Abstract
Ivonescimab (依达方®) is a first-in-class, humanized, tetravalent bispecific monoclonal antibody targeting programmed cell death protein 1 (PD-1) and vascular endothelial growth factor (VEGF)-A being developed by Akeso Biopharma for the treatment of non-small cell lung cancer (NSCLC) and other solid tumours, including breast cancer, liver cancer and gastric cancer. Ivonescimab simultaneously blocks the binding of PD-1 to its ligand (PD-L1), thereby relieving PD-1/PD-L1-mediated immunosuppression, and blocks the binding of VEGF-A to its receptor (VEGFR2), thus blocking tumour angiogenesis in the tumour microenvironment. In May 2024, ivonescimab, in combination with pemetrexed and carboplatin, received its first approval in China for the treatment of patients with EGFR-mutated locally advanced or metastatic non-squamous NSCLC who have progressed after tyrosine kinase inhibitor (TKI) therapy. Clinical studies of ivonescimab are underway in multiple countries worldwide. This article summarizes the milestones in the development of ivonescimab leading to this first approval for EGFR-mutated locally advanced or metastatic non-squamous NSCLC who have progressed after TKI therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.26228597. |
A first-in-class anti-PD-1 and anti-VEGF-A bispecific antibody being developed by Akeso Biopharma for the treatment of NSCLC and other solid tumours |
Received its first approval on 24 May 2024 in China |
Approved, in combination with pemetrexed and carboplatin, for the treatment of patients with EGFR-mutated locally advanced or metastatic non-squamous NSCLC who have progressed after TKI therapy |
1 Introduction
The development of targeted therapies over the last few decades has revolutionized the treatment of oncogene-driven non-small cell lung cancer (NSCLC). EGFR-tyrosine kinase inhibitors (TKIs) are standard treatment for patients with classical EGFR alterations (exon 21 L858R or exon 19 deletion), with the third-generation EGFR-TKI osimertinib, as monotherapy (USA and EU) or in combination with chemotherapy (USA), the preferred first-line treatment for advanced EGFR-mutated NSCLC [1, 2]. However, most patients eventually develop resistance to osimertinib and there are few treatment options available for patients progressing on third-generation TKIs [1, 2], highlighting the need for novel therapies.

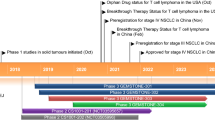
Key milestones in the development of ivonescimab, focusing on its use in the treatment of NSCLC. EGFR epidermal growth factor receptor, NMPA National Medical Products Administration, NSCLC non-small cell lung cancer, PD-1 programmed cell death protein 1, PD-L1 programmed death ligand 1, TKI tyrosine kinase inhibitor
Ivonescimab (依达方®) is a first-in-class, humanized, bispecific monoclonal antibody targeting programmed cell death protein 1 (PD-1) and vascular endothelial growth factor (VEGF)-A being developed by Akeso Biopharma for the treatment of NSCLC and other advanced solid tumours, including breast cancer, liver cancer and gastric cancer. Ivonescimab is a novel tetravalent molecule that simultaneously blocks the binding of PD-1 to its ligand (PD-L1), thereby relieving PD-1/PD-L1-mediated immunosuppression, and blocks the binding of VEGF-A to its receptor (VEGFR2), thus blocking tumour angiogenesis in the tumour microenvironment. On 24 May 2024 [3], ivonescimab, in combination with pemetrexed and carboplatin, received its first approval in China for the treatment of patients with EGFR-mutated locally advanced or metastatic non-squamous NSCLC who have progressed after TKI therapy [4]. The recommended dosage of ivonescimab in this approved setting is 20 mg/kg once every 3 weeks administered as an intravenous infusion over 60 min until disease progression or unacceptable toxicity occurs. Ivonescimab is to be administered first, followed by chemotherapy, with an interval of at least 30 min between the two treatments. Temporary interruptions and permanent discontinuation of ivonescimab may be required to manage associated adverse reactions [4]. Clinical studies of ivonescimab are underway in multiple countries worldwide.
1.1 Company Agreements
In December 2022, Akeso Biopharma and Summit Therapeutics entered into a collaboration and licensing agreement, under the terms of which Summit Therapeutics was responsible for the development and commercialization of ivonescimab in the USA, Canada, Europe and Japan, and Akeso retained development and commercialization rights for the rest of the world including China [5]. In January 2023, Summit Therapeutics closed the previously announced collaboration and licensing agreement with Akeso Inc. [6]. In June 2024, Summit announced an amendment to the agreement with Akeso Inc., under the terms of which Summit Therapeutics expanded its license territories for ivonescimab to include Latin America, including Mexico and all countries in Central America and South America, in addition to the Middle East and Africa [7].
2 Scientific Summary
2.1 Pharmacodynamics
Ivonescimab is a bispecific anti-PD-1/VEGF-A antibody designed to inhibit PD-1-mediated immunosuppression and block tumour angiogenesis in the tumour microenvironment [8, 9]. Ivonescimab binds to PD-1 and VEGF with high affinity (dissociation constant 2.46 × 10−10 and 3.3 × 10−10 mol/L, respectively) and blocks their binding to PD-L1 and VEGFR (half maximal inhibitory concentration 0.06 and 0.36 nmol/L), respectively, resulting in the inhibition of PD-1/PD-L1 and VEGF/VEGFR signalling and dose-dependent inhibition of VEGF-mediated proliferation of human umbilical vein endothelial cells in vitro [8, 9]. In the presence of VEGF, ivonescimab forms soluble complexes with VEGF dimers, resulting in a > 10-fold increase in the affinity of ivonescimab to PD-1, increased internalization of PD-1 and more potent blockade of PD-1/PD-L1 signalling, which in turn enhances T-cell activation [9]. Through cooperative binding, PD-1 also enhances the binding affinity of ivonescimab to VEGF, resulting in more potent blockade of VEGF/VEGFR signalling [9].
Additionally, the fragment crystallizable (Fc) region of ivonescimab has been engineered to reduce Fcγ receptor (FcγR) binding that could cause adverse events (AEs) and immune-related AEs [9]. Ivonescimab has silencing mutations in the Fc region that abrogate FcγR/FcγIIIa binding, resulting in significantly reduced antibody-dependent cellular cytotoxicity (ADCP), complement dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP) and cytokine release [interleukin (IL)-10 and IL-6] in in vitro studies (all p-values nonsignificant vs negative control). In contrast, penpulimab (anti-PD-1 antibody) showed significantly higher ADCC and CDC (both p < 0.001) and nivolumab (anti-PD-1 antibody) had significantly higher ADCP (p < 0.001) relative to negative control [9]
The antitumour activity of ivonescimab was demonstrated in a mouse model of EGFR-mutated NSCLC, as evidenced by dose-dependent inhibition of tumour growth in mice treated with ivonescimab [9]. Ivonescimab showed enhanced antitumour activity when used in combination with anti-CD47 [ligufalimab (AK117); p < 0.01 vs ivonescimab) or anti-CD73 [dresbuxelimab (AK119)] antibodies, relative to ivonescimab alone, in xenograft models of breast cancer and colon cancer, respectively [9].
In the clinical setting, the pharmacodynamic activity of ivonescimab may be affected by systemic corticosteroids, other immunosuppressants and angiogenesis inhibitors; therefore, administration of these agents prior to initiating ivonescimab treatment should be avoided [4]. Over an ivonescimab dose range of 0.3–30 mg/kg, no increase in the corrected QT interval was observed with increasing ivonescimab exposure [4].
2.2 Pharmacokinetics
The pharmacokinetic properties of ivonescimab are based on data from 647 patients who received ivonescimab 0.3–30 mg/kg once every 3 weeks or every 2 weeks in seven clinical studies [4].
After a single intravenous infusion, a dose-proportional increase in ivonescimab exposure was observed across a dose range of 3–30 mg/kg [4]. Steady state was reached by week 15 after multiple infusions of ivonescimab. Based on population pharmacokinetic analysis, the mean volume of distribution of ivonescimab was 4.57 L. The baseline clearance of ivonescimab decreased over time, with a clearance of 0.461 L/day at baseline and a steady-state clearance of 0.334 L/day; the maximum decrease in clearance was 26%. The elimination half-life of ivonescimab was 9.85 days [4].
The pharmacokinetics of ivonescimab have not been assessed in special populations [4]. In a population pharmacokinetic analysis, baseline body weight (31–155 kg), age and gender had no clinically relevant effects on the pharmacokinetics of ivonescimab. Mild [creatinine clearance (CLCR) 60 to < 90 mL/min] or moderate (CLCR 30 to < 60 mL/min) renal impairment and mild hepatic impairment [upper limit of normal (ULN) < total bilirubin ≤ 1.5 x ULN, or total bilirubin ≤ ULN and aspartate aminotransferase (AST) > ULN] did not affect the pharmacokinetics of ivonescimab to a clinically relevant extent in the population pharmacokinetic analysis. The effects of severe renal impairment and moderate or severe hepatic impairment on the pharmacokinetics of ivonescimab have not been determined [4].
Drug interaction studies have not been conducted for ivonescimab [4]. As ivonescimab is a monoclonal antibody and not metabolized by cytochrome P450 enzymes or other drug-metabolizing enzymes, the pharmacokinetics of ivonescimab are not expected to be influenced by coadministered drugs that inhibit or induce these enzymes [4].
Features and properties of ivonescimab
Alternative names | AK112; PD-1/VEGF bi-specific antibody—Akeso Biopharma; SMT112; ivonescimab |
Class | Antineoplastics; bispecific antibodies; immunotherapies |
Mechanism of action | Simultaneously and competitively blocks binding of PD-1 to PD-L1 (relieving PD-1/PD-L1-mediated immunosuppression) and blocks binding of VEGF-A to VEGFR2 (blocking tumour angiogenesis in the TME) |
Route of administration | Intravenous infusion |
Pharmacodynamics | Binds to PD-1 and VEGF with high affinity and blocks their binding to PD-L1 and VEGFR, respectively |
Inhibits PD-1/PD-L1 and VEGF/VEGFR signalling and dose-dependently inhibits VEGF-mediated proliferation of human umbilical vein endothelial cells in vitro | |
Through cooperative binding, VEGF binding to ivonescimab enhances affinity to PD-1, and PD-1 binding enhances ivonescimab binding to VEGF | |
Fc region of ivonescimab is engineered to reduce Fcγ receptor binding that could cause adverse events and immune-related adverse events | |
Inhibits tumour growth in a mouse model of EGFR-mutated NSCLC | |
Antitumour activity is enhanced when combined with anti-CD47 (AK117) or anti-CD73 (AK119) antibodies in xenograft models of breast cancer and colon cancer, respectively | |
Pharmacokinetics | Steady state reached by week 15; mean volume of distribution 4.57 L |
Clearance decreased over time from 0.461 L/day at baseline to 0.334 L/day at steady state; maximum decrease in clearance 26%; elimination half-life 9.85 days | |
Adverse events in NSCLC | |
Most frequent grade ≥ 3 | Decreased neutrophil counts, decreased white blood cell counts, decreased platelet counts, anaemia |
Serious | Disease progression, decreased platelet counts, anaemia, infectious pneumonia, COVID-19 |
ATC codes | |
WHO ATC code | L01X (other antineoplastic agents) |
EphMRA ATC code | L1X (all other antineoplastics) |
2.3 Therapeutic Trials
2.3.1 Non-Small Cell Lung Cancer
Pivotal Phase 3 HARMONi-A Study Ivonescimab in combination with chemotherapy significantly improved progression-free survival (PFS) in patients with relapsed advanced or metastatic NSCLC with an EGFR variant who were participating in the ongoing, randomized, double-blind, placebo-controlled, phase 3 HARMONI-A trial (NCT05184712) being conducted in China [10]. The study enrolled patients 18–75 years of age with locally advanced (stage IIIB or IIIC) or metastatic (stage IV) NSCLC that has progressed after first- or second-generation EGFR-TKIs and who have a confirmed EGFR Thr790Met-negative variant or who have progressed after third-generation EGFR-TKI as first- or second-line treatment. Patients were randomized 1:1 to receive intravenous ivonescimab 20 mg/kg (n = 161) or placebo (n = 161) in combination with pemetrexed and carboplatin on day 1 of a 3-weekly cycle for 4 cycles, followed by maintenance therapy with ivonescimab or placebo in combination with pemetrexed until disease progression, unacceptable tolerability, investigator decision, patient withdrawal of consent or death. Randomization was stratified according to prior treatment with third-generation EGFR-TKI (received or not received; n = 276 and 46, respectively) and brain metastases (present or absent; n = 72 and 250). The primary endpoint was progression-free survival (PFS) in the intent-to-treat population as assessed by the independent radiology review committee (IRRC) using Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) criteria [10].
After a median follow-up of 7.9 months (data cutoff 10 March 2023), the IRRC-assessed median PFS (primary endpoint) was significantly prolonged by 2.3 months with ivonescimab plus chemotherapy compared with placebo plus chemotherapy [median 7.1 vs 4.8 months; hazard ratio (HR) 0.46; 95% CI 0.34–0.62; p < 0.001] [10]. Overall survival (OS) data were immature at the data cutoff, and at the time of PFS analysis, 20% of patients in the ivonescimab group and 23% of patients in the placebo group had died. The IRRC-assessed objective response rate (ORR) in the ivonescimab versus placebo groups was 51% vs 35% (p = 0.006) and the median duration of response (DOR) was 6.6 vs 4.2 months. In prespecified subgroups based on demographic and disease characteristics, consistent PFS benefit was seen across most subgroups, including patients who had (ivonescimab vs placebo HR 0.48; 95% CI 0.35–0.66) and had not (HR 0.40; 95% CI 0.17–0.95) received previous third-generation EGFR-TKI therapy, and patients with (HR 0.40; 95% CI 0.22–0.73) or without (0.48; 95% CI 0.34–0.69) baseline brain metastases [10].
After a median follow-up of 17. 6months (data cutoff of 31 December 2023), OS data were 52% mature, with 77 patients having died in the ivonescimab group and 90 patients in the placebo group (median OS 17.1 vs 14.5 months; HR 0.80; 95% CI 0.59–1.08) [4, 11]. If patients treated with new immune checkpoint inhibitors were censored, the risk of death was reduced by 23% in patients receiving ivonescimab compared with those receiving placebo (HR 0.77; 95% CI 0.56–1.07) [4].
Phase 3 HARMONi-2 Study Ivonescimab monotherapy significantly improved PFS in patients with locally advanced or metastatic NSCLC who were participating in the randomized, multicentre phase 3 HARMONi-2 study (NCT05499390) being conducted in China [12]. The study enrolled patients aged ≥ 18 years with previously untreated stage IIIB/C or IV NSCLC with positive PD-L1 expression in tumour tissue [13]. Patients were randomized to receive intravenous ivonescimab at a selected dose or intravenous pembrolizumab 200 mg once every 3 weeks. The primary endpoint was PFS as assessed by the IRRC using RECIST v1.1 criteria [13].
In a prespecified interim analysis, ivonescimab demonstrated statistically significant superiority over pembrolizumab in terms of PFS [12]. The PFS benefit of ivonescimab was observed across patient subgroups, including those with PD-L1 low expression [PD-L1 tumour proportion score (TPS) 1-49%], PD-L1 high expression (PD-L1 TPS > 50%), squamous and nonsquamous histologies, as well as other high-risk clinical features [12].
Phase 2 Study Ivonescimab in combination with chemotherapy showed promising antitumour activity in patients with advanced NSCLC who were participating in the ongoing, open-label, multicentre, phase 2 study (NCT04736823) being conducted in China [14]. The study enrolled patients 18–75 years of age with locally advanced (stage IIIB or IIIC) or metastatic (stage IV) NSCLC who were categorized into three cohorts. In cohort 1, patients without driver mutations (n = 44) received first-line treatment with intravenous ivonescimab 10 or 20 mg/kg in 3-weekly cycles: in combination with pemetrexed plus carboplatin for 4 cycles, followed by ivonescimab plus pemetrexed maintenance therapy (non-squamous NSCLC); or in combination with paclitaxel plus carboplatin for 4 cycles, followed by ivonescimab maintenance therapy (squamous NSCLC). In cohort 2, patients with EGFR-sensitive mutations who failed previous EGFR-TKI therapy (n = 19) received intravenous ivonescimab 10 or 20 mg/kg in combination with pemetrexed and carboplatin in 3-weekly cycles for 4 cycles, followed by ivonescimab plus pemetrexed maintenance therapy. In cohort 3, patients who failed prior PD-1/PD-L1 inhibitor and platinum-based chemotherapy (n = 20) received intravenous ivonescimab 10 or 20 mg/kg in combination with docetaxel in 3-weekly cycles. Treatment was continued up to 24 months or until there was no clinical benefit, unacceptable toxicity occurred or other criteria for treatment discontinuation were met [14].
At last data cutoff (10 October 2023), the median follow-up durations in cohorts 1, 2 and 3 were 21.3, 25.8 and 24.7 months, respectively [15]. Across the two doses, the ORR as per RECIST v1.1 criteria (primary endpoint), in cohort 1 (n = 135) was 62% (84/135), with ORRs of 54% and 71% in non-squamous and squamous NSCLC. The disease control rates (DCRs) were 96% and 91%, the median DORs were 15.4 and 12.7 months and the median PFS were 13.3 and 11.1 months; median OS was not reached in either patient group after a median follow-up of 22.1 months. In cohorts 2 (n = 19) and 3 (n = 20), the ORRs were 68% and 40%, the DCRs were 95% and 80%, median DORs were 8.7 and 12.7 months, median PFS were 8.5 and 7.1 months and median OS were 22.5 and 17.1 months, respectively [15].
Phase 1b/2 Study Ivonescimab monotherapy showed promising efficacy in patients with immunotherapy-naïve advanced or metastatic NSCLC who were participating in the ongoing, open-label, multicentre, dose-escalation and -expansion study (NCT04900363) being conducted in China [16]. The study enrolled 108 patients aged 18–75 years with unresectable locally advanced (stage IIIB or IIIC) NSCLC not suitable for chemoradiation, or metastatic NSCLC without EGFR, anaplastic lymphoma kinase (ALK) or ROS1 mutations. Patients received ivonescimab 10, 20 or 30 mg/kg every 3 weeks or 20 mg/kg every 2 weeks until clinical benefits were observed, unacceptable toxicity occurred or other criteria for treatment discontinuation were met. After a median follow-up of 10.4 months (data cutoff 5 October 2022), the overall ORR as assessed by RECIST v1.1 (primary efficacy outcome) was 40% (43/108), with an ORR of 38% (20/53) in patients with nonsquamous and 42% (23/55) in those with squamous histology. The median PFS was 11.4 months and the median OS was not reached. In patients with PD-1 TPS < 1%, ≥ 1% and ≥ 50% the ORRs were 15%, 51% and 57%, respectively. Among the 67 patients with PD-L1 TPS ≥ 1% receiving first-line ivonescimab, the ORRs in patients receiving doses of 10, 20, and 30 mg/kg every 3 weeks were 33%, 60% and 75%, respectively, and in patients receiving 20 mg/kg every 2 weeks was 53% [16].
Pooled Analysis of Intracranial Activity Ivonescimab with or without platinum doublet chemotherapy resulted in intracranial responses in patients with advanced NSCLC and untreated, asymptomatic brain metastases (n = 35) in a pooled analysis of the phase 2 (NCT04736823; 28/174 patients) and phase 1b/2 (NCT04900363; 7/108 patients) studies [17]. Intracranial responses, as assessed by Response Assessment in Neuro-Oncology (RANO) criteria, were achieved by 34% (12/35) patients, including seven complete responses (CRs) in 11 patients who received ivonescimab plus chemotherapy (phase 2 study) and one CR among seven patients treated with ivonescimab monotherapy (phase 1b/2 study). Median PFS was 19.3 months, and no patient with baseline brain metastases had intracranial bleeding within 3 months of ivonescimab with or without chemotherapy [17].
2.3.2 Other Indications
Advanced Solid Tumours Ivonescimab showed antitumour activity in patients with advanced solid tumours who were participating in an ongoing, first-in-human, multicentre, dose-escalation and -expansion phase 1 study (NCT04047290) being conducted in Australia [18]. Eligible patients had advanced or metastatic solid tumours that had relapsed or were refractory to standard therapies or for which there were no available effective therapies. Patients (n = 51) received intravenous ivonescimab 0.3–30 mg/kg every 2 weeks, with a starting dose of 0.3 mg/kg in a single-subject cohort, followed by a 3 + 3 + 3 dose-escalation design (n = 1–10 per group). In the dose-escalation phase, two dose-limiting toxicities (grade 1 myocarditis and grade 3 hypertension) occurred in the ivonescimab 30 mg/kg group; the maximum tolerated dose of ivonescimab was determined to be 20 mg/kg every 2 weeks. Ivonescimab 10 and 20 mg/kg doses were selected for dose expansion. Antitumour responses were observed in patients receiving ivonescimab ≥ 3 mg/kg every 2 weeks. After a median follow-up of 12.8 months (data cutoff 7 June 2022), the ORR in the evaluable population was 26% (12/47) and disease control rate was 64% (30/47) [18].
Advanced Biliary Tract Cancer Ivonescimab in combination with chemotherapy showed antitumour activity in a cohort of patients with advanced or metastatic biliary tract cancer who were participating in an ongoing, open-label, multicentre phase 1b/2 study (NCT05214482) in patients with advanced malignant tumours being conducted in China [19]. One cohort in phase 2 of this trial enrolled 22 patients with locally advanced or metastatic biliary tract cancer, including 12 patients with intrahepatic cholangiocarcinoma, one with extrahepatic cholangiocarcinoma and nine with gall bladder cancer. Patients received ivonescimab 20 or 30 mg/kg every 3 weeks in combination with gemcitabine and cisplatin for up to 8 cycles, followed by ivonescimab monotherapy until disease progression or unacceptable toxicity. After a median duration of 13.8 months (31 January 2024), the overall ORR as assessed by RECIST v1.1 was 64% (14/22), with all partial responses; eight patients had stable disease. The median PFS was 8.5 months and the median OS was 16.8 months [19].
Key clinical trials of ivonescimab
Drug(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor |
|---|---|---|---|---|---|---|
Ivonescimab, carboplatin, pemetrexed | Nsq-NSCLC | 3 | Recruiting | China | NCT05184712; HARMONi-A; AK112-301 | Akeso |
Ivonescimab, carboplatin, pemetrexed | Nsq-NSCLC | 3 | Recruiting | Multinational | NCT06396065; HARMONi; AK112-301 | Summit Therapeutics |
Ivonescimab, pembrulizumab | NSCLC | 3 | Active, not recruiting | China | NCT05499390; HARMONi-2; AK112-303 | Akeso |
Ivonescimab, tislelizumab, carboplatin, paclitaxel | Sq-NSCLC | 3 | Recruiting | China | NCT05840016; AK112-306 | Akeso |
Ivonescimab, pembrolizumab, chemotherapy | Sq-NSCLC | 3 | Recruiting | Canada, USA | NCT05899608; HARMONi-3; SMT112-3003 | Summit Therapeutics |
Ivonescimab, chemotherapy | NSCLC | 2 | Recruiting | China | NCT04736823; AK112-201 | Akeso |
Ivonescimab, ligufalimab (AK117), chemotherapy | TNBC | 2 | Recruiting | China | NCT05227664; AK117-203 | Akeso |
Ivonescimab | Gynaecological tumours | 2 | Active, not recruiting | China | NCT04870177; AK112-203 | Akeso |
Ivonescimab, ligufalimab (AK117), chemotherapy | CRC | 2 | Recruiting | China | NCT05382442; AK112-206 | Akeso |
2.4 Adverse Events
Ivonescimab in combination with chemotherapy had a manageable tolerability profile in patients with relapsed advanced or metastatic NSCLC with an EGFR variant who were participating in the ongoing, randomized, double-blind, placebo-controlled, phase 3 HARMONI-A trial (NCT05184712; Sect. 2.3.1) [10]. Over a median of 8.8 cycles in patients receiving ivonescimab plus chemotherapy (n = 161) and 7.3 cycles in those receiving placebo plus chemotherapy (n = 161), treatment-emergent AEs occurred in 99% and 98% of patients in the respective groups, with 62% and 49% being of grade ≥ 3 severity. The most common any-grade treatment-emergent AEs in patients receiving ivonescimab plus chemotherapy were chemotherapy related, including any-grade decreased white blood cell count (66% vs 65% with placebo plus chemotherapy), anaemia (63% vs 73%), decreased neutrophil count (60% vs 58%), decreased platelet count (48% vs 41%), increased AST (44% vs 31%) and increased alanine aminotransferase (37% vs 37%). The most common (incidence > 10% of patients) grade ≥ 3 treatment-emergent AEs with ivonescimab combination therapy were decreased neutrophil count (31% vs 19% with placebo plus chemotherapy), decreased white blood cell count (21% vs 17%), decreased platelet count (16% vs 12%) and anaemia (16% vs 14%). Treatment emergent AEs led to ivonescimab and placebo discontinuation in 6% and 3% of patients, respectively [10].
Immune-related AEs were reported in 24% and 6% of patients in the ivonescimab and placebo groups [10]. Grade ≥ 3 immune-related AEs occurred in 6% and 3% of patients in the ivonescimab and placebo groups, with the most common being rash (3% vs 1%), dermatitis (1% vs 0%) and interstitial lung disease (1% vs 1%). AEs of special interest (AESIs) related to VEGF blocking occurred in 30% of patients in the ivonescimab and 16% of patients in the placebo group, with the events being mostly mild or moderate in severity. Grade ≥ 3 AESIs occurred in 3% of patients in the ivonescimab group and 2% of patients in the placebo group, with the most common being hypertension (2% vs 2%), proteinuria (1% vs 0%) and congestive heart failure (1% vs 0%) [10].
Pooled Analysis The tolerability profile of ivonescimab plus chemotherapy was confirmed in a pooled analysis of a phase 1b study (NCT05116007), a phase 2 study (NCT04736823) and the phase 3 HARMONi-A study (NCT05184712) [4]. The pooled analysis included tolerability data for 316 patients treated with ivonescimab 20 mg/kg every 3 weeks plus chemotherapy, including 295 patients with NSCLC and 21 patients with small-cell lung cancer. The median treatment duration was 201 days (median of 9 treatment cycles), with 86% of patients having received ivonescimab combination therapy for ≥ 3 months, 57% for ≥ 6 months and 9% for ≥ 12 months. Adverse reactions with ivonescimab plus chemotherapy occurred in 93% of patients, with the most common (incidence > 15% of patients) adverse reactions being anaemia (35%), leukopenia (30%), neutropenia (29%), increased AST (25%), decreased platelet count (23%) and proteinuria (20%). Grade ≥ 3 adverse reactions occurred in 48% of patients treated with ivonescimab plus chemotherapy, with neutropenia (19%), leukopenia (12%), anaemia (11%), and decreased platelet count (9%) reported most frequently (incidence > 5%) [4].
2.5 Ongoing Clinical Trials
In addition to the ongoing trials discussed earlier in Sect. 2.3, the randomized, double-blind, multinational, phase 3 HARMONi study (NCT06396065) is recruiting patients to evaluate the efficacy and safety of ivonescimab versus placebo in combination with pemetrexed and carboplatin in ≈ 420 patients with EGFR-mutated locally advanced of metastatic NSCLC who have progressed following EGFR-TKI therapy. The primary outcome measures are PFS and OS and secondary outcome measures include ORR and the incidence and severity of AEs.
Recruitment is underway for a randomized, multicentre phase 3 study (NCT05840016) to evaluate the efficacy and safety of ivonescimab plus chemotherapy versus PD-1 inhibitor plus chemotherapy as first-line treatment in ≈ 396 patients with advanced squamous NSCLC. The primary outcome is PFS and secondary outcomes include OS, ORR and DOR. Patients are also being recruited for the randomized, multicentre, phase 3 HARMONi-3 study (NCT05899608) to evaluate the efficacy and safety of ivonescimab plus chemotherapy versus pembrolizumab plus chemotherapy as first-line treatment in ≈ 400 patients with metastatic squamous NSCLC. The primary outcome is OS and secondary outcomes include PFS and the incidence and severity of AEs.
A phase 2 study (NCT05227664) is recruiting patients to assess the efficacy, safety and pharmacokinetics of ivonescimab and ligufalimab (AK117) plus chemotherapy versus ivonescimab plus chemotherapy and ligufalimab plus chemotherapy in ≈ 80 patients with locally advanced or metastatic triple-negative breast cancer who have not received prior systemic therapy. Also recruiting patients is a phase 2 study (NCT05382442) to assess the efficacy, safety and pharmacokinetics of ivonescimab (monotherapy or combination therapy with chemotherapy) with or without ligufalimab in ≈ 104 patients with metastatic colorectal cancer who are not suitable for surgery. A phase 2 study (NCT04870177) is underway to assess the efficacy and safety of ivonescimab in ≈ 270 patients with advanced gynaecological tumours. Also underway is an open-label, multicentre, phase 1b/2 study (NCT05229497) to evaluate the efficacy and safety of ivonescimab and ligufalimab in ≈ 114 patients with advanced malignant tumours.
Other phase 2 studies are underway, including studies that will evaluate ivonescimab monotherapy in patients with unresectable hepatocellular carcinoma (NCT05432492) and ivonescimab combination therapy in patients with NSCLC (NCT05247684). With the approval of ivonescimab, many investigator-initiated studies have also been planned.
References
Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–57.
Network NCC. NCCN clinical practice guidelines in oncology: non small cell lung cancer (version 7.2024). 2024. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 10 July 2024.
PR Newswire. Ivonescimab in combination with chemotherapy approved in China by NMPA for 2L+ EGFRm NSCLC based on HARMONi-A clinical trial: positive trend observed in overall survival towards ivonescimab plus chemotherapy [media release]. 1 June 2024. https://www.prnewswire.com/news-releases/ivonescimab-in-combination-with-chemotherapy-approved-in-china-by-nmpa-for-2l-egfrm-nsclc-based-on-harmoni-a-clinical-trial-positive-trend-observed-in-overall-survival-towards-ivonescimab-plus-chemotherapy-302161109.html.
Akeso Biopharma. Ivonescimab (依达方): Chinese prescribing information. Zhongshan City: Kangfang Sino Pharmaceutical Co; 2024.
Akeso Biopharma. Akeso Inc. announces collaboration and license agreement for up to US$5 billion with Summit Therapeutics to accelerate global development and commercialization of its breakthrough bispecific antibody, ivonescimab (PD-1/VEGF) [media release]. 6 Dec 2022. https://www.akesobio.com/en/media/akeso-news/20221206/.
Summit T. Summit Therapeutics closes deal with Akeso Inc. to in-license breakthrough innovative bispecific antibody [media release]. 20 Jan 2023. https://www.smmttx.com/pressrelease/summit-therapeutics-closes-deal-with-akeso-inc-to-in-license-breakthrough-innovative-bispecific-antibody/.
Summit Therapeutics. Summit raises $200 million; also expands license territories for ivonescimab [media release]. 3 June 2024. https://www.smmttx.com/pressrelease/summit-raises-200-million-also-expands-license-territories-for-ivonescimab/.
Zhong TT, Huang ZL, Pang XH, et al. AK112, a tetravalent bispecific antibody targeting PD-1 and VEGF, enhances binding avidity and functional activities and elicits potent anti-tumor efficacy in pre-clinical studies [abstract no. 521 plus poster]. J Immunother Cancer. 2022;10(Suppl 2):A546–7.
Zhong TT, Huang ZL, Pang XH, et al. Mechanism of action of ivonescimab (AK112/SMT112): a first-in-class tetravalent Fc-silent bispecific antibody with dual blockade of PD-1 and VEGF that promotes cooperative biological effects [abstract no. B123 plus poster]. Mol Cancer Ther. 2023;22(12_Suppl).
Fang W, Zhao Y, Luo Y, et al. Ivonescimab plus chemotherapy in non-small cell lung cancer with EGFR variant: a randomized clinical trial. JAMA. 2024. https://doi.org/10.1001/jama.2024.10613.
Zhang L, Fang W, Zhao Y, et al. Ivonescimab combined with chemotherapy in patients with EGFR-mutant non-squamous non-small cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor treatment (HARMONi-A): a randomized, double-blind, multi-center, phase 3 trial [abstract no. 8508 plus oral presentation]. J Clin Oncol. 2024;42(16_suppl).
Summit Therapeutics. Ivonescimab monotherapy decisively beats pembrolizumab monotherapy head-to-head, achieves statistically significant superiority in PFS in first-line treatment of patients with PD-L1 positive NSCLC in China [media release]. 30 May 2024. https://www.smmttx.com/pressrelease/ivonescimab-monotherapy-decisively-beats-pembrolizumab-monotherapy-head-to-head-achieves-statistically-significant-superiority-in-pfs-in-first-line-treatment-of-patients-with-pd-l1-positive-nsclc-in/.
US National Institutes of Health. ClinicalTrials identifier NCT05499390. 2022. https://clinicaltrials.gov/study/NCT05499390. Accessed 11 July 2024.
Zhao Y, Chen G, Chen J, et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinicalMedicine. 2023;62: 102106.
Zhang L, Fang WF, Zhao Y, et al. Phase II results of ivonescimab (ivo) a novel PD-1/VEGF bispecific in combination with chemotherapy for first-line treatment of patients (pts) with advanced/metastatic squamous (sq) non-small cell lung cancer (NSCLC) [abstract no. 68P plus poster]. ESMO Open. 2024;9(Suppl 3):36.
Wang L, Luo Y, Ren S, et al. A phase 1b study of ivonescimab, a programmed cell death protein-1 and vascular endothelial growth factor bispecific antibody, as first- or second-line therapy for advanced or metastatic immunotherapy-naive NSCLC. J Thorac Oncol. 2024;19(3):465–75.
Zhang L, Zhou C, Fang WF, et al. Intracranial (IC) activity of ivonescimab (ivo) alone or in combination with platinum doublet chemotherapy (PC) in patients (pts) with advanced non-small cell lung cancer (aNSCLC) and brain metastases (BMs) [abstract no. 174P plus poster]. ESMO Open. 2024;9(S3):3–4.
Frentzas S, Austria Mislang AR, Lemech C, et al. Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors. J Immunother Cancer. 2024;12(4): e008037.
Yung J, Xie Y, Xu Q, et al. The safety and efficacy of ivonescimab in combination with chemotherapy as first-line treatment for advanced biliary tract cancer [abstract no. 4095 plus poster]. J Clin Oncol. 2024;42(16_suppl).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Ivonescimab: First Approval. Drugs (2024). https://doi.org/10.1007/s40265-024-02073-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s40265-024-02073-w