Abstract
Purpose
The sodium–glucose cotransporter-2 (SGLT2) inhibitors have changed the treatment of type 2 diabetes mellitus. Several studies evaluated SGLT2 inhibitor-related acute kidney injury (AKI), but pharmacoepidemiology studies are needed to compare the adverse events in different SGLT2 inhibitors (SGLT2i).
Methods
We used disproportionality analysis and Bayesian analysis in data mining to screen the AKI cases after initiating different SGLT2i among diabetic patients, based on the FDA’s Adverse Event Reporting System (FAERS) updated to December 2020. We also investigated the onset time and fatality rates of SGLT2i-associated AKI, which was based on preferred terms (PTs) coded for the renal adverse events in the structure of the FARES database.
Results
We identified 2483 cases of AKI following SGLT2i regimens among diabetic patients. Most of them were 45–64 years old (58.46%) and > 65 years old (28.67%). Canagliflozin generated the largest number of AKI reports (n = 1650, 66.45%) in our study. Canagliflozin showed the strongest association among SGLT2i, evidenced by the highest reporting odds ratio (ROR = 3.70, two-sided 95% CI 3.51–3.91), proportional reporting ratio (PRR = 3.39, χ2 = 2635.06), and empirical Bayes geometric mean (EBGM = 3.18, one-sided 95% CI 3.04). The median onset time to AKI was 72.0 (interquartile range [IQR] 21.0–266.0) days after SGLT2i initiation. The general hospitalization rate of SGLT2i-associated AKI was 63.50%, and the fatality rate was 1.59%. The deceased patients (62.94 ± 10.69 years) were significantly older than the survived ones (57.82 ± 11.84 years) (P = 0.011).
Conclusion
We compared AKI events in the real-world practice of various SGLT2i among diabetic cases from the FAERS database. It is essential to monitor kidney function during the early administration of SGLT2i. Concern should be paid for AKI in patients older than 65 taking SGLT2i.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The debut of sodium–glucose cotransporter-2 (SGLT2) inhibitors has changed the landscape of treating type 2 diabetes since 2013. Currently, the United States Food and Drug Administration (FDA) has approved four SGLT2 inhibitors (SGLT2i), including canagliflozin, dapagliflozin, empagliflozin, ertugliflozin single regimens; and their combination prescriptions with metformin or dipeptidyl peptidase-4 inhibitors (Table 1). In the kidney, the SGLT2 actively transports glucose against the concentration gradient at the tubular apical membrane accompanied by sodium passage. The SGLT2 channel manages 80–90% of glucose reabsorption in proximal renal tubules, and its inhibition can effectively lower plasma glucose levels by coupling with increased urinary glucose excretion [1, 2]. The SGLT2i also show beneficial effects on reducing urine protein [3, 4], lowering uric acid [5], blood pressure control [6, 7], bodyweight management [8], and cardiovascular protection [9].
Despite the favorable effects, there is a conflicting concern that SGLT2i may cause acute kidney injury (AKI) due to volume depletion, a disproportionately decline in intraglomerular pressure, and hypoxemia-induced renal medullar injury [10]. On the one hand, SGLT2-associated AKI has been reported in cases [11, 12], clinical studies [13, 14], and meta-analyses [15, 16]. The FDA also released a warning in June 2016 towards SGLT2i due to the emerging AKI reports [17]. On the other hand, SGLT2i regimens have shown prevention of renal function deterioration in diabetic patients, as shown in the EMPA-REG [18], CANVAS [19], and CREDENCE [20] trials. Some meta-analyses claimed that AKI incidence was even fewer in SGLT2i users than non-SGLT2i users.
Since the discrepancy in clinical observation, the post-marketing AE monitoring is essential to expand our understanding of the potential AKI of SGLT2i. There was one pharmacovigilance study describing various SGLT2i-associated AEs [21] and one analysis on SGLT2i-induced AKI [22] updated till 2016. Since then, ertugliflozin has been developed as a new member of SGLT2i and accumulating clinical trials [19, 20, 23, 24] have been conducted. Knowledge is still lacking to detail the safety profile of renal adverse events following SGLT2i in everyday clinical practice. Therefore, we intended to evaluate the links between various SGLT2i regimens and AKI in a large population by researching the FDA’s Adverse Event Reporting System (FAERS) updated to December 2020. We further examined the onset times of AKI events after different SGLT2i and the fatality rates after AKI.
Methods
Data source
We implemented a pharmacovigilance study using data from the FAERS database dated from January 2004 to December 2020. The FAERS is a spontaneous reporting system (SRS) that contains data about adverse drug reports provided by doctors, pharmacists, patients, and manufacturers globally. Therefore, the FAERS demonstrates the association between drugs and various reporting adverse effects. The files in FAERS describes demographic and administrative information (DEMO), drug information (DRUG), report sources (RPSR), preferred terms (PTs) coded for the adverse events (REAC), patient outcomes (OUTC), therapy periods for reported drugs (THER), indications for drug administration (INDI), and deleted cases (DELE).
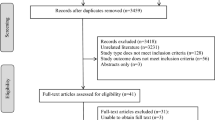
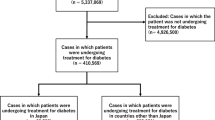
We screened 15,406,797 cases from the FAERS database. We first removed the deduplicated records (2,486,008) by choosing the latest FDA_DT when the CASEIDs were the same and selecting the higher PRIMARYID when the FDA_DT and CASEID were the same and then removed non-diabetic reports (12,285,594). We finally included 635,195 diabetic cases for further analysis (Fig. 1).
Date mapping
We inspected the REAC files for comprehensive Medical Dictionary for Regulatory Activities (MedDRA) V24.0, and preferred terms linked to AKI were defined as following: “acute kidney injury”, “blood creatinine increased”, “blood urea abnormal”, “glomerular filtration rate decreased”, “renal impairment”, “oliguria”, “anuria”, “dialysis”, “hemodialysis”, “peritoneal dialysis”, “renal tubular injury”, “nephropathy toxic”, “tubulointerstitial nephritis”. We chose the generic and brand names of SGLT2i regimes (Table 1), according to MICROMEDEX (Index Nominum), in the process of data mining.
Data mining
Based on the logic of Bayesian analysis and disproportionality analysis, we utilized the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS) algorithms to compare the association between SGLT2i and AKI. We listed the equations for the four algorithms in Table 2. We investigated the ROLE_COD field of DRUG files, which presented the role of suspected drugs in specified AEs. We first analyzed the associations between SGLT2i and AKI when an SGLT2i was identified as “primary suspect” in the ROLE_COD field of DRUG files. We also compared SGLT2i-associated AKI signals when SGLT2i was identified as “primary or secondary suspects”.
We compared the onset times of AKI after various SGLT2i, which was defined as the interval between the EVENT_DT (onset date of AKI) and the START_DT (start date of the SGLT2i initiation). We excluded the records with incorrect or erred inputs (START_DT later than EVETN_DT). Additionally, we analyzed the reports with fatal events and hospitalization events due to AKI. The fatality and hospitalization rates were calculated by dividing deaths and hospitalization events by the total number of reported SGLT2i-associated AKI.
Statistical analysis
We summarized the clinical features of the patients with SGLT2i-associated AKI from the FAERS database. The time to onset of AKI among different SGLT2i was compared using non-parametric tests (Kruskal–Wallis test when there were more than two subgroups of respondents). We used Pearson’s chi-square test to compare the fatality rates between different SGLT2i. The statistical significance was determined at P < 0.05. Data mining and statistical analysis were performed by SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Descriptive analysis
We identified 21,192 reports associated with AKI among the 635,195 diabetic cases recorded in the FAERS database from January 2004 to December 2020. Meanwhile, the database recorded 31,618 AEs related to SGLT2i (Fig. 1). We have screened 2483 reports with suspected SGLT2i-associated AKI and summarized the clinical features of related patients in Table 3. There is no reported AKI event associated with the combination compound of ertugliflozin/sitagliptin in the current FAERS database. Other SGLT2i single or combination prescriptions have been informed AKI in the database. Most cases were reported from North America (84.74%), followed by Europe (8.86%) and Asia (4.59%). The healthcare professionals reported 38.99% of the cases, and non-health-care professionals contributed 55.90% of the reports. The SGLT2i-related AKI cases have generally increased since 2013, with a peak of 36.69% of total reports in 2018. The average age for all patients was 57.90 (± 11.84) years. Most of the affected patients were 45–64 years old (58.46%) and the > 65 years old (28.67%). Excluding the unspecified data, 56.68% of the cases were male patients. And the male subjects with AKI (58.45 ± 11.78) tended to be older than the females (57.17 ± 11.84) (P = 0.014). The most common SGLT2i-associated AKI cases are related to canagliflozin (n = 1650, 66.45%), followed by empagliflozin (n = 374, 15.06%) and dapagliflozin (n = 325, 13.09%). Among the selected diabetic cases, SGLT2i has been dominantly administrated in type 2 diabetes mellitus (71.41%).
The disproportionality analysis and Bayesian analysis
We detected AKI signals for different types of single prescriptions of SGLT2i based on the “primary suspects” and recorded the results in Table 4. In the analysis, canagliflozin was particularly remarkable for the connection to renal AEs due to its highest ROR (3.70, 95% CI 3.51, 3.91), PRR (3.39, χ2 2635.06), IC (1.67, IC025 1.58), and EBGM (3.18, EBGM05 3.04). Dapagliflozin and empagliflozin was positive in ROR and IC025. Ertugliflozin signals were currently not powerful enough to demonstrate a definite conclusion. Similarly, we detected the SGLT2i-associated AKI signals by comparing the RORs based on “primary suspects” and “primary and secondary suspects” for different SGLT2i. In Table S1, the ROR signals for AKI were stable among the SGLT2i across the different analyses.
Time to onset of SGLT2i-associated AKI
Overall, the median time to onset of SGLT2i-associated AKI was 72.0 (interquartile range [IQR] 21.0–266.0) days after administering drugs. In Fig. 2, we presented the general accumulated AKI rates for all SGLT2i, and we also described the AKI rates in different time intervals for canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Almost one-third (32.29%) of the AKI cases occurred in the first month, and almost half (45.56%) occurred in the first two months. We noticed that the AKI could develop as soon as the initiation of several SGLT2i regimens. The quick onsets took place in 5.63% of all SGLT2i-associated AKI cases and respectively have occurred in 4.97%, 8.96%, and 5.00% of patients initiated with canagliflozin, dapagliflozin, and empagliflozin. Kruskal–Wallis test detected a significant difference in time to onset of AKI among various SGLT2i (P < 0.01), with the shortest median time (23.0, IQR 18.0–28.0 days) in ertugliflozin and the longest (98.0, IQR 27.0–295.0 days) in canagliflozin.
Time to event onset of AKI following SGLT2i initiation. Accumulated AKI rates for all SGLT2i were presented as a survival curve. Other AKI rates for canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin were not accumulated. AKI acute kidney injury, SGLT2i sodium–glucose cotransporter-2 inhibitor
Fatality due to SGLT2i-associated AKI
To investigate the prognosis of SGLT2i-associated AKI, we evaluated fatality due to SGLT2i-associated AKI available in the FAERS database. Generally, SGLT2i-associated AKI resulted in 63.50% initial or prolonged hospitalization. The fatality rate among all AKI cases was 1.59%. We did not find a significant difference in the fatality rate among various SGLT2i (Pearson’s chi-square test for overall comparison, P = 0.553). The fatality rate of dapagliflozin-associated AKI ranked the highest (2.12%). The deceased patients (62.94 ± 10.69 years) were significantly older than the survived ones (57.82 ± 11.84 years) (P = 0.011). Among the deceased cases, males composited 58.33%; there was no age difference between males (63.81 ± 11.88 years) and females (61.64 ± 8.89 years) (P = 0.565).
Discussion
By investigating the FAERS pharmacovigilance database, our study presented an extensive analysis to compare the association, onset times, and prognosis of AKI after different SGLT2i in real-world practice. All four members of SGLT2i demonstrated a relationship with AKI and presented diverse characteristics.
The mechanism of SGLT2i predisposes the potential to develop AKI in diabetic patients. SGLT2i induces osmotic diuresis and increases the possibility of hypovolemia [25], a well-recognized risk factor for acute prerenal failure. SGLT2i leads to the decline of eGFR due to the shrinking of the afferent arteriole resulting from augmented sodium delivery to macula densa at the distal tubule [26]. SGLT2i reduces glucose reabsorption but otherwise enhances fructose metabolism in the S3 segment of the proximal tubule and further induces oxidative stress, uric acid toxicity, inflammation, and tubular injury [10, 12]. Moreover, diabetes mellitus tends to develop in 45–64-year-old or > 65-year-old patients, and their comorbidities and concurrent medications may further increase the risk of tubular injury. Clinical evidence has revealed AKI after SGLT2i application in such patient groups [11,12,13,14,15,16]. By performing analyses among diabetic patients, the real-world data also confirmed the association between SGLT2i and AKI.
Recent years have witnessed the heated debate over the “friend or foe” of SGLT2i effects on the kidney after the FDA labeled AKI warning for SGLT2i [27]. Indeed, patients receiving SGLT2i experienced a rapid decline in estimated glomerular filtration rate (eGFR) during the early phase in several clinical trials [20]; however, the trajectory was reversible after SGLT2i withdrawal [24], and some trials even proved the long-term beneficial effect in slowing eGFR decline if SGLT2i was continued [19, 20]. This phenomenon was consistent with our findings, which revealed that almost half of the AKI cases had occurred within the first two months; after that, the proportion of reported AKI events declined. From this viewpoint, some AKI reports flagged in the FAERS database may represent an expected decline in eGFR due to known hemodynamic effects, similar to the renin-angiotensin system (RAS) inhibitors [28]. A previous study demonstrated a trend to a lower baseline eGFR in patients who developed AKI after SGLT2i compared with patients without AKI [14]. We should keep in mind that the RAS inhibitor could be harmful in > 65-year-old patients with impaired kidney function [29]. A similar situation could result in particular populations, leading to harmful AKI.
Despite the possible confounding results of temporary eGFR decline, we summarized the potential applications of our analysis of this FAERS dataset. First, it was noted that the immediate AKI could occur in more than 5% of all affected diabetic patients. The average time to onset of AKI was around 70 days for all SGLT2i, and differences in onset times could be detected among the regimens. Combined with the observation of declined eGFR within six weeks in trials [24], it reminds us to trace the changes in kidney function during the early administration of SGLT2i. Second, we noted that canagliflozin and dapagliflozin, which were especially alerted to AKI by the FDA in 2016 [17], demonstrated a generally stronger association with AKI compared with empagliflozin and ertugliflozin. Similarly, meta-analyses also listed canagliflozin and dapagliflozin with a greater chance of AKI compared to empagliflozin [15, 16]. The discrepancy may be driven by empagliflozin's pharmacological characteristics, featured by more than 2,500 times of affinity for SGLT2 over SGLT1. The specificity of empagliflozin is tenfold and twofold more remarkable than that in canagliflozin and dapagliflozin, respectively [30]. Sparse data yet exist regarding the pharmacokinetics of ertugliflozin [30]. Therefore, canagliflozin should be used cautiously in patients who tend to develop AKI based on the current findings. Third, despite the relatively modest fatality rate, the > 60% of AKI cases contributed hospitalization rate is concerning. Admittedly, cases with more severe outcomes were inclined to be reported in real-world SRS; still, the AKI-associated hospital stay was remarkable. More than 70% of AKI cases were middle-aged and elderly patients, and older age was a significant risk factor of consequent death. We should carefully monitor SGLT2i-associated AKI in elderly patients with less-preserved kidney function [31].
We noticed the possible Notoriety Bias during our interpretation of the FAERS data [32], which showed the tendency of intensified reports following the publishing of FDA cautions towards SGLT2i-associated AKI in 2016. The modern FAERS database is stable enough to wean the effects of reporting bias [33]. Our study strengthens the claim of harmful effects on the kidney in a previous pharmacovigilance analysis [22]. Compared to previous pharmacovigilance analysis, we utilized four algorithms to compare the association between SGLT2i and AKI. We also compared fatality and time to the onset of SGLT2i-associated AKI, which generated more convincing data. Still, we neutrally interpreted our data after reviewing a growing body of evidence indicating the renal protective effects of SGLT2i in the long term [18,19,20]. We can expect the EMPA-KIDNEY [34] and DAPA-CKD [35] to address this issue more clearly.
Admittedly, this study has some limitations despite the advantages of data mining. First, the AEs described in the FAERS database depend on the reporting quality. We noticed the inadequacy of information during data mining, such as incomplete inputs, resulting in a bias in the analysis. Second, the FAERS database only records patients with adverse effects. Some statistics, such as the incidence rate of AKI for each SGLT2i regimen, cannot be explored due to the deficiency of the total number of patients receiving treatments. Third, it is problematic to profile risk factors between SGLT2i and AKI since the lack of baseline kidney conditions, comorbidities, and concomitant drugs that may contribute to AKI. Fourth, we lacked the original data to distinguish AKI and hemodynamic effect due to SGLT2i. Although some inherited imperfection in the FAERS database, it hints at some critical aspects of SGLT2i-associated AKI and provides evidence for further studies.
Conclusion
We identified AKI cases after different SGLT2i regimens among diabetic patients based on the FAERS database indicating the real-world practice. We signaled the association between SGLT2i regimens and AKI. Moreover, most AKI cases after SGLT2i occurred within the first two months, and we should monitor some immediate decline in eGFR following the prescription of SGLT2i regimens. Besides, diabetic patients with more advanced ages may be more sensitive to SGLT2i-associated AEs, and we should regularly monitor kidney function in some particular populations. Our findings justified the continued pharmacovigilance investigation, and we expect further clinical trials to identify the boundary to apply SGLT2i in patients who are more fragile in kidney function.
Availability of data and materials
All necessary data have been presented as tables and figures in the manuscript. Related information is accessible under request to the corresponding author.
Abbreviations
- AKI:
-
Acute kidney injury
- SGLT2:
-
Sodium–glucose cotransporter-2
- SGLT2i:
-
SGLT2 inhibitor
- AE:
-
Adverse event
- FAERS:
-
The Food and Drug Administration’s Adverse Event Reporting System
- ROR:
-
Reporting odds ratio
- PRR:
-
Proportional reporting ratio
- EBGM:
-
Empirical Bayes geometric mean
- IQR:
-
Interquartile range
- FDA:
-
The Food and Drug Administration
- SRS:
-
Spontaneous reporting system
- BCPNN:
-
Bayesian confidence propagation neural network
- MGPS:
-
Multi-item gamma Poisson shrinker
- RAS:
-
Renin–angiotensin system
References
Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 66:255–270
DeFronzo RA, Norton L, Abdul-Ghani M (2017) Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 13:11–26
Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M (2016) The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 59:1860–1870
Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD (2016) Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia 59:2036–2039
Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L (2018) Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 20:458–462
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB (2014) Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 8:262–75.e9
Muskiet MHA, van Bommel EJ, van Raalte DH (2016) Antihypertensive effects of SGLT2 inhibitors in type 2 diabetes. Lancet Diabetes Endocrinol 4:188–189
Cefalu WT, Stenlöf K, Leiter LA, Wilding JP, Blonde L, Polidori D, Xie J, Sullivan D, Usiskin K, Canovatchel W, Meininger G (2015) Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia 58:1183–1187
Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61:2108–2117
Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ (2016) Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol 12:711–712
Shrivastava S, Srivastava N, Alfanso-Jaume M (2019) Acute renal failure with cocaine and SGLT-2 inhibitor. Am J Ther 26:e762–e763
Phadke G, Kaushal A, Tolan DR, Hahn K, Jensen T, Bjornstad P, Roncal-Jimenez C, Hernando AA, Lanaspa MA, Alexander MP, Kukla A, Johnson RJ (2020) Osmotic nephrosis and acute kidney injury associated with SGLT2 inhibitor use: a case report. Am J Kidney Dis 76:144–147
Usiskin K, Kline I, Fung A, Mayer C, Meininger G (2014) Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase 3 study results. Postgrad Med 126:16–34
Darawshi S, Yaseen H, Gorelik Y, Faor C, Szalat A, Abassi Z, Heyman SN, Khamaisi M (2020) Biomarker evidence for distal tubular damage but cortical sparing in hospitalized diabetic patients with acute kidney injury (AKI) while on SGLT2 inhibitors. Ren Fail 42:836–844
Tang H, Li D, Zhang J, Li Y, Wang T, Zhai S, Song Y (2017) Sodium-glucose co-transporter-2 inhibitors and risk of adverse renal outcomes among patients with type 2 diabetes: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab 19:1106–1115
Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A (2013) Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 159:262–274
FDA. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). Available from: https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm. 2016.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Investigators CT (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380:2295–2306
Raschi E, Parisotto M, Forcesi E, La Placa M, Marchesini G, De Ponti F, Poluzzi E (2017) Adverse events with sodium-glucose co-transporter-2 inhibitors: a global analysis of international spontaneous reporting systems. Nutr Metab Cardiovasc Dis 27:1098–1107
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A (2017) Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis 27:1108–1113
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380:347–357
Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, Vervloet MG, Wong MG, Gansevoort RT, Heerspink HJL (2020) Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8:582–593
Menne J, Dumann E, Haller H, Schmidt BMW (2019) Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med 16:e1002983
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375:323–334
Sridhar VS, Tuttle KR, Cherney DZI (2020) We can finally stop worrying about SGLT2 inhibitors and acute kidney injury. Am J Kidney Dis 76:454–456
Desai M, Yavin Y, Balis D, Sun D, Xie J, Canovatchel W, Rosenthal N (2017) Renal safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus. Diabetes Obes Metab 19:897–900
Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, Teo KK, Yusuf S, Mann JF (2011) Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 123:1098–1107
Garcia-Ropero A, Badimon JJ, Santos-Gallego CG (2018) The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicol 14:1287–1302
O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal aging: causes and consequences. J Am Soc Nephrol 28:407–420
Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E (2021) Existence of notoriety bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm 56:152–158
Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM (2014) The Weber effect and the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf 37:283–294
Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, George JT, Green JB, Landray MJ, Baigent C, Wanner C (2018) The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 11:749–761
Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC (2020) Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35:274–282
Acknowledgements
This work has been made possible through an ISN Sister Renal Centers Grant.
Funding
This study was supported by Thrombocytopenia Funding from Yeehong School of Shenyang Pharmaceutical University (TCP funding).
Author information
Authors and Affiliations
Contributions
GC designed the study, analyzed and interpreted data, generated figures/tables, and drafted the manuscript. XL analyzed and interpreted data, and drafted the manuscript. QC contributed to manuscript drafting. BZ designed the study and directed the data mining in the FAERS database. QC, YZ, DM, and XL reviewed and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, G., Li, X., Cui, Q. et al. Acute kidney injury following SGLT2 inhibitors among diabetic patients: a pharmacovigilance study. Int Urol Nephrol 54, 2949–2957 (2022). https://doi.org/10.1007/s11255-022-03211-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03211-7