Abstract
Background
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a class of oral anti-hyperglycemic agents that have been available on the market in Japan since 2014. Although safety information has accumulated alongside the clinical use, the warnings issued by each country based on adverse events associated with the drug are different and examination of the safety of the drug is insufficient.
Objective
This study examined the safety of SGLT2 inhibitors by using a Japanese spontaneous reporting database and focusing on the cautions issued in each country and the disparities within existing research into the occurrence of the adverse events of acute renal failure (ARF), ketoacidosis, and urogenital tract infections (UTIs).
Patients and Methods
We analyzed data recorded on the Japanese Adverse Drug Event Report database (JADER) between April 2014 and February 2019. We calculated the reporting odds ratio (ROR) and 95% confidence interval (CI) with sex and age as adjustment factors.
Results
JADER contained 366,501 cases with the adverse events of interest; 4322 involved SGLT2 inhibitors. The ROR for SGLT2 inhibitors was calculated as 1.0 (95% CI 0.9–1.2) for ARF, 72.2 (95% CI 59.3–87.8) for ketoacidosis, and 14.0 (95% CI 11.0–17.8) for UTIs. Analysis of only subjects receiving treatment for diabetes showed a similar trend.
Conclusion
The results suggested a correlation between SGLT2 inhibitors and the onset of ketoacidosis and UTIs, but not between SGLT2 inhibitors and ARF. Further verification of the safety of SGLT2 inhibitors, through continued risk assessments and large-scale clinical studies, are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We analyzed a Japanese spontaneous reporting database, the Japanese Adverse Drug Event Report database (JADER), and observed a correlation between the sodium-glucose cotransporter-2 (SGLT2) inhibitors and the onset of ketoacidosis and urogenital tract infections, but not between SGLT2 inhibitors and acute renal failure. |
The trends confirmed in this study are in accordance with the recent revisions of precautions by the Pharmaceutical and Medical Devices Agency (PMDA) of Japan. |
1 Introduction
Sodium-glucose-cotransporter-2 (SGLT2) inhibitors are a relatively new class of oral anti-hyperglycemic agents that entered the market in the USA in 2013 and in Japan in 2014. Their mechanism of action is to facilitate the elimination of glucose through the urine by inhibiting the reabsorption of glucose through the SGLT2 located in the renal proximal tubules. As the clinical use of SGLT2 inhibitors has increased worldwide, the emerging adverse event reports have aided clarification their safety data. Disparities have emerged in the response of each country to case reports of the various adverse events. The results from clinical trials and adverse event reports also revealed differing trends. For example, based on the accumulated assessments of spontaneously reported adverse events, the United States Food and Drug Administration (FDA) issued a warning for the possible onset of ketoacidosis and urinary tract infections resulting from the use of canagliflozin, dapagliflozin, or empagliflozin, which were mentioned on drug labels beginning from May 15, 2015 [1], in addition to the existing warning for the possible onset of genital infections. They also issued a further warning on June 14, 2016, based on the accumulated assessments of spontaneously reported adverse events, about the possible onset of acute renal failure resulting from the use of canagliflozin or dapagliflozin [2]. Meanwhile, the Pharmaceutical and Medical Devices Agency (PMDA) of Japan issued an alert for the risk of dehydration resulting from the use of all six types of SGLT2 inhibitors (ipragliflozin, dapagliflozin, luseogliflozin, tofogliflozin, canagliflozin, and empagliflozin) currently approved for use in Japan on January 9, 2015. They also issued an alert on the revision of the precautions on the inhibitors to include the risk of ketoacidosis and urogenital tract infections on September 15, 2015. However, the PMDA did not issue alerts with regard to the risk of acute renal failure as a separate event, and so the Japan Diabetes Society has maintained an alert for this adverse event as a type of dehydration-related event [3,4,5,6,7].
Even clinical studies have revealed diverse observations on the correlation between these adverse events and SGLT2 inhibitors. A placebo-controlled, randomized, double-blind study (the EMPA-REG study) of patients with type 2 diabetes at a high risk of a cardiovascular event suggested that empagliflozin could potentially suppress the progression of renal failure [8]. Although a meta-analysis of randomized controlled trials (RCTs) showed that SGLT2 inhibitors tended to diminish the risk of acute renal failure [relative risk (RR) 0.59, 95% CI 0.39–0.89], this result was strongly influenced by the previously mentioned EMPA-REG study. When the EMPA-REG study was excluded from the analysis, it was concluded that the significant difference was eliminated, with an RR 0.48 (95% CI 0.14–1.64) [9]. The meta-analysis conducted during this study also examined ketoacidosis and urinary tract infections. No clear correlation between SGLT2 inhibitors and ketoacidosis was determined (RR 0.66, 95% CI 0.30–1.45) [9]. Concerning urogenital tract infections, only urinary tract infections were examined in the previously mentioned meta-analysis. Although the analysis did not find a clear correlation between SGLT2 inhibitors and urinary tract infections (RR 1.02, 95% CI 0.95–1.09), it did yield the positive result of RR 1.21 (95% CI 1.02–1.43) when focusing only on dapagliflozin, which suggested a correlation between this particular inhibitor and urinary tract infections [8, 9]. Another study using a network meta-analysis, which examined urinary tract infections and genital infections, found no clear correlation with SGLT2 inhibitors [urinary tract infection odds ratio (OR) 1.00, (95% CI 0.84–1.20); genital infection OR 0.93, (95% CI 0.70–1.25)]. However, a positive correlation was observed for both urinary tract infections and genital infections when focused solely on dapagliflozin [urinary tract infection OR 1.28, (95% CI 1.06–1.54); genital infection OR 4.51, (95% CI 3.37–6.04)]. Regarding genital infections, it was also suggested that canagliflozin and empagliflozin were correlated with adverse events (OR 4.99, 95% CI 3.74–6.67 and OR 3.64, 95% CI 2.87–4.63 for canagliflozin and empagliflozin, respectively) [10]. Further examination on the safety of the practical use of SGLT2 inhibitors following accumulation of further usage data is necessary.
Signal detection is a post-marketing drug safety monitoring method that uses spontaneous reporting databases. Public spontaneous reporting databases include the Eudra Vigilance from the European Medicine Agency (EMA) [11], the Canada Vigilance Adverse Reaction Online Database from the Canadian government [12], and VigiBase from the World Health Organization (WHO) [13], in addition to the previously mentioned Japanese Adverse Drug Event Report Database (JADER) from the PMDA [14] and the FDA Adverse Event Reporting System (FAERS) [15]. FAERS is the largest spontaneous reporting database, containing over 1,000,000 recorded reports from every country in the world. However, as approximately 50,000 Japanese domestic reports are recorded each year on JADER, it is most suitable for a safety assessment conducted within Japan [16].
Until now, examinations of the correlation between SGLT2 inhibitors and acute renal failure, ketoacidosis, and urogenital tract infections performed using FAERS have shown a positive correlation between SGLT2 inhibitors and each of these adverse events [17,18,19]. There has been no examination of the use of SGLT2 inhibitors and the onset of acute renal failure, ketoacidosis, and urogenital tract infections that used JADER and were conducted within Japan.
The purpose of this study was to examine the risk of onset of acute renal failure, ketoacidosis, and urogenital tract infections when taking SGLT2 inhibitors in Japan by using signal detection in JADER.
2 Methods
JADER is available to the public and can be downloaded from the official PMDA website [14]. JADER consists of four types of tables: patient demographic information (DEMO), drug information (DRUG), ADR information (REAC), and primary disease information (HIST). This study downloaded and analyzed records from April 2014 to February 2019, the period in which ipragliflozin went on sale as the first SGLT2 inhibitors in Japan.
After the information was downloaded, we combined the patient demographic information (DEMO) and the drug information (DRUG) with the ADR information (REAC) using the identification numbers. We categorized the drug information (DRUG) as either “suspected drug”, “drug interactions”, or “companion drug”, and then restricted the analysis to the “suspected drug” category. Any cases in which information on the patient’s sex (male or female) was missing and cases where their age which was entered as “teens” were marked as “details unclear” and excluded from the analysis.
During the extraction of the adverse events, we used preferred terms (PT) collected from the Medical Dictionary for Regulatory Activities (MedDRA). The following definitions were selected for our Japanese terms in English. We chose 50 events under the category “acute renal failure”, as determined by the Standardized MedDRA Query (SMQ) broad terminology (Supplementary Table 1). We chose “ketoacidosis” and “diabetic ketoacidosis” under the category “ketoacidosis”. We chose “urinary tract infection” and “genitourinary tract infection” under the category “urogenital tract infections”. For the SGLT2 inhibitors, we chose those that are currently approved for sale in Japan: “ipragliflozin”, “dapagliflozin”, “luseogliflozin”, “tofogliflozin”, “canagliflozin”, and “empagliflozin”.
As to whether an onset occurred for any of the adverse events while using SGLT2 inhibitors, we calculated the reporting odds ratio (ROR) and the 95% CI by using a logistic regression analysis with sex and age (70 years of age or older/69 years of age or younger) as adjustment factors. We calculated the ROR after combining “presence or absence of specific drugs” and “presence or absence of specific adverse events” to yield an index for signal detection in the spontaneous reporting database. This has been applied by the Netherlands Pharmacovigilance Centre Lareb as an index for signal detection [20]. We then limited the cases to patients using SGLT2 inhibitors and receiving treatment for diabetes and then calculated the ROR. To refine the analysis subjects to only the patients receiving treatment for diabetes, we reviewed the reason for using the drug and identified any records that indicated that the patient was undergoing treatment for diabetes from among the records with diabetes listed. We used the software program IBM SPSS Statistics 26 (IBM, NYC) to compute the analyses.
3 Results
3.1 Data Summary
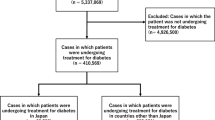
There were 425,668 cases of adverse events recorded on JADER between April 2014 and February 2019. Of these, 366,501 remained to be analyzed in this study after exclusion of cases in which the suspected drug or sex or age of the patient were unknown. Of these cases, 4322 cases (1.2%) were patients taking SGLT2 inhibitors (Fig. 1). From among these 4322 cases and the remaining 362,179 cases of patients who were not taking the inhibitors, 2594 (60.0%) and 185,139 (51.1%), respectively, were male patients. The number of cases in which patients were aged ≥ 70 years and taking SGLT2 inhibitors, and the number of remaining cases in which patients were aged ≤ 70 years and not taking SGLT2 inhibitors was 1537 (35.6%) and 156,885 (43.3%), respectively (Table 1).
The number of cases remaining after the total number of analysis subjects (366,501 cases) was refined to include only cases in which patients were receiving treatment for diabetes was 13,672. From among those cases, the number of cases in which patients were taking SGLT2 inhibitors was 4077 (29.8%) (Fig. 1). From among these cases and the remaining 9595 cases in which patients were not taking inhibitors, 2433 cases (59.7%) and 5853 cases (61.0%), respectively, were male patients. The number of cases in which patients were aged ≥ 70 years and taking SGLT2 inhibitors and the remaining number of cases who were not taking the inhibitor was 1440 (35.3%) and 5552 (57.9%), respectively (Table 2).
3.2 Acute Renal Failure
In the analysis subject group, there were 126 reported cases of acute renal failure from among the subjects taking SGLT2 inhibitors. The ROR was estimated to be 1.0 (95% CI 0.9–1.2). Cases in which patients with diabetes who were taking a particular SGLT2 inhibitor showed similar trends (Table 3).
3.3 Ketoacidosis
From among the cases in which patients were taking SGLT2 inhibitors, 194 cases were of an onset of ketoacidosis, ROR 72.2 (95% CI 59.3–87.8). Similarly, the ROR for cases of diabetes was 10.9 (95% CI 7.6–15.7). These results were suggestive of a correlation between SGLT2 inhibitors and ketoacidosis (Table 4). Cases in which patients were taking a particular SGLT2 inhibitor revealed a similar trend. In particular, cases in which patients were taking canagliflozin or empagliflozin revealed a tendency for a high ROR.
3.4 Urogenital Tract Infections
From among the cases taking SGLT2 inhibitors, 78 cases experienced an onset of urogenital tract infection, ROR 14.0 (95% CI 11.0–17.8). Similarly, the ROR for the cases in patients with diabetes was 19.4 (95% CI 9.9–38.0). These results suggested a correlation between SGLT2 inhibitors and urogenital tract infections (Table 5). Although cases in which patients were taking a particular SGLT2 inhibitor revealed a similar trend, cases in which patients were taking ipragliflozin or luseogliflozin revealed a tendency for a somewhat lower ROR. Furthermore, cases in which patients had diabetes did not show a correlation between luseogliflozin and the onset of urogenital tract infection.
4 Discussion
4.1 Acute Renal Failure
Among the adverse events reported on JADER, a correlation between SGLT2 inhibitors and the onset of ketoacidosis and urogenital tract infections was suggested; however, a correlation between SGLT2 inhibitors and the onset of acute renal failure was not suggested. These trends were the same in cases in which patients with diabetes. No existing studies that used FAERS found data on ipragliflozin, luseogliflozin, or tofogliflozin. This may be attributed to the fact that the data generated in the USA accounts for about two-thirds of the data submitted to FAERS, and that the three medicines mentioned above are not approved for use in the USA [16]. Our study investigated the safety of the three above-mentioned medicines and showed these had same tendencies with regard to safety as the other SGLT2 inhibitors.
Prior studies that used FAERS suggested a significant correlation between SGLT2 inhibitors and acute renal failure, regardless of whether patients were receiving treatment for diabetes [17]. Furthermore, a report indicated that canagliflozin has stronger correlation with adverse events than empagliflozin and dapagliflozin [17]. In contrast, in clinical studies that examined the safety of canagliflozin and empagliflozin in patients with type 2 diabetes, canagliflozin and empagliflozin significantly suppressed the exacerbation of albuminuria in comparison with placebo [21, 22].
As this study did not suggest a correlation between SGLT2 inhibitors and acute renal failure, regardless of whether the patient was receiving treatment for diabetes, there was not a large difference in the ROR for each inhibitor. This was supported by the results of the previous meta-analysis that was conducted excluding the EMPA-REG study [9]. It is assumed that co-administered drugs were used in most of the data employed in this study. Among these, it is possible that the combined use of angiotensin-converting-enzyme (ACE) inhibitors and angiotensin 2 receptor blockers (ARBs), which have a renal protective effect, influenced the results. One study suggests that ACE inhibitors and ARBs may be more effective in Asians than in non-Asians [23,24,25]. By using JADER, which exclusively consists of events from Japan, as a primary factor of the difference in the incidence tendency of acute renal failure between our study and those using FAERS, we believe that the renal protective effect of ACE inhibitors and ARBs may have strongly manifested in an indirect manner and influenced the results. The possibility that the signal could not be detected due to the limited data cannot be excluded. However, there are less data in JADER than in FAERS. In the studies that used FAERS, the number of cases in which patients were taking SGLT2 inhibitors and experienced an onset of acute renal failure was 1224/18915 [17]; in contrast, in JADER there were 126/4322 cases. We hope that further studies will be conducted using more data and will account for the usage status of co-administered drugs.
4.2 Ketoacidosis
Previous studies using FAERS suggested a correlation between SGLT2 inhibitors and the onset of ketoacidosis, regardless of whether the case was undergoing treatment for diabetes by performing signal detection with the proportional reporting ratio (PRR) as an index [18]. Even previous studies that used the VigiBase published by WHO suggest a correlation between SGLT2 inhibitors and the onset of ketoacidosis with ROR as an index [26]. However, as previous meta-analyses have not suggested this correlation, a divergence of views has emerged [9].
The results of this study suggested a correlation between SGLT2 inhibitors and the onset of ketoacidosis, regardless of whether the patient was undergoing treatment for diabetes, which supported the results of previous studies conducted using FAERS and VigiBase [18, 26]. The onset of ketoacidosis due to SGLT2 inhibitors was considered to be the cause of the fat metabolism and production of ketone bodies that accompany the excretion of glucose [27]. Furthermore, there is a concern that patients may delay diagnosis and treatment as most of these cases of ketoacidosis were not accompanied by a clear elevation in blood glucose. There was an especially large number of reports from patients with type 1 diabetes [28].
Although ipragliflozin, one type of SGLT2 inhibitor, was approved in Japan for the treatment of type 1 diabetes in December 2018, it had not been approved in the USA as of December 2019. Of the adverse events used in this study reported on JADER during the 3 months between December 2018 and March 2019, in four cases patients used ipragliflozin for type 1 diabetes and all four experienced an onset of ketoacidosis.
Although there is possibly a fixed number of cases in the JADER spontaneous reporting database of using SGLT2 inhibitors to treat type 1 diabetes off label, we recommend the inclusion of these cases in the analysis of any safety assessment on how the inhibitor is used. If the use of SGLT2 inhibitors for type 1 diabetes expands as the inhibitor gains further approval in the future, we believe it is necessary to examine more precisely the correlation between SGLT2 inhibitors and ketoacidosis by further refining the subjects in the analysis, considering the higher risk of ketoacidosis faced by patients with type 1 diabetes.
4.3 Urogenital Tract Infections
Previous studies that used FAERS suggested a correlation between SGLT2 inhibitors and urogenital tract infections [19]. Although previous meta-analyses have not suggested this correlation, they have suggested a correlation between dapagliflozin only and the onset of urinary tract infections and genital infections [10]. This study suggested a correlation between SGLT2 inhibitors and urogenital tract infections, regardless of whether the patient was receiving treatment for diabetes. Furthermore, when examining the individual types of SGLT2 inhibitors, this study suggested a correlation between all SGLT2 inhibitors approved for use in Japan (except luseogliflozin) and the onset of urogenital tract infections, regardless of whether the patient was receiving treatment for diabetes. We believe the reason this study did not show a significant difference with luseogliflozin is because of the small data pool. The aforementioned meta-analysis [9, 10] detected a signal only for dapagliflozin, and this study also showed a comparatively high ROR for that inhibitor. Although the onset of urogenital tract infections due to SGLT2 inhibitors is considered to be the cause of an increase of bacteria accompanied by the excretion of glucose in the urine, it is unclear why dapagliflozin increases the risk of a urogenital tract infection.
Focusing on urinary tract infections and genital infections separately, we concluded that some existing studies suggested a correlation between SGLT2 inhibitors and genital infections only, and not with urinary tract infections [29, 30]. A meta-analysis comparing Asians and non-Asians suggested that SGLT2 inhibitors were correlated with genital infections regardless of race, but urinary tract infections were only correlated with non-Asians [31]. Similar to previous studies using FAERS [19], the current study targeted urogenital tract infections, so it is not possible to compare the difference in the degree of the association of SGLT2 inhibitors with urinary tract infections and genital infections. However, in this study, it was suggested that SGLT2 inhibitors were correlated with urogenital tract infections, and the FDA and PMDA alerted not only genital infections but also urinary tract infections. Based on these facts, we believe that the continuous monitoring of events such as urinary tract infections and genital infections in clinical settings is important for risk management [1, 3, 4].
4.4 Limitations
This study faced the following limitations. First, one of the limitations of studies that use spontaneous reporting databases is that these databases may include duplicate reports from different users or incomplete/false reports. Second, another limitation of this type of study was the inability to conduct a detailed analysis due to the lack of laboratory values for renal function and HbA1c. Third, the data recorded on JADER do not amount to even 1/20th of the data recorded on FAERS. As this study analyzed only the data on JADER, the small volume of data may have affected the results. Due to these constraints, the signal detection research method used in this study was unable to determine the causal relationships between the medicines and adverse events. Further clinical trials and a comprehensive interpretation of existing clinical trials are needed to show the causal relationship between the medicines and adverse events. On the other hand, the effects of co-administered drugs and complications in actual use do not necessarily cause the same adverse events as in the clinical studies conducted according to protocols. Therefore, in terms of drug risk management, we believe that risk should be minimized by using the signal detection research methods and early careful monitoring of signal-detected adverse events, even if the causal relationship is not clear.
5 Conclusion
This study examined domestic data from Japan by using JADER to determine the risk of the onset of acute renal failure, ketoacidosis, and urogenital tract infections associated with taking SGLT2 inhibitors. Although this study suggested a correlation between SGLT2 inhibitors and the onset of ketoacidosis and urogenital tract infections, it did not suggest a correlation between SGLT2 inhibitors and the onset of acute renal failure. Recently, the PMDA called for revision to the precautions concerning ketoacidosis and urogenital tract infections. This response is in accordance with the trends confirmed in this study. However, further verification through continued risk assessments and large-scale clinical studies following the accumulation of emerging usage data is desirable.
References
FDA. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 15 May 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about. Accessed 19 Dec 2019.
FDA. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). 14 Jun 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Accessed 19 Dec 2019.
PMDA. PMDA revises labels of ipragliflozin, luseogliflozin and tofogliflozin. 15 Sep 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150915A001/02.pdf. Accessed 19 Dec 2019.
PMDA. PMDA revises labels dapagliflozin, canagliflozin and empagliflozin. 15 Sep 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150915A001/03.pdf. Accessed 19 Dec 2019.
PMDA. PMDA revises labels ipragliflozin, dapagliflozin, luseogliflozin, canagliflozin, and empagliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/02.pdf. Accessed 19 Dec 2019.
PMDA. PMDA revises a label tofogliflozin. 9 Jan 2015. (In Japanese). https://www.info.pmda.go.jp/kaiteip/20150109A002/03.pdf. Accessed 19 Dec 2019.
Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. Diabetol Int. 2019;11(1):1–5.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–288.
Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9:e022577.
Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19(3):348–55.
EMA. EudraVigilance. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance. Accessed 19 Dec 2019.
Government of Canada. Adverse Reaction Database. https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/adverse-reaction-database/medeffect-canada-caveat-privacy-statement-interpretation-data-search-canada-vigilance-adverse-reaction-online-database.html. Accessed 19 Dec 2019.
Uppsala Monitoring Centre. VigiBase. https://www.who-umc.org/vigibase/vigibase/. Accessed 19 Dec 2019.
PMDA. JADER. (In Japanese). https://www.pmda.go.jp/safety/info-services/drugs/adr-info/suspected-adr/0004.html. Accessed 17 Jun 2019.
FDA. FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Accessed 19 Dec 2019.
Narukawa M. Expectations for pharmacoepidemiology studies on drug risk management. Regul Sci Med Prod. 2016;6:335–43 (in Japanese).
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27:1108–13.
Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385–9.
Shen J, Yang J, Zhao B. A Survey of the FDA’s Adverse Event Reporting system database concerning urogenital tract infections and sodium glucose cotransporter-2 inhibitor use. Diabetes Ther. 2019;10:1043–50.
Fujita T. Signal detection of adverse drug reactions. Japan J Pharmacoepidemiol/Yakuzai ekigaku. 2009;14:27–36.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Kadowaki T, Nangaku M, Hantel S, Okamura T, von Eynatten M, Wanner C, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from the EMPA-REG OUTCOME(®) trial. J Diabetes Investig. 2019;10(3):760–70.
Chan JC, Wat NM, So WY, Lam KS, Chua CT, Wong KS, et al. Renin angiotensin aldosterone system blockade and renal disease in patients with type 2 diabetes. An Asian perspective from the RENAAL Study. Diabetes Care. 2004;27(4):874–9.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving H-H, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
Ado Moumouni AN, Robin P, Hillaire-Buys D, Faillie JL. SGLT-2 inhibitors and ketoacidosis: a disproportionality analysis in the World Health Organization's adverse drug reactions database. Fundam Clin Pharmacol. 2018;32:216–26.
Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–52.
McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. 2019;124:S45–S52.
Yang Y, Chen S, Pan H, Zou Y, Wang B, Wang G, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(21):e6944.
Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503–14.
Cai X, Gao X, Yang W, Chen Y, Zhang S, Zhou L, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J Diabetes Investig. 2018;9(4):850–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this study.
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Katsuhara, Y., Ogawa, T. Acute Renal Failure, Ketoacidosis, and Urogenital Tract Infections with SGLT2 Inhibitors: Signal Detection Using a Japanese Spontaneous Reporting Database. Clin Drug Investig 40, 645–652 (2020). https://doi.org/10.1007/s40261-020-00925-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00925-3