Abstract
Jiangsu Chia-Tai Tianqing Pharmaceutical and Advenchen Laboratories are co-developing anlotinib (Focus V®) for the treatment of advanced cancer. Anlotinib is an oral small molecule inhibitor of multiple receptor tyrosine kinases, with a broad spectrum of inhibitory effects on tumour angiogenesis and growth. Anlotinib is approved in China for the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have undergone progression or recurrence after ≥ 2 lines of systemic chemotherapy. Anlotinib is also undergoing phase II and/or III clinical development for various sarcomas and carcinomas in China, USA and Italy. This article summarizes the milestones in the development of anlotinib leading to this first approval for NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anlotinib (Focus V®) is an oral small molecule inhibitor of multiple receptor tyrosine kinases that is being co-developed by Jiangsu Chia-Tai Tianqing Pharmaceutical (a subsidiary of Sino Biopharmaceutical) and Advenchen Laboratories for the treatment of advanced cancer. Aberrant or constitutive activation of receptor tyrosine kinases is associated with increased cell proliferation, survival and metastasis in a wide range of cancers [1]. Anlotinib is designed to primarily inhibit vascular endothelial growth factor receptors (VEGFR) 2 and 3, fibroblast growth factor receptors (FGFR) 1–4, platelet-derived growth factor receptors (PDGFR) α and β, c-Kit and Ret; thus, the drug has a broad spectrum of inhibitory effects on tumour angiogenesis and growth [2].
Availability of effective drugs for first- and second-line and maintenance therapy in NSCLC has led to increased number of patients requiring third-line treatment, but options are limited [3]. Anlotinib is approved in China as a single drug therapy for patients with locally advanced or metastatic NSCLC who have undergone progression or recurrence after ≥ 2 lines of systemic chemotherapy [4]. Patients with EGFR- or ALK-positive tumours should have received appropriate targeted drug therapy prior to receiving chemotherapy [4]. The recommended dosage of anlotinib is 12 mg once daily taken orally continuously for 2 weeks of each 3-week cycle until disease progression or unacceptable tolerability [4].
Anlotinib is undergoing phase II and/or III clinical development in China, USA and Italy for soft tissue sarcomas and a wide range of carcinomas.
1.1 Company Agreements
Advenchen Laboratories has an alliance with Jiangsu Chia-Tai Tianqing Pharmaceutical for the clinical development of anlotinib in cancer [5].

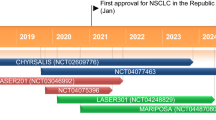
Key development milestones in the development of anlotinib, focusing on its use in NSCLC. GC gastric cancer, IND investigational new drug, mCRC metastatic colorectal cancer, MTC medullary thyroid cancer, NSCLC non-small cell lung cancer, STS soft tissue sarcoma
2 Scientific Summary
2.1 Pharmacodynamics
Anlotinib has high selectivity for VEGFR 2 and 3 (IC50 values 0.2 and 0.7 nmol/L) and was 20-fold more potent than sunitinib in inhibiting these receptor kinases [6]. In vitro, anlotinib selectively inhibited VEGF-stimulated receptor phosphorylation and proliferation of HUVEC, and inhibited angiogenesis [6]. Anlotinib inhibited VEGF-, PDGF BB- and FGF2-induced angiogenesis in vitro and in vivo, potentially by inhibiting the activation of VEGFR 2, PDGFR β and FGFR 1 and their common downstream ERK signalling [7]. In vivo, the anti-angiogenic effect of anlotinib appeared to be superior to sunitinib, sorafenib and nintedanib [7]. In cell line-derived [8] and human [6] xenograft models, anlotinib showed antitumour effects, which appeared to be superior to carboplatin and paclitaxel [8] or sunitinib [6].
2.2 Pharmacokinetics
Following a single oral dose of anlotinib 12 mg in patients with advanced solid tumours, the time to reach the maximum plasma concentration of the drug was 7.3 h [2]. In vitro studies indicated that anlotinib was primarily metabolized by CYP-mediated hydroxylation and dealkylation, with its oxidized metabolites excreted directly into the bile or excreted after glucuronidation. Anlotinib has poor renal clearance (0.004 L/h/kg), with ≈ 0.9% of the dose excreted into urine. Anlotinib is eliminated slowly, with a long terminal elimination half-life (t1/2; 98 h after a single 12 mg dose), which appeared to be dose-dependent. The long t1/2 results in marked accumulation of the drug over time (mean accumulation ratio 12). Following a once-daily on days 1–14 of each 21-day cycle regimen, plasma concentrations of anlotinib reached maximum on day 14 and decreased subsequently with the 7-day rest period until the start of the next treatment cycle [2].
2.3 Therapeutic Trials
In the first-in-human, open-label phase I study (NCT01833923) in patients with advanced refractory solid tumours, anlotinib 12 mg once-daily on days 1–14 of each 21-day cycle showed promising antitumour activity and subsequently, this dosage was selected for further studies [2]. All studies discussed further in this section used this dosage.

Features and properties of anlotinib
Alternative names | AL-3818; ALTN; anlotinib hydrochloride; FOCUS V |
Class | Antineoplastics; Cyclopropanes; Indoles; Quinolines; Small molecules |
Mechanism of action | VEGFR antagonists; FGFR antagonists; PDGFR antagonists; c-Kit inhibitors; Ret inhibitors |
Route of administration | Oral |
Pharmacodynamics | Selective for VEGFR 2/3; inhibits angiogenesis; shows antitumour effects in xenograft models |
Pharmacokinetics | Tmax 7.3 h after a single 12 mg oral dose; metabolized by CYP enzymes, with oxidized metabolites excreted directly into the bile or excreted after glucuronidation; long t1/2 (98 h after the 12 mg dose), poor renal clearance (0.004 L/h/kg) and marked drug accumulation (ratio 12) |
Most frequent adverse events | Hypertension, fatigue, hand-foot skin reactions, gastrointestinal reactions, abnormal liver function, abnormal thyroid function, high blood lipids and proteinuria |
ATC codes | |
WHO ATC code | L01X-E (Protein kinase inhibitors) |
EphMRA ATC code | L1H (Protein Kinase Inhibitor Antineoplastics) |
Chemical name | 1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine |
2.3.1 Non-Small Cell Lung Cancer
The efficacy of anlotinib in patients with stage 3B/4 NSCLC who progressed after ≥ 2 lines of previous therapies was demonstrated in the randomised, double-blind, placebo-controlled, multicentre phase II/III ALTER-0303 trial (NCT02388919) [9]. Patients were randomized to anlotinib (n = 294) or placebo (n = 143) until disease progression or unacceptable toxicity. Anlotinib significantly prolonged median overall survival (OS) versus placebo [9.63 vs. 6.30 months; hazard ratio (HR) 0.68; p = 0.0018; primary endpoint]. Anlotinib also provided significant (p < 0.0001) progression-free survival (PFS; 5.37 vs. 1.40 months; HR 0.25), overall response rate (ORR; 9% vs. 1%) and disease control rate (DCR; 81% vs. 37%) benefits versus placebo [9].
In ALTER-0303, anlotinib improved OS and/or PFS in patients with wild-type EGFR gene [10] as well as in those with EGFR-sensitizing mutations [10, 11] or T790 M [11]. Among anlotinib recipients, patients with EGFR amplification had shorter PFS compared to those with normal EGFR copy number, although this observation requires further verification using larger samples [11]. In anlotinib recipients, the independent risk factors were ratio of granulocytes to lymphocytes at progressive disease and baseline sum of longest diameters of target lesions for PFS and OS, and elevated alkaline phosphatase levels for PFS [12]. The independent protective factors were elevated thyroid-stimulating hormone (TSH) or low density lipoprotein cholesterol levels, hypercholesteremia, hypertension, hand-foot skin reaction (HFSR) and age for PFS, and elevated TSH level, hypertriglyceridemia, rash and female gender for OS [12]. A decrease in activated circulating endothelial cell counts during initial periods of anlotinib therapy may predict longer PFS and good response [13].
Anlotinib significantly prolonged PFS (primary endpoint) versus placebo in patients with metastatic or recurrent advanced NSCLC who progressed after ≥ 2 lines of previous therapies in the randomized, double-blind, multicentre phase II ALTER-0302 trial (NCT01924195) [14]. Patients were randomized to anlotinib (n = 60) or placebo (n = 57) until disease progression or unacceptable toxicity. PFS was significantly longer with anlotinib than with placebo (4.8 vs. 1.2 months; HR 0.32; p < 0.0001). The PFS benefit was seen in all subgroups by age, sex, smoking history, disease stage 4, efficacy of previous treatments, histology, number of metastases (with the exception of ≤ 3 metastases subgroup) and EGFR mutation status. Anlotinib was also associated with a significantly higher ORR (10.0% vs. 0.0%; p < 0.05) and DCR (83.3% vs. 31.6%; p < 0.0001) versus placebo. The median OS did not differ significantly between the anlotinib and placebo groups (9.3 vs. 6.3 months) [14].
2.3.2 Other Cancers
Favourable efficacy data for anlotinib in other cancers are available as abstracts from ongoing, multicentre phase II trials [15,16,17,18]. In a single-arm trial (NCT02072044), anlotinib showed efficacy in patients with metastatic renal cancer (mRCC) who progressed on or were intolerant to previous tyrosine kinase inhibitor (sorafenib or sunitinib) therapy [15]. In 42 evaluable patients, the median PFS was 11.8 months (95% CI 8.5 to not estimable; primary endpoint), ORR was 19.1% (8.60–34.12) and 6-week DCR was 90.5% (77.4–97.3%) (data cut-off 15 May 2015) [15]. In a randomized trial (NCT02072031), the efficacy of first-line anlotinib in patients with mRCC was similar to that of sunitinib [17]. There were no significant differences between anlotinib (n = 93) and sunitinib (n = 40) groups for PFS (11.3 vs. 11.0 months; primary endpoint), ORR (24.4% vs. 23.3%) or 6-week DCR (97.8% vs. 93.0%) (data cut-off 15 May 2015) [17].
In a single-arm trial (NCT01874873) in 55 evaluable patients with medullary thyroid carcinoma treated with anlotinib, the mean PFS was 12.8 months (median PFS still not reached) (primary endpoint), ORR was 48.28% and 48-week DCR was 84.53% (data cut-off May 2015) [18]
In a single-arm trial (NCT01878448) in 154 evaluable patients with soft tissue sarcoma treated with anlotinib, 12-week progression-free rate was 57.23% (primary endpoint), median PFS was 5.63 months and ORR was 11.45% [16]. Anlotinib was effective across many pathological subtypes of soft tissue sarcoma, with the highest 12-week progression-free rate (76.92%) seen for alveolar soft part sarcoma [16].
Key clinical trials of anlotinib (sponsored by Jiangsu Chia-tai Tianqing Pharmaceutical unless stated otherwise)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Anlotinib, PL | Differentiated thyroid cancer | II/III | Recruiting | China | NCT02586337; ALTN-01-IIDTC |
Anlotinib, PL | Gastric cancer (≥ 3rd line) | II/III | Recruiting | China | NCT02461407; ALTN-05-II |
Anlotinib | GNT (grade 3) | II | Recruiting | China | NCT03457844; ALTN-13-II |
Anlotinib | Hepatocellular carcinoma | II | Recruiting | China | NCT02809534; ALTN-08-II |
Anlotinib, PL | Medullary thyroid carcinoma | II/III | Recruiting | China | NCT02586350; ALTN-01-IIMTC |
Anlotinib, PL | Metastatic colorectal cancer | II/III | Ongoing | China | NCT02332499; ALTN-07-IIB |
Anlotinib, PL | Non-small cell lung cancer | II | Completed | China | NCT01924195; ALTER0302 |
Anlotinib, PL | Non-small cell lung cancer | II/III | Completed | China | NCT02388919; ALTER0303 |
Anlotinib, PL | Non-small cell lung cancer | II/III | Ongoing | China | NCT02029209; ALTN-03-II 1.2-2 |
Anlotinib, PL | Oesophageal SCC | II | Recruiting | China | NCT02649361; ALTN-11-II |
Anlotinib | Ovarian cancera | I/II | Recruiting | USA | NCT02558348; AL3818-US-001b |
Anlotinib + CHEMO | Ovarian cancerc | I/II | Recruiting | USA | NCT02584478; AL3818-US-002b |
Anlotinib | Renal cell carcinoma | II | Ongoing | China | NCT02072044; ALTN-06-IIB |
Anlotinib, PL | Small-cell lung cancer (≥ 3rd line) | II | Recruiting | China | NCT03059797; ALTN-12-II |
Anlotinib, DAC | Soft tissue sarcomad (≥ 2nd line) | III | Recruiting | USA, Italy | NCT03016819; APROMISSb |
Anlotinib, PL | Soft tissue sarcoma (≥ 2nd line) | II/III | Ongoing | China | NCT02449343; ALTN-02-IIB |
2.4 Adverse Events
Oral anlotinib (12 mg once-daily on days 1–14 of each 21-day cycle) had a manageable tolerability profile in patients with NSCLC in the ALTER-0302 trial [14]. All adverse events (AEs) occurred during the trial were managed with dose reduction or symptomatic treatments, and there was no treatment-related death. Anlotinib was associated with significantly (p ≤ 0.0140) higher incidences of any grade AEs (91.7% vs. 70.2%) and treatment-related grade 3 or 4 AEs (21.7% vs. 5.3%) than placebo. AEs (any grade) that occurred significantly (p value not reported) more frequently with anlotinib than with placebo were: hypertension (55.0% vs. 5.3%), elevated TSH (36.7% vs. 1.8%), HFSR (28.3% vs. 1.8%), elevated thyroglobulin (26.7% vs. 5.3%), elevated total cholesterol (25.0% vs. 5.3%) and diarrhoea (23.3% vs. 5.3%). The most common treatment-related grade 3 or 4 AEs with anlotinib were hypertension (10.0% vs. 0% with placebo), elevated thyroglobulin (5.0% vs. 1.8%), and HFSR (3.3% vs. 0%) [14]. The tolerability profile of anlotinib in ALTER-0303 was generally similar to that in ALTER-0302 [9].
The most common treatment-related AEs (any grade) with anlotinib in patients with mRCC (NCT02072044) were hypertension (45.2%), HFSR (42.9%), fatigue (35.7%), proteinuria (35.7%), anorexia (33.3%), hypothyroidism (28.6%), hoarseness (23.8%), hypertriglyceridemia (21.3%), hypercholesterolemia (21.4%), oral mucositis (21.4%), skin rash (21.4%) and thrombocytopenia (14.3%) [15]. The most common grade 3 or 4 AEs included lymphopenia (7.1%), hypertension (4.8%) and hypothyroidism (4.8%) [15].
In patients with mRCC (NCT02072031), anlotinib had better tolerability profile than sunitinib [17]. The incidences of the following AEs (any grade) were significantly (p ≤ 0.015) lower with anlotinib than with sunitinib: hand-foot syndrome (41.1% vs. 65.1%), eyelid oedema (2.2% vs. 25.4%), hair depigmentation (0.0% vs. 14.0%), skin yellowing (0.0% vs. 37.2%), neutropenia (3.3% vs. 44.2%), thrombocytopenia (11.1% vs. 58.1%) and anaemia (4.4% vs. 34.9%). The incidence of grade 3 or 4 AEs was also significantly (p < 0.01) lower with anlotinib than with sunitinib (28.9% vs. 55.8%), especially for thrombocytopenia (0% vs. 11.6%) and neutropenia (0.0% vs. 9.3%). Moreover, although there were no significant between-group differences, anlotinib was associated with less frequent hypertension (50% vs. 67.4%), increase of creatinine (14.4% vs. 27.9%) and hypophosphatemia (13.3% vs. 27.9%) than sunitinib. On the other hand, hypercholesterolemia appeared to occur more frequently with anlotinib than with sunitinib (25.6% vs. 11.6%) [17].
The types of AEs with anlotinib in patients with medullary thyroid cancer (NCT01874873) [18] or soft tissue sarcoma (NCT01878448) [16] were broadly similar to that seen in patients with NSCLC or mRCC.
2.5 Ongoing Clinical Trials
A phase III trial in the USA and Italy is recruiting patients to evaluate the safety and efficacy of anlotinib in metastatic or advanced alveolar soft part sarcoma (single-arm, open-label), or leiomyosarcoma or synovial sarcoma (vs. dacarbazine) (NCT03016819; APROMISS). A phase II/III trial in China is evaluating changes in circulating endothelial cells and blood perfusion parameters as predictive markers of anlotinib efficacy in patients with advanced NSCLC (NCT02029209). Phase II/III trials in China are ongoing or recruiting patients to evaluate the efficacy and safety of anlotinib in differentiated thyroid cancer (NCT02586337), medullary thyroid carcinoma (NCT02586350), gastric cancer (NCT02461407), metastatic colorectal cancer (NCT02332499) or soft tissue sarcoma (NCT02449343).
Phase II trials in China are ongoing or recruiting patients to evaluate the safety and efficacy of anlotinib in oesophageal squamous cell carcinoma (NCT02649361), advanced gastroenteropancreatic neuroendocrine tumour (NCT03457844), hepatocellular carcinoma (NCT02809534), renal cell carcinoma (NCT02072044) and small-cell lung cancer (NCT03059797). A phase I/II trial in the USA is recruiting patients to evaluate the safety and efficacy of anlotinib in recurrent or metastatic endometrial, ovarian or cervical cancer (NCT02558348). Another phase I/II trial in USA is recruiting patients to evaluate the safety and efficacy of adding anlotinib to standard platinum-based chemotherapy (carboplatin and paclitaxel) concurrently and continued as a maintenance therapy for up to 12 months (NCT02584478).
3 Current Status
Anlotinib received its first global approval in China on 10 May 2018 as a single drug therapy for patients with locally advanced or metastatic NSCLC who have undergone progression or recurrence after ≥ 2 lines of systemic chemotherapy [4].
Change history
06 August 2018
Following a once-daily on days 1–14 of each 21-day cycle regimen, plasma concentrations of anlotinib reached maximum on day 14 and decreased subsequently with the 7-day rest period until the start of the next treatment cycle [2].
References
Regad T, Targeting RTK. Signaling pathways in cancer. Cancers. 2015;7(3):1758–84.
Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105.
Syrigos KN, Saif MW, Karapanagiotou EM, et al. The need for third-line treatment in non-small cell lung cancer: an overview of new options. Anticancer Res. 2011;31(2):649–59.
Chia Tai Tianqing Pharmaceutical. Anlotinib hydrochloride capsules. 2018. https://www.cttq.com Accessed 29 May 2018.
Advenchen Laboratories. 2018. http://advenchen.com. Accessed 30 May 2018.
Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–19.
Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene. 2018;654:77–86.
Taurin S, Yang CH, Reyes M, et al. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer. 2018;28(1):152–60.
Han B, Li K, Wang Q, et al. Efficacy and safety of third-line treatment with anlotinib in patients with refractory advanced non-small-cell lung cancer (ALTER-0303): a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2017;18(Suppl 1):S3.
Han B, Li K, Wang Q, et al. Efficiency of anlotinib as 3rd line treatment in patients with different EGFR gene status, an exploratory subgroup analysis of ALTER0303 trial [abstract no. P3.03-006]. J Thorac Oncol. 2017;12(11 Suppl 2):S2275.
Han B, Zhao Y, Li K, et al. Blood samples NGS for baseline molecular signature of anotinib treated advanced NSCLC patients in ALTER0303 trial [abstract no. P3.03-017]. J Thorac Oncol. 2017;12(11 Suppl 2):S2279.
Li K, Wang J, Han B, et al. Impact factor analysis for efficacy and prognosis of anlotinib in NSCLC as third-line treatment: data from trial alter 0303 [abstract no. P3.01-087]. J Thorac Oncol. 2017;12(11 Suppl 2):S2236.
Li K, Wang J, Wang X, et al. Level of activated circulating endothelial cells to predict progression-free survival of anlotinib treatment in patients with NSCLC: analysis on ALTER-0303 study [abstract no. e20530]. J Clin Oncol. 2017;35(15 Suppl):e20530.
Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–61.
Zhou AP, Bai Y, Song Y, et al. Anlotinib in metastatic renal cell carcinoma (mRCC) with a previous anti-VEGFR TKI: preliminary results from a multicenter, phase II trial [abstract no. e16082]. J Clin Oncol. 2016;34(15 Suppl):e16082.
Chi Y, Sun Y, Cai J, et al. Phase II study of anlotinib for treatment of advanced soft tissues sarcomas [abstract no. 11005]. J Clin Oncol. 2016;34(Suppl 15):11005.
Zhou AP, Ma J, Bai Y, et al. Anlotinib versus sunitinib as first line treatment for metastatic renal cell carcinoma (mRCC): preliminary results from a randomized phase II clinical trial [abstract no. 4565]. J Clin Oncol. 2016;34(15 Supplement):4565.
Sun Y, Chi Y, Tang P, et al. Phase II study of anlotinib for treatment of advanced medullary thyroid carcinoma [abstract no. 6015]. J Clin Oncol. 2016;34(15 Suppl):6015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Yahiya Y. Syed is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Anlotinib: First Global Approval. Drugs 78, 1057–1062 (2018). https://doi.org/10.1007/s40265-018-0939-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0939-x