Abstract
Background
Previous studies have shown conflicting observations regarding the correlation between sodium-glucose-cotransporter-2 inhibitors (SGLT2i) and acute renal failure. Although wide use has contributed to the accumulation of safety information on SGLT2i, the examination of the countries reporting cases of SGLT2i use and influence of concomitant drugs has been insufficient in studies using spontaneous adverse event reporting databases.
Objective
We aimed to re-examine the correlation between SGLT2i and acute renal failure using the latest United States Food and Drug Administration’s Adverse Event Reporting System (FAERS) records and to conduct a stratified analysis for the reporting countries (Japan or other countries), as well as the concomitant use of drugs such as angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) with SGLT2i.
Patients and Methods
The reporting odds ratio (ROR) and 95% confidence interval (CI) for cases recorded on FAERS from January 2013 to March 2020 were calculated. We then limited the cases to patients using SGLT2i and receiving treatment for diabetes mellitus and then calculated the ROR. A stratified analysis was performed for reporting countries (Japan or other countries), and the presence or absence of concomitant use of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) to examine their influence on the correlation between SGLT2i and acute renal failure.
Results
Of the 5,337,069 cases of adverse events recorded on FAERS, 410,569 were cases in which patients had received treatment for diabetes. The ROR for SGLT2i calculated from the total analysis subjects was 4.16 (95% CI 4.01–4.31), suggesting its correlation with acute renal failure. Similar results were obtained for the cases in which patients had received treatment for diabetes. However, the stratified analysis of these diabetes-treatment cases for reporting countries showed no correlation between SGLT2i and acute renal failure in cases reported in Japan with ROR 0.58 (95% CI 0.49–0.69). In contrast, a correlation was suggested in cases reported in countries other than Japan with ROR 1.91 (95% CI 1.83–1.98). Moreover, the stratified analysis for the concomitant use of an ACEi or ARB showed that the ROR tended to be low in the cases with one of these drugs.
Conclusion
Examination with the signal detection method using FAERS suggested the correlation between SGLT2i and the onset of acute renal failure. However, when focusing on the cases reported in Japan, such a correlation was not suggested. In addition, this study indicated that the signal of acute renal failure tends to be reduced in cases with the concomitant use of either an ACEi or ARB. Through this study we suggest that patients should be closely monitored when they take SGLT2i without an ACEi or ARB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We analyzed the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS), and observed a correlation between the sodium-glucose cotransporter-2 (SGLT2) inhibitors and the onset of acute renal failure (ARF). |
The stratified analysis for reporting countries showed that such a correlation was not observed when focusing on the cases reported in Japan. |
The stratified analysis for concomitant drugs showed that the signal for ARF tended to be reduced in cases with the use of either an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker. |
1 Introduction
Sodium-glucose-cotransporter-2 inhibitors (SGLT2i) are a relatively new class of oral glucose-lowering drugs, and their mechanism of action is to facilitate urinary excretion of glucose by inhibiting its SGLT2-mediated reabsorption in the renal proximal tubule. With the worldwide increase in the clinical use of SGLT2i, disparities regarding the correlation between SGLT2i and acute renal failure have emerged. The US Food and Drug Administration (FDA), for example, issued a warning for the onset of acute renal failure owing to canagliflozin and dapagliflozin on 14 June 2016 [1]. In addition, the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan revised the precautions to add the risk of dehydration to the section on serious side effects of canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, luseogliflozin, and tofogliflozin on 9 January 2015. However, it did not issue clear alerts with regard to the risk of acute renal failure, and the Japan Diabetes Society only calls attention to these as a type of dehydration-related event [2,3,4].
Furthermore, existing clinical studies have presented conflicting observations on the correlation between SGLT2i and acute renal failure. The EMPA-REG study, which was a placebo-controlled, randomized, double-blind study of patients with type 2 diabetes with a high risk of a cardiovascular event, found that empagliflozin suppressed the progression of renal failure [5]. In addition, a meta-analysis that reviewed 109 randomized clinical trials (RCTs) showed that SGLT2i reduced the risk of acute renal failure (relative risk 0.59; 95% confidence interval (CI) 0.39–0.89); however, this was because of the strong influence of the EMPA-REG study mentioned above. When the EMPA-REG study was excluded from the analysis, no significant trend was observed with relative risk = 0.48 (95% CI 0.14–1.64) [6]. Moreover, the latest retrospective cohort study of patients with type 2 diabetes, which was published in October 2020, did not report a significant increase in acute renal failure in patients receiving SGLT2i compared to that in patients receiving other anti-diabetic drugs [7].
Signal detection using adverse event report databases is widely used for post-marketing monitoring of drug safety [8]. The adverse event report databases include the FDA Adverse Event Reporting System (FAERS) [9] published by the FDA, the Japanese Adverse Drug Event Report (JADER) database [10] published by the PMDA, VigiBase [11] from the World Health Organization (WHO), and the Eudra Vigilance [12] from the European Medicine Agency (EMA). FAERS is the largest adverse event report database, containing more than 1 million reports recorded from all over the world every year. In contrast, approximately 50,000 Japanese reports are recorded on JADER every year [13]. The reports recorded on FAERS include events that occurred in Japan. However, a study examining the overlap between the two databases by matching the information on the first suspected drug, adverse event, age group, gender, and date (event occurrence date, administration start date, or end date of the first suspected drug) found that out of 53,210 Japanese reports recorded on FAERS, 14,448 reports were also recorded on JADER. This indicates that a considerable number of Japanese reports not recorded on JADER are included in FAERS [14]. The differences in characteristics between the two databases are thought to result from the different reporting subjects owing to distinct regulations in Japan and the USA, lack of detailed information owing to insufficient pharmacovigilance agreements between Japanese and US pharmaceutical companies, and accompanying under-reporting. Therefore, in order to examine adverse events in Japan, the use of these databases in a complementary manner is desired [14, 15].
A previous study suggested a correlation between SGLT2i and acute renal failure by signal detection using the data recorded on FAERS from January 2013 to September 2016 [16]. However, our previous study using the data recorded on JADER from April 2014 to February 2019 did not suggest such a correlation, indicating that there are disparities in the observations of the correlation between the studies using spontaneous reporting databases [17]. There are some studies using the data recorded on VigiBase and the Eudra Vigilance. For instance, a study shows that VigiBase contained more reports for SGLT2i compared to other databases [18]. Furthermore, by analyzing VigiBase, one study showed that canagliflozin and empagliflozin were associated with lower limb amputation [19]. As with acute kidney injury, lower limb amputation is partly due to a decrease in fluid volume, which may affect the onset of acute kidney injury. The objectives of our study are to re-examine the correlation between SGLT2i and acute renal failure using the latest FAERS records and to examine the differences in the observations between FAERS and JADER in existing studies by conducting a stratified analysis for the reporting countries (Japan or other countries), as well as the concomitant use of drugs such as angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) with SGLT2i.
2 Methods
The data recorded on FAERS from January 2013 to March 2020, published by the FDA, was downloaded for analysis [9]. FAERS consists of Demographic, Drug, Therapy, Indication, Reaction, Outcome, and Report Sources files. The Drug and Reaction files were integrated into the Demographic file using the primary ID. The Preferred Term (PT) obtained from the Medical Dictionary for Regulatory Activities (MedDRA) was used for the extraction of the adverse events. Acute renal failure was defined as 50 types of events determined by the Standardized MedDRA Query (SMQ) broad terminology (Online Supplementary Material (OSM) Table 1). MedDRA is a rich and highly specific standardized medical terminology database that was developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) [20]. SGLT2i was defined as one of the product or generic names of canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, luseogliflozin, and tofogliflozin (OSM Table 2). The Drug files were classified into Primary Suspect Drug, Secondary Suspect Drug, Concomitant, and Interacting. The analysis subjects of this study were Primary Suspect Drug and Secondary Suspect Drug, and the cases in which patients had received SGLT2i were defined as the cases in which the Drug files contained the names of the drugs mentioned above. We first calculated the reporting odds ratio (ROR) and 95% CI for the presence or absence of acute renal failure in SGLT2i using a logistic regression model. A positive signal was determined by the lower limit of the 95% CI exceeding 1.
Subsequently, the ROR was calculated by limiting the analysis subjects to the cases in which patients had received treatment for diabetes. The cases in which patients had received treatment for diabetes were defined as cases in which the Drug files contained the product or generic names (OSM Table 3) of glucose-lowering drugs currently approved in the USA or Japan. Then, we calculated the ROR after the diabetes-treatment cases were divided into those reported in Japan and those reported in countries other than Japan.
Furthermore, in order to examine the influence of the concomitant use of an ACEi or ARB, which have renal protective effects, we conducted a stratified analysis of cases of diabetes reported in Japan and those reported outside Japan for the presence or absence of an ACEi or ARB prescription. The cases with an ACEi or ARB prescription were defined as cases in which the Drug files contained the product or generic names (OSM Tables 4, 5) of ACEis or ARBs currently approved in the USA or Japan. The software programs IBM SPSS Statistics 26 and 27 (IBM, NYC) were used for the analysis in this study.
3 Results
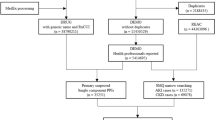
There were 5,337,069 cases of adverse events recorded on FAERS between January 2013 and March 2020. Of these, 410,569 were cases in which patients had received treatment for diabetes. From among these, 20,588 were cases reported in Japan and 389,981 were cases reported in countries other than Japan (Fig. 1). Of the 389,981 cases reported in countries other than Japan, 257,281 (66%) were cases reported in the USA. The number of cases in which patients had received ertugliflozin, which is not approved in Japan was 0, in cases of diabetes reported in Japan. Also, the number of cases in which patients had received ipragliflozin, luseogliflozin, and tofogliflozin, which are not approved in the US was 0, in cases of diabetes reported outside Japan (OSM Table 6). The details of demographic distribution of cases with treatment for diabetes is shown in Table 1.
The ROR for all SGLT2i calculated from all cases was 4.16 (95% CI 4.01–4.31). When focusing on each drug, the lower limit of 95% CI exceeded 1 for all drugs except for ertugliflozin with an ROR of 1.72 (95% CI 0.94–3.16), suggesting a correlation with acute renal failure (Table 2). When limited to the cases in which patients had received treatment for diabetes, ROR was 1.75 (95% CI 1.69–1.82) for all SGLT2i, suggesting a correlation with acute renal failure. In contrast, focusing on each drug, no significant correlation was observed, with the lower limit of 95% CI ranging below 1 for all drugs except for canagliflozin, with an ROR of 2.57 (95% CI 2.46–2.69) (Table 2).
The stratified analysis for reporting countries showed that ROR for all SGLT2i was 0.58 (95% CI 0.49–0.69) in the cases reported in Japan, suggesting no correlation with acute renal failure. In contrast, ROR for all SGLT2i was 1.91 (95% CI 1.83–1.98) in the cases reported in countries other than Japan, suggesting a correlation with acute renal failure (Table 3). Owing to their different approval status in each country, ROR for some SGLT2i, for which the number of cases is indicated as 0, was not calculated.
Furthermore, as a result of the stratified analysis for the concomitant use of ACEis or ARBs, 118 cases (3.8%) were prescribed ACEis and 1141 cases (37%) were prescribed ARBs in patients who were undergoing treatment for diabetes prescribed with SGLT2i in Japan. On the other hand, 3099 cases (11.9%) were prescribed ACEis and 2,111 cases (8.1%) were prescribed ARBs of patients who were undergoing treatment for diabetes prescribed with SGLT2i in countries other than Japan (OSM Table 7). In the cases reported in Japan, RORs for all SGLT2i were 0.48 (95% CI 0.23–1.00) with ACEi, 0.59 (95% CI 0.49–0.70) without ACEi, 0.49 (95% CI 0.38–0.64) with ARB, and 0.61 (95% CI 0.48–0.77) without ARB (Table 4). In contrast, in the cases reported in countries other than Japan, RORs for all SGLT2i were 1.70 (95% CI 1.55–1.87) with ACEi, 2.12 (95% CI 2.03–2.21) without ACEi, 1.12 (95% CI 0.99–1.27) with ARB, and 2.20 (95% CI 2.11–2.30) without ARB (Table 4). Therefore, ROR tended to be low in the cases with ACEis or ARBs. The analysis using cases with diabetes reported outside Japan suggested a correlation with adverse events. However, when limited to the cases with ARBs reported outside Japan, the analysis showed no significant trend between SGLT2i and the onset of acute renal failure.
4 Discussion
In this study, the correlation between SGLT2i and the onset of acute renal failure was suggested by signal detection using FAERS. Consistent with the findings of a previous study [16], this trend was observed even when focusing on the cases in which patients had received treatment for diabetes. In addition, the correlation of each SGLT2i, except for ertugliflozin, with acute renal failure was suggested in all adverse events recorded on FAERS. However, when focusing on the cases in which patients had received treatment for diabetes, only canagliflozin showed a correlation with acute renal failure. Because diabetes, which is the primary disease, poses a risk of renal failure, examination of the results by focusing on the diabetes-treatment cases was considered appropriate. The previous study, which analyzed data recorded from January 2013 to September 2016, suggested that canagliflozin, empagliflozin, and dapagliflozin were correlated with acute renal failure when focusing on the cases in which patients had received treatment for diabetes [16]. The lack of signal detected for empagliflozin and dapagliflozin in the present study may indicate that a more accurate ROR was calculated by including approximately 3.5 years of recent reports for analysis. In addition, as with the previous study, a correlation of canagliflozin with acute renal failure was suggested, and its ROR tended to be higher than that of other drugs [16]. Moreover, the previous study did not investigate ertugliflozin, ipragliflozin, luseogliflozin, and tofogliflozin. Although the correlation of these drugs with acute renal failure was not observed in the present study, their calculated ROR may not be accurate owing to the small amount of data on these drugs, as ipragliflozin, luseogliflozin, and tofogliflozin have not been approved in the USA and ertugliflozin was approved in the USA in 2017.
When focusing on each SGLT2i, only canagliflozin was suggested to be associated with acute renal failure. This was believed to be because of its strong affinity to SGLT1. While SGLT2 is predominantly expressed in the kidneys, SGLT1 is expressed not only in the kidneys but also in the small intestine and the heart. It is known that inhibiting SGLT1 may cause diarrhea and volume depletion [21]. Canagliflozin has a relatively strong SGLT1 inhibitory action, followed by dapagliflozin and empagliflozin [22]. Due to its relatively strong SGLT1 inhibitory action, canagliflozin is believed to facilitate acute renal failure induced by volume depletion. In addition, lower extremity amputation was also considered to be due to volume depletion. A meta-analysis has reported that canagliflozin alone significantly increased the incidence of lower extremity amputation compared to empagliflozin and dapagliflozin, and that there was no significant difference in the incidence of lower extremity amputation between empagliflozin and dapagliflozin [23]. This meta-analysis suggested that canagliflozin poses the highest risk of lower extremity amputation, followed by dapagliflozin and empagliflozin, which corresponds with the strength of their SGLT1 inhibitory action [23]. Additionally, it cannot be ruled out that the pharmacodynamic interaction of concomitant drugs may have affected the RORs. For instance, a study reported that the combined use of diuretics increased the risk of lower extremity amputation [24]. It has also been reported that pharmacodynamic interaction from concomitant use of dipeptidyl peptidase-4 inhibitors (DPP4i) reduced the incidence of genitourinary tract infections (GUTIs) [25]. Moreover, it has been found that drugs that induce canagliflozin-metabolizing enzymes (UGT1A9 and UGT2B4) such as rifampin, phenytoin, phenobarbital, and ritonavir reduce the efficacy of canagliflozin [26].
The stratified analysis for reporting countries suggested no correlation between SGLT2i and acute renal failure in the cases reported in Japan. However, the correlation was suggested in the cases reported in countries other than Japan. Even when focusing on each SGLT2i drug, each of them was not correlated with acute renal failure in the cases reported in Japan. In contrast, only canagliflozin was suggested to be correlated with acute renal failure in the cases reported in countries other than Japan. This trend was similar to the result of analysis that limited the total FAERS to the cases in which patients had received treatment for diabetes. When focusing on the cases reported in Japan, no correlation with acute renal failure was suggested, supporting the result of our previous study using JADER [17]. One of the reasons why there is a difference in the correlation between SGLT2i and acute renal failure in the cases reported in Japan and other countries is due to the difference in the proportion of prescriptions of ARBs, which have nephroprotective effects.
The stratified analysis for the presence or absence of ACEi or ARB prescription found that the cases with ACEis or ARBs tended to have lower RORs than the cases without the prescription. Focusing on the cases with ARB prescription, in particular, no significant trend in correlation with acute renal failure was observed in cases reported in countries other than Japan. The ROR of the cases with ACEi prescription in Japan may not be accurate, owing to the small amount of data on these drugs. A meta-analysis using RCTs suggested that ARBs suppress end-stage renal failure more effectively than ACEis [27]. This is likely reflected in the present study, since the ROR of the cases with ARB prescription in countries other than Japan (ROR 1.12 [95% CI 0.99–1.27]) was lower than that of the cases with ACEi prescription (ROR 1.70 [95% CI 1.55–1.87]).
The cases reported in Japan reflect prescription trends in Japan. As shown in a study on the prescription situation in Japan, ARBs are commonly prescribed to Japanese patients with hypertension and diabetes. This prescription situation is likely reflected in the present study, as the cases of diabetes reported in Japan included more cases with ARBs than those with ACEis [28]. In contrast, a study on the actual prescription situation in patients with hypertension and diabetes in the USA showed that ACEis were prescribed more frequently than ARBs in the USA [29]. The cases reported in the USA account for 66% of the cases included in the cases of diabetes reported outside Japan, and the higher number of cases with ACEis than those with ARBs in the present study likely reflects the prescription trend in the USA mentioned above. Furthermore, there are two reasons for the difference in the prescription trend of ACEis and ARBs between Japan and the USA. First, because the dose of ACEis approved in Japan is lower than that in the USA, the antihypertensive effect of ACEis is smaller than that of ARBs in Japan [30]. Second, dry cough, a characteristic side effect of ACEis, is known to be more likely to occur in Asians [31]. Based on this background, ARBs are more likely to be prescribed than ACEis in Japan. As a result, the relatively strong nephroprotective effect of ARBs likely affects the ROR in the analysis of cases of diabetes reported in Japan, resulting in no correlation with acute renal failure.
On the other hand, it cannot be ruled out that the concomitant use of drugs that may reduce renal function may have affected the RORs. A study by Szelat et al. [32] suggested that the following three mechanisms are involved when SGLT2i leads to acute renal failure. First, excessive diuresis leads to volume depletion, particularly in hemodynamically unstable and volume-depleted patients. Second, the concomitant action of SGLT2i and RAAS blockers lead to a greater reduction in trans-glomerular pressure. Finally, administration of molecules such as non-steroidal anti-inflammatory drugs (NSAIDs) and radiocontrast agents may lead to renal medullary hypoxic injury. This study conducted a stratified analysis focusing on ACEis and ARBs, as they are RAAS (renin-angiotensin-aldosterone system) blockers considered to affect trans-glomerular pressure. It should be noted that the ROR calculated in this study may be greater than in actuality due to the concomitant use of drugs that may reduce renal function such as NSAIDs, antibiotics, small-molecule protein kinase inhibitors, aprotinin, sodium phosphate, and furosemide [33,34,35].
Another reason for the difference in the relationship between SGLT2i and acute renal failure between cases reported in Japan and those reported outside Japan was because there was an impact of notoriety bias. A study that reported on the impact of notoriety bias stated that the number of adverse event reports for a drug increases when a warning for adverse events associated with the drug is issued [36]. The study explained that this was because people pay more attention to the drug and adverse events when they are aware of the warning. In fact, there is a report that notoriety bias was observed regarding the association between anti-diabetic drugs and pancreatitis [37]. The majority of FAERS entries have been reported from the USA. In June 2016, the USA issued a warning for SGLT2i-induced acute renal failure. A comparison of calculations before and after the issuance of this warning showed that the ROR after the issuance was increased [16]. It cannot be ruled out that the RORs for cases reported outside Japan were affected by notoriety bias. Meanwhile, in Japan, regulatory authorities do not directly issue warnings for acute renal failure. For this reason, compared to cases reported outside Japan, those reported in Japan were less affected by notoriety bias.
Although the the FDA has issued a warning for SGLT2i-induced acute renal failure, PMDA has not issued such a warning as an individual item. This difference in correspondence is seen in the difference in trends between RORs calculated by all cases with diabetes and RORs calculated when focusing on the cases reported in Japan in this study. However, when risk management is performed in clinical practice, the effect of the concomitant use of ARBs or ACEis, as shown in this study, should be considered. The results of the previous study using JADER [17] as well as the present study, which focused on Japanese cases in FAERS, were not able to exclude the possibility that the signal for developing acute renal failure is underestimated because of the concomitant use of ARBs or ACEis. Therefore, we propose that patients who do not take these drugs concomitantly be monitored more carefully.
5 Limitations
This study had the following limitations. First, analyses using adverse event report databases may contain duplicate reports and incorrect information and may be affected by the pharmacodynamic interactions of concomitant drugs and notoriety bias. Second, the detailed analysis could not be performed because of the lack of data such as clinical laboratory test values. Third, FAERS mainly consists of reports from the USA. Thus, the results may be affected by the small volume of data in the RORs of drugs not approved in the USA and the RORs calculated from the stratified analysis for reporting countries. Because of these limitations, the signal detection method used in this study was not able to determine the causal relationship between drugs and adverse events. Therefore, further clinical studies are needed to determine the causal relationship between drugs and adverse events, and regular assessment using the adverse event report databases is necessary. Lastly, although this study conducted an analysis with a focus on the concomitant use of other drugs, future studies would need to examine the times when adverse events occurred so as to help monitor patients at more appropriate times in clinical settings.
6 Conclusion
Our study suggested a correlation of SGLT2i, especially canagliflozin, with the onset of acute renal failure. However, no correlation between SGLT2i and the onset of acute renal failure was observed when focusing on the cases reported in Japan, and this trend was consistent with the findings of our previous study using JADER. Furthermore, this study revealed that the concomitant use of ARBs or ACEis tends to reduce the signal of acute renal failure in SGLT2i. When conducting risk management based on the accumulated assessment of adverse events such as alerts from the authorities in clinical practice, we believe that the risk can be managed more appropriately by closely monitoring patients who take SGLT2i without ACEis or ARBs.
References
FDA. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). 14 Jun 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Accessed 9 July 2020.
The Committee on the Proper Use of SI. Recommendations on the proper use of SGLT2 inhibitors. Diabetology International. 2019 2019/11/15.
PMDA. PMDA revises a label tofogliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/03.pdf. Accessed 9 July 2020.
PMDA. PMDA revises labels ipragliflozin, dapagliflozin, luseogliflozin, canagliflozin, and empagliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/02.pdf. Accessed 9 July 2020.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Donnan JR, Grandy CA, Chibrikov E, Marra CA, Aubrey-Bassler K, Johnston K, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e022577.
Rampersad C, Kraut E, Whitlock RH, Komenda P, Woo V, Rigatto C, et al. Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: a retrospective cohort study. Am J Kidney Dis. 2020;76(4):471–91.
Fujita T. Signal detection of adverse drug reactions. Jpn J Pharmacoepidemiol. 2009;14(1):27–36 (in Japanese).
FDA. FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Accessed 9 July 2020.
PMDA. JADER. (In Japanese). https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0004.html. Accessed 9 July 2020.
Uppsala Monitoring Centre. VigiBase. https://www.who-umc.org/vigibase/vigibase/. Accessed 20 Dec 2020.
EMA. EudraVigilance. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance. Accessed 20 Dec 2020.
Narukawa M. Expectations for pharmacoepidemiology studies on drug risk management. Regul Sci Med Prod. 2016;6:335–43 (In Japanese).
Hinomura Y, Tadenuma H, Uehara K, Hidaka T, Murakami T, Narukawa M. Comparison of the characteristics of data contained in the drug side effect database by Japanese and US regulators. Japanese Society for Pharmacoepidemiology, 19th Annual Scientific Meeting. November 16th and 17th, 2013. (In Japanese). https://www.japic.or.jp/service/information/pdf/FAERS_JADER_data_compare.pdf. Accessed 9 July 2020.
Nomura K, Takahashi K, Hinomura Y, Kawaguchi G, Matsushita Y, Marui H, et al. Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des Dev Ther. 2015;9:3031–41.
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27(12):1108–13.
Katsuhara Y, Ogawa T. Acute renal failure, ketoacidosis, and urogenital tract infections with SGLT2 inhibitors: signal detection using a Japanese spontaneous reporting database. Clin Drug Investig. 2020;40(7):645–52.
Raschi E, Parisotto M, Forcesi E, La Placa M, Marchesini G, De Ponti F, et al. Adverse events with sodium-glucose co-transporter-2 inhibitors: a global analysis of international spontaneous reporting systems. Nutr Metab Cardiovasc Dis. 2017;27(12):1098–107.
Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab. 2018;20(6):1531–4.
MedDRA. About MedDRA. https://www.meddra.org/how-to-use/support-documentation/english/welcome. Accessed 20 Dec 2020.
Tsimihodimos V, Filippas-Ntekouan S, Elisaf M. SGLT1 inhibition: pros and cons. Eur J Pharmacol. 2018;5(838):153–6.
Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213–25.
Heyward J, Mansour O, Olson L, Singh S, Alexander GC. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: a systematic review and meta-analysis. PLoS ONE. 2020;15(6):e0234065.
Potier L, Roussel R, Velho G, Saulnier PJ, Bumbu A, Matar O, et al. Lower limb events in individuals with type 2 diabetes: evidence for an increased risk associated with diuretic use. Diabetologia. 2019;62(6):939–47.
Fadini GP, Bonora BM, Mayur S, Rigato M, Avogaro A. Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2018;20(3):740–4.
FDA. Highlights of prescribing information invokana (Canagliflozin). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204042s027lbl.pdf. Accessed 20 Dec 2020.
Wang K, Hu J, Luo T, Wang Y, Yang S, Qing H, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res. 2018;43(3):768–79.
Ishida T, Oh A, Hiroi S, Shimasaki Y, Tsuchihashi T. Current use of antihypertensive drugs in Japanese patients with hypertension: analysis by age group. Geriatr Gerontol Int. 2018;18(6):899–906.
Gu A, Farzadeh SN, Chang YJ, Kwong A, Lam S. Patterns of antihypertensive drug utilization among US adults with diabetes and comorbid hypertension: the National Health and Nutrition Examination Survey 1999–2014. Clin Med Insights Cardiol. 2019;13:1179546819839418.
Yamazaki T. A new topic for renin–angiotensin system inhibitors angiotensin converting enzyme inhibitor. Angiol Front. 2011;10(1):19–23.
McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332(7551):1177–81.
Szalat A, Perlman A, Muszkat M, Khamaisi M, Abassi Z, Heyman SN. Can SGLT2 inhibitors cause acute renal failure? Plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 2018;41(3):239–52.
Patek TM, Teng C, Kennedy KE, Alvarez CA, Frei CR. Comparing acute kidney injury reports among antibiotics: a pharmacovigilance study of the FDA adverse event reporting system (FAERS). Drug Saf. 2020;43(1):17–22.
Fan Q, Ma J, Zhang B, Li Q, Liu F, Zhao B. Assessment of acute kidney injury related to small-molecule protein kinase inhibitors using the FDA adverse event reporting system. Cancer Chemother Pharmacol. 2020;86(5):655–62.
Welch HK, Kellum JA, Kane-Gill SL. Drug-associated acute kidney injury identified in the United States Food and Drug administration adverse event reporting system database. Pharmacotherapy. 2018;38(8):785–93.
Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases the notoriety bias. Drug Saf. 2007;30(10):891–8.
Raschi E, Piccinni C, Poluzzi E, Marchesini G, De Ponti F. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 2013;50(4):569–77.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this study.
Conflict of interest
Yukari Katsuhara is an employee of Takeda Pharmaceutical Company Limited and had been on leave during the research period. Shunya Ikeda has no conflict of interest.
Ethics approval
This study used anonymized information from the database, which is open to the public; therefore, in accordance with the 1964 Helsinki Declaration (and its amendments), institutional ethics approval was not required.
Consent to participate
This study used anonymized information from the database, which is open to the public; therefore, in accordance with the 1964 Helsinki Declaration (and its amendments), institutional ethics approval was not required.
Consent for publication
Not applicable.
Availability of data and material
The datasets analyzed in this study are available in the FDA Adverse Event Reporting System (FAERS): https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files
Code availability
Not applicable.
Authors contributions
Both authors were investigators in the study and participated in the study design, interpretation of the study results, and in the drafting, critical revision, and approval of the final version of the manuscript.
Acknowledgements
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Katsuhara, Y., Ikeda, S. Correlations Between SGLT-2 Inhibitors and Acute Renal Failure by Signal Detection Using FAERS: Stratified Analysis for Reporting Country and Concomitant Drugs. Clin Drug Investig 41, 235–243 (2021). https://doi.org/10.1007/s40261-021-01006-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01006-9