Abstract
Eli Lilly is developing necitumumab (Portrazza™), an intravenously administered fully human IgG monoclonal antibody directed against the epidermal growth factor receptor (EGFR), which is expressed in a variety of solid tumours and has been implicated in promoting oncogenesis and tumour progression. Necitumumab is approved as a part of combination therapy (with gemcitabine and cisplatin) in the USA for the first-line treatment of metastatic squamous non-small cell lung cancer (NSCLC), and regulatory submissions have been made in the EU for this same indication. Necitumumab was derived from the proprietary phage display library of Dyax Corp, and originated with ImClone Systems, which was acquired by Eli Lilly in November 2008. Necitumumab was also under phase II development for colorectal cancer in Belgium and Spain; however, no recent development has been reported for this indication. This article summarizes the milestones in the development of necitumumab leading to this first approval for the first-line treatment of metastatic squamous NSCLC, in combination with gemcitabine and cisplatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Most advances in the treatment of non-small cell lung cancer (NSCLC) have been for adenocarcinomas; treatment options for metastatic squamous NSCLC have not developed at the same rate, with no real advances in first-line therapy for the last 15 years [1]. Of the growth factor receptors being investigated as potential targets for the treatment of NSCLC, the epidermal growth factor receptor (EGFR) has been seen as particularly promising; the binding of ligands to EGFR is associated with cell proliferation, invasion, metastasis, angiogenesis and decreased apoptosis [2, 3]. The majority of patients with squamous NSCLC have relatively high EGFR protein expression [1].
Necitumumab (Portrazza™) is a fully human monoclonal IgG1κ antibody that binds to the EGFR [3]. In November 2015, the US FDA granted approval for necitumumab in combination with gemcitabine and cisplatin as first-line treatment of patients with metastatic squamous NSCLC [3, 4]. The approval was based on the results from the phase III SQUIRE trial [4]. Necitumumab has been granted orphan drug designation by the US FDA [5]. Eli Lilly announced in January 2015 that it had completed its rolling submission for the biological license application (BLA) to the US FDA and the European submission for necitumumab in squamous NSCLC [6]. The company announced in July 2015 that the US FDA’s Oncologic Drugs Advisory Committee discussed the data supporting the regulatory application in July 2015 [7]. Necitumumab was granted fast track status by the US FDA [8]. Necitumumab is not indicated for treatment of non-squamous NSCLC [3].

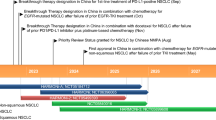
Key milestones in the development of necitumumab for the first-line treatment of metastatic squamous non-small cell lung cancer
The recommended dosage is intravenous necitumumab 800 mg (absolute dose), infused over 60 minutes on days 1 and 8 of a 3-week cycle, prior to intravenous gemcitabine and cisplatin infusion [3]. Treatment should continue until disease progression or unacceptable toxicity. The US prescribing information contains a boxed warning regarding an increased risk of cardiopulmonary arrest and/or sudden death, and of hypomagnesaemia [3]. Close monitoring is advised [3]. Dosage modifications or treatment discontinuation may be required under certain circumstances [3].
Necitumumab was also under development for colorectal cancer and non-squamous non-small cell lung cancer; however, no recent development has been reported for the former (preliminary results were reported in 2008 [9]), and enrolment of patients with the latter disease into the phase III INSPIRE trial was stopped in February 2011, following an independent Data Monitoring Committee (DMC) recommendation that no new or recently enrolled patients continue treatment, due to lack of efficacy and safety concerns related to thromboembolism in the experimental arm [10].
1.1 Company Agreements
Necitumumab originated with ImClone Systems, which was acquired by Eli Lilly in November 2008 [11]. In April 2003, ImClone and Dyax Corp. entered into an antibody library licence agreement [12]. Under the terms of the agreement, ImClone (a wholly owned subsidiary of Eli Lilly) had a non-exclusive licence to Dyax’s antibody phage display library and patent rights. In return, Dyax received an upfront licence fee and for up to 4 years will continue to receive annual technology licence fee payments [12]. Dyax will also receive clinical milestone payments and royalties on sales of any products arising from use of the library [12].
Eli Lilly has the exclusive rights to develop and commercialise necitumumab outside the US and Canada, according to a binding decision in a dispute between ImClone and Merck KGaA over the rights to IMC 11F8 [13]. In the US, Canada and Japan, ImClone and Bristol-Myers Squibb made an updated agreement to co-develop and co-commercialise necitumumab [14]. Under this agreement, Bristol-Myers Squibb was to share the cost in development, and potential commercialisation [14]. However, in the last quarter of 2012, Eli Lilly received 18 months’ notice from Bristol-Myers Squibb of the termination of the collaboration for necitumumab in North America and Japan [15, 16]. This left the worldwide development and commercialisation rights to necitumumab with Eli Lilly [15].
In January 2015, Eli Lilly announced that it entered into an oncology clinical trial collaboration with Merck to evaluate the safety, tolerability and preliminary efficacy of necitumumab with pembrolizumab [6].
Eli Lilly announced in October 2015 that they entered into a clinical trial collaboration agreement with AstraZeneca, to investigate the safety and efficacy of the combination therapy of osimertinib with necitumumab for the treatment of patients with solid tumours [17]. Under the agreement, Lilly will lead the execution of the studies, and both companies will contribute resources [17]. Further details of the agreement, including tumours to be studied and financial terms, were undisclosed. This agreement was an expansion of a previous agreement entered into by the companies in May 2015, for the evaluation of the combination of durvalumab with ramucirumab [17, 18].
1.2 Patent Information
Eli Lilly owns a US patent covering necitumumab as composition of matter that expires in 2025 [19].
2 Scientific Summary
2.1 Pharmacodynamics
Necitumumab specifically binds to the EGF binding site of human EGFR with high affinity (dissociation constant of 0.32 nmol/L [20]), blocking the binding of ligands (half maximal inhibition concentration (IC50) of 1–2 nmol/L [20]) [3, 20, 21], neutralizing ligand-induced EGFR phosphorylation (IC50 of 1.5–3 nmol/L) and the resulting downstream signaling, and inhibiting EGFR-dependent colorectal tumour cells (IC50 of 0.8–1.0 nmol/L) [20]. In vitro, necitumumab induced the internalization and degradation of EGFR, and led to antibody-dependent cellular cytotoxicity in EGFR-expressing cells [3].
In animal xenograft models of human cancer, necitumumab with and without other cancer drugs was associated with antitumour activity in multiple cancer cell lines [3, 20, 21]. For example, necitumumab + gemcitabine cisplatin administration was associated with significantly (p < 0.05) increased antitumour activity, compared with gemcitabine + cisplatin in mice with NSCLC xenografts [3, 21].
2.2 Pharmacokinetics
Population pharmacokinetic studies indicate that necitumumab has dose-dependent pharmacokinetics [3]. The predicted time to reach steady state is ≈100 days. Following intravenous administration of necitumumab 800 mg on days 1 and 8 of a 3-week cycle, the steady-state volume of distribution was 7.0 L, the estimated mean total systemic clearance at steady state was 14.1 L/h, and the elimination half-life was ≈14 days [3].
The systemic exposure of necitumumab is not affected by patient age, race or sex, and body weight is not expected to significantly decreased exposure variability [3]. No correlation was found between necitumumab exposure and renal or mild to moderate hepatic impairment in the population pharmacokinetic analysis. In patients who tested positive for anti-necitumumab antibodies post-treatment, the average necitumumab concentration at steady state was lower and the total systemic clearance was higher than in patients who tested negative for these antibodies [3].
Coadministration of necitumumab + gemcitabine + cisplatin was associated with an increase in gemcitabine exposure compared with gemcitabine + cisplatin alone, and cisplatin exposure was not altered by the presence of necitumumab [3]. Necitumumab exposure was not altered by the presence of the other drugs [3].
Features and properties of necitumumab
Alternative names | IMC-11F8; LY 3012211; Portrazza™ |
Class | Antineoplastics; Fab fragments; monoclonal antibodies |
Mechanism of action | Epidermal growth factor inhibitors |
Route of administration | Intravenous |
Pharmacodynamics | Anti-EGFR fully human monoclonal IgG1κ antibody Binds to the EGF binding site of human EGFR Demonstrates anti-tumour activity in vitro and in vivo |
Pharmacokinetics | Dose-dependent pharmacokinetic profile Predicted time to reach steady state: ≈100 days Steady-state volume of distribution: 7.0 L Estimated mean total systemic clearance at steady state: 14.1 L/h Elimination half-life: ≈14 days |
Most frequent adverse events | |
All grades | Rash, vomiting, diarrhoea, dermatitis acneiform |
Grades 3 or 4 | Venous thromboembolic events, rash, vomiting, diarrhoea |
Electrolyte abnormalities | Hypomagnesaemia, hypocalcaemia, albumin-corrected hypocalcaemia, hypophosphataemia, hypokalaemia |
ATC codes | |
WHO ATC code | L01X-C (monoclonal antibodies) |
EphMRA ATC code | L1G (monoclonal antibody antineoplastics) |
2.3 Therapeutic Trials
2.3.1 Squamous Non-Small Cell Lung Cancer
In the phase III SQUIRE trial (NCT00981058), median overall survival (OS; primary endpoint) was significantly longer in patients receiving necitumumab + gemcitabine + cisplatin than in patients receiving gemcitabine + cisplatin alone [11.5 vs. 9.9 months; HR 0.84 (95 % CI 0.74–0.96); p = 0.01], after a median follow up of 25.2 and 24.8 months, respectively [22]. The 1-year OS rate was 48 and 43 %; the 2-year OS rate was 20 and 17 %. The median progression-free survival (PFS) was 5.7 versus 5.5 months in necitumumab versus control recipients (HR 0.85; 95 % CI 0.74–0.98; p = 0.02); the 3-month PFS rate was 79 and 73 % and the 6-month PFS rate was 45 and 37 %. Median time to treatment failure was 4.3 versus 3.6 months (p = 0.006), 31 and 29 % of patients achieved an overall response, and 82 versus 77 % (p = 0.043) achieved disease control. In this open-label, multicentre trial, patients with treatment-naïve, stage IV squamous NSCLC in both treatment groups received a maximum of six 3-week cycles (median of six cycles) of intravenous gemcitabine 1250 mg/m2 on days 1 and 8 and intravenous cisplatin 75 mg/m2 on day 1; 545 patients were randomized to receive intravenous necitumumab 800 mg on days 1 and 8 (before gemcitabine administration) and 548 patients to receive no additional treatment. At the end of chemotherapy, 275 necitumumab recipients with no disease progression continued to receive necitumumab on the same schedule until disease progression, toxicity or study withdrawal (median of four further cycles) [22].
2.3.2 Non-Squamous Non-Small Cell Lung Cancer
No significant difference in median OS (primary endpoint) was observed between recipients of necitumumab + pemetrexed + cisplatin (n = 315) and those receiving pemetrexed + cisplatin alone (n = 318) in the phase III INSPIRE trial (NCT00982111) [11.3 vs. 11.5 months; HR 1.01 (95 % CI 0.84–1.21)], after a median follow up of 24.5 and 25.6 months, respectively [23]. No significant treatment differences were observed for PFS, objective response or disease control. In this open-label, multicentre trial, patients with treatment-naïve, stage IV non-squamous NSCLC in both treatment groups received a maximum of six 3-week cycles of intravenous pemetrexed 500 mg/m2 plus intravenous cisplatin 75 mg/m2, both on day 1; patients randomized to the necitumumab group received intravenous doses of 800 mg on days 1 and 8. At the end of chemotherapy, necitumumab recipients with no disease progression continued to receive necitumumab on the same schedule until disease progression, toxicity or study withdrawal (median of four further cycles) [23]. Lack of efficacy contributed to the premature closing of the study [3].
2.3.3 Colorectal Cancer
Preliminary data from a phase II study in patients with metastatic colorectal cancer (NCT00835185) showed that, of 23 recipients of necitumumab + mFOLFOX-6 with at least one response assessment at follow-up, 15 patients had a partial response and 8 had stable disease [9]. The primary endpoint was the objective response rate in this noncomparative, multicentre trial. Patients received intravenous necitumumab 800 mg on day 1, followed by intravenous oxaliplatin 85 mg/m2, intravenous folinic acid 400 mg/m2 and intravenous 5-fluorouracil (400 mg bolus then 2400 mg/m2), in a 2-week cycle, repeated until disease progression or toxicity [9].
2.3.4 Solid Tumours
In phase I trials (NCT00801177; NCT01088464), administration of necitumumab was associated with early signs of anti-tumour activity in patients with advanced solid tumours [24, 25].
2.4 Adverse Events
In SQUIRE, 72 % of patients with squamous NSCLC receiving necitumumab + gemcitabine + cisplatin and 62 % of those receiving gemcitabine + cisplatin had ≥1 adverse event of grade 3 or higher [22]. The most common (incidence ≥2 %) grade-3 or -4 adverse events in necitumumab recipients versus recipients of gemcitabine and cisplatin alone were venous thromboembolic events (VTEs) [5 vs. 3 %; mostly pulmonary embolism (4 vs. 2 %)], rash (4 vs. 0.2 %), vomiting (3 vs. 0.9 %) and diarrhoea (2 vs. 1 %) [3]. The most common (incidence ≥15 %) adverse events (all grades) observed in necitumumab recipients were rash (44 vs. 6 % in recipients of gemcitabine and cisplatin alone), vomiting (29 vs. 25 %), diarrhoea (16 vs. 11 %) and dermatitis acneiform (15 vs. 0.6 %) [3].
The most common electrolyte abnormalities in patients receiving necitumumab + gemcitabine + cisplatin (incidence for all grades of >10 and >2 % greater than control recipients) versus gemcitabine + cisplatin alone were hypomagnesaemia (all grades: 83 vs. 70 %; grade 3 or 4: 20 vs. 7 %), hypocalcaemia (45 vs. 30 %; 6 vs. 2 %), albumin-corrected hypocalcaemia (36 vs. 23 %; 4 vs. 2 %), hypophosphataemia (31 vs. 23 %; 8 vs. 6 %) and hypokalaemia (28 vs. 18 %; 5 vs. 3 %) [3]. Adverse events that led to discontinuation of at least one study drug occurred in 31 % of patients in the necitumumab group and 25 % of patients in the control group; neutropenia and thrombocytopenia were the most common adverse events leading to treatment discontinuation [22]. Study drug-related adverse events leading to death occurred in 3 and 2 % of patients, and serious adverse events occurred in 48 and 38 % of patients [22].
Cardiopulmonary arrest or sudden death occurred in 3 % of patients in the necitumumab group and <1 % of patients in the control group of SQUIRE [3]. Most of these patients had comorbid conditions, and died within 30 days of the last dose of necitumumab. In the patients with hypomagnesaemia in this trial, the median time to development of hypomagnesaemia and accompanying electrolyte abnormalities was 6 weeks after necitumumab treatment initiation. The incidence of VTEs of any grade was 9 versus 5 % of patients; VTEs were more common in elderly patients than younger patients. Arterial thromboembolic events (ATEs) occurred in 5 versus 4 % (any grade) and 4 versus 2 % (grade 3 or higher); the most common ATE was cerebral stroke and ischaemia. The overall rate of dermatological toxicities was 79 % in the necitumumab group; these were severe in 8 % of patients. In the necitumumab group, a total of 1.5 % of patients had infusion-related reactions of any grade (0.4 % had grade 3 infusion-related reactions) [3].
Animal data indicate that necitumumab can cause fetal harm in pregnant women, impairing embryofetal development, and has resulted in embryolethality and postnatal death in animals [3].
In INSPIRE, more patients with non-squamous NSCLC receiving necitumumab + pemetrexed + cisplatin than those receiving pemetrexed + cisplatin had serious adverse events (51 vs. 41 %), fatal toxicities (16 vs. 10 %) and cardiopulmonary arrest/sudden death within 30 days of the last study-drug dose (3.3 vs. 1.3 %); this contributed to the premature closing of the study [3].
Key clinical trials of necitumumab (Eli Lilly and Company)
Drugs(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Necitumumab + gemcitabine + cisplatin vs. gemcitabine + cisplatin | Squamous non-small cell lung cancer (stage IV; first-line treatment) | III | Ongoing | Multinational | NCT00981058, 13909, CP11-0806, I4X-IE-JFCC, 2009-013838-25, SQUIRE |
Necitumumab + paclitaxel + carboplatin vs. paclitaxel + carboplatin | Squamous non-small cell lung cancer (stage IV; first-line treatment) | II | Ongoing | Multinational | NCT01769391, 14790, I4X-MC-JFCL, 2012-003214-13 |
Necitumumab + gemcitabine + cisplatin | Squamous non-small cell lung cancer (stage IV; first-line treatment) | II | Ongoing | Multinational | NCT01788566, 14789, I4X-MC-JFCK |
Necitumumab + nab-paclitaxel + carboplatin | Squamous non-small cell lung cancer (stage IV; first-line treatment) | II | Recruiting | USA | NCT02392507, 15529, I4X-MC-JFCP |
Necitumumab + gemcitabine + cisplatin vs. gemcitabine + cisplatin | Squamous non-small cell lung cancer (stage IV; first-line treatment) | Ib/II | Recruiting | Japan | NCT01763788, 14461, I4X-JE-JFCM |
Necitumumab + LY3023414 | Squamous non-small cell lung cancer (stage IV; second-line therapy) | II | Recruiting | USA | NCT02443337, 15799, I6A-MC-CBBE |
Necitumumab + pemetrexed + cisplatin vs. pemetrexed + cisplatin | Non-squamous non-small cell lung cancer (stage IV; first-line therapy) | III | Ongoing | Multinational | NCT00982111, 13908, 2009-012574-12, CP11-0805, I4X-IE-JFCB, INSPIRE |
Necitumumab + mFOLFOX-6 | Locally advanced unresectable or metastatic colorectal cancer (first-line therapy) | II | Completed | Belgium, Spain | NCT00835185, 13926, 2006-003147-23, CP11-0602, I4X-IE-JFCD |
2.4.1 Immunogenicity
Anti-necitumumab antibodies were detected in 4.1 % and neutralizing antibodies in 1.4 % of recipients of necitumumab in clinical trials [3].
2.5 Ongoing Clinical Trials
A randomized, open-label, phase Ib/II trial to investigate the safety and efficacy of necitumumab in combination with gemcitabine and cisplatin versus gemcitabine plus cisplatin alone in the first-line treatment of patients with stage IV squamous NSCLC (NCT01763788) was initiated by Eli Lilly in May 2013. The primary outcomes are the number of patients with dose-limiting toxicities (phase Ib) and OS (phase II). The trial will enrol an estimated 189 patients in Japan.
In October 2015, Eli Lilly initiated a noncomparative phase II trial to evaluate the efficacy and safety of necitumumab in combination with albumin-bound paclitaxel and carboplatin chemotherapy as first-line treatment in patients with stage IV squamous NSCLC (NCT02392507). The primary endpoint is the objective response rate, and the trial will involve an estimated 50 patients in the USA.
Eli Lilly initiated a noncomparative phase II trial to evaluate the safety and activity of necitumumab in combination with LY3023414 as second-line treatment in patients with metastatic, stage IV squamous NSCLC in July 2015 (NCT02443337). The primary endpoint is the disease control rate. The trial will enrol an estimated 48 patients in the USA.
Several phase I studies are also currently under way in patients with NSCLC, investigating the combination of necitumumab with pembrolizumab (in stage IV disease) [NCT02451930], abemaciclib (in stage IV disease) [NCT02411591], and mereletinib (in EGFR-mutant disease) [NCT02496663].
3 Current Status
Necitumumab received its first global approval on November 24, 2015 for first-line treatment of metastatic squamous NSCLC, in combination with gemcitabine and cisplatin, in the USA [4].
References
Eastman P. Oncology Drugs Advisory Committee generally favors approval of necitumumab for lung cancer. Oncol Times. 2015;37(15):6–8.
Pirker R. EGFR-directed monoclonal antibodies in non-small cell lung cancer. Targ Oncol. 2013;8(1):47–53.
Eli Lilly and Company. Prescribing information for Portrazza™ (necitumumab). 2015. http://pi.lilly.com/us/portrazza-uspi.pdf. Accessed 25 Nov.
US FDA. FDA approves Portrazza to treat advanced squamous non-small cell lung cancer [media release]. 24 Nov 2015. http://www.fda.gov.
Eli Lilly and Company. FDA approves Portrazza™ (necitumumab) for specific type of lung cancer [media release]. 24 Nov 2015. http://lilly.mediaroom.com.
Eli Lilly and Company. Lilly reports fourth-quarter and full-year 2014 results, updates 2015 guidance [media release]. 30 Jan 2015. http://www.lilly.com.
Eli Lilly and Company. Lilly statement on FDA Advisory Committee review of necitumumab [media release]. 9 July 2015. http://www.lilly.com.
Dyax Corp. Dyax Corp. highlights recent progress in licensing and funded research portfolio [media release]. 22 Sep 2014. http://www.dyax.com.
Tabernero J, Sastre Valera J, Delaunoit T, et al. A phase II multicenter study evaluating the efficacy and safety of IMC-11F8, a recombinant human IgG1 anti- epidermal growth factor receptor (EGFR) monoclonal antibody (Mab), combined with 5-FU/FA and oxaliplatin (mFOLFOX-6) as first- line therapy [abstract no. 4066]. J Clin Oncol. 2008;26(15 Suppl).
Eli Lilly and Company. INSPIRE trial enrollment stopped; enrollment in lung cancer trial SQUIRE continues [media release]. 2 Feb 2011. http://www.bms.com.
Eli Lilly and Company. Lilly completes acquisition of ImClone Systems [media release]. 24 Nov 2008. http://www.lilly.com.
Dyax Corp. Dyax enters into antibody library license agreement with ImClone Systems Incorporated [media release]. 8 Apr 2003. http://www.dyax.com.
ImClone Systems Incorporated. ImClone Systems announces favorable outcome in binding arbitration over rights to IMC-11F8 [media release]. 4 Apr 2006.
Bristol-Myers Squibb. Strong operational and strategic performance in fourth quarter caps transformative 2009 [media release]. 28 Jan 2010. http://www.bms.com.
Eli Lilly and Company. Lilly reports fourth-quarter and full-year 2012 results, revises 2013 EPS guideance [media release]. 29 Jan 2013. http://www.lilly.com.
Eli Lilly and Company. Eli Lilly and Company 2012 annual report. 2013. http://www.lilly.com. Accessed 7 Dec 2015.
Eli Lilly and Company. Lilly and Astrazeneca expand immuno-oncology research collaboration with new combinations [media release]. 22 Oct 2015. http://www.lillyoncology.com.
Eli Lilly and Company. Lilly and Astrazeneca to collaborate on immuno-oncology combination clinical trial in solid tumours [media release]. 29 May 2015. http://www.lilly.com.
US Securities and Exchange Commission. Bristol-Myers Squibb Company Form 10-K: annual report. 2010. http://www.sec.gov/. Accessed 2 Dec 2015.
Kuenen B, Witteveen PO, Ruijter R, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res. 2010;16(6):1915–25.
Li S, Kussie P, Ferguson KM. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure. 2008;16(2):216–27.
Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–74.
Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol. 2015;16:328–37.
Nokihara H, Yamamoto N, Tamura Y, et al. A phase 1 study of necitumumab (anti-EGFR monoclonal antibody) in Japanese patients with advanced solid tumors [abstract no. O3-9-2]. Ann Oncol. 2014;25(Suppl 5):v70.
Kuenen B, Witteveen PO, Ruijter R, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res. 2010;16(6):1915–23.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. K. P. Garnock-Jones is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Necitumumab: First Global Approval. Drugs 76, 283–289 (2016). https://doi.org/10.1007/s40265-015-0537-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0537-0