypothesis, and these have been reviewed recently [3].
4.1 Targets of Antiretroviral Therapy in the CNS
In order to understand the pharmacodynamics of antiretroviral treatment in the CNS it is necessary to establish the markers of compartmental efficacy. However, this is particularly difficult given the complexity and multi-factorial nature of HAND pathogenesis, the non-linear relationship between these markers and the possible emergence of drug-associated neurotoxicity (Table 1). Each of these targets may warrant study into specific interventions.
Some of these markers are purely hypothetical and biopsies are not feasible except in cases of unexplained rapid worsening of cognitive function. Those markers normally used in clinical practice have significant pitfalls, including the low sensitivity and practice effect of neurocognitive tests, the lack of validation of CSF biomarkers, the non-reversibility of brain atrophy and white matter abnormalities on MRIs [69]. The use of CSF as a surrogate marker for brain tissue needs further discussion; its composition has been deemed to originate both from brain extracellular fluid (two-thirds) and plasma (one-third) [70]. In the pre-cART era, autoptic brain tissue HIV RNA showed significant regional variations but good correlation with CSF HIV RNA; compartmental efficacy is currently based on this biomarker [71]. The most interesting tissue data in the cART era have been published by Gelman and colleagues [72]: brain HIV RNA was higher in subjects with HAND plus HIV encephalitis (HIVE) but not in those without HIVE or microglial nodule encephalitis (MGNE) [72]. Interestingly, worse neurocognitive scores correlated significantly with higher HIV RNA in brain specimens but not with HIV RNA levels in pre-mortem blood plasma or CSF.
The optimal level of viral suppression is, however, uncertain: low-level CSF HIV RNA has been associated with a higher prevalence of neurocognitive impairment and higher neopterin concentrations (as discussed in sect. 3.1) but its relevance and targeted interventions are not known. For instance, guidelines provide recommendations if CSF escape occurs, but no recommendation is provided for symptomatic patients with suppressed CSF HIV RNA when receiving cART [73].
4.2 Pharmacokinetics and the Concentration Penetration Efficacy (CPE) Score
Antiretrovirals reach the CSF with significant variability, and concentrations of some antiretrovirals do not exceed the inhibitory concentration for wild-type HIV replication in CSF. Several factors may influence the passage of ARVs in the CSF. Some are patient related (age, meningeal inflammation, CSF flow alterations and BBB damage) and some are drug related (molecular size, lipophilicity, binding to plasma proteins, ionisation and affinity to transporter enzymes) or variable according to patients and drugs (plasma concentrations and concomitant drugs) [74].
Several attempts have been made to create a score relating to the neuropenetration/neuroeffectiveness of ARVs following the observation that drugs estimated to have good effectiveness on the CNS are associated with lower levels of HIV RNA in CSF and better cognitive function [75]. The largest and most structured analysis was performed by the CHARTER (CNS HIV Antiretroviral Therapy Effects Research) group, a large US-based collaborative research group that enrolled more than 1000 patients and followed them prospectively. The last version of the Concentration Penetration Efficacy (CPE) score ranked ARVs into four categories (from 1 to 4), where those in the higher group are associated with the highest efficacy in the CSF (Table 3): this was obtained using the properties of the drugs, CSF concentrations and, in a few cases, compartmental viral response in monotherapy studies. This scoring system was published in 2014 and a few co-factors were found to be associated with an undetectable CSF HIV RNA—plasma HIV RNA levels, ethnicity, ongoing depression, incomplete adherence and duration of cART [76]. All of these modifiers are associated with socioeconomic status, adherence to medication and the amount of HIV in body reservoirs and they had already been identified in previous studies as determinants of HAND and CSF escape. Besides the initial CHARTER analysis, several other studies have found an association between a higher CPE score and a lower prevalence of CSF viral replication; in contrast, an association with better neurocognitive function is still controversial [74]. Two longitudinal studies found a protective effect of cART, with higher CPE scores on the prospective changes in neurocognitive function [77, 78]. The aggregate results of four randomized clinical trials that compared regimens with different CPE scores suggest better or equal neurocognitive outcomes with more “neuroefficacious” drugs and worse ones with efavirenz-containing regimens [79–81]. In the prevention trial, conducted in China, the magnitude of neurocognitive decline was directly associated with efavirenz CSF concentrations (suggesting potential neurotoxicity) and inversely associated with tenofovir CSF concentrations (implying incomplete drug penetration) [82].
A single study (involving 61,938 individuals) found an unexpected result: patients starting antiretroviral treatment with high CPE regimens had a higher incidence of dementia [83]. However, the large sample size is counterbalanced by some methodological pitfalls such as a substantial channelling bias and an arbitrary CPE cut-off (10; not achieved by currently recommended regimens). A large body of evidence supports the usefulness of this approach, even if some controversies regarding its use need to be acknowledged (Table 2).
Two further issues also exist: CSF HIV RNA is a surrogate marker of tissue replication and the optimal inhibitory concentration has not been clearly defined. As opposed to bacterial meningitis (where bacteria or fungi replicate in the CSF and meninges), CSF contains viral particles released from cells in ‘deep’ brain parenchyma. Furthermore, concentrations are judged as adequate according to their ability to overcome the 50% inhibitory concentration (IC50), although a theoretical risk of residual viral production exists. Our group found that individual concentrations (as opposed to drugs’ ranks) above the 95% inhibitory concentration (IC95) (as opposed to IC50) were associated with a lower prevalence of CSF escape: this observation supports potential benefits with higher CSF concentrations in some patients [87].
4.3 Cell Types
While the major target of ARVs is the pool of activated and resting lymphocytes, all cells in the CNS are macrophage derived (microglia, perivascular macrophages and astrocytes). Several pieces of evidence support a major role of microglial activation and astrocytosis in the pathogenesis of HAND and BBB damage. An in vivo study using positron emission tomography with [11C]-PK11195, a marker of translocator protein (TSPO) expressed by activated microglia, found several areas of activated microglia despite optimal antiretroviral treatment [88]. Furthermore, greater [11C]-PK11195 binding in certain brain areas (anterior cingulate, corpus callosum and posterior cingulate) was associated with poorer executive function performance. The role of astrocytosis in the pathogenesis of BBB damage has already been discussed—an additional study found that patients with higher CSF S100β protein had a deficit in their verbal fluency [89].
In vitro data suggest that ARVs may have different activity (and intracellular concentrations) in activated and resting macrophages. This may be due to the different pattern of protein expressed (according to the cellular state) and to the endogenous nucleotide pool (lower than that measured in lymphocytes) [90]. Interestingly Shikuma et al. [91] found that drugs with a lower macrophage activity score (calculated according to their in vitro macrophage inhibitory concentration) were associated with a higher prevalence and severity of neurocognitive disorders. This line of research is very interesting but data are limited and standardised macrophage activity scores are mostly unavailable (Table 3). A recent study applied patients’ withdrawn CSF to three cell lines (PBMCs, neuro-derived glial [U87] and astrocyte [373] cells) and developed an infectivity model with IC50 [92]. Antiviral efficacy was higher in patients receiving tenofovir/emtricitabine plus lopinavir/ritonavir plus maraviroc than in those receiving tenofovir/emtricitabine/rilpivirine: the antiviral effect on astrocyte cell lines directly correlated with rilpivirine and lopinavir CSF concentrations. These data support the idea that compartmental efficacy with currently available drugs may be incomplete, as already suggested in lymph nodes [93]. Further data suggest that some ARVs may have reduced inhibitory effects in infected astrocytes: lamivudine, stavudine and, surprisingly, zidovudine had insufficient HIV-1 inhibitory activity, with 90% effective concentrations that were significantly greater than the achievable CSF concentrations [94] (Table 3).
5 Adjunctive Therapies
Several adjunctive treatments have been tested in HIV-positive patients with neurocognitive impairment, although none had a significant effect. The most striking results have been observed with cART initiation in patients with dementia. In the early period of the HIV epidemic, the introduction of zidovudine was able to improve severe neurological conditions [95]. To date, two studies have demonstrated non-ARV drugs to provide some benefit in HIV-positive patients. A randomized, double-blind, placebo-controlled crossover trial tested the use of oral rivastigmine (up to 12 mg/day for 20 weeks) in 17 aviraemic patients with HAND [96]; although no effect was observed on the primary endpoint (Alzheimer’s Disease Assessment Scale-Cognitive subscale), patients taking rivastigmine showed improvements in processing speed and executive functioning. In addition, a recently presented trial compared the effect of adding paroxetine, fluconazole or both in 24 highly adherent cART-treated individuals with HAND: paroxetine was associated with neurocognitive improvements (after adjusting for depression) while fluconazole was associated with a decrease in inflammatory markers [97].
In addition to its antiviral activity, maraviroc, a CCR5 antagonist, has several favourable immunological properties; preliminary evidence supports further study in the setting of progressive multifocal leukoencephalopathy and CNS inflammatory disorders [98]. In HIV-positive patients, the effect of maraviroc is partially mediated by entry inhibition since most CSF viruses are R5-tropic; cellular interactions are, however, more complex and HIV-loaded lymphocytes may infect astrocytes via CXCR4 [99, 100]. Maraviroc treatment has been found to be protective for CNS infection in Simian immunodeficiency virus in macaques [101]. In addition, maraviroc intensification has been associated with better neuronal integrity (increase in spectroscopy-MRI N-acetylaspartate to creatine ratio [Naa/Cr]), lower CSF immune activation (lower interferon-γ-induced protein 10 [IP-10] and CD16+ monocytes), lower monocyte-associated HIV DNA and better cognitive function in HIV-infected subjects and with the improvement of CNS MRI abnormalities in an HIV-positive child: better effects were reported in patients with higher maraviroc plasma concentrations [102–105]. Finally, a recent small randomised controlled trial has been conducted in 14 virally suppressed (blood and CSF) HIV-positive males receiving stable cART who had recent progression to HAND: patients in the maraviroc arm showed significant cognitive improvement [106]. However, no neurocognitive benefit was found in naïve patients starting a maraviroc versus a tenofovir-based regimen [107].
Given the incomplete effectiveness of these interventions, cognitive rehabilitation protocols have been developed. Three studies have used a restorative approach (which aims to restore the neural circuits underlying impaired cognitive processes by means of practice and focused training exercises)—they reported positive effects on visual learning and speed of information processing [108–111]. In a recent study, our group developed a cognitive rehabilitation treatment for HAND in HIV-positive adults, combining a restorative and compensatory approach. We reported significant improvements in patients with HAND undergoing the rehabilitation protocol; furthermore, such benefits persisted for at least 6 months after the end of the intervention [112].
6 Conclusions
The pathogenesis of neurocognitive disorders in HIV-positive patients is complex and multifactorial. Although current knowledge does not allow clear recommendations to be formulated, we propose a pragmatic flowchart of potential management strategies (Fig. 1). In the future we will probably observe a different clinical scenario involving universal testing and early access to ARVs, thus preventing late presentation and the observed neurological effect of years of immune depression and viral replication. The identification and treatment of patients with PHI will theoretically be beneficial, although it will remain a challenging task. The prevention and treatment of HAND will also be part of a comprehensive care of HIV-positive patients going forward: managing age-, virus- and drug-associated co-morbidities is a primary objective of HIV outpatient management due to the ‘greying’ of the HIV epidemic. Vascular abnormalities are common in HIV-infected patients for several reasons (lifestyle, viral-associated chronic inflammation, drug-induced toxicities) and have been recognised as a key risk factor for HAND. Small-vessel cerebral disease and vascular abnormalities need to be assessed and managed adequately. Reducing the direct and indirect toxicities of antiretrovirals is now possible with the availability of efficacious, well-tolerated and safe drugs and should be actively pursued. The contribution of HCV to neurocognitive disorders has been recognised but we still have no data on the consequences of eradicating this infection; however, given the undeniable benefits of directly active anti-HCV agents it is imperative to treat as many patients as possible. No evidence is currently available on the consequences of interventions targeted at co-morbidities on neurocognitive function in HIV-positive subjects.
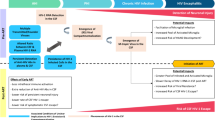
Flowchart of potential management in HIV-positive subjects diagnosed with HIV-associated neurocognitive disorders (HAND). Although the suggested interventions are derived from studies identifying risk factors for HAND, no randomised clinical trial has proven their efficacy. The management of patients with controlled plasma and cerebrospinal fluid HIV RNA is controversial and the interventions proposed in the grey box are based on the authors’ expert opinion. C NS central nervous system, CSF cerebrospinal fluid, GRT genotype resistance test, HAART highly active antiretroviral therapy, SVR sustained virological response
However, despite all of these efforts, we will still observe patients with acute neurological symptoms, CSF escape and HAND. Besides optimising antiretroviral regimens according to resistance-associated mutations (both in plasma and CSF) and enhancing patients’ adherence to medication, no other clear recommendation can be formulated. Several factors may affect cognitive function in those patients with undetectable plasma and CSF HIV RNA, including residual viral replication, immune activation and, potentially, neurotoxicity of ARVs; further studies are needed in order to understand the efficacy of targeted interventions in this complicated scenario.
References
González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81.
Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–60.
Nightingale S, Winston A, Letendre S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13:1139–51.
McDonnell J, Haddow L, Daskalopoulou M, Cognitive Impairment in People with HIV in the European Region (CIPHER) Study Group, et al. Minimal cognitive impairment in UK HIV-positive men who have sex with men: effect of case definitions and comparison with the general population and HIV-negative men. J Acquir Immune Defic Syndr. 2014;67:120–7.
Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–60.
Suh J, Sinclair E, Peterson J, et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J Neuroinflammation. 2014;11:199.
Wright PW, Pyakurel A, Vaida FF, et al. Putamen volume and its clinical and neurological correlates in primary HIV infection. AIDS. 2016;30:1789–94.
Kore I, Ananworanich J, Valcour V, RV254/SEARCH 010 Study Group, et al. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70:393–9.
Heaton RK, Franklin DR Jr, Deutsch R, CHARTER Group, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60:473–80.
Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep. 2015;12:16–24.
Campbell JH, Ratai EM, Autissier P, et al. Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10:e1004533.
Maubert ME, Pirrone V, Rivera NT, et al. Interaction between Tat and drugs of abuse during HIV-1 infection and central nervous system disease. Front Microbiol. 2015;6:1512.
Dahl V, Gisslen M, Hagberg L, et al. An example of genetically distinct HIV type 1 variants in cerebrospinal fluid and plasma during suppressive therapy. J Infect Dis. 2014;209:1618–22.
Peterson J, Gisslen M, Zetterberg H, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PloS One. 2014;9:e116081.
Eugenin EA, Clements JE, Zink MC, et al. Human immunodeficiency virus infection of human astrocytes disrupts blood–brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31:9456–65.
Awan FM, Anjum S, Obaid A, et al. In-silico analysis of claudin-5 reveals novel putative sites for post-translational modifications: insights into potential molecular determinants of blood–brain barrier breach during HIV-1 infiltration. Infect Genet Evol. 2014;27:355–65.
Petito CK, Cash KS. Blood–brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–66.
Calcagno A, Alberione MC, Romito A, et al. Prevalence and predictors of blood–brain barrier damage in the HAART era. J Neurovirol. 2014;20:521–5.
Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99.
Yilmaz A, Yiannoutsos CT, Fuchs D, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62.
Anthony IC, Bell JE. The neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24.
Lescure FX, Moulignier A, Savatovsky J, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013;57:101–8.
Yilmaz A, Price RW, Spudich S, et al. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–73.
Dahl V, Peterson J, Fuchs D, et al. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28:2251–8.
Yilmaz A, Verhofstede C, D’Avolio A, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–6.
Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–25.
Edén A, Nilsson S, Hagberg L, et al. Asymptomatic cerebrospinal fluid HIV-1 viral blips and viral escape during antiretroviral therapy: a longitudinal study. J Infect Dis. 2016;214:1822–5.
Nightingale S, Geretti AM, Beloukas A, et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J Neurovirol. 2016;22:852–60.
Ferretti F, Gisslen M, Cinque P, et al. Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep. 2015;12:280–8.
Canestri A, Lescure FX, Jareguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8.
Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–74.
Dravid AN, Kulkarni MV, Mahajan U, et al. Cerebrospinal fluid (CSF) HIV escape is associated with progressive neurologic deterioration in patients on virologically suppressive antiretroviral therapy(ART) in Western India. In: 15th European AIDS Conference, Barcelona, October 21–24, 2015.
Pérez-Valero I, González-Baeza A, Estébanez M, et al. A prospective cohort study of neurocognitive function in aviremic HIV-infected patients treated with 1 or 3 antiretrovirals. Clin Infect Dis. 2014;59:1627–34.
Antinori A, Clarke A, Svedhem-Johansson V, et al. Week 48 efficacy and central nervous system analysis of darunavir/ritonavir monotherapy versus darunavir/ritonavir with two nucleoside analogues. AIDS. 2015;29:1811–20.
Tiraboschi J, Hamzah L, Siddiqui A, et al. Cerebrospinal fluid viral escape and acute encephalitis in a patient on boosted protease inhibitor monotherapy. Antivir Ther. 2016;21:461–4.
Spudich SS. Immune activation in the central nervous system throughout the course of HIV infection. Curr Opin HIV AIDS. 2016;11:226–33.
Heaton RK, Clifford DB, Franklin DR Jr, CHARTER Group, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96.
Heaton RK, Franklin DR, Ellis RJ, CHARTER Group, HNRC Group, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16.
Blackstone K, Moore DJ, Heaton RK, CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18:79–88.
Grant I, Franklin DR Jr, Deutsch R, CHARTER Group, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82:2055–62.
Obermeit LC, Beltran J, Casaletto KB, et al. Evaluating the accuracy of self-report for the diagnosis of HIV-associated neurocognitive disorder (HAND): defining ‘symptomatic’ versus ‘asymptomatic’ HAND. J Neurovirol. 2016. doi:10.1007/s13365-016-0474-z.
Patton DE, Woods SP, Franklin D Jr, et al. Relationship of Medication Management Test-Revised (MMT-R) performance to neuropsychological functioning and antiretroviral adherence in adults with HIV. AIDS Behav. 2012;16:2286–96.
Edén A, Marcotte TD, Heaton RK, et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PloS One. 2016;11:e0157160.
Shiramizu B, Williams AE, Shikuma C, et al. Amount of HIV DNA in peripheral blood mononuclear cells is proportional to the severity of HIV-1-associated neurocognitive disorders. J Neuropsychiatry Clin Neurosci. 2009;21:68–74.
Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80:1792–9.
Cysique LA, Hey-Cunningham WJ, Dermody N, et al. Peripheral blood mononuclear cells HIV DNA levels impact intermittently on neurocognition. PloS One. 2015;10:e0120488.
Monaco S, Mariotto S, Ferrari S, et al. Hepatitis C virus-associated neurocognitive and neuropsychiatric disorders: advances in 2015. World J Gastroenterol. 2015;21:11974–83.
Clifford DB, Vaida F, Kao YT, CHARTER Group, et al. Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology. 2015;84:241–50.
Ballocca F, Gili S, D’Ascenzo F, et al. HIV infection and primary prevention of cardiovascular disease: lights and shadows in the HAART era. Prog Cardiovasc Dis. 2016;58:565–76.
Wright EJ, Grund B, Robertson K, INSIGHT SMART Study Group, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75:864–73.
McCutchan JA, Marquie-Beck JA, Fitzsimons CA, CHARTER Group, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–92.
Grima P, Fabbiani M, Ciccarelli N, et al. Increased ophthalmic artery resistance index is associated with cognitive impairment in HIV-infected patients. J Infect. 2012;65:439–46.
Bladowska J, Knysz B, Zimny A, et al. Value of perfusion-weighted MR imaging in the assessment of early cerebral alterations in neurologically asymptomatic HIV-1-positive and HCV-positive patients. PloS One. 2014;9:e102214.
Mäkitalo S, Mellgren Å, Borgh E, et al. The cerebrospinal fluid biomarker profile in an HIV-infected subject with Alzheimer’s disease. AIDS Res Ther. 2015;12:23.
Tovar-y-Romo LB, Bumpus NN, Pomerantz D, et al. Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther. 2012;343:696–703.
Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18:388–99.
Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20:39–53.
Giunta B, Ehrhart J, Obregon DF, et al. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain. 2011;4:23.
Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403–9.
Dickinson L, Amin J, Else L, ENCORE1 Study Group, et al. Pharmacokinetic and pharmacodynamic comparison of once-daily efavirenz (400 mg vs. 600 mg) in treatment-naïve HIV-infected patients: results of the ENCORE1 study. Clin Pharmacol Ther. 2015;98:406–16.
Robertson KR, Su Z, Margolis DM, A5170 Study Team, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–6.
DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 9 Dec 2016.
Gelman BB, Chen T, Lisinicchia JG, National NeuroAIDS Tissue Consortium, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS One. 2012;7:e46178.
Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–30.
Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28:1297–306.
Arribas JR, Girard PM, Paton N, et al. Efficacy of protease inhibitor monotherapy vs. triple therapy: meta-analysis of data from 2303 patients in 13 randomized trials. HIV Med. 2016;17:358–67.
Wright E, Grund B, Robertson K, Price R (INSIGHT START Neurology Substudy Group). No Difference between the Effects of Immediate versus Deferred ART on Neuropsychological Test Performance in HIV-positive Adults with CD4+ Cell Counts above 500 cells/μL: The Strategic Timing of Anti Retroviral Treatment (START) Neurology Substudy. 15th European AIDS Conference. Barcelona, 21–24 October, 2015.
Peterson J, Lee E, Fuchs D, et al. Early antiretroviral therapy appears to normalize intrathecal markers of immune activation [abstract no. 30]. Conference on Retroviruses and Opportunistic Infections (CROI); 3–6 Mar 2014; Boston.
Corrêa DG, Zimmerman N, Tukamoto G, et al. Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging. 2016;44:1262–9.
de Lange ECM. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn. 2013;40:315–26.
Kumar AM, Borodowsky I, Fernandez B, et al. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol. 2007;13:210–24.
Gelman BB, Lisinicchia JG, Morgello S, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr. 2013;62:487–95.
Antinori A, Marcotullio S, Andreoni M, Italian HIV Guidelines Working Group, et al. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. Update 2015. New Microbiol. 2016;39:93–109.
Calcagno A, Di Perri G, Bonora S. Pharmacokinetics and pharmacodynamics of antiretrovirals in the central nervous system. Clin Pharmacokinet. 2014;53:891–906.
Antinori A, Perno CF, Giancola ML, et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41:1787–93.
Hammond ER, Crum RM, Treisman GJ, CHARTER Group, et al. The cerebrospinal fluid HIV risk score for assessing central nervous system activity in persons with HIV. Am J Epidemiol. 2014;180:297–307.
Cross HM, Combrinck MI, Joska JA. HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J. 2013;103:758–62.
Vassallo M, Durant J, Biscay V, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28:493–501.
Robertson K, Jiang H, Kumwenda J, AIDS Clinical Trials Group, et al. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis. 2012;55:868–76.
Winston A, Duncombe C, Li PC, et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin. Infect. Dis. 2010;50:920–9.
Ellis RJ, Letendre S, Vaida F, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58:1015–22.
Ma Q, Liu X, Heaton R, et al. Neurocognitive decline is associated with antiretroviral concentrations in plasma and cerebrospinal fluid (CSF) [abstract no. 444]. Conference on Retroviruses and Opportunistic Infections (CROI); 23–26 Feb 2015; Seattle.
Caniglia EC, Cain LE, Justice A, HIV-CAUSAL Collaboration, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83:134–41.
Fabbiani M, Grima P, Milanini B, et al. Antiretroviral neuropenetration scores better correlate with cognitive performance of HIV-infected patients after accounting for drug susceptibility. Antivir Ther. 2015;20:441–7.
Force G, Hahn V, Defferriere H, et al. Week48 cognitive improvement in HAND after switch to HAART based on CHARTER score +3 [abstract no. 420]. Conference on Retroviruses and Opportunistic Infections (CROI); 22–23 Feb 2016; Boston.
Hammond ER, Crum RM, Treisman GJ, CHARTER Group, et al. Persistent CSF but not plasma HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms; a prospective cohort study. J Neurovirol. 2016;22:479–87.
Calcagno A, Simiele M, Alberione MC, et al. Cerebrospinal fluid inhibitory quotients of antiretroviral drugs in HIV-infected patients are associated with compartmental viral control. Clin Infect Dis. 2015;60:311–7.
Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28:67–72.
Woods SP, Iudicello JE, Dawson MS, HIV Neurobehavioral Research Center (HNRC) Group, et al. HIV-associated deficits in action (verb) generation may reflect astrocytosis. J Clin Exp Neuropsychol. 2010;32:522–7.
Gavegnano C, Detorio MA, Bassit L, et al. Cellular pharmacology and potency of HIV-1 nucleoside analogs in primary human macrophages. Antimicrob Agents Chemother. 2013;57:1262–9.
Shikuma CM, Nakamoto B, Shiramizu B, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17:1233–42.
Mora-Peris B, Winston A, Garvey L, et al. HIV-1 CNS in vitro infectivity models based on clinical CSF samples. J Antimicrob Chemother. 2016;71:235–43.
Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA. 2014;111:2307–12.
Gray LR, Tachedjian G, Ellett AM, et al. The NRTIs lamivudine, stavudine and zidovudine have reduced HIV-1 inhibitory activity in astrocytes. PloS One. 2013;8:e62196.
Reinvang I, Frøland SS, Karlsen NR, et al. Only temporary improvement in impaired neuropsychological function in AIDS patients treated with zidovudine. AIDS. 1991;5:228–9.
Simioni S, Cavassini M, Annoni JM, et al. Rivastigmine for HIV-associated neurocognitive disorders: a randomized crossover pilot study. Neurology. 2013;80:553–60.
Sacktor N, Skolasky RL, Haughey N, et al. Paroxetine and fluconazole therapy for HAND: a double-blind, placebo-controlled trial [abstract no. 146]. Conference on Retroviruses and Opportunistic Infections (CROI); 22–23 Feb 2016; Boston.
Martin-Blondel G, Brassat D, Bauer J, et al. CCR5 blockade for neuroinflammatory diseases–beyond control of HIV. Nat Rev Neurol. 2016;12:95–105.
Soulié C, Tubiana R, Simon A, et al. Presence of HIV-1 R5 viruses in cerebrospinal fluid even in patients harboring R5X4/X4 viruses in plasma. J Acquir Immune Defic Syndr. 2009;51:60–4.
Li G-H, Anderson C, Jaeger L, et al. Cell-to-cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4. AIDS. 2015;29:755–66.
Kelly KM, Beck SE, Metcalf Pate KA, et al. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS. 2013;27:F21–8.
Garvey L, Nelson M, Latch N, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother. 2012;67:206–12.
Vera JH, Garvey LJ, Allsop JM, et al. Alterations in cerebrospinal fluid chemokines are associated with maraviroc exposure and in vivo metabolites measurable by magnetic resonance spectroscopy. HIV Clin Trials. 2012;13:222–7.
Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20:571–82.
Ball C, Sudhanva M, Jarosz J, et al. Is there a role for maraviroc to treat HIV-associated central nervous system white matter disease? AIDS. 2016;30:334–6.
Gates TM, Cysique LA, Siefried KJ, et al. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016;30:591–600.
Robertson KR, Miyahara S, Lee A, AIDS Clinical Trials Group (ACTG) 5303 team, et al. Neurocognition in maraviroc compared to tenofovir in HIV. AIDS. 2016;30:2315–21.
Boivin MJ, Busman RA, Parikh SM, et al. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24:667–73.
Vance DE, Fazeli PL, Ross LA, et al. Speed of processing training with middle-age and older adults with HIV: a pilot study. J Assoc Nurses AIDS Care. 2012;23:500–10.
Becker JT, Dew MA, Aizenstein HJ, et al. A pilot study of the effects of internet-based cognitive stimulation on neuropsychological function in HIV disease. Disabil Rehabil. 2012;34:1848–52.
Boivin MJ, Nakasujja N, Sikorskii A, et al. A randomized controlled trial to evaluate if computerized cognitive rehabilitation improves neurocognition in Ugandan children with HIV. AIDS Res Hum Retroviruses. 2016;32:743–55.
Livelli A, Orofino GC, Calcagno A, et al. Evaluation of a cognitive rehabilitation protocol in HIV patients with associated neurocognitive disorders: efficacy and stability over time. Front Behav Neurosci. 2015;9:306.
Acknowledgements
We thank Ms. Gloria Toledano Calderon for proofreading and language editing assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the present study.
Conflict of interest
AC has received honoraria from Abbvie, BMS, Gilead, Janssen-Cilag, MSD and Viiv and is currently receiving research grants from BMS, Gilead and Viiv. GDP and SB have received honoraria from Abbvie, BMS, Gilead, Janssen-Cilag, MSD and Viiv.
Rights and permissions
About this article
Cite this article
Calcagno, A., Di Perri, G. & Bonora, S. Treating HIV Infection in the Central Nervous System. Drugs 77, 145–157 (2017). https://doi.org/10.1007/s40265-016-0678-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0678-9