Abstract
Background
Filgrastim is a recombinant, non-glycosylated form of human granulocyte colony-stimulating factor, used to stimulate leukocyte proliferation in patients suffering from neutropenia. Since the expiration of patents associated with Amgen’s filgrastim biopharmaceutical, Neupogen®, in 2006, a number of filgrastim products have been marketed; however, a detailed characterization and comparison of variants associated with these products have not been publically reported.
Objective
The objective of this study was to identify and quantify product-related variants in filgrastim reference products and biosimilars thereof that are presently available in highly regulated markets.
Methods
In this study, we used intact and top–down mass spectrometry to identify and quantify product-related variants in filgrastim products. Mass spectrometry has become the method of choice for physicochemical characterization of biopharmaceuticals, allowing accurate and sensitive characterization of product-related variants.
Results
In addition to modifications ubiquitously present in biopharmaceuticals, such as methionine oxidation and asparagine/glutamine deamidation, we identified six different low-level, product-related variants present in some, but not all, of the tested products. Two variants, an acetylated filgrastim variant and a filgrastim variant containing an additional C-terminal tryptophan extension, are newly identified variants.
Conclusion
This study demonstrates that filgrastim products already in widespread clinical use in highly regulated markets differ in low-level, product-related variants present at levels mostly below 1 % relative abundance. This study provides a comprehensive catalog of minor differences between filgrastim products and suggests that the filgrastim product-related variants described here are not clinically relevant when present at low abundance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The available filgrastim products differ in low-level, product-related variants. |
The identified product-related variants are mostly <1 % in relative abundance. |
All tested products are in widespread clinical use. |
1 Introduction
One of the first biopharmaceuticals to be commercialized in the mid-1990s was Amgen’s Neupogen®, which is a recombinant, non-glycosylated form of human granulocyte colony-stimulating factor (G-CSF). G-CSF is a hematopoietic growth factor and cytokine, which stimulates production of neutrophils and affects neutrophil progenitor proliferation, differentiation, and functional activation [1, 2]. The biopharmaceutical is composed of a 175–amino acid polypeptide with an additional N-terminal methionine residue not found in the native endogenous human protein. Filgrastim is largely used to restore production of neutrophils in patients undergoing chemotherapy [3, 4]. A potentially life-threatening consequence of loss of neutrophils is febrile neutropenia, the risk of which is increased in the case of non-adherence to treatment guidelines—for example, in a situation where patient access to filgrastim is limited because of high treatment costs [5–7]. Febrile neutropenia not only presents a serious health concern for patients but also results in extensive utilization of healthcare resources and consequently high costs [8, 9]. One possible solution to reduce healthcare costs and improve patient access is use of biosimilars [10]. The introduction of biosimilar filgrastims has led to significant cost savings and increased patient access in countries within the European Union (EU), exemplified by a shift in treatment practice from secondary prophylaxis to increased primary prophylaxis in some countries, especially in patients at high risk of developing febrile neutropenia [5].
The active ingredients of a biosimilar and its reference medicine are essentially the same biological substance, though there may be minor differences due to their complex nature and production methods [11]; as defined by the US Food and Drug Administration (FDA), biosimilars “are highly similar to the reference product notwithstanding minor differences in clinically inactive components,” which may be confirmed by documenting “no clinically meaningful difference between the biological product and the reference product in terms of the safety, purity, and potency of the product” [10, 12, 13]. Following the loss of exclusivity of Amgen’s Neupogen®, a number of biosimilar filgrastim products have been approved in highly regulated markets. In 2015, the FDA approved Zarxio™ (Sandoz) as the first biosimilar in the USA, but in the EU and Japan, biosimilars were already in widespread clinical use and demonstrated clinical performance comparable to that of the reference product in terms of safety, purity, and potency [5, 14–19]. Because of the unique diversity of the marketed products and substantial use of these products in clinical practice, we reasoned that it might be of interest to perform a characterization of the microheterogeneity in the available filgrastim products to provide a catalog of low-level, product-related variants.
Reversed-phase high-performance liquid chromatography (RP-HPLC) with ultraviolet or fluorescence detection is used to detect product-related variants in filgrastim on a routine basis. However, for physicochemical characterization, mass spectrometry (MS) has become the method of choice, as it can provide increased sensitivity, as well as the ability to determine the type and location of the modification [20–22]. We have previously demonstrated the applicability of top–down MS for characterization of oxidized variants in filgrastim, with sensitivity of 0.1 % [20]. In this study, we used an intact/top–down approach for highly sensitive identification and quantification of product-related variants present in the available filgrastim products.
2 Methods
2.1 Chemicals and Study Material
All chemicals that were purchased for this study were of the highest purity available. Trifluoroacetic acid (TFA) was obtained from Sigma-Aldrich (Steinheim, Germany). HPLC-grade water and acetonitrile (ACN) were purchased from J.T. Baker (Deventer, the Netherlands). Products were sourced from the pharmaceutical companies listed in Table 1 and were stored under the intended storage conditions.
2.2 HPLC Separation and MS Analysis
Nine micrograms of filgrastim were separated on a Zorbax 300SB-C18 column (4.6 × 150 mm, 3.5 μm particle size) with a gradient of solutions A (0.1 % TFA in water) and B (0.1 % TFA in ACN) at a flow rate of 1 mL/min: 25 min from 25 % B to 54 % B, followed by a 32 min gradient from 54 % B to 73 % B. After ultraviolet and fluorescence detection, the flow was split 1:5 and then electrosprayed into the Exactive MS.
For intact mass measurements, the Exactive MS was operated with the following settings: spray voltage 4 kV, capillary temperature 275 °C, sheath gas 20, auxiliary gas 8, scan range 300–2200 m/z, resolution ultra-high, automatic gain control (AGC) target 1e6, maximum inject time 100 ms, and microscans 10. For all-ion fragmentation (AIF) measurements, the higher-energy collision dissociation (HCD) fragmentation energy was set at 35 eV. For data evaluation, the spectra acquired within a time frame of approximately 10 s were averaged and deconvoluted using the Xtract algorithm implemented into Xcalibur 2.1 software (Thermo Scientific, Bremen, Germany). The following parameters were used for deconvolution: S/N threshold 2, fit factor 44 %, remainder 25 %, and maximum charge 20. Deconvoluted spectra were manually compared against the raw data and checked for plausibility to exclude artifacts from the deconvolution algorithm. The assignment of deconvoluted AIF spectra was done manually using GPMAW 9.02 software (Lighthouse data, Odense, Denmark) to predict the masses of the individual fragment ions. Relative quantification of product-related variants was performed on the basis of the extracted ion chromatograms (EICs) of the native and modified variants. Ion chromatograms were extracted in Xcalibur 2.1, using the theoretical masses of the +12 to +17 charged molecules with a mass window of 0.5 Da.
3 Results
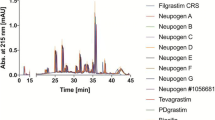
Figure 1 shows the RP-HPLC fluorescence traces of the filgrastim products listed in Table 1. The product-related variants typically observed in RP-HPLC analyses are methionine oxidation and glutamine deamidation. These species are the most prominent product-related variants typically identified in filgrastim products, and identical oxidized and deamidated species were observed in all of the tested products (Fig. 1). The relative abundance of these variants is dependent on the age of the product and, thus, they are shelf life–limiting factors. We have previously reported site-specific characterization of oxidized variants [19]. Oxidized and deamidated variants are well-known degradation products, which are commonly present in protein-based drugs, and thus they are not discussed in more detail in this study [20].
Reversed-phase high-performance liquid chromatography fluorescence traces of filgrastim products (also listed in Table 1). Product-related variants discussed in this study are shown in bold text, and variants present in not all tested products are shown in parentheses. Co-eluting species, e.g. deamidation/M→Nle/D→E, can be identified and quantified only by mass spectrometry. D→E aspartate-to-glutamate misincorporation, fMet N-formylmethionine, M→Nle methionine-to-norleucine (sum of four variants), Met1ox methionine oxidation at position 1, Met122ox methionine oxidation at position 122, Met127ox methionine oxidation at position 127, Met138ox methionine oxidation at position 138, Trp C-terminal tryptophan. N-terminal truncation variant (sum of five variants) co-elutes with native Filgrastim
In addition to oxidized and deamidated species, which can easily be detected and monitored using RP-HPLC, several low-level, product-related variants were detected in the products, using MS. Ribosomal translation in bacteria starts with N-formylmethionine (fMet), which is a derivative of the amino acid methionine [23]. fMet is efficiently deformylated to methionine by an enzyme called peptide deformylase [24]. As shown in Table 2, some of the products exhibited remnant levels of fMet of up to 0.3 %. Interestingly, fMet levels in the filgrastim product Gran® reached levels of 1.8 % (see Table 2). The fMet species eluted as a post-peak in the RP-HPLC assay (Fig. 1) and showed a typical +28 Da mass shift in MS analysis (Fig. 2). fMet, as a product-related variant in filgrastim, has already been reported in the literature [25, 26].
The N-terminal first 10 amino acids form an unstructured region in filgrastim, which may explain their susceptibility to cleavage by aminopeptidases [27]. N-terminal heterogeneity was observed in all tested products to a similar extent; the relative abundances of the sum of N-terminal truncated species ranged from 1.4 to 2.4 % (Table 2). Figure 3 shows the EICs used for quantification of the individual truncated species in a representative sample (Zarxio™ batch 600314). In all tested products, the most abundant truncated species was the -M variant, followed by the -MTPLG variant. The N-terminal truncated variants co-eluted (-M and -MT) or eluted as a pre-shoulder (-MTPL to -MTPLGP) of the native molecule in the RP-HPLC assay.
Misincorporation of amino acids in therapeutic and endogenous proteins has been reported to occur at a rate of 10−3 to 10−4 per codon (i.e. 0.01–0.1 % of relative abundance) [28, 29]. Misincorporation events are inherent to biological systems because of inaccuracies in the cellular gene expression machinery; their abundance, however, may be influenced by factors such as the selection of the coding DNA triplet, the expression strain, and the fermentation conditions [30–33]. A well-documented case in recombinant proteins is misincorporation of norleucine (Nle) at methionine positions (M→Nle), which may result from methionine deficiency or other factors [34, 35]. Norleucine is a structural analog of methionine, where the sulfur atom is replaced by a methylene group. Trace levels of the M→Nle variant of up to 0.4 % (the sum of four variants) were identified in the tested filgrastim products (Table 2). The M→Nle variants showed a typical −18 Da shift in MS analysis (not shown) and eluted as post-peaks in the RP-HPLC assay (Fig. 1). Another frequently observed misincorporation in recombinant proteins is the aspartate-to-glutamate (D→E) misincorporation variant, which can be explained by the wobble-base theory [32]. We identified this variant in several of the tested products at levels of up to 0.7 % (Table 2). The D→E variant showed a typical +14 Da shift in the MS analysis (Fig. 2) and eluted as a post-peak in the RP-HPLC assay (Fig. 1).
In three of the tested products (Filgrastim NK, Ratiograstim®, and Granix®) we identified a low-level, product-related variant of filgrastim, which has not been previously reported in the literature (Fig. 4a–c). Figure 4b illustrates representative raw spectra of the 16-fold charged molecule. Deconvolution of the summed raw spectra resulted in identification of three species: deamidated filgrastim, its TFA adduct and a species with a mass difference of +186.1 Da relative to the native molecule. The variant eluted as a post-peak relative to the native molecule in the RP-HPLC assay, with a maximum relative abundance of approximately 0.3 % in a subset of the tested products (see Table 2).
Identification of a product-related variant with a mass shift of +186.1 Da corresponding to a C-terminal tryptophan extension. a The variant elutes as a post-peak in the reversed-phase high-performance liquid chromatography assay. b Summed raw spectra of the +16 charge state of the deamidated filgrastim and its trifluoroacetic acid (TFA) adduct (highlighted in gray) and the +186.1 Da modified molecule (highlighted in red). Products representative of the absence (Neupogen® US and Zarxio™) and of the presence (Filgrastim NK and Ratiograstim®) of this variant are shown. c Deconvoluted spectrum showing the deamidated filgrastim and its TFA adduct, as well as the +186.1 Da variant (highlighted in red). The identified TFA adduct represents an analytical artifact frequently observed in mass spectrometry (MS). The deconvoluted masses are [M+H]+
Because this variant is chromatographically separated from the native molecule, we were able to perform top–down MS using the AIF mode of the Exactive mass spectrometer, as has been described previously [20]. In the top–down experiments, we observed that the +186.1 Da mass shift was associated with the y-ion series but not with the b-ion series (Fig. 5a), demonstrating that the modification resides on the C-terminus of the protein rather than on the N-terminus. The closest C-terminal fragment showing the +186.1 Da modification was the y43 ion, demonstrating that the modification resides within the last 43 amino acids of the protein. A mass shift of +186.1 Da corresponds to the mass of an additional tryptophan (Trp) residue being incorporated into the C-terminal region of the molecule. Figure 5b illustrates the EICs of this variant used for the relative quantification.
Site assignment and relative quantification of the +186.1 Da modification corresponding to the C-terminal tryptophan extension variant. a Top–down spectrum from all-ion fragmentation of the molecules eluting at 31.0 min in the Ratiograstim® N41250 sample. The +186.1 Da mass shift is observed in the y-ion series but not in the b-ion series. Trifluoroacetic acid adducts have been removed for ease of reading. b Extracted ion chromatograms of the C-terminal tryptophan extension variant used for quantification. The peak indicating the C-terminal tryptophan extension variant is highlighted in red
The variant can most likely be explained by differences in the selected stop codon. Translational termination can be achieved by using one of the three codons UGA, UAA and UAG. A leaky UGA stop codon due to the presence of the suppressor transfer RNA Trp (tRNATrp) has been reported in the literature [36, 37]. In this process, called UGA readthrough, the suppressor tRNATrp binds to the stop codon UGA, leading to elongation of the polypeptide chain by a tryptophan residue rather than translational termination [38, 39]. Depending on the genetic context, tryptophan levels of up to 3 % can be incorporated by this mechanism at the UGA site [36, 40]. Therefore, the most likely explanation for the observed +186.1 Da species is C-terminal extension of the protein with a tryptophan residue due to the readthrough of the first stop codon (usually, two stop codons are used to ensure translational termination). The use of different stop codons (UGA versus UAA and UAG) might therefore also explain the presence/absence of this variant in the different products (Fig. 5b).
Another hitherto unreported product-related variant in filgrastim products was observed in four of the tested products (Gran®, Filgrastim Mochida/Fuji, Grastofil®, and Nivestim®). The variant eluted as a post-peak relative to the native molecule in the RP-HPLC assay (Fig. 6a), with a maximum relative abundance of 0.5 % in the tested products. Figure 6b illustrates representative raw spectra of the +16 charge state. Deconvolution of the summed raw spectra resulted in identification of two species: native filgrastim and a species with a mass difference of +42.0 Da, which corresponds to an acetylation modification. As this species was not successfully separated by chromatography from native filgrastim, we could not perform AIF for site assignment on the Exactive instrument. Subsequent characterization using PepMap™ technology coupled with MS detection (not shown) confirmed that this variant was an acetylated filgrastim species, which is in agreement with the +42 Da mass shift observed in the intact measurement.
Identification of a product-related variant with a mass shift of +42.0 Da corresponding to an acetylation modification. a The variant elutes as a post-peak in the reversed-phase high-performance liquid chromatography assay. b Summed raw spectra of the +16 charge state of native filgrastim (highlighted in gray) and the +42.0 Da modified molecule (highlighted in red). Products representative of the absence (Neupogen® US and Zarxio™) and of the presence (Filgrastim Mochida and Grastofil®) of this variant are shown. c Deconvoluted spectrum showing native filgrastim and the +42.0 Da variant, which corresponds to an acetylation modification. The deconvoluted masses are [M+H]+. MS mass spectrometry
4 Discussion
Microheterogeneity of biopharmaceuticals is caused by posttranslational modifications introduced during manufacturing in living cells. Filgrastim is a comparatively simple biopharmaceutical. It has a size of only 19 kDa and is produced in an expression system (Escherichia coli) that lacks the ability to perform complex posttranslational modifications, such as glycosylation, allowing for a highly homogenous product. However, even in this simple drug, the presence of a number of low-level variants is commonly observed. Ubiquitously identified variants in biopharmaceuticals are methionine oxidation and glutamine/asparagine deamidation. Previously reported variants that are more specific to filgrastim are fMet, truncated variants, D→E, and M→Nle [25, 26]. In this study, we identified previously unreported, low-abundance variants, including an acetylated filgrastim variant and a variant with an additional C-terminal tryptophan residue, which were present in some, but not all, filgrastim products that were analyzed.
Most of the products that were analyzed in this study are biosimilars of a filgrastim reference product. For biosimilar products, the approval pathway in highly regulated markets requires an in-depth comparative analytical characterization [11, 41]. Conceptually, the high sensitivity and resolution of state-of-the-art analytical technologies, such as MS, allow for a tailored clinical program if the analytical data can provide evidence that the biosimilar candidate and the reference product contain essentially the same active ingredient. In such a situation, the analytical and functional comparative data can serve as surrogates for prediction of the clinical properties of safety, immunogenicity and efficacy.
When a biosimilar product is being developed, the presence of product-related variants that are not present in the reference product requires special attention before the clinical stage to ensure patient safety and to address the issue of immunogenicity. The data from this study revealed the identities and levels of product-related variants in filgrastim products from different manufacturers, which had already been approved for commercialization. As summarized in Table 2, in this study we identified a total of six product-related variants (not including oxidized and deamidated variants), demonstrating that the composition of low-level variants differs between filgrastim products. This variability is most likely a characteristic of the different manufacturing processes. However, on the basis of the low abundance of those variants of mostly less than 1 %, and even smaller quantitative differences between products, a clinical impact on safety, immunogenicity, and efficacy is unlikely. This is supported by the clinical studies performed for approval of the products and by the current clinical experience with these products [5, 14–19]. Although not all of these products have been tested in comparative clinical trials, no apparent clinical differences with regard to safety, immunogenicity, and efficacy have been reported. Furthermore, using filgrastim as a model, our data support the notion that currently available analytical technologies, such as MS, are more capable and sensitive than clinical studies for identifying differences between products.
5 Conclusion
Mass spectrometry has become the method of choice for physicochemical characterization of biopharmaceuticals. In this study, we used a combination of native and top–down MS for sensitive identification and relative quantification of product-related variants in filgrastim products that are presently available in highly regulated markets. In addition to modifications that are ubiquitously present in biopharmaceuticals, such as methionine oxidation and asparagine/glutamine deamidation, we identified a total of six different low-level, product-related variants with relative abundances of mostly <1 %. As the composition of these variants differs between products, this study provides a comprehensive catalog of minor differences between filgrastim products. However, the low abundance combined with the current clinical experience suggest that the observed minor differences in the filgrastim product-related variants are not clinically relevant.
References
Metcalf D. The granulocyte-macrophage colony stimulating factors. Cell. 1985;43(1):5–6.
Nemunaitis J. A comparative review of colony-stimulating factors. Drugs. 1997;54(5):709–29.
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
Ye SG, Ding YI, Li L, et al. Colony-stimulating factors for chemotherapy-related febrile neutropenia are associated with improved prognosis in adult acute lymphoblastic leukemia. Mol Clin Oncol. 2015;3(3):730–6.
Gascon P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925–32.
Renner P, Milazzo S, Liu JP, et al. Primary prophylactic colony-stimulating factors for the prevention of chemotherapy-induced febrile neutropenia in breast cancer patients. Cochrane Database Syst Rev. 2012;10:CD007913.
Verpoort K, Mohler TM. A non-interventional study of biosimilar granulocyte colony-stimulating factor as prophylaxis for chemotherapy-induced neutropenia in a community oncology centre. Ther Adv Med Oncol. 2012;4(6):289–93.
Michels SL, Barron RL, Reynolds MW, et al. Costs associated with febrile neutropenia in the US. Pharmacoeconomics. 2012;30(9):809–23.
Wang XJ, Lopez SE, Chan A. Economic burden of chemotherapy-induced febrile neutropenia in patients with lymphoma: a systematic review. Crit Rev Oncol Hematol. 2015;94(2):201–12.
Aapro M, Cornes P, Abraham I. Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract. 2012;18(2):171–9.
European Medicines Agency [EMA]. Questions and answers on biosimilar medicines (similar biological medicinal products). London: EMA; 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf. Accessed 25 Mar 2016.
Weise M, Kurki P, Wolff-Holz E, et al. Biosimilars: the science of extrapolation. Blood. 2014;124(22):3191–6.
US Food and Drug Administration [FDA]. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product: guidance for industry. Silver Spring: FDA; 2015. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/General/ucm444891.htm. Accessed 17 Mar 2016.
Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26(9):1948–53.
Sagara Y, Sato K, Fukuma E, et al. The efficacy and safety of FSK0808, filgrastim biosimilar: a multicenter, non-randomized study in Japanese patients with breast cancer. Jpn J Clin Oncol. 2013;43(9):865–73.
Brito M, Esteves S, André R, et al. Comparison of effectiveness of biosimilar filgrastim (Nivestim™), reference Amgen filgrastim and pegfilgrastim in febrile neutropenia primary prevention in breast cancer patients treated with neo(adjuvant) TAC: a non-interventional cohort study. Support Care Cancer. 2016;24(2):597–603.
Waller CF, Semiglazov VF, Tjulandin S, et al. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504–11.
del GA, Eniu A, Ganea-Motan D, et al. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332.
Gascon P. Presently available biosimilars in hematology–oncology: G-CSF. Target Oncol. 2012;7(Suppl 1):S29–34.
Holzmann J, Hausberger A, Rupprechter A, et al. Top-down MS for rapid methionine oxidation site assignment in filgrastim. Anal Bioanal Chem. 2013;405(21):6667–74.
Berkowitz SA, Engen JR, Mazzeo JR, et al. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 2012;11(7):527–40.
Beck A, Wagner-Rousset E, Ayoub D, et al. Characterization of therapeutic antibodies and related products. Anal Chem. 2013;85(2):715–36.
Meinnel T, Mechulam Y, Blanquet S. Methionine as translation start signal: a review of the enzymes of the pathway in Escherichia coli. Biochimie. 1993;75(12):1061–75.
Mazel D, Pochet S, Marliere P. Genetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 1994;13(4):914–23.
Skrlin A, Radic I, Vuletic M, et al. Comparison of the physicochemical properties of a biosimilar filgrastim with those of reference filgrastim. Biologicals. 2010;38(5):557–66.
Sorgel F, Schwebig A, Holzmann J, et al. Comparability of biosimilar filgrastim with originator filgrastim: protein characterization, pharmacodynamics, and pharmacokinetics. BioDrugs. 2015;29(2):123–31.
Hill CP, Osslund TD, Eisenberg D. The structure of granulocyte-colony-stimulating factor and its relationship to other growth factors. Proc Natl Acad Sci USA. 1993;90(11):5167–71.
Loftfield RB, Vanderjagt D. The frequency of errors in protein biosynthesis. Biochem J. 1972;128(5):1353–6.
Edelmann P, Gallant J. Mistranslation in E. coli. Cell. 1977;10(1):131–7.
Ulrich AK, Li LY, Parker J. Codon usage, transfer RNA availability and mistranslation in amino acid starved bacteria. Biochim Biophys Acta. 1991;1089(3):362–6.
Kramer EB, Farabaugh PJ. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA. 2007;13(1):87–96.
Zhang Z, Shah B, Bondarenko PV. G/U and certain wobble position mismatches as possible main causes of amino acid misincorporations. Biochemistry. 2013;52(45):8165–76.
Parker J, Friesen JD. “Two out of three” codon reading leading to mistranslation in vivo. Mol Gen Genet. 1980;177(3):439–45.
Ni J, Gao M, James A, et al. Investigation into the misincorporation of norleucine into a recombinant protein vaccine candidate. J Ind Microbiol Biotechnol. 2015;42(6):971–5.
Bogosian G, Violand BN, Dorward-King EJ, et al. Biosynthesis and incorporation into protein of norleucine by Escherichia coli. J Biol Chem. 1989;264(1):531–9.
Buckingham RH, Kurland CG. Codon specificity of UGA suppressor tRNATrp from Escherichia coli. Proc Natl Acad Sci USA. 1977;74(12):5496–8.
Engelberg-Kulka H. UGA suppression by normal tRNA Trp in Escherichia coli: codon context effects. Nucl Acids Res. 1981;9(4):983–91.
Eggertsson G, Soll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol Rev. 1988;52(3):354–74.
Hirsh D, Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA’s. J Mol Biol. 1971;58(2):459–68.
MacBeath G, Kast P. UGA read-through artifacts—when popular gene expression systems need a pATCH. Biotechniques. 1998;24(5):789–94.
US Food and Drug Administration [FDA]. Scientific considerations in demonstrating biosimilarity to a reference product. Silver Spring: FDA; 2015. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm290967.htm. Accessed 17 Mar 2016.
European Medicines Agency [EMA]. Grastofil: EPAR—public assessment report. London: EMA; 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002150/human_med_001688.jsp&mid=WC0b01ac058001d124. Accessed 17 Mar 2016.
European Medicines Agency [EMA]. Accofil: EPAR—public assessment report. London: EMA; 2014. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003956/human_med_001798.jsp&mid=WC0b01ac058001d124. Accessed 17 Mar 2016.
Acknowledgments
The authors would like to thank Martin Schiestl for his thorough review and support.
Author contributions
All authors made substantial contributions to the conception or design of the work and/or the acquisition, analysis, or interpretation of the data. All authors were involved in developing and critically revising the content of the manuscript, and all provided final approval of the version submitted for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Anna Hausberger, William C. Lamanna, Hansjoerg Toll, and Johann Holzmann are employees of Sandoz GmbH. Martin Hartinger and Andreas Seidl are employees of Hexal AG.
Funding
This study was funded by Sandoz GmbH.
Rights and permissions
About this article
Cite this article
Hausberger, A., Lamanna, W.C., Hartinger, M. et al. Identification of Low-Level Product-Related Variants in Filgrastim Products Presently Available in Highly Regulated Markets. BioDrugs 30, 233–242 (2016). https://doi.org/10.1007/s40259-016-0169-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0169-2