Abstract
Vitiligo is a chronic autoimmune disease characterized by loss of pigment of the skin, affecting 0.5–2% of the population worldwide. It can have a significant impact on patients’ quality of life. In recent years, there has been significant progress in our understanding of the pathogenesis of vitiligo. It is believed that vitiligo develops due to a complex combination of genetics, oxidative stress, inflammation, and environmental triggers. Conventional treatments include camouflage, topical corticosteroids, topical calcineurin inhibitors, oral corticosteroids, phototherapy, and surgical procedures, with the treatment regimen dependent on the patient’s preferences and characteristics. With increased understanding of the importance of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway in the pathogenesis of vitiligo, treatment has expanded to include the first US FDA-approved cream to repigment patients with vitiligo. This review summarizes our understanding of the major mechanisms involved in the pathogenesis of vitiligo and its most common available treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Vitiligo is a chronic autoimmune disease leading to white patches, which can have a profound impact on a patient’s quality of life. |
The T-helper (Th) 1 pathway is overactive with interferon-γ driving the pathogenesis and signaling via Janus kinase (JAK) 1 and JAK2. |
Topical corticosteroids, topical calcineurin inhibitors, oral corticosteroids, and phototherapy are among the most common treatments. The US FDA has approved the JAK1 and JAK2 inhibitor ruxolitinib cream as the first treatment to repigment patients with vitiligo. |
1 Introduction
Approximately 0.5–2% of the population worldwide is affected by vitiligo, a chronic autoimmune disease characterized by the selective loss of melanocytes, resulting in depigmented patches of skin [1, 2]. The disease affects both males and females and all races and ethnicities [3]. About 50% of patients show clinically apparent depigmented lesions before age 20 years, and nearly 70–80% before age 30 years, but the disease can manifest itself at any age [4]. Although patients with vitiligo are not in physical pain, the psychological burden of the disease can be devastating, especially for darker-skinned individuals in whom depigmented areas are more easily detectable, particularly if present on visible areas of the skin. Thus, psychologically, vitiligo can lead to negative self-esteem, depression, social isolation, stigmatization, and overall decreased quality of life [3, 5,6,7,8,9,10]. Vitiligo is classified as either nonsegmental or segmental, as determined by the 2011 Vitiligo Global Issues Consensus Conference, and treatment and prognosis for both classifications vary [11]. Until recently, there have been no US FDA-approved treatments for repigmenting vitiligo. Therapeutic options are based on several published consensus guidelines [12,13,14,15], but available options are not always effective, may have adverse effects, and disease often returns after discontinuing therapy. Additionally, acral areas, such as the hands and feet, are notoriously difficult to repigment with conventional methods compared with areas such as the face and trunk [16]. In general, first-line treatment consists of topical corticosteroids (TCSs) and topical calcineurin inhibitors (TCIs), second-line therapies consist of phototherapy and systemic corticosteroids, and third-line treatment consists of surgical procedures and depigmenting therapy [14, 15, 17,18,19,20], although an element of personalization must be kept in mind when determining a treatment approach. There is a strong need for targeted, safe therapies that are effective and long-lasting, and this goal is within reach given our increased knowledge of the specific pathways involved in the pathogenesis of vitiligo. For this narrative review, PubMed was searched using terms including, but not limited to, vitiligo pathogenesis, vitiligo treatment, and the relevant vitiligo treatment modalities included in this article (TCSs, TCIs, oral corticosteroids, phototherapy, surgical treatment, depigmentation therapy, minocycline, methotrexate, azathioprine, levamisole, apremilast, Ginkgo biloba (GB), Polypodium leucotomos (PL), and topical/oral Janus kinase (JAK) inhibitors). This paper aims to provide an overview of the pathogenesis of vitiligo and a comprehensive review of the therapeutic options available for vitiligo.
2 Pathogenesis
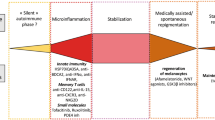
There has been much debate over the underlying pathogenesis of vitiligo, but recent research has led to a more concrete understanding of the mechanisms behind this disease. There is now a consensus that vitiligo is an autoimmune disease driven by the combination of stress and immune responses, together with genetic predisposition and environmental exposures [11, 21].
2.1 Genetics
There have been several genome-wide association studies (GWAS) in European and Chinese populations that have discovered nearly 50 genetic loci associated with vitiligo, confirming this genetic contribution [22,23,24,25,26,27,28,29,30]. Several of these genes are involved in other autoimmune diseases, such as thyroid disease, which is a common comorbidity among patients with vitiligo, in addition to type I diabetes and rheumatoid arthritis [26, 31,32,33]. Furthermore, the risk of developing vitiligo increases to 6.1% if a sibling has vitiligo, and is as high as 23% in identical twins [34]. There are reports of familial clusters of cases [35], and among patients with vitiligo, about 20% have at least one first-degree relative with the disease [36].
2.2 Oxidative Stress
There is still discussion over what first initiates the onset of vitiligo, but one widely accepted hypothesis is the role of oxidative stress [37,38,39,40]. In response to stress, reactive oxygen species (ROS) are released from melanocytes. ROS generation can be triggered by many different stimuli [41], including mitochondria, which have proven to be altered in vitiligo patients [42, 43], membrane lipid defects [44], and the synthesis of melanin itself [45]. The melanin biosynthesis pathway is an energy-consuming process that is directly toxic to melanocytes, generating a pro-oxidant state in the skin [45]. Melanogenesis, which requires the production of numerous proteins, increases the risk of forming misfolded proteins and activating the unfolded protein response stress pathway, and the production of ROS from energy metabolism in mitochondria [45,46,47]. Melanocytes in patients with vitiligo may be more sensitive to pro-oxidant stimuli [38, 39]. Ultraviolet (UV) light, while a well-established treatment for vitiligo, can also induce the production of ROS, hydrogen peroxide (H2O2), and superoxide anions [48]. The melanocytes of patients with vitiligo may also have intrinsic defects, which is suggested by findings that melanocytes cultured from non-lesional skin of patients with vitiligo are more difficult to grow in vitro compared with healthy controls [49] and require the addition of growth factors [50].
2.3 T-helper 1 Pathway, Interferon-γ, and CXC Chemokine Ligand (CXCL) 9/CXCL10
Cytotoxic CD8+ T cells that target melanocytes are present in both the serum of vitiligo patients and the epidermis and dermis of vitiligo lesions [51, 52]. A key study showed definitive evidence of a T-cell cytotoxic effect on melanocytes, causing targeted autoimmune destruction. T cells that were reactive to melanocyte antigen-specific stimulation were obtained from perilesional skin and were transferred to areas of normal skin pigmentation, where they induced depigmentation, effectively killing melanocytes [53].
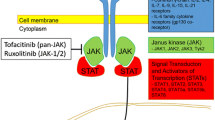
CD8+ T cells, which are involved in the T-helper (Th) 1 pathway of the immune system, produce several cytokines, including interferon (IFN)-γ, among others, which are upregulated in vitiligo lesions [53,54,55]. IFNγ directly induces melanocyte apoptosis and inhibits melanogenesis in vitro [56], and also induces several chemokines including CXC chemokine ligand (CXCL) 9, CXCL10, and CXCL11, all of which have been reported to be increased in the serum and lesions of patients with vitiligo [57]. CD8+ T cells are recruited to melanocytes in the epidermis via these IFNγ-induced chemokines, making IFNγ central to this process [54]. IFNγ activates JAK1 and 2, which are intracellular signaling enzymes used to exert its effect [54, 57, 58]. Thus, the JAK/signal transducer and activator of transcription (STAT) pathway has been found to be a major contributor to the pathogenesis of vitiligo, and an appealing therapeutic target. Additionally, disease activity has been found to correlate with levels of CXCL10 in vitiligo patients [59, 60]. In a study by Rashighi and colleagues, it was found that CXCL9 is primarily involved in the recruitment of T cells to the skin, whereas CXCL10 is required for localization of the CD8 T+ cells within the epidermis. The study also showed that blocking CXCL10 both prevents and reverses vitiligo [57].
2.4 Tissue-Resident Memory T Cells
After a T-cell-mediated immune response occurs, tissue-resident memory T cells (TRMs) develop and persist in nonlymphoid tissues, including the skin [61], explaining the recurrence of depigmentation in the same location after therapies are stopped. In vitiligo lesions, autoreactive CD8+CD69+CD49a+CD103+ TRMs require interleukin (IL)-15 for their maintenance [62,63,64]. Several studies have shown the presence of CD8+ TRMs in active and stable vitiligo lesions [63], in addition to elevated levels of IL-15 in the serum of vitiligo patients [65]. Due to the ability of these TRMs to secrete perforin, IFNγ, and granzyme B after being stimulated by IL-15, they have a cytotoxic effect on melanocytes, causing the depigmentation seen in vitiligo [66]. One study found that treating lesions in a mice model with anti-CD122 (an antibody against the CD122 subunit of the IL-15 receptor) led to repigmentation [67]. On the other hand, treatments that aim to inhibit TRMs but not deplete them from the skin are not durable [61, 68].
2.5 WNT Signaling
The WNT pathway is important for melanocyte differentiation [69], and there is an alteration of the WNT/beta (β) catenin pathway in vitiligo lesions [70]. After oxidative stress in pre-melanocytes following ex vivo stimulation with WNT activators, there is a decrease in lymphoid enhancer-binding factor/T-cell factor (LEF/TCF) expression [71]. Additionally, the WNT pathway is involved in the regulation of E-cadherin expression, which is decreased in vitiligo lesions [72]. When using an ex vivo model of vitiligo, there is increased expression of melanocyte markers when treatment with WNT agonists or GSK3beta was applied [71]. A more recent study showed that micropigmentation in a vitiligo mouse model induced a repair process mediated by the WNT/β-catenin pathway in which the micro-injury stimulated hair follicle melanocyte stem cells to move towards the epidermis [73].
3 Treatments
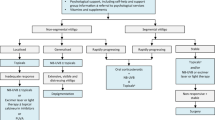
The treatment of vitiligo depends on many factors, including the type of vitiligo (nonsegmental versus segmental), the stage of disease, and patient preference. This, combined with several different published therapeutic consensus guidelines, makes the treatment of vitiligo particularly challenging. Our vitiligo treatment algorithm can be found in Fig. 1.
Therapeutic algorithm for vitiligo. NB-UVB narrow-band ultraviolet-B, PUVA psoralen plus UVA, JAK Janus kinase. 1Can consider methotrexate, minocycline, azathioprine, or levamisole. 2Includes NB-UVB, excimer laser/lamp, PUVA. Can consider the addition of Ginkgo biloba or Polypodium leucotomos. 3Includes topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors. 4Can consider apremilast. 5Includes tissue grafts (suction blister, split-thickness, full-thickness punch/minipunch) and cellular grafts (cultured epidermal graft, cultured melanocyte transplantation, noncultured epidermal or noncultured hair follicle suspensions). Can also consider platelet rich plasma or micropigmentation for refractory disease. 6Includes NB-UVB, PUVA. Can consider the addition of Ginkgo biloba or Polypodium leucotomos. 7Includes topical corticosteroids, topical calcineurin inhibitors. 8Can include methotrexate, minocycline, azathioprine, levamisole, apremilast, supplements. 9Includes bleaching creams, laser, cryotherapy, or phenols.
3.1 Topical Therapies
3.1.1 Cosmetic Camouflage
One strategy to lessen the burden of vitiligo is the use of camouflage via concealers and self-tanning products, which can be used throughout the long-term treatment process, often as an adjunct to medical therapy [74]. These products can significantly improve the self-esteem and quality of life of vitiligo patients [75,78,79,78]. Dihydroxyacetone (DHA)-containing camouflage, the active ingredient in sunless tanning agents, including creams and spray tans, has been found to not interfere with the underlying medical management of vitiligo lesions, making it a safe option for these patients [79]. It is also convenient, allowing patients to camouflage their vitiligo lesions temporarily [80,83,84,83] without requiring medical treatment [84], and different concentrations can be used for different skin types to achieve color matching [82].
3.1.2 Topical Corticosteroids
TCSs are considered a first-line treatment for vitiligo due to their ability to dampen the immune response, and are effective as monotherapy or in combination therapy. The primary endpoint of TCS use is disease stabilization. Although repigmentation can also occur, this is particularly true with concomitant UV exposure [85]. In a meta-analysis that assessed the effectiveness of nonsurgical options for vitiligo, 56% and 55% of patients receiving class 3 and class 4 corticosteroids, respectively, achieved ≥ 75% repigmentation, with more adverse effects occurring in the class 4 corticosteroid group [86]. Adverse effects, which are more common when used for long periods of time in sensitive areas of the skin such as the face, axilla, and genitals, include local skin atrophy, telangiectasia, acneiform eruptions, hypopigmentation, striae, and hypertrichosis [87]. In a retrospective study evaluating the efficacy and safety of high-potency corticosteroid use in children with vitiligo, 64% showed repigmentation of the lesions (45/70), demonstrating efficacy, but cortisol levels were abnormal in 29% of patients, suggesting possible systemic absorption, particularly when used in sensitive areas of the skin such as the head or neck [88]. However, it is important to note that the amount of TCS was not quantified, and 75% of patients were prescribed TCSs three times daily, compared with the more standard once-daily regimen. Of the two patients who were diagnosed with corticosteroid-induced adrenal suppression, laboratory abnormalities resolved after corticosteroid discontinuation [88].
Thus, although effective in inducing repigmentation, concern regarding the possible adverse effects of TCSs has led to the use of TCIs, which have become a standard therapy for vitiligo patients, especially for sensitive areas, such as the face, and in pediatric populations.
3.1.3 Topical Calcineurin Inhibitors
TCIs, such as tacrolimus, are commonly used as treatments for vitiligo. TCIs have been proven to be effective as monotherapy or in combination therapy, especially when used concomitantly with phototherapy [89]. They have mild adverse effects, which can include a burning sensation, erythema, or pruritus after application, and they have been found to be as effective as TCSs, providing a reliable alternative for long-term therapy [89,92,93,94,95,94]. In a randomized controlled study that compared the efficacy of topical tacrolimus 0.03% ointment compared to 1% hydrocortisone acetate ointment, the 24-week Vitiligo Area Scoring Index (VASI) score was significantly lower for those receiving tacrolimus, and repigmentation rates were 45.2% compared with 0.0% in the hydrocortisone group [95]. Topical tacrolimus is especially effective as a monotherapy in those patients with vitiligo lesions on the face and neck [95,98,97]. Cavalié and colleagues conducted a randomized, double-blind study to determine the efficacy of a twice-weekly application of 0.1% tacrolimus ointment as maintenance therapy and found this regimen to be successful in preventing relapses [98].
Topical tacrolimus 0.03% ointment and pimecrolimus 1% cream are suitable for childhood vitiligo and for infants with vitiligo under 2 years of age, with a low incidence of adverse effects consisting mainly of mild local redness and burning [99, 100]. In a study to evaluate the safety and efficacy of tacrolimus 0.03% ointment in infants under 2 years of age, repigmentation was 100% and there was no evidence of any metabolic or physical changes after 6 months of treatment [101]. This provides reassuring evidence for the use of TCIs for even the youngest vitiligo patients.
Since oral calcineurin inhibitors have an increased risk of malignancy, including lymphomas, there is a black-box warning for TCIs. A recent multicenter retrospective cohort study of 25,694 vitiligo patients who received TCIs, phototherapy for 6 weeks or more, or a combination of the two, found no substantial increased risk of skin cancer or lymphoma [102].
3.1.4 Depigmentation Therapies
Depigmentation can be achieved with bleaching creams such as monobenzone (monobenzyl ether of hydroquinone [MBEH]), laser, cryotherapy, or phenols. This route is generally an option for patients with extensive vitiligo lesions (vitiligo universalis). MBEH cream, with the most common concentration at 20%, is highly effective at inducing depigmentation of unaffected vitiligo skin, with minimal adverse effects, consisting mainly of local skin irritation [103,105,106,106]. MBEH is supposedly a permanent depigmenting agent, but patients should be counseled that there is a risk for repigmentation [106, 107]. Trichloroacetic acid (TCA) 100% concentration [108] and 88% phenol [109, 110] have also been shown to be effective for depigmentation. Lasers and cryotherapy have also been used to induce local depigmentation [104, 111,113,114,115,116,117,118,119,119].
3.2 Oral Therapies
3.2.1 Oral Corticosteroids
Oral corticosteroids, which suppress the immune response, are used primarily in cases of rapidly progressive vitiligo. They are particularly helpful in halting the disease process, and, in some cases, can lead to repigmentation of active vitiligo lesions [120, 121]. Long-term therapy with daily oral corticosteroids is not recommended due to the adverse effect profile, which includes skin atrophy, striae, weight gain, hyperglycemia, hypertension, Cushing’s syndrome, suppression of the hypothalamic-pituitary axis, and osteoporosis [121, 122]. Because of this risk, low dose or short pulses of oral corticosteroids, known as oral mini-pulse (OMP) therapy, is recommended. One study evaluated betamethasone/dexamethasone 5 mg as a single dose by mouth on 2 consecutive days per week in 40 patients with extensive and/or fast-spreading vitiligo, with patients evaluated every 2–4 months. 89% of the 36 patients with active disease had arrest of progression of disease, and 2 patients needed an increase in dose to 7.5 mg per day to achieve suppression of disease, over the first 1–3 months of treatment. There was almost complete repigmentation (> 90%) in three patients and < 10% response in 14 patients, and treatment resulted in tolerable adverse effects. [123]. Another study that evaluated the efficacy of 10 mg dexamethasone pulse therapy (2 consecutive days per week for a maximum period of 24 weeks) in 29 patients found similar results. Vitiligo progression was halted in 88% of patients with active disease, with marked repigmentation in 2 patients (6.9%), moderate or slight repigmentation in 3 patients (10.3%), and no repigmentation in 21 patients (72.4%), with mild–moderate adverse effects consisting of weight gain, insomnia, acne, menstrual disturbance, hypertrichosis, and agitation in 69% of the patients [124]. Similar results were seen in studies that examined 100 patients taking prednisolone [125] and 444 patients taking dexamethasone 2.5 mg [126]. However, a systematic review evaluating the efficacy of OMP monotherapy compared with other treatments for vitiligo found no definitive conclusion due to the heterogeneity of the four randomized controlled studies included [127], suggesting that further studies are needed to evaluate OMP monotherapy.
3.3 Phototherapy
It is well-established that phototherapy is a validated treatment for vitiligo. The different phototherapies are defined based on the wavelengths of light used: narrow-band UV-B light (NB-UVB; 311–313 nm), 308 nm excimer laser or lamp, and psoralen plus UVA (PUVA; 320–380 nm). All formulations are effective and well-tolerated in adults as well as children [128,130,131,131].
3.3.1 Narrow Band UV-B (NB-UVB)
There have been several studies showing the efficacy and safety of NB-UVB therapy for the treatment of vitiligo in various patient populations. In a systematic review, 62.1% of patients receiving NB-UVB had ≥ 25% response at 3 months, 74.2% at 6 months, and 75% at 12 months, and ≥ 75% response in 13.0%, 19.2%, and 35.7% of patients at 3, 6, and 12 months, respectively [131]. NB-UVB is also more effective in younger patients [132], when treating early lesions, and in patients with non-segmental vitiligo compared with segmental vitiligo [133]. Additionally, NB-UVB leads to high levels of patient and physician satisfaction [134] and has been shown to improve quality of life [135]. NB-UVB is well-tolerated, with adverse effects consisting of erythema, burning, xerosis, pruritus, and photodamage after treatments [136].
Phototherapy is very time-intensive, requiring multiple treatments per week, and it can be challenging for patients to have access to the necessary equipment. A systematic review evaluating the effectiveness of home-based phototherapy found no significant difference in repigmentation rates among the groups, although only three studies were included, which varied in terms of quality and treatment regimens. Home-based therapy did afford more adherence to treatment due to its convenience, but more studies need to be conducted to determine the efficacy and safety of home-based treatments, given the possible advantages [137]. Additionally, a topical band-pass filter cream that selectively filters solar radiation to allow the spectrum of NB-UVB to pass toward vitiligo lesions has shown promise in treating segmental vitiligo lesions [138].
3.3.2 Excimer Laser and Lamp
The 308 nm monochromatic excimer light (MEL) is effective in the treatment of vitiligo [139,141,141]. Formulations can be in the form of a lamp or laser, with the lamp able to treat a larger surface area compared with the more localized laser. In a systematic review and meta-analysis that included six studies, no significant difference was found in efficacy between the excimer lamps and excimer lasers, or the excimer lamps and NB-UVB, suggesting that all are effective treatments for vitiligo, with mild adverse effects consisting of a local burning sensation, dryness, and pruritus [130]. Similar results were seen in another systematic review [142] and in a prospective analysis that examined the efficacy of the excimer lamp in the treatment of refractory vitiligo lesions [143].
For patients with localized lesions, the excimer laser is promising [144,146,147,148,149,150,151,152,152], particularly on the face [153,155,156,157,157]. There is more effective repigmentation when the excimer laser is used on lesions earlier in the disease process [155], particularly in patients with segmental vitiligo [158, 159]. In a recent chart review of pediatric patients with vitiligo treated with the 308 nm laser, after an average of 3.38 years, repigmentation was stable in 80% of facial, 40% of body, and 20% of extremity lesions [160].
In a retrospective study that evaluated the efficacy of targeted phototherapy with excimer light (EL) compared with targeted UVB, it was found that patients treated with EL had more significant repigmentation [161], which is similar to the results from a study by Poolsuwan and colleagues [162]. Of note, EL therapy was recently shown to be not effective in treating residual depigmentation after whole-body NB-UVB therapy [163].
Although repigmentation occurs fastest with laser treatments three times weekly, repigmentation overall seems to depend on the overall number of treatments rather than the frequency of treatments [164, 165], suggesting that the cumulative UV dose is most important [158]. There have been studies showing repigmentation with even weekly treatment [166] for 6 months, and pigmentation persistence in the majority of patients after 2 years [167].
Laser treatments have shown improvement in the quality of life of patients with vitiligo [166, 168, 169]. In terms of safety, there is no increased risk of skin cancer or premalignant skin lesions in patients treated with the excimer laser [170]. Due to the plateau effect of treatment [146], a cyclic treatment option using the excimer laser was studied which showed that it may be a promising algorithm leading to more patient compliance, although larger studies must be conducted [171].
3.3.3 Psoralen Plus UVA (PUVA)
PUVA requires the administration of a photosensitizer, known as psoralen, either topically or orally, followed by the administration of UV light. However, there are concerns regarding the long-term adverse effects, which can include eye toxicity, photoaging, and cutaneous malignancy, in addition to short-term adverse effects, including erythema, pruritus, xerosis, hyperpigmentation, headache, dizziness, bronchoconstriction, and depression, among others [172]. Several studies have shown PUVA to be effective in repigmenting, particularly of the face and trunk [173,175,176,176], with acral areas most resistant to treatment. After treatment with PUVA, on a histopathological level, there is an increase in active melanocytes that leads to reduced levels of melanocyte and keratinocyte degeneration [177], and treatment creates a favorable environment for the growth of melanocytes [178]. Although repigmentation can occur, relapse is common, especially in older patients [179]. In a systematic review, ≥ 25% repigmentation occurred in 51.4% of patients at 6 months, 61.6% of patients at 12 months after receiving PUVA therapy, and ≥ 75% repigmentation occurred in 8.5% of patients at 6 months and 13.6% of patients at 12 months, suggesting longer treatments result in improved results [131]. PUVA has fallen out of favor due to NB-UVB’s decreased adverse effect profile, its increased efficacy [180, 181], and its ability to induce disease stability (80% for NB-UVB vs. 40% for PUVA in one study) [182].
3.4 Combination Therapy
Combination treatments, particularly those with phototherapy [183], are extremely effective for patients with vitiligo, including children [184]. Tacrolimus in combination with a TCS is also a proven therapy [185].
Several meta-analyses have found TCIs to be effective when combined with phototherapy [89, 96, 186,188,188]. This conclusion is also supported by a randomized double-blind trial that found topical tacrolimus 0.1% combined with NB-UVB to be more effective than NB-UVB monotherapy [189]. More successful repigmentation has been documented with combined treatment of vitiligo lesions with the excimer laser and topical tacrolimus, as documented in a systematic review [190], and with microneedling in combination with topical pimecrolimus 1% [191]. Two systematic reviews found the combination of topical vitamin D analogs such as calcipotriol or tacalcitol and NB-UVB may enhance the treatment response [192, 193]. Furthermore, a recent study showed promising results for the combination of EL and topical calcipotriol for the treatment of acral vitiligo [194]. A three-arm randomized controlled trial evaluating the effectiveness of hand-held NB-UVB monotherapy, TCS monotherapy, or combination treatment found the combination to be more effective than TCS monotherapy [195]. There have also been several studies showing increased response to treatment and disease arrest in patients receiving combination OMP and NB-UVB compared with NB-UVB monotherapy [196,198,199,199].
3.5 Procedures
3.5.1 Surgical Grafting
There are several surgical procedures that can be performed in patients with refractory and stable vitiligo. Disease stability refers to a period of disease inactivity ranging from 6 months to 2 years, with no koebnerization [12,13,14,15, 200, 201]. The goal is to transfer healthy melanocytes to the depigmented lesion. These techniques include tissue grafts (suction blister [SBG], split-thickness and full-thickness punch/minipunch grafts) and cellular grafts (cultured epidermal graft [CEG], cultured melanocyte transplantation [CMT], noncultured epidermal suspension [NES] or noncultured hair follicle suspensions [NHFS]) [200, 201]. In a systematic review by Ju and colleagues that evaluated surgical techniques, the rate of pigmentation > 50% after surgical intervention was 81.01% in 92 studies and > 90% in 52.69% of patients in 106 studies [202].
In minigraft and punch grafting, 1 mm and 1.5–2 mm full-thickness punches are taken from non-lesional skin and implanted in lesional skin, respectively. These procedures are widely available, easy to perform in the outpatient setting, and are inexpensive, with many devices available for use. After about 2–3 weeks, repigmentation usually appears, with coalescing of individual lesions over the subsequent 4–6 months [200, 201, 203,205,205]. However, there is risk for cobblestone appearance, but newer devices have been created to reduce these risks [206, 207]. A recent study has shown that the combination of mini-punch grafting with weekly transverse needling has fast increased repigmentation compared with mini-punch grafting alone [208].
In split-thickness skin grafting, thin donor grafts are obtained using a dermatome, are de-epithelialized, and then transferred to vitiligo lesions. This procedure is difficult to perform on large areas of skin, can cause uneven pigmentation, can lead to scarring of the donor site, and can cause a peripheral halo due to contraction of the graft [200,202,203,203, 209].
For suction blister grafting, blisters at the dermoepidermal junction are created on pigmented skin using negative pressure, with the resultant roofs of the blisters transferred to vitiligo lesions [202, 203, 209]. This procedure reduces the risk of scarring compared with other surgical techniques, has more uniform color matching results, and has shown to be effective in most areas of the body, including the lips and eyelids [210,212,212], however melanocytes do not always thrive when transplanted and there is a risk for hemorrhagic blisters [201].
For larger areas affected by vitiligo, NES, NHFS, CMT, or CEG can be used, although the process for each is time-consuming. NES, also known as the melanocyte keratinocyte transplant procedure, which cannot be used on the palms and soles, involves obtaining a thin donor sample, followed by cellular separation of the dermis and epidermis, with the resulting epidermis cellular pellet placed over the depigmented areas. Studies have shown good color matching results [200, 203]. In NHFS, 1 mm punch biopsies are performed on the scalp to obtain hair follicles, which tend to have numerous melanocytes. The resulting cellular pellet is then applied to the depigmented area [200, 203, 213, 214]. For CMT, the epidermis is isolated from donor grafts and melanocytes are cultured with growth factors for about 15–30 days, after which they are applied to the depigmented areas [200, 203, 215, 216]. CEG is similar, but both melanocytes and keratinocytes are cultured. Cultured grafts require more specialized equipment and time [200].
In a systematic review evaluating the efficacy of surgical treatment combined with phototherapy, limited evidence was found to suggest that phototherapy enhances surgical techniques when it comes to vitiligo [217].
3.5.2 Platelet-Rich Plasma (PRP)
Platelet-rich plasma (PRP) is an alternative surgical technique that has gained interest for the treatment of vitiligo. In a systematic review that examined the utility of PRP in dermatology, two vitiligo studies were included, both showing an adjunctive benefit of PRP in stable vitiligo [218]. A recent prospective study of 10 patients with refractory stable vitiligo who were treated with PRP showed improvement after a mean of 1.5 sessions, which were well tolerated by patients [219]. PRP is most useful as an adjunct to treatment [220,222,223,224,225,226,227,228,229,230,231,232,232].
3.5.3 Micropigmentation
Micropigmentation, more commonly known as medical tattooing, can be a treatment option for patients who are resistant to conventional therapy. In this procedure, pigment is injected into the dermis manually or via an electrically driven needle [233], and there has been success in treating vitiligo [234, 235]. It is particularly useful for acral [236] and mucosal areas [237, 238], which are typically most resistant to traditional treatments, with minimal local reactions consisting of erythema and swelling that resolve after a few days. While difficult to achieve color matching, excellent results are possible with experienced experts, with one study showing color matching in 80% (20/25) of lesions in patients with Fitzpatrick skin types III and IV who underwent the procedure [239].
3.6 Alternate Oral Therapies
3.6.1 Methotrexate
Methotrexate, a folate antagonist used in the treatment of autoimmune diseases, has been proposed as a treatment for vitiligo. In a randomized comparative study examining the efficacy of methotrexate compared with OMP therapy, there was no difference in the number of patients who developed new lesions and there was a comparable reduction in the vitiligo disease activity score, suggesting no difference in efficacy between the two treatments [240]. There are limited case reports of patients repigmenting with methotrexate [241,243,243]. This suggests that methotrexate may be an alternative, corticosteroid-sparing treatment for vitiligo patients in whom corticosteroids are contraindicated or phototherapy is not feasible, although additional larger-scale, randomized trials need to be conducted.
3.6.2 Minocycline
Minocycline has been proposed as a treatment for vitiligo due to its antioxidant and anti-inflammatory activity, in addition to its ability to attenuate oxidative stress [244]. A study assessing the effect of minocycline 100 mg daily in 32 patients with gradually progressive vitiligo was completed, with 29 patients showing arrest of disease progression at study completion and 10 patients showing arrest of depigmentation after just 4 weeks of treatment [245]. Additionally, minocycline and OMP therapy are equally effective in halting disease activity in vitiligo [246], however NB-UVB is suggested to be superior to minocycline in inducing disease stability and repigmentation [247]. Most recently, a randomized, double-blind, placebo-controlled trial was conducted to determine the efficacy and safety of minocycline and NB-UVB, which showed that the combination therapy does not augment the results of NB-UVB monotherapy and may cause hyperpigmentation of the skin [248]. However, in general, studies evaluating the effect of minocycline need to be conducted on a larger scale with randomized controlled trials to see if there is any beneficial effect of adding this antibiotic to the vitiligo treatment regimen.
3.6.3 Azathioprine
Azathioprine, an immunomodulator that is used in the treatment of several other autoimmune diseases, has been proposed as a treatment for vitiligo. In a study that evaluated the efficacy of low-dose azathioprine (0.6–0.75 mg/kg per day with a maximum single dose of 50 mg) combined with oral PUVA versus oral PUVA monotherapy, combination therapy resulted in earlier perifollicular repigmentation, and the mean total repigmentation rate was 58.4% for the combination group compared to 24.8% for the oral PUVA monotherapy group at 4 months. Although no validated vitiligo assessment measures were used, azathioprine was well tolerated among study participants [249]. In a randomized study comparing the effect of OMP betamethasone therapy and oral azathioprine in halting disease progression and inducing repigmentation in patients with progressive nonsegmental vitiligo, oral azathioprine therapy was found to be inferior to OMP, but may have fewer adverse effects [250], providing a possible alternative for patients with active disease, although additional large-scale studies must be conducted to validate these results.
3.6.4 Levamisole
Levamisole, an antiparasitic agent with immunomodulating properties that is generally well tolerated, has also been postulated as a treatment option for vitiligo [251, 252]. A randomized controlled trial assessed the efficacy of levamisole (150 mg on 2 consecutive days per week for adults; 100 mg on 2 consecutive days per week in children aged 6–12 years) compared with placebo in the treatment of slowly spreading vitiligo. Although the proportion of patients who stopped developing new lesions was higher for the levamisole group, this difference was only significant at month 4 after 6 months of treatment [252]. It is important to note that although this study suggests that levamisole may not be as effective in inducing cessation of disease activity as previously reported in an open trial study [253], these patients were also concomitantly applying topical mometasone furoate 0.1% cream once daily, and the study lacked sufficient power [252]. Thus, additional placebo-controlled studies with larger patient populations must be conducted to determine levamisole’s efficacy in treating vitiligo.
3.6.5 Apremilast
Apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for the treatment of psoriasis, has potential as a therapeutic modality for vitiligo that is generally well tolerated, with adverse effects consisting primarily of gastrointestinal disturbances and headache. A case series of 13 patients treated with oral apremilast controlled progression of disease and induced repigmentation in 61.5% of patients [254]. A subsequent randomized controlled study that evaluated the efficacy of apremilast in combination with NB-UVB versus placebo and NB-UVB in patients with vitiligo found no added benefit of apremilast combination therapy compared with NB-UVB monotherapy in a study population that included a higher proportion of lighter skin types [255]. In a more recent randomized split-body pilot study that compared the combination of apremilast and NB-UVB with NB-UVB monotherapy in skin types IV–VI in the treatment of vitiligo found that apremilast may potentiate the effects of NB-UVB in inducing repigmentation [256]. Tissue samples of patients with darker skin types treated with this combination therapy resulted in decreased levels of CD8+ T cells, among other markers, and increased levels of melanogenesis markers, again supporting the findings that apremilast may increase the repigmentation effects of NB-UVB in patients with skin types IV–VI with vitiligo [257]. Despite these findings, apremilast has not been studied as monotherapy in a randomized trial, therefore larger trials must be conducted to confirm the efficacy of apremilast.
3.7 Supplements
The use of antioxidants, GB, PL, lipoic acid, and vitamin C/E has shown promising results, as demonstrated by a systematic review and meta-analysis that showed antioxidants in combination with phototherapy are more effective than phototherapy monotherapy for the treatment of vitiligo [258, 259].
3.7.1 Ginkgo biloba
GB is an antioxidant with immunomodulatory properties. Parsad and colleagues found that among patients with limited and slow-spreading vitiligo who were given GB extract 40 mg three times daily compared with placebo, there was cessation of active progression of disease [260]. In a similar study, the administration of 60 mg of GB twice daily showed significant improvement in total VASI and Vitiligo European Task Force (VETF) spread [261]. These studies suggest that GB may be an effective therapy to halt disease progression in patients with vitiligo, although additional larger, randomized studies are needed to evaluate this effect.
3.7.2 Polypodium l eucotomos
PL is a tropical fern that has beneficial properties for skin, used by Native Americans to treat several inflammatory disorders. PL extracts from fern leaves can be given orally or topically and have been shown to decrease levels of free radicals, lipid peroxidation, and ROS, thereby demonstrating an immunomodulating effect [262, 263]. In two randomized placebo-controlled studies, oral PL (480 mg twice daily or 250 mg three times daily) combined with NB-UVB showed a statistically significant increase in the repigmentation rate compared with NB-UVB and placebo in patients with vitiligo [264, 265]. However, no studies have demonstrated efficacy of PL monotherapy in the absence of concomitant phototherapy.
3.8 Janus Kinase Inhibitors
There has been increasing evidence for success of both oral and topical JAK inhibitors [266]. With increased understanding of the involvement of the JAK/STAT pathway in the pathogenesis of vitiligo, treatments with JAK inhibitors have led to key new therapeutic options for patients. The JAK1/2 inhibitor ruxolitinib cream is the first FDA-approved treatment to repigment patients 12 years of age and older with nonsegmental vitiligo affecting ≤ 10% of total body surface area (BSA).
3.8.1 Topical
Two 52-week phase III studies were conducted in 674 adolescent and adult patients to evaluate the efficacy and safety of ruxolitinib cream in patients with nonsegmental vitiligo affecting ≤ 10% of total BSA, including facial and nonfacial depigmented areas. Patients were randomized to receive either ruxolitinib 1.5% cream twice daily or placebo cream for 24 weeks, followed by a 28-week open-label extension. By week 24, ruxolitinib cream was found to be superior to placebo in terms of the primary endpoint, with nearly 30% of patients achieving ≥ 75% improvement from baseline in the facial Vitiligo Area Scoring Index (F-VASI75). By week 52, approximately 50% of patients who applied ruxolitinib cream from day 1 achieved F-VASI75, and approximately 30% of crossover patients who received ruxolitinib cream for 28 weeks achieved F-VASI75, which is consistent with the week-24 data for patients who applied the cream from day 1. Approximately 30% of patients who received ruxolitinib for 52 weeks achieved F-VASI90, 75% achieved F-VASI50, and 50% achieved total body (T)-VASI50. A response on the Vitiligo Noticeability Scale (VNS) of ‘a lot less noticeable’ or ‘no longer noticeable’ was achieved in 39.9% and 32.8%, respectively, of patients in each of the two phase III trials. Importantly, ruxolitinib cream was well tolerated, with no clinically significant application site reactions or serious adverse events. The most common adverse effects consisted of application site acne present in about 6.3–6.6% of patients and application site pruritus present in about 5.3–5.4% of patients [267, 268]. The phase II trial showed similar results, however the trial did not include adolescents, included segmental vitiligo patients, and allowed up to 20% BSA use of ruxolitinib cream. Additionally, although longer duration of therapy was associated with increased repigmentation, low numbers of patients by week 156 limited the available data [269, 270].
There were increased F-VASI75 response rates for ruxolitinib cream compared with placebo at week 24 across all age groups, regardless of sex, geographic region, race, Fitzpatrick skin phototype, and baseline clinical characteristics (facial BSA, disease stability, and prior therapy) [271]. Compared with adults, adolescents who received ruxolitinib cream achieved similar results regarding F-VASI75 at weeks 24 and 52. However, T-VASI50 was 25.4% and 60% at weeks 24 and 52, respectively, for adolescents, which is numerically higher than the T-VASI50 for adults (22.9% and 49.7%, respectively). VNS response was different for adolescents, with 56.0% achieving response compared with 33% for adults at week 52. Ruxolitinib cream was well tolerated in adolescents, with no serious treatment-related adverse events [272].
It is important to note that ruxolitinib cream produced clinically meaningful repigmentation of all body regions in adults and adolescents through week 52 in both phase III trials. The body regions specifically examined were head and neck (excluding face), hands, upper extremities, trunk (including genitals), lower extremities, feet, and total body (excluding face). By week 24, VASI50 was achieved in higher percentages of patients applying ruxolitinib cream versus placebo regardless of the body region, and by week 52, this percentage increased, including in those patients who crossed over to ruxolitinib from vehicle after week 24. Although there was a slight taper in VASI50 response after 46 weeks for the hands and feet, which are two areas that are historically the most resistant to repigmentation with conventional treatment modalities, the response rates were still clinically meaningful [273]. The results of the phase II trials were similar [274].
Maintenance of pigmentation in patients who received ruxolitinib for 52 weeks or more in the phase II trial after discontinuation showed maintenance for up to 6 months, although this portion of the 156-week study included low numbers of patients [270, 275]. Additionally, CXCL9, CXCL10, and IL-15 levels do not seem to be predictive of maintenance response [275]. Since up to 40% of patients can experience relapse after discontinuing conventional therapy [276], further studies as to the durability and whether ruxolitinib cream can be used as a maintenance drug need to be conducted.
The combination of topical JAK inhibitors and phototherapy may also lead to more significant repigmentation [266, 277,279,279]. In the phase II ruxolitinib trial, 19 patients received add-on NB-UVB during the open-label treatment period, resulting in some additional benefit in terms of facial and total body repigmentation. This study was part of an open-label design, included a low number of patients, and the phototherapy regimens were heterogeneous [279]. However, concomitant use of phototherapy needs to be evaluated in larger scale studies to make a definitive statement regarding the efficacy of this combination.
3.8.2 Oral
Data for the use of oral JAK inhibitors consists mainly of case series and case reports. Craiglow and colleagues reported the first use of oral tofacitinib, a JAK1/3 inhibitor, to treat generalized vitiligo. After 5 months, the patient significantly repigmented on the face and upper extremities [280]. Since this report, additional case reports have shown promising results for the use of tofacitinib [281,283,283], the JAK1/2 inhibitor baricitinib [284, 285], and oral ruxolitinib [286]. Importantly, when oral JAK inhibitors have been discontinued after successful repigmentation, there are reports of loss of response [284, 286].
A phase IIb study evaluating the efficacy and safety of ritlecitinib, an oral JAK 3/TEC inhibitor, has been conducted. Patients with active, non-segmental vitiligo were randomized in a 24-week dose-ranging period in which patients received either placebo or ritlecitinib 10, 30, or 50 mg with or without a 4-week induction period of 100 or 200 mg, followed by a 24-week open-label extension period. After 24 weeks, ritlecitinib 50 mg daily with or without induction met the primary endpoint, the percentage change from baseline (%CFB) in F-VASI, and met the key secondary endpoint F-VASI75. After 48 weeks, the %CFB for all treatment regimens was larger between week 24 and week 48 compared with day 1 and week 24, which was a similar trend for centrally read F-VASI, locally read F-VASI, locally read T-VASI, and patient global impression of change (PGIC), suggesting continued improvements over the course of the study. Although ritlecitinib was well tolerated in patients who completed the study, it should be noted that several patients dropped out of the study, and the extension period was not placebo-controlled [287]. Thus, although promising, larger randomized controlled studies must be conducted in order to verify the efficacy and safety of ritlecitinib.
It has been suggested that oral tofacitinib used together with low-dose phototherapy may provide enhanced repigmentation. Five of 10 patients who received tofacitinib 5–10 mg daily or twice daily for an average of 9.9 months achieved some degree of repigmentation in sun-exposed areas or while receiving concomitant NB-UVB. Suction blister sampling of one patient before and after treatment revealed decreased T-cell numbers and CXCL9 and CXCL10 in the skin [288]. An additional two cases reported similar results [289]. The NB-UVB dose used in these series is much lower than the dose required for repigmentation when used as monotherapy. The theory behind this combination strategy is that tofacitinib suppresses the immune response, while low-dose NB-UVB or sunlight stimulates melanocyte regeneration [288, 289]. Similar results of increased repigmentation with tofacitinib/NB-UVB combination therapy were seen in an additional study [290] and a case report [291].
It is important to note that JAK inhibitors, both the oral and topical formulation, have black-box warnings for malignancy, infection, major adverse cardiovascular events, thrombosis, and mortality. There are also warnings for cytopenias, gastrointestinal perforation, hepatotoxicity, lipid abnormalities, interstitial lung disease, and tuberculosis [292]. For vitiligo patients treated with oral JAK inhibitors, the most common treatment-related adverse events have consisted of upper respiratory tract infections, headaches, nasopharyngitis, hyperlipidemia, weight gain, arthralgia, and diarrhea [266, 287].
3.9 Emerging Therapies
In addition to further work into the development of JAK inhibitors, there are several other areas of investigation that are promising based on our increased understanding of vitiligo pathogenesis. WNT agonists and other treatments that target different aspects of this pathway have been proposed [71, 73, 293, 294]. Other potential therapeutic targets include targeting the IL-15 pathway [67], HSP70 proteins [295], and NK cells and CD8+ T cells [296]. Increasing the Treg pool [297] and the development of anti-CXCR3B antibodies to prevent the initial melanocyte apoptosis [47, 298] are also possibilities.
4 Conclusion
Vitiligo is a chronic autoimmune disease characterized by loss of melanocytes, leading to depigmentation that can have a significant impact on mental health. Although many treatments are available for vitiligo, most are off-label and carry varying risks of adverse effects. Over recent years, there has been significant progress in our understanding of the pathogenesis of vitiligo, specifically the involvement of the JAK/STAT pathway. Although there is an overall algorithm for treatment, strategies to combat vitiligo are highly personalized and must consider the goals of the patient, how bothersome the disease is to the patient, the areas of involvement, and the type of vitiligo present. The approval of ruxolitinib cream for repigmentation is a monumental milestone for the vitiligo community. Continued research into the pathogenesis of vitiligo will expand upon these advances, leading to the development of novel therapeutics.
References
Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51(10):1206–12. https://doi.org/10.1111/j.1365-4632.2011.05377.x.
Taïeb A, Picardo M. Clinical practice Vitiligo. N Engl J Med. 2009;360(2):160–9. https://doi.org/10.1056/NEJMcp0804388.
Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–91. https://doi.org/10.1016/j.jaad.2010.11.061.
Sehgal VN, Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Indian J Dermatol Venereol Leprol. 2007;73(3):149–56. https://doi.org/10.4103/0378-6323.32708.
Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149(2):159–64. https://doi.org/10.1001/jamadermatol.2013.927.
Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, Pandya AG, Vitiligo Working Group. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73(5):883–5. https://doi.org/10.1016/j.jaad.2015.07.039.
Ezzedine K, Grimes PE, Meurant JM, Seneschal J, Léauté-Labrèze C, Ballanger F, Jouary T, Taïeb C, Taïeb A. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol. 2015;173(2):607–9. https://doi.org/10.1111/bjd.13839.
Osinubi O, Grainge MJ, Hong L, Ahmed A, Batchelor JM, Grindlay D, Thompson AR, Ratib S. The prevalence of psychological comorbidity in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2018;178(4):863–78. https://doi.org/10.1111/bjd.16049.
Hamidizadeh N, Ranjbar S, Ghanizadeh A, Parvizi MM, Jafari P, Handjani F. Evaluating prevalence of depression, anxiety and hopelessness in patients with Vitiligo on an Iranian population. Health Qual Life Outcomes. 2020;18(1):20. https://doi.org/10.1186/s12955-020-1278-7.
Bae JM, Kim JE, Lee RW, Ju HJ, Han JH, Lee JH, Woo YR, Lee JH, Bang CH, Park CJ, Ezzedine K, Kim M. Beyond quality of life: a call for patients’ own willingness to pay in chronic skin disease to assess psychosocial burden—a multicenter, cross-sectional, prospective survey. J Am Acad Dermatol. 2021;85(5):1321–4. https://doi.org/10.1016/j.jaad.2020.09.088.
Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, Goh BK, Anbar T. Vitiligo Global Issue Consensus Conference Panelists. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25(3):E1–13. https://doi.org/10.1111/j.1755-148X.2012.00997.x.
Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20(1):27–35. https://doi.org/10.1111/j.1600-0749.2006.00355.x.
Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J, Young K, Therapy Guidelines and Audit Subcommittee, British Association of Dermatologists; Clinical Standards Department, Royal College of Physicians of London; Cochrane Skin Group. Vitiligo Society Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159(5):1051–76. https://doi.org/10.1111/j.1365-2133.2008.08881.x.
Taieb A, Alomar A, Böhm M, Dell’anna ML, De Pase A, Eleftheriadou V, Ezzedine K, Gauthier Y, Gawkrodger DJ, Jouary T, Leone G, Moretti S, Nieuweboer-Krobotova L, Olsson MJ, Parsad D, Passeron T, Tanew A, van der Veen W, van Geel N, Whitton M, Wolkerstorfer A, Picardo M, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europeenne des MedecinsSpecialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168(1):5–19. https://doi.org/10.1111/j.1365-2133.2012.11197.x.
Oiso N, Suzuki T, Wataya-Kaneda M, Tanemura A, Tanioka M, Fujimoto T, Fukai K, Kawakami T, Tsukamoto K, Yamaguchi Y, Sano S, Mitsuhashi Y, Nishigori C, Morita A, Nakagawa H, Mizoguchi M, Katayama I. Guidelines for the diagnosis and treatment of vitiligo in Japan. J Dermatol. 2013;40(5):344–54. https://doi.org/10.1111/1346-8138.12099.
Esmat SM, El-Tawdy AM, Hafez GA, Zeid OA, Abdel Halim DM, Saleh MA, Leheta TM, Elmofty M. Acral lesions of vitiligo: why are they resistant to photochemotherapy? J Eur Acad Dermatol Venereol. 2012;26(9):1097–104. https://doi.org/10.1111/j.1468-3083.2011.04215.x.
Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J, Young K. Vitiligo: concise evidence based guidelines on diagnosis and management. Postgrad Med J. 2010;86(1018):466–71. https://doi.org/10.1136/pgmj.2009.093278.
Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;1: CD003263. https://doi.org/10.1002/14651858.CD003263.pub4.
Whitton ME, Pinart M, Batchelor J, Leonardi-Bee J, González U, Jiyad Z, Eleftheriadou V, Ezzedine K. Interventions for vitiligo. Cochrane Database Syst Rev. 2015;2: CD003263. https://doi.org/10.1002/14651858.CD003263.pub5.
Whitton M, Pinart M, Batchelor JM, Leonardi-Bee J, Gonzalez U, Jiyad Z, Eleftheriadou V, Ezzedine K. Evidence-based management of vitiligo: summary of a Cochrane systematic review. Br J Dermatol. 2016;174(5):962–9. https://doi.org/10.1111/bjd.14356.
Picardo M, Dell’Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, Taieb A. Vitiligo. Nat Rev Dis Primers. 2015;1:15011. https://doi.org/10.1038/nrdp.2015.11.
Spritz RA, Andersen GH. Genetics of Vitiligo. Dermatol Clin. 2017;35(2):245–55. https://doi.org/10.1016/j.det.2016.11.013.
Spritz RA. Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol. 2013;40(5):310–8. https://doi.org/10.1111/1346-8138.12147.
Spritz RA. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid. 2010;20(7):745–54. https://doi.org/10.1089/thy.2010.1643.
Czajkowski R, Męcińska-Jundziłł K. Current aspects of vitiligo genetics. Postepy Dermatol Alergol. 2014;31(4):247–55. https://doi.org/10.5114/pdia.2014.43497.
Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–25. https://doi.org/10.1056/NEJMoa061592.
Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, Holland PJ, Mailloux CM, Sufit AJ, Hutton SM, Amadi-Myers A, Bennett DC, Wallace MR, McCormack WT, Kemp EH, Gawkrodger DJ, Weetman AP, Picardo M, Leone G, Taïeb A, Jouary T, Ezzedine K, van Geel N, Lambert J, Overbeck A, Spritz RA. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362(18):1686–97. https://doi.org/10.1056/NEJMoa0908547.
Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, Holland PJ, Birlea SA, Siebert J, Hartmann A, Lienert A, van Geel N, Lambert J, Luiten RM, Wolkerstorfer A, Wietze van der Veen JP, Bennett DC, Taïeb A, Ezzedine K, Kemp EH, Gawkrodger DJ, Weetman AP, Kõks S, Prans E, Kingo K, Karelson M, Wallace MR, McCormack WT, Overbeck A, Moretti S, Colucci R, Picardo M, Silverberg NB, Olsson M, Valle Y, Korobko I, Böhm M, Lim HW, Hamzavi I, Zhou L, Mi QS, Fain PR, Santorico SA, Spritz RA. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. 2016;48(11):1418–24. https://doi.org/10.1038/ng.3680.
Jin Y, Birlea SA, Fain PR, Mailloux CM, Riccardi SL, Gowan K, Holland PJ, Bennett DC, Wallace MR, McCormack WT, Kemp EH, Gawkrodger DJ, Weetman AP, Picardo M, Leone G, Taïeb A, Jouary T, Ezzedine K, van Geel N, Lambert J, Overbeck A, Spritz RA. Common variants in FOXP1 are associated with generalized vitiligo. Nat Genet. 2010;42(7):576–8. https://doi.org/10.1038/ng.602.
Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, Cole JB, Gowan K, Holland PJ, Bennett DC, Luiten RM, Wolkerstorfer A, van der Veen JP, Hartmann A, Eichner S, Schuler G, van Geel N, Lambert J, Kemp EH, Gawkrodger DJ, Weetman AP, Taïeb A, Jouary T, Ezzedine K, Wallace MR, McCormack WT, Picardo M, Leone G, Overbeck A, Silverberg NB, Spritz RA. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44(6):676–80. https://doi.org/10.1038/ng.2272.
Spritz RA. The genetics of generalized vitiligo: autoimmune pathways and an inverse relationship with malignant melanoma. Genome Med. 2010;2(10):78. https://doi.org/10.1186/gm199.
Birlea SA, Jin Y, Bennett DC, Herbstman DM, Wallace MR, McCormack WT, Kemp EH, Gawkrodger DJ, Weetman AP, Picardo M, Leone G, Taïeb A, Jouary T, Ezzedine K, van Geel N, Lambert J, Overbeck A, Fain PR, Spritz RA. Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J Investig Dermatol. 2011;131(2):371–81. https://doi.org/10.1038/jid.2010.337.
Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J Investig Dermatol. 2012;132(2):268–73. https://doi.org/10.1038/jid.2011.321.
Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16(3):208–14. https://doi.org/10.1034/j.1600-0749.2003.00032.x.
Millington GW, Levell NJ. Vitiligo: the historical curse of depigmentation. Int J Dermatol. 2007;46(9):990–5. https://doi.org/10.1111/j.1365-4632.2007.03195.x.
Nath SK, Majumder PP, Nordlund JJ. Genetic epidemiology of vitiligo: multilocus recessivity cross-validated. Am J Hum Genet. 1994;55(5):981–90.
Dell’Anna ML, Maresca V, Briganti S, Camera E, Falchi M, Picardo M. Mitochondrial impairment in peripheral blood mononuclear cells during the active phase of vitiligo. J Investig Dermatol. 2001;117(4):908–13. https://doi.org/10.1046/j.0022-202x.2001.01459.x.
Maresca V, Roccella M, Roccella F, Camera E, Del Porto G, Passi S, Grammatico P, Picardo M. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Investig Dermatol. 1997;109(3):310–3. https://doi.org/10.1111/1523-1747.ep12335801.
Jimbow K, Chen H, Park JS, Thomas PD. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol. 2001;144(1):55–65. https://doi.org/10.1046/j.1365-2133.2001.03952.x.
Speeckaert R, Dugardin J, Lambert J, Lapeere H, Verhaeghe E, Speeckaert MM, van Geel N. Critical appraisal of the oxidative stress pathway in vitiligo: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1089–98. https://doi.org/10.1111/jdv.14792.
Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol. 2013;25(6):676–82. https://doi.org/10.1016/j.coi.2013.10.010.
Dell’Anna ML, Urbanelli S, Mastrofrancesco A, Camera E, Iacovelli P, Leone G, Manini P, D’Ischia M, Picardo M. Alterations of mitochondria in peripheral blood mononuclear cells of vitiligo patients. Pigment Cell Res. 2003;16(5):553–9. https://doi.org/10.1034/j.1600-0749.2003.00087.x.
Kang P, Zhang W, Chen X, Yi X, Song P, Chang Y, Zhang S, Gao T, Li C, Li S. TRPM2 mediates mitochondria-dependent apoptosis of melanocytes under oxidative stress. Free Radic Biol Med. 2018;126:259–68. https://doi.org/10.1016/j.freeradbiomed.2018.08.022.
Dell’Anna ML, Ottaviani M, Bellei B, Albanesi V, Cossarizza A, Rossi L, Picardo M. Membrane lipid defects are responsible for the generation of reactive oxygen species in peripheral blood mononuclear cells from vitiligo patients. J Cell Physiol. 2010;223(1):187–93. https://doi.org/10.1002/jcp.22027.
Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel-Malek ZA. Melanocytes as instigators and victims of oxidative stress. J Investig Dermatol. 2014;134(6):1512–8. https://doi.org/10.1038/jid.2014.65.
Zhong J, Rao X, Xu JF, Yang P, Wang CY. The role of endoplasmic reticulum stress in autoimmune-mediated beta-cell destruction in type 1 diabetes. Exp Diabetes Res. 2012;2012: 238980. https://doi.org/10.1155/2012/238980.
Bergqvist C, Ezzedine K. Vitiligo: a focus on pathogenesis and its therapeutic implications. J Dermatol. 2021;48(3):252–70. https://doi.org/10.1111/1346-8138.15743.
Meyskens FL Jr, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14(3):148–54. https://doi.org/10.1034/j.1600-0749.2001.140303.x.
Puri N, Mojamdar M, Ramaiah A. In vitro growth characteristics of melanocytes obtained from adult normal and vitiligo subjects. J Investig Dermatol. 1987;88(4):434–8. https://doi.org/10.1111/1523-1747.ep12469795.
Puri N, Mojamdar M, Ramaiah A. Growth defects of melanocytes in culture from vitiligo subjects are spontaneously corrected in vivo in repigmenting subjects and can be partially corrected by the addition of fibroblast-derived growth factors in vitro. Arch Dermatol Res. 1989;281(3):178–84. https://doi.org/10.1007/BF00456389.
Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188(6):1203–8. https://doi.org/10.1084/jem.188.6.1203.
Wańkowicz-Kalińska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Investig. 2003;83(5):683–95. https://doi.org/10.1097/01.lab.0000069521.42488.1b.
van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, Vyth-Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Investig Dermatol. 2009;129(9):2220–32. https://doi.org/10.1038/jid.2009.32.
Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Investig Dermatol. 2012;132(7):1869–76. https://doi.org/10.1038/jid.2011.463.
Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taieb A, Ezzedine K, Seneschal J. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27(3):398–407. https://doi.org/10.1111/pcmr.12219.
Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, Li M. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: a pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol. 2015;95(6):664–70. https://doi.org/10.2340/00015555-2080.
Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, Zhou Y, Deng A, Hunter CA, Luster AD, Harris JE. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6(223): 223ra23. https://doi.org/10.1126/scitranslmed.3007811.
Rashighi M, Harris JE. Interfering with the IFN-γ/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med. 2015;3(21):343. https://doi.org/10.3978/j.issn.2305-5839.2015.11.36.
Wang XX, Wang QQ, Wu JQ, Jiang M, Chen L, Zhang CF, Xiang LH. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016;174(6):1318–26. https://doi.org/10.1111/bjd.14416.
Abdallah M, El-Mofty M, Anbar T, Rasheed H, Esmat S, Al-Tawdy A, Fawzy MM, Abdel-Halim D, Hegazy R, Gawdat H, Bassiouny D, Ibrahim MA, Sany I, El-Bassiouny M, Khalil M, Abdel-Aziz A, El Maadawi ZM, Mostafa WZ, Egyptian Vitiligo Group. CXCL-10 and Interleukin-6 are reliable serum markers for vitiligo activity: a multicenter cross-sectional study. Pigment Cell Melanoma Res. 2018;31(2):330–6. https://doi.org/10.1111/pcmr.12667.
Riding RL, Harris JE. The role of memory CD8+ T cells in vitiligo. J Immunol. 2019;203(1):11–9. https://doi.org/10.4049/jimmunol.1900027.
Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Pathophysiology of skin resident memory T cells. Front Immunol. 2021;11: 618897. https://doi.org/10.3389/fimmu.2020.618897.
Boniface K, Jacquemin C, Darrigade AS, Dessarthe B, Martins C, Boukhedouni N, Vernisse C, Grasseau A, Thiolat D, Rambert J, Lucchese F, Bertolotti A, Ezzedine K, Taieb A, Seneschal J. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Investig Dermatol. 2018;138(2):355–64. https://doi.org/10.1016/j.jid.2017.08.038.
Cheuk S, Schlums H, GallaisSérézal I, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, Höög C, Tjernlund A, Michaëlsson J, Folkersen L, Mjösberg J, Blomqvist L, Ehrström M, Ståhle M, Bryceson YT, Eidsmo L. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity. 2017;46(2):287–300. https://doi.org/10.1016/j.immuni.2017.01.009.
Atwa MA, Ali SMM, Youssef N, Mahmoud Marie RE. Elevated serum level of interleukin-15 in vitiligo patients and its correlation with disease severity but not activity. J Cosmet Dermatol. 2021;20(8):2640–4. https://doi.org/10.1111/jocd.13908.
Frączek A, Owczarczyk-Saczonek A, Placek W. The role of TRM cells in the pathogenesis of vitiligo—a review of the current state-of-the-art. Int J Mol Sci. 2020;21(10):3552. https://doi.org/10.3390/ijms21103552.
Richmond JM, Strassner JP, Zapata L Jr, Garg M, Riding RL, Refat MA, Fan X, Azzolino V, Tovar-Garza A, Tsurushita N, Pandya AG, Tso JY, Harris JE. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med. 2018;10(450): eaam7710. https://doi.org/10.1126/scitranslmed.aam7710.
Azzolino V, Zapata L Jr, Garg M, Gjoni M, Riding RL, Strassner JP, Richmond JM, Harris JE. Jak inhibitors reverse vitiligo in mice but do not deplete skin resident memory T cells. J Investig Dermatol. 2021;141(1):182-184.e1. https://doi.org/10.1016/j.jid.2020.04.027.
Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, Nakata S, Matsunaga K, Akamatsu H. Wnt/β-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Investig Dermatol. 2013;133(12):2753–62. https://doi.org/10.1038/jid.2013.235.
Zhao SJ, Jia H, Xu XL, Bu WB, Zhang Q, Chen X, Ji J, Sun JF. Identification of the role of Wnt/β-catenin pathway through integrated analyses and in vivo experiments in vitiligo. Clin Cosmet Investig Dermatol. 2021;14:1089–103. https://doi.org/10.2147/CCID.S319061.
Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, Mounier C, Rival Y, Piwnica D, Cavalié M, Chignon-Sicard B, Ballotti R, Voegel J, Passeron T. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Investig Dermatol. 2015;135(12):3105–14.
Wagner RY, Luciani F, Cario-André M, Rubod A, Petit V, Benzekri L, Ezzedine K, Lepreux S, Steingrimsson E, Taieb A, Gauthier Y, Larue L, Delmas V. Altered E-cadherin levels and distribution in melanocytes precede clinical manifestations of vitiligo. J Investig Dermatol. 2015;135(7):1810–9. https://doi.org/10.1038/jid.2015.25.
Han X, Chang L, Qiu Z, Lin M, Wang Y, Liu D, Diao Q, Zhong JL, Xu W. Micro-injury induces hair regeneration and vitiligo repigmentation through Wnt/β-catenin pathway. Stem Cells Dev. 2022;31(5–6):111–8. https://doi.org/10.1089/scd.2021.0276.
Tedeschi A, Dall’Oglio F, Micali G, Schwartz RA, Janniger CK. Corrective camouflage in pediatric dermatology. Cutis. 2007;79(2):110–2.
Ongenae K, Dierckxsens L, Brochez L, van Geel N, Naeyaert JM. Quality of life and stigmatization profile in a cohort of vitiligo patients and effect of the use of camouflage. Dermatology. 2005;210(4):279–85. https://doi.org/10.1159/000084751.
Tanioka M, Yamamoto Y, Kato M, Miyachi Y. Camouflage for patients with vitiligo vulgaris improved their quality of life. J Cosmet Dermatol. 2010;9(1):72–5. https://doi.org/10.1111/j.1473-2165.2010.00479.x.
Bassiouny D, Hegazy R, Esmat S, Gawdat HI, Ahmed Ezzat M, Tawfik HA, Hegazy AA, Ibrahim S. Cosmetic camouflage as an adjuvant to vitiligo therapies: effect on quality of life. J Cosmet Dermatol. 2021;20(1):159–65. https://doi.org/10.1111/jocd.13459.
Morales-Sánchez MA, Laguna-Meraz JP, Peralta-Pedrero ML, Jurado-Santa CF. Effect of cosmetic camouflage in adults with vitiligo. Actas Dermosifiliogr. 2022;113(3):316–8. https://doi.org/10.1016/j.ad.2020.04.018(English, Spanish).
Li M, Wang F, Ding X, Xu Q, Du J. Evaluation of the potential interference of camouflage on the treatment of vitiligo: an observer-blinded self-controlled study. Dermatol Ther. 2021;34(1): e14545. https://doi.org/10.1111/dth.14545.
Fesq H, Brockow K, Strom K, Mempel M, Ring J, Abeck D. Dihydroxyacetone in a new formulation: a powerful therapeutic option in vitiligo. Dermatology. 2001;203(3):241–3. https://doi.org/10.1159/000051757.
Suga Y, Ikejima A, Matsuba S, Ogawa H. Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol. 2002;47(3):436–8. https://doi.org/10.1067/mjd.2002.119670.
Rajatanavin N, Suwanachote S, Kulkollakarn S. Dihydroxyacetone: a safe camouflaging option in vitiligo. Int J Dermatol. 2008;47(4):402–6. https://doi.org/10.1111/j.1365-4632.2008.03356.x.
Steuer AB, Zampella JG. Camouflaging vitiligo using a spray tan. Dermatol Online J. 2020;26(7): 13030/qt63j996qx.
Hsu S. Camouflaging vitiligo with dihydroxyacetone. Dermatol Online J. 2008;14(8):23.
Speeckaert R, van Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol. 2017;18(6):733–44. https://doi.org/10.1007/s40257-017-0298-5.
Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo Meta-analysis of the literature. Arch Dermatol. 1998;134(12):1532–40. https://doi.org/10.1001/archderm.134.12.1532.
Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416–25. https://doi.org/10.4103/2229-5178.142483.
Kwinter J, Pelletier J, Khambalia A, Pope E. High-potency steroid use in children with vitiligo: a retrospective study. J Am Acad Dermatol. 2007;56(2):236–41.
Arora CJ, Rafiq M, Shumack S, Gupta M. The efficacy and safety of tacrolimus as mono- and adjunctive therapy for vitiligo: a systematic review of randomised clinical trials. Australas J Dermatol. 2020;61(1):e1–9. https://doi.org/10.1111/ajd.13096.
Lepe V, Moncada B, Castanedo-Cazares JP, Torres-Alvarez MB, Ortiz CA, Torres-Rubalcava AB. A double-blind randomized trial of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol. 2003;139(5):581–5. https://doi.org/10.1001/archderm.139.5.581.
Coskun B, Saral Y, Turgut D. Topical 0.05% clobetasol propionate versus 1% pimecrolimus ointment in vitiligo. Eur J Dermatol. 2005;15(2):88–91.
Mumtaz H, Anis S, Akhtar A, Rubab M, Zafar A, Niazi N, Bahadur H, Talpur AS, Shafiq MA, Fatima T. Efficacy of tacrolimus versus clobetasol in the treatment of vitiligo. Cureus. 2020;12(12): e11985. https://doi.org/10.7759/cureus.11985.
Ho N, Pope E, Weinstein M, Greenberg S, Webster C, Krafchik BR. A double-blind, randomized, placebo-controlled trial of topical tacrolimus 0.1% vs. clobetasol propionate 0.05% in childhood vitiligo. Br J Dermatol. 2011;165(3):626–32. https://doi.org/10.1111/j.1365-2133.2011.10351.x.
Chang HC, Hsu YP, Huang YC. The effectiveness of topical calcineurin inhibitors compared with topical corticosteroids in the treatment of vitiligo: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82(1):243–5. https://doi.org/10.1016/j.jaad.2019.07.108.
Saleh R, Ahmed AA, M Abd-Elmagid W. Efficacy of topical tacrolimus 003% monotherapy in the treatment of non-segmental vitiligo: a randomized, controlled trial. J Cosmet Dermatol. 2021;20(12):3943–52. https://doi.org/10.1111/jocd.14041.
Lee JH, Kwon HS, Jung HM, Lee H, Kim GM, Yim HW, Bae JM. Treatment outcomes of topical calcineurin inhibitor therapy for patients with vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(8):929–38. https://doi.org/10.1001/jamadermatol.2019.0696.
Seneschal J, Duplaine A, Maillard H, Passeron T, Andreu N, Lassalle R, Favary C, Droitcourt C, Taïeb A, Ezzedine K. Efficacy and safety of tacrolimus 0.1% for the treatment of facial vitiligo: a multicenter randomized, double-blinded vehicle-controlled study. J Investig Dermatol. 2021;141(7):1728–34. https://doi.org/10.1016/j.jid.2020.12.028.
Cavalié M, Ezzedine K, Fontas E, Montaudié H, Castela E, Bahadoran P, Taïeb A, Lacour JP, Passeron T. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Investig Dermatol. 2015;135(4):970–4. https://doi.org/10.1038/jid.2014.527.
Shim WH, Suh SW, Jwa SW, Song M, Kim HS, Ko HC, Kim BS, Kim MB. A pilot study of 1% pimecrolimus cream for the treatment of childhood segmental vitiligo. Ann Dermatol. 2013;25(2):168–72. https://doi.org/10.5021/ad.2013.25.2.168.
Hu W, Xu Y, Ma Y, Lei J, Lin F, Xu AE. Efficacy of the topical calcineurin inhibitors tacrolimus and pimecrolimus in the treatment of vitiligo in infants under 2 years of age: a randomized open-label pilot study. Clin Drug Investig. 2019;39(12):1233–8. https://doi.org/10.1007/s40261-019-00845-x.
Hu W, Lin F, Lei J, Xu AE. Impacts of exposure to topical calcineurin inhibitors on metabolism in vitiligo infants. Pediatr Res. 2022. https://doi.org/10.1038/s41390-022-02133-5.
Ju HJ, Han JH, Kim MS, Lee SH, Shin JW, Choi M, Jeong KH, Han TY, Choi CW, Lee HJ, Oh SH, Lee SH, Kim DH, Shin J, Lee JH, Kim SS, Kang HY, Chang SE, Kim JS, Lee DY, Choi GS, Suh DH, Chan Kim Y, Park CJ, Kim KH, Lee AY, Chan Park K, Lee MH, Bae JM, Korean Society for Vitiligo and the Korean Society for Photomedicine. The long-term risk of lymphoma and skin cancer did not increase after topical calcineurin inhibitor use and phototherapy in a cohort of 25,694 patients with vitiligo. J Am Acad Dermatol. 2021;84(6):1619–27. https://doi.org/10.1016/j.jaad.2021.01.067.
Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone. A retrospective study of treatment of 18 vitiligo patients and a review of the literature. Br J Dermatol. 1977;97(6):669–79. https://doi.org/10.1111/j.1365-2133.1977.tb14275.x.
Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol. 2000;42(5 Pt 1):760–9. https://doi.org/10.1067/mjd.2000.103813.
Rordam OM, Lenouvel EW, Maalo M. Successful treatment of extensive vitiligo with monobenzone. J Clin Aesthet Dermatol. 2012;5(12):36–9.
Tan ES, Sarkany R. Topical monobenzyl ether of hydroquinone is an effective and safe treatment for depigmentation of extensive vitiligo in the medium term: a retrospective cohort study of 53 cases. Br J Dermatol. 2015;172(6):1662–4. https://doi.org/10.1111/bjd.13642.
Oakley AM. Rapid repigmentation after depigmentation therapy: vitiligo treated with monobenzyl ether of hydroquinone. Australas J Dermatol. 1996;37(2):96–8. https://doi.org/10.1111/j.1440-0960.1996.tb01014.x.
Nofal A, Fawzy MM, Alakad R. The use of trichloroacetic acid as a depigmenting therapy in universal vitiligo. J Dtsch Dermatol Ges. 2021;19(2):241–6. https://doi.org/10.1111/ddg.14316.
Zanini M, Machado Filho CD. Depigmentation therapy for generalized vitiligo with topical 88% phenol solution. An Bras Dermatol. 2005;80(4):415–6.
Mahmood F, Beach RA. Can it make me white again? A case report of 88% phenol as a depigmenting agent in vitiligo. SAGE Open Med Case Rep. 2021;9: 2050313X21993307. https://doi.org/10.1177/2050313X21993307.
Kim YJ, Chung BS, Choi KC. Depigmentation therapy with Q-switched ruby laser after tanning in vitiligo universalis. Dermatol Surg. 2001;27(11):969–70. https://doi.org/10.1046/j.1524-4725.2001.01101.x.
Komen L, Zwertbroek L, Burger SJ, van der Veen JP, de Rie MA, Wolkerstorfer A. Q-switched laser depigmentation in vitiligo, most effective in active disease. Br J Dermatol. 2013;169(6):1246–51. https://doi.org/10.1111/bjd.12571.
Majid I, Imran S. Depigmentation therapy with Q-switched Nd:YAG laser in universal vitiligo. J Cutan Aesthet Surg. 2013;6(2):93–6. https://doi.org/10.4103/0974-2077.112670.
Rao J, Fitzpatrick RE. Use of the Q-switched 755-nm alexandrite laser to treat recalcitrant pigment after depigmentation therapy for vitiligo. Dermatol Surg. 2004;30(7):1043–5. https://doi.org/10.1111/j.1524-4725.2004.30313.x.
Radmanesh M. Depigmentation of the normally pigmented patches in universal vitiligo patients by cryotherapy. J Eur Acad Dermatol Venereol. 2000;14(3):149–52. https://doi.org/10.1046/j.1468-3083.2000.00038.x.
van Geel N, Depaepe L, Speeckaert R. Laser (755 nm) and cryotherapy as depigmentation treatments for vitiligo: a comparative study. J Eur Acad Dermatol Venereol. 2015;29(6):1121–7. https://doi.org/10.1111/jdv.12762.
Kavuossi H. Induction of depigmentation in a universal vitiligo patient with combination of cryotherapy and phenol. J Pak Assoc Dermatol. 2009;19:112–4.
Di Nuzzo S, Masotti A. Depigmentation therapy in vitiligo universalis with cryotherapy and 4-hydroxyanisole. Clin Exp Dermatol. 2010;35(2):215–6. https://doi.org/10.1111/j.1365-2230.2009.03412.x.
AlGhamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011;25(7):749–57. https://doi.org/10.1111/j.1468-3083.2010.03876.x.
Kim SM, Lee HS, Hann SK. The efficacy of low-dose oral corticosteroids in the treatment of vitiligo patients. Int J Dermatol. 1999;38(7):546–50. https://doi.org/10.1046/j.1365-4362.1999.00623.x.
Imamura S, Tagami H. Treatment of vitiligo with oral corticosteroids. Dermatologica. 1976;153(3):179–85. https://doi.org/10.1159/000251114.
Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–29. https://doi.org/10.1016/j.clinthera.2017.09.011.
Pasricha JS, Khaitan BK. Oral mini-pulse therapy with betamethasone in vitiligo patients having extensive or fast-spreading disease. Int J Dermatol. 1993;32(10):753–7. https://doi.org/10.1111/j.1365-4362.1993.tb02754.x.
Radakovic-Fijan S, Fürnsinn-Friedl AM, Hönigsmann H, Tanew A. Oral dexamethasone pulse treatment for vitiligo. J Am Acad Dermatol. 2001;44(5):814–7. https://doi.org/10.1067/mjd.2001.113475.
Banerjee K, Barbhuiya JN, Ghosh AP, Dey SK, Karmakar PR. The efficacy of low-dose oral corticosteroids in the treatment of vitiligo patient. Indian J Dermatol Venereol Leprol. 2003;69(2):135–7.
Kanwar AJ, Mahajan R, Parsad D. Low-dose oral mini-pulse dexamethasone therapy in progressive unstable vitiligo. J Cutan Med Surg. 2013;17(4):259–68. https://doi.org/10.2310/7750.2013.12053.
Chavez-Alvarez S, Herz-Ruelas M, Raygoza-Cortez AK, Suro-Santos Y, Ocampo-Candiani J, Alvarez-Villalobos NA, Villarreal-Martinez A. Oral mini-pulse therapy in vitiligo: a systematic review. Int J Dermatol. 2021;60(7):868–76. https://doi.org/10.1111/ijd.15464.
Koh MJ, Mok ZR, Chong WS. Phototherapy for the treatment of vitiligo in Asian children. Pediatr Dermatol. 2015;32(2):192–7. https://doi.org/10.1111/pde.12506.
Seccombe E, Wynne MD, Clancy C, Godfrey KM, Fityan A. A retrospective review of phototherapy in children, at a tertiary paediatric dermatology unit. Photodermatol Photoimmunol Photomed. 2021;37(1):34–8. https://doi.org/10.1111/phpp.12604.
Lopes C, Trevisani VF, Melnik T. Efficacy and safety of 308-nm monochromatic excimer lamp versus other phototherapy devices for vitiligo: a systematic review with meta-analysis. Am J Clin Dermatol. 2016;17(1):23–32. https://doi.org/10.1007/s40257-015-0164-2.
Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH, Kim GM. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(7):666–74. https://doi.org/10.1001/jamadermatol.2017.0002.
Brazzelli V, Antoninetti M, Palazzini S, Barbagallo T, De Silvestri A, Borroni G. Critical evaluation of the variants influencing the clinical response of vitiligo: study of 60 cases treated with ultraviolet B narrow-band phototherapy. J Eur Acad Dermatol Venereol. 2007;21(10):1369–74. https://doi.org/10.1111/j.1468-3083.2007.02278.x.
Anbar TS, Westerhof W, Abdel-Rahman AT, El-Khayyat MA. Evaluation of the effects of NB-UVB in both segmental and non-segmental vitiligo affecting different body sites. Photodermatol Photoimmunol Photomed. 2006;22(3):157–63. https://doi.org/10.1111/j.1600-0781.2006.00222.x.
Abdulla SJ, Desgroseilliers JP. Treatment of vitiligo with narrow-band ultraviolet B: advantages and disadvantages. J Cutan Med Surg. 2008;12(4):174–9. https://doi.org/10.2310/7750.2008.07054.
Khojah HMJ, Alharbi AG, Alshaeri AA, Alahmadi YM, Elbadawy HM. Impact of narrow-band ultraviolet B radiation therapy on the quality of life of patients with vitiligo. J Taibah Univ Med Sci. 2021;16(6):843–8. https://doi.org/10.1016/j.jtumed.2021.04.012.
Agarwal K, Podder I, Kassir M, Vojvodic A, Schwartz RA, Wollina U, Valle Y, Lotti T, Rokni GR, Grabbe S, Goldust M. Therapeutic options in vitiligo with special emphasis on immunomodulators: a comprehensive update with review of literature. Dermatol Ther. 2020;33(2): e13215. https://doi.org/10.1111/dth.13215.
Ashraf AZ, Azurdia RM, Cohen SN. The effectiveness of home-based phototherapy for vitiligo: a systematic review of randomised controlled trials. Photodermatol Photoimmunol Photomed. 2021. https://doi.org/10.1111/phpp.12766.
Sonthalia S. Topical Band-pass Filter Cream (TBFC)-assisted home-based NB-UVB: a must-know Alternative to artificial phototherapy. J Cosmet Dermatol. 2021;20(7):2141–7. https://doi.org/10.1111/jocd.14215.
Leone G, Iacovelli P, Paro Vidolin A, Picardo M. Monochromatic excimer light 308 nm in the treatment of vitiligo: a pilot study. J Eur Acad Dermatol Venereol. 2003;17(5):531–7. https://doi.org/10.1046/j.1468-3083.2003.00818.x.
Cheng YP, Chiu HY, Jee SH, Tsai TF. Excimer light photototherapy of segmental and non-segmental vitiligo: experience in Taiwan. Photodermatol Photoimmunol Photomed. 2012;28(1):6–11. https://doi.org/10.1111/j.1600-0781.2011.00628.x.
Abdel Latif AA, Ibrahim SM. Monochromatic excimer light versus combination of topical steroid with vitamin D3 analogue in the treatment of nonsegmental vitiligo: a randomized blinded comparative study. Dermatol Ther. 2015;28(6):383–9. https://doi.org/10.1111/dth.12289.
Sun Y, Wu Y, Xiao B, Li L, Li L, Chen HD, Gao XH. Treatment of 308-nm excimer laser on vitiligo: a systemic review of randomized controlled trials. J Dermatolog Treat. 2015;26(4):347–53. https://doi.org/10.3109/09546634.2014.991268.
Tran AK, Vaidya S. Treatment of refractory vitiligo with the 308-nm excimer lamp—an Australian prospective analysis of clinical efficacy and safety. Australas J Dermatol. 2020;61(3):289–91. https://doi.org/10.1111/ajd.13259.
Spencer JM, Nossa R, Ajmeri J. Treatment of vitiligo with the 308-nm excimer laser: a pilot study. J Am Acad Dermatol. 2002;46(5):727–31. https://doi.org/10.1067/mjd.2002.121357.
Taneja A, Trehan M, Taylor CR. 308-nm excimer laser for the treatment of localized vitiligo. Int J Dermatol. 2003;42(8):658–62. https://doi.org/10.1046/j.1365-4362.2003.01997.x.
Choi KH, Park JH, Ro YS. Treatment of Vitiligo with 308-nm xenon-chloride excimer laser: therapeutic efficacy of different initial doses according to treatment areas. J Dermatol. 2004;31(4):284–92. https://doi.org/10.1111/j.1346-8138.2004.tb00674.x.
Esposito M, Soda R, Costanzo A, Chimenti S. Treatment of vitiligo with the 308 nm excimer laser. Clin Exp Dermatol. 2004;29(2):133–7. https://doi.org/10.1111/j.1365-2230.2004.01477.x.
Hadi SM, Spencer JM, Lebwohl M. The use of the 308-nm excimer laser for the treatment of vitiligo. Dermatol Surg. 2004;30(7):983–6. https://doi.org/10.1111/j.1524-4725.2004.30302.x.
Hadi S, Tinio P, Al-Ghaithi K, Al-Qari H, Al-Helalat M, Lebwohl M, Spencer J. Treatment of vitiligo using the 308-nm excimer laser. Photomed Laser Surg. 2006;24(3):354–7. https://doi.org/10.1089/pho.2006.24.354.
Hofer A, Hassan AS, Legat FJ, Kerl H, Wolf P. The efficacy of excimer laser (308 nm) for vitiligo at different body sites. J Eur Acad Dermatol Venereol. 2006;20(5):558–64. https://doi.org/10.1111/j.1468-3083.2006.01547.x.
Chimento SM, Newland M, Ricotti C, Nistico S, Romanelli P. A pilot study to determine the safety and efficacy of monochromatic excimer light in the treatment of vitiligo. J Drugs Dermatol. 2008;7(3):258–63.
Cho S, Zheng Z, Park YK, Roh MR. The 308-nm excimer laser: a promising device for the treatment of childhood vitiligo. Photodermatol Photoimmunol Photomed. 2011;27(1):24–9. https://doi.org/10.1111/j.1600-0781.2010.00558.x.
Greve B, Raulin C, Fischer E. Excimer-Laser bei Vitiligo—Kritische Wertung eigener retrospektiver Behandlungsergebnisse und Literaturübersicht [Excimer laser treatment of vitiligo—critical retrospective assessment of own results and literature overview]. J Dtsch Dermatol Ges. 2006;4(1):32–40. https://doi.org/10.1111/j.1610-0387.2006.05879.x(German).
Al-Otaibi SR, Zadeh VB, Al-Abdulrazzaq AH, Tarrab SM, Al-Owaidi HA, Mahrous R, Kadyan RS, Najem NM. Using a 308-nm excimer laser to treat vitiligo in Asians. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18(1):13–9.
Zhang XY, He YL, Dong J, Xu JZ, Wang J. Clinical efficacy of a 308 nm excimer laser in the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2010;26(3):138–42. https://doi.org/10.1111/j.1600-0781.2010.00509.x.
Noborio R, Nakamura M, Yoshida M, Nakamura R, Oshima R, Kubo R, Kato H, Morita A. Monotherapy for vitiligo using a 308-nm xenon-chloride excimer laser: colorimetric assessment of factors that influence treatment efficacy. J Dermatol. 2012;39(12):1102–3. https://doi.org/10.1111/j.1346-8138.2012.01633.x.
Fa Y, Lin Y, Chi XJ, Shi WH, Wang JL, Guo X, Geng JH, Liu HX, Zhang FR. Treatment of vitiligo with 308-nm excimer laser: our experience from a 2-year follow-up of 979 Chinese patients. J Eur Acad Dermatol Venereol. 2017;31(2):337–40. https://doi.org/10.1111/jdv.13917.
Do JE, Shin JY, Kim DY, Hann SK, Oh SH. The effect of 308 nm excimer laser on segmental vitiligo: a retrospective study of 80 patients with segmental vitiligo. Photodermatol Photoimmunol Photomed. 2011;27(3):147–51. https://doi.org/10.1111/j.1600-0781.2011.00587.x.
Majid I, Imran S. Excimer light therapy in childhood segmental vitiligo: early treatment gives better results. Dermatol Ther. 2020;33(3): e13408. https://doi.org/10.1111/dth.13408.
Sethi S, Silverberg N. Short and long-term outcomes of 308-nm laser for pediatric vitiligo. J Drugs Dermatol. 2022;21(7):773–5. https://doi.org/10.36849/JDD.6895.
Tabassum H, Majid I, Imran S. Is targeted UVB as effective as excimer light phototherapy in treatment of vitiligo? Dermatol Ther. 2021;34(5): e15058. https://doi.org/10.1111/dth.15058.
Poolsuwan P, Churee C, Pattamadilok B. Comparative efficacy between localized 308-nm excimer light and targeted 311-nm narrowband ultraviolet B phototherapy in vitiligo: a randomized, single-blind comparison study. Photodermatol Photoimmunol Photomed. 2021;37(2):123–30. https://doi.org/10.1111/phpp.12619.
Yadav D, Khandpur S, Bhari N. Targeted phototherapy with excimer light is not efficacious in the management of residual vitiligo patches following whole-body narrowband ultraviolet B light therapy: results of a retrospective case series. Indian J Dermatol Venereol Leprol. 2022;88(2):249–51. https://doi.org/10.25259/IJDVL_8_2020.
Hofer A, Hassan AS, Legat FJ, Kerl H, Wolf P. Optimal weekly frequency of 308-nm excimer laser treatment in vitiligo patients. Br J Dermatol. 2005;152(5):981–5. https://doi.org/10.1111/j.1365-2133.2004.06321.x.
Shen Z, Gao TW, Chen L, Yang L, Wang YC, Sun LC, Li CY, Xiao Y, Liu YF. Optimal frequency of treatment with the 308-nm excimer laser for vitiligo on the face and neck. Photomed Laser Surg. 2007;25(5):418–27. https://doi.org/10.1089/pho.2007.2086.
Ayob S, Cockayne SE, Gawkrodger DJ. Once weekly targeted excimer light produced modest repigmentation of vitiligo over a 20-week period. J Eur Acad Dermatol Venereol. 2018;32(8):e307–8. https://doi.org/10.1111/jdv.14853.
Xiang L. Once-weekly treatment of vitiligo with monochromatic excimer light 308 nm in Chinese patients. J Eur Acad Dermatol Venereol. 2008;22(7):899–900. https://doi.org/10.1111/j.1468-3083.2007.02518.x.
Al-Shobaili HA. Correlation of clinical efficacy and psychosocial impact on vitiligo patients by excimer laser treatment. Ann Saudi Med. 2014;34(2):115–21. https://doi.org/10.5144/0256-4947.2014.115.
Al-Shobaili HA. Treatment of vitiligo patients by excimer laser improves patients’ quality of life. J Cutan Med Surg. 2015;19(1):50–6. https://doi.org/10.2310/7750.2014.14002.
Bae JM, Eun SH, Oh SH, Shin JH, Kang HY, Kim KH, Lee SC, Choi CW. The 308-nm excimer laser treatment does not increase the risk of skin cancer in patients with vitiligo: a population-based retrospective cohort study. Pigment Cell Melanoma Res. 2019;32(5):714–8. https://doi.org/10.1111/pcmr.12781.
Sung JM, Bae JM, Kang HY. Comparison of cyclic and continuous 308-nm excimer laser treatments for vitiligo: a randomized controlled noninferiority trial. J Am Acad Dermatol. 2018;78(3):605-607.e1. https://doi.org/10.1016/j.jaad.2017.09.048.
Shenoi SD, Prabhu S, Indian Association of Dermatologists, Venereologists and Leprologists. Photochemotherapy (PUVA) in psoriasis and vitiligo. Indian J Dermatol Venereol Leprol. 2014;80(6):497–504. https://doi.org/10.4103/0378-6323.144143.
Wildfang IL, Jacobsen FK, Thestrup-Pedersen K. PUVA treatment of vitiligo: a retrospective study of 59 patients. Acta Derm Venereol. 1992;72(4):305–6.
Chuan MT, Tsai YJ, Wu MC. Effectiveness of psoralen photochemotherapy for vitiligo. J Formos Med Assoc. 1999;98(5):335–40.
Sahin S, Hindioğlu U, Karaduman A. PUVA treatment of vitiligo: a retrospective study of Turkish patients. Int J Dermatol. 1999;38(7):542–5. https://doi.org/10.1046/j.1365-4362.1999.00654.x.
Tallab T, Joharji H, Bahamdan K, Karkashan E, Mourad M, Ibrahim K. Response of vitiligo to PUVA therapy in Saudi patients. Int J Dermatol. 2005;44(7):556–8. https://doi.org/10.1111/j.1365-4632.2004.02014.x.
Anbar TS, El-Sawy AE, Attia SK, Barakat MT, Moftah NH, El-Ammawy TS, Abdel-Rahman AT, El-Tonsy MH. Effect of PUVA therapy on melanocytes and keratinocytes in non-segmental vitiligo: histopathological, immuno-histochemical and ultrastructural study. Photodermatol Photoimmunol Photomed. 2012;28(1):17–25. https://doi.org/10.1111/j.1600-0781.2011.00631.x.
Wu CS, Lan CC, Wang LF, Chen GS, Wu CS, Yu HS. Effects of psoralen plus ultraviolet A irradiation on cultured epidermal cells in vitro and patients with vitiligo in vivo. Br J Dermatol. 2007;156(1):122–9. https://doi.org/10.1111/j.1365-2133.2006.07584.x.
Kwok YK, Anstey AV, Hawk JL. Psoralen photochemotherapy (PUVA) is only moderately effective in widespread vitiligo: a 10-year retrospective study. Clin Exp Dermatol. 2002;27(2):104–10. https://doi.org/10.1046/j.1365-2230.2002.00984.x.
Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A therapy vs Narrowband-UV-B therapy. Arch Dermatol. 2007;143(5):578–84. https://doi.org/10.1001/archderm.143.5.578.
El-Mofty M, Mostafa WZ, Bosseila M, Youssef R, Esmat S, El Ramly A, Fawzi M, Mahgoub D, Nagui N, Mashaly HM, El-Fangary M, Fathy M. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23(4):428–34. https://doi.org/10.1111/j.1529-8019.2010.01345.x.
Bhatnagar A, Kanwar AJ, Parsad D, De D. Psoralen and ultraviolet A and narrow-band ultraviolet B in inducing stability in vitiligo, assessed by vitiligo disease activity score: an open prospective comparative study. J Eur Acad Dermatol Venereol. 2007;21(10):1381–5. https://doi.org/10.1111/j.1468-3083.2007.02283.x.
Sakhiya J, Sakhiya D, Virmani N, Gajjar T, Kaklotar J, Khambhati R, Daruwala F, Dudhatra N. A retrospective study of 3,000 Indian patients with vitiligo treated with phototherapy or topical monotherapy. J Clin Aesthet Dermatol. 2021;14(2):46–9.
Liu YY, Zhou JF, Zhen Y, Cui Y, Song Y, Yao L, Li SS. Clinical efficacy analysis of 110 cases of childhood vitiligo with non-surgical combined therapy. J Dermatolog Treat. 2022. https://doi.org/10.1080/09546634.2022.2104443.
Roy P, Saha SK, Paul PC, Reza AK, Nandi AK, Sultana S, Saha S, Akhter SM, Khatun S, Habibunnahar M. Effectiveness of topical corticosteroid, topical calcineurin inhibitors and combination of them in the treatment of vitiligo. Mymensingh Med J. 2016;25(4):620–7.
Dang YP, Li Q, Shi F, Yuan XY, Liu W. Effect of topical calcineurin inhibitors as monotherapy or combined with phototherapy for vitiligo treatment: a meta-analysis. Dermatol Ther. 2016;29(2):126–33. https://doi.org/10.1111/dth.12295.
Chang HC, Sung CW. Efficacy of combination therapy of narrowband-ultraviolet B phototherapy or excimer laser with topical tacrolimus for vitiligo: an updated systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2021;37(1):74–7. https://doi.org/10.1111/phpp.12593.
Li R, Qiao M, Wang X, Zhao X, Sun Q. Effect of narrow band ultraviolet B phototherapy as monotherapy or combination therapy for vitiligo: a meta-analysis. Photodermatol Photoimmunol Photomed. 2017;33(1):22–31. https://doi.org/10.1111/phpp.12277.
Nordal EJ, Guleng GE, Rönnevig JR. Treatment of vitiligo with narrowband-UVB (TL01) combined with tacrolimus ointment (0.1%) vs. placebo ointment, a randomized right/left double-blind comparative study. J Eur Acad Dermatol Venereol. 2011;25(12):1440–3. https://doi.org/10.1111/j.1468-3083.2011.04002.x.
Bae JM, Hong BY, Lee JH, Lee JH, Kim GM. The efficacy of 308-nm excimer laser/light (EL) and topical agent combination therapy versus EL monotherapy for vitiligo: a systematic review and meta-analysis of randomized controlled trials (RCTs). J Am Acad Dermatol. 2016;74(5):907–15. https://doi.org/10.1016/j.jaad.2015.11.044.
Iraji F, Asilian A, Talebzadeh Z, Saber M, Mokhtari F, Siadat A, Hosseini SM. Microneedling in combination with topical pimecrolimus 1% versus topical pimecrolimus 1% for the treatment of refractory stable vitiligo: a randomized clinical trial. Dermatol Res Pract. 2021;2021:5652140. https://doi.org/10.1155/2021/5652140.
Hu M, Liao K, Lei W, Zhang R, Tu C. The addition of topical calcipotriol to phototherapy enhance the efficacy of treatment in patients with vitiligo: a systematic review and meta-analysis. Int Immunopharmacol. 2021;98: 107910. https://doi.org/10.1016/j.intimp.2021.107910.
Liu X, Yao Z, Wang Y, Chai L, Zhou X. Vitamin D analogs combined with different types of phototherapy in the treatment of vitiligo: a systematic review of randomized trials and within-patient studies. Int Immunopharmacol. 2022;109: 108789. https://doi.org/10.1016/j.intimp.2022.108789.
Juntongjin P, Sangganjanavanich P. Efficacy of the combined excimer light and topical calcipotriol for acral vitiligo: a randomized double-blind comparative study. Dermatol Ther. 2021;34(2): e14886. https://doi.org/10.1111/dth.14886.
Batchelor JM, Thomas KS, Akram P, Azad J, Bewley A, Chalmers JR, Cheung ST, Duley L, Eleftheriadou V, Ellis R, Ferguson A, Goulding JM, Haines RH, Hamad H, Ingram JR, Laguda B, Leighton P, Levell N, Makrygeorgou A, Meakin GD, Millington A, Ogboli M, Rajasekaran A, Ravenscroft JC, Rogers A, Sach TH, Santer M, Stainforth J, Tan W, Wahie S, White J, Whitton ME, Williams HC, Wright A, Montgomery AA. Home-based narrowband UVB, topical corticosteroid or combination for children and adults with vitiligo: HI-Light Vitiligo three-arm RCT. Health Technol Assess. 2020;24(64):1–128. https://doi.org/10.3310/hta24640.
El Mofty M, Essmat S, Youssef R, Sobeih S, Mahgoub D, Ossama S, Saad A, El Tawdy A, Mashaly HM, Saney I, Helal R, Shaker O. The role of systemic steroids and phototherapy in the treatment of stable vitiligo: a randomized controlled trial. Dermatol Ther. 2016;29(6):406–12. https://doi.org/10.1111/dth.12384.
Esmat SM, El-Mofty M, Rasheed H, Mostafa WZ, Anbar TS, Abdallah M, Bassiouny D, Abdel-Halim D, Hegazy R, Eid AA, Nassar A, Abdel-Aziz RT, Fawzy MM, Gawdat HI, El Hawary M, Sany I, Shalaby S, Ragab N, Abdel-Gaber RM, Tawfik YM, El-Bassiouny M, El-Husseiny R, Attia MS, Farid C, Genedy RM, Mogawer RM. Efficacy of narrow band UVB with or without OMP in stabilization of vitiligo activity in skin photo-types (III-V): a double-blind, randomized, placebo-controlled, prospective, multicenter study. Photodermatol Photoimmunol Photomed. 2022;38(3):277–87. https://doi.org/10.1111/phpp.12749.
Tovar-Garza A, Hinojosa JA, Hynan LS, Pandya AG. Addition of oral minipulse dexamethasone to narrowband ultraviolet B phototherapy and topical steroids helps arrest disease activity in patients with vitiligo. Br J Dermatol. 2019;180(1):193–4. https://doi.org/10.1111/bjd.17150(Erratum in: Br J Dermatol. 2020;182(5):1318).
Lee J, Chu H, Lee H, Kim M, Kim DS, Oh SH. A Retrospective study of methylprednisolone mini-pulse therapy combined with narrow-band UVB in non-segmental vitiligo. Dermatology. 2016;232(2):224–9. https://doi.org/10.1159/000439563.
Mulekar SV, Isedeh P. Surgical interventions for vitiligo: an evidence-based review. Br J Dermatol. 2013;169(Suppl 3):57–66. https://doi.org/10.1111/bjd.12532.
Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. Current and emerging treatments for vitiligo. J Am Acad Dermatol. 2017;77(1):17–29. https://doi.org/10.1016/j.jaad.2016.11.010.
Ju HJ, Bae JM, Lee RW, Kim SH, Parsad D, Pourang A, Hamzavi I, Shourick J, Ezzedine K. Surgical interventions for patients with vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2021;157(3):307–16. https://doi.org/10.1001/jamadermatol.2020.5756.
Mohammad TF, Hamzavi IH. Surgical therapies for vitiligo. Dermatol Clin. 2017;35(2):193–203. https://doi.org/10.1016/j.det.2016.11.009.
Feetham HJ, Chan JL, Pandya AG. Characterization of clinical response in patients with vitiligo undergoing autologous epidermal punch grafting. Dermatol Surg. 2012;38(1):14–9. https://doi.org/10.1111/j.1524-4725.2011.02171.x.
Fongers A, Wolkerstorfer A, Nieuweboer-Krobotova L, Krawczyk P, Tóth GG, van der Veen JP. Long-term results of 2-mm punch grafting in patients with vitiligo vulgaris and segmental vitiligo: effect of disease activity. Br J Dermatol. 2009;161(5):1105–11. https://doi.org/10.1111/j.1365-2133.2009.09367.x.
Bae JM, Lee JH, Kwon HS, Kim J, Kim DS. Motorized 0.8-mm micropunch grafting for refractory vitiligo: a retrospective study of 230 cases. J Am Acad Dermatol. 2018;79(4):720-727.e1. https://doi.org/10.1016/j.jaad.2018.06.016.
Kim JC, Kim DC, Kang HY, Kim DS. Treatment outcomes and prognostic factors of motorized 0.5-mm micropunch grafting with a skin-seeding technique for 83 cases of vitiligo in children. J Am Acad Dermatol. 2022. https://doi.org/10.1016/j.jaad.2022.07.021.
Ragab M, El Zagh O, Farid C. Transverse needling after autologous mini-punch grafts improves repigmentation in stable non-segmental vitiligo. Clin Cosmet Investig Dermatol. 2021;14:827–35. https://doi.org/10.2147/CCID.S315407.
McCrary MR, Gibbs DC, Alharthi M, Krueger LD. Utilization of our toolkit: a systematic review and meta-analysis of surgical therapies in vitiligo treatment. Dermatol Surg. 2022;48(8):815–21. https://doi.org/10.1097/DSS.0000000000003503.
Nanda S, Relhan V, Grover C, Reddy BS. Suction blister epidermal grafting for management of eyelid vitiligo: special considerations. Dermatol Surg. 2006;32(3):387–91. https://doi.org/10.1111/j.1524-4725.2006.32078.x.
Gupta S, Goel A, Kanwar AJ, Kumar B. Autologous melanocyte transfer via epidermal grafts for lip vitiligo. Int J Dermatol. 2006;45(6):747–50. https://doi.org/10.1111/j.1365-4632.2006.02694.x.
Kar BR, Raj C. Suction blister epidermal grafting for vitiligo involving angles of lip: experience of 112 patients. J Cutan Aesthet Surg. 2018;11(1):13–9. https://doi.org/10.4103/JCAS.JCAS_111_15.
Kumar A, Mohanty S, Sahni K, Kumar R, Gupta S. Extracted hair follicle outer root sheath cell suspension for pigment cell restoration in vitiligo. J Cutan Aesthet Surg. 2013;6(2):121–5. https://doi.org/10.4103/0974-2077.112679.
Mohanty S, Kumar A, Dhawan J, Sreenivas V, Gupta S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br J Dermatol. 2011;164(6):1241–6. https://doi.org/10.1111/j.1365-2133.2011.10234.x.
Chen YF, Yang PY, Hu DN, Kuo FS, Hung CS, Hung CM. Treatment of vitiligo by transplantation of cultured pure melanocyte suspension: analysis of 120 cases. J Am Acad Dermatol. 2004;51(1):68–74. https://doi.org/10.1016/j.jaad.2003.12.013.
Hong WS, Hu DN, Qian GP, McCormick SA, Xu AE. Treatment of vitiligo in children and adolescents by autologous cultured pure melanocytes transplantation with comparison of efficacy to results in adults. J Eur Acad Dermatol Venereol. 2011;25(5):538–43. https://doi.org/10.1111/j.1468-3083.2010.03824.x.
Lommerts JE, Uitentuis SE, Bekkenk MW, de Rie MA, Wolkerstorfer A. The role of phototherapy in the surgical treatment of vitiligo: a systematic review. J Eur Acad Dermatol Venereol. 2018;32(9):1427–35. https://doi.org/10.1111/jdv.14950.
Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: a systematic review. J Am Acad Dermatol. 2019;81(3):834–46. https://doi.org/10.1016/j.jaad.2019.04.037.
Rekik M, Mseddi M, Kammoun N, Sellami K, Turki H. Efficacy of autologous platelet-rich plasma in the treatment of vitiligo: a 10-patient prospective study. J Cosmet Dermatol. 2022. https://doi.org/10.1111/jocd.15050.
Afify AA, Zuelfakkar NM, Eshafi MA. Fractional CO2 laser, platelet rich plasma and narrow band ultraviolet B in the treatment of Vitiligo (a randomized clinical trial). Lasers Med Sci. 2021;36(7):1479–86. https://doi.org/10.1007/s10103-020-03195-9.
Chen J, Wan Y, Lin Y, Jiang H. Current art of combination therapy with autologous platelet-rich plasma for stable vitiligo: a meta-analysis. Int Wound J. 2021;18(3):251–60. https://doi.org/10.1111/iwj.13524.
Deng Y, Li J, Yang G. 308-nm excimer laser plus platelet-rich plasma for treatment of stable vitiligo: a prospective, randomized case-control study. Clin Cosmet Investig Dermatol. 2020;13:461–7. https://doi.org/10.2147/CCID.S260434.
Khattab FM, Abdelbary E, Fawzi M. Evaluation of combined excimer laser and platelet-rich plasma for the treatment of nonsegmental vitiligo: a prospective comparative study. J Cosmet Dermatol. 2020;19(4):869–77. https://doi.org/10.1111/jocd.13103.
Kadry M, Tawfik A, Abdallah N, Badawi A, Shokeir H. Platelet-rich plasma versus combined fractional carbon dioxide laser with platelet-rich plasma in the treatment of vitiligo: a comparative study. Clin Cosmet Investig Dermatol. 2018;11:551–9. https://doi.org/10.2147/CCID.S178817.
Abdelghani R, Ahmed NA, Darwish HM. Combined treatment with fractional carbon dioxide laser, autologous platelet-rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: a prospective, randomized comparative trial. J Cosmet Dermatol. 2018;17(3):365–72. https://doi.org/10.1111/jocd.12397.
Raizada A, Panda M, Singh BS, Kar BR. Fractional carbon dioxide laser versus fractional carbon dioxide laser with autologous intralesional platelet-rich plasma in the treatment of stable, non-segmental vitiligo: a randomized comparative study. J Cutan Aesthet Surg. 2021;14(1):55–63. https://doi.org/10.4103/JCAS.JCAS_188_19.
Mercuri SR, Di Nicola MR, Brianti P, Bianchi VG, Paolino G. Pilot study on the use of the “monocyte-rich” platelet-rich plasma in combination with 1927 nm fractional and 308 nm excimer lasers for the treatment of vitiligo. Medicina (Kaunas). 2021;57(9):904. https://doi.org/10.3390/medicina57090904.
Salem SAM, Fezeaa TA, El Khazragy N, Soltan MY. Effect of platelet-rich plasma on the outcome of mini-punch grafting procedure in localized stable vitiligo: clinical evaluation and relation to lesional basic fibroblast growth factor. Dermatol Ther. 2021;34(2): e14738. https://doi.org/10.1111/dth.14738.
Ibrahim ZA, El-Ashmawy AA, El-Tatawy RA, Sallam FA. The effect of platelet-rich plasma on the outcome of short-term narrowband-ultraviolet B phototherapy in the treatment of vitiligo: a pilot study. J Cosmet Dermatol. 2016;15(2):108–16. https://doi.org/10.1111/jocd.12194.
Yin L, Adotama P, Svigos K, Gutierrez D, Lo SK. Platelet-rich plasma, a promising adjunctive treatment for vitiligo: a case report. JAAD Case Rep. 2020;6(12):1320–2. https://doi.org/10.1016/j.jdcr.2020.09.021.
Parambath N, Sharma VK, Parihar AS, Sahni K, Gupta S. Use of platelet-rich plasma to suspend noncultured epidermal cell suspension improves repigmentation after autologous transplantation in stable vitiligo: a double-blind randomized controlled trial. Int J Dermatol. 2019;58(4):472–6. https://doi.org/10.1111/ijd.14286.
Albalat W, Elsayed M, Salem A, Ehab R, Fawzy M. Non-cultured epidermal cells suspended in either platelet-rich plasma or ringer lactate for stable vitiligo: a prospective comparative study. J Cosmet Dermatol. 2022;21(7):3102–9. https://doi.org/10.1111/jocd.14576.
De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol. 2008;26(1):30–4. https://doi.org/10.1016/j.clindermatol.2007.10.009.
Halder RM, Pham HN, Breadon JY, Johnson BA. Micropigmentation for the treatment of vitiligo. J Dermatol Surg Oncol. 1989;15(10):1092–8. https://doi.org/10.1111/j.1524-4725.1989.tb03129.x.
Mahajan BB, Garg G, Gupta RR. Evaluation of cosmetic tattooing in localised stable vitiligo. J Dermatol. 2002;29(11):726–30. https://doi.org/10.1111/j.1346-8138.2002.tb00210.x.
Eun SH, Lee HN, Kim SH, et al. Micropigmentation for acral vitiligo: an open-label pilot study of 12 patients. Korean J Dermatol. 2020;58:20–5.
Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg. 2010;63(6):988–91. https://doi.org/10.1016/j.bjps.2009.03.013.
Francis A, Criton S, Shojan A, Philip R. Micropigmentation in vitiligo of lateral lower lip. J Cutan Aesthet Surg. 2013;6(4):236–7. https://doi.org/10.4103/0974-2077.123416.
Ju HJ, Eun SH, Lee HN, Lee JH, Kim GM, Bae JM. Micropigmentation for vitiligo on light to moderately colored skin: updated evidence from a clinical and animal study. J Dermatol. 2020;47(5):464–9. https://doi.org/10.1111/1346-8138.15282.
Singh H, Kumaran MS, Bains A, Parsad D. A Randomized comparative study of oral corticosteroid minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatology. 2015;231(3):286–90. https://doi.org/10.1159/000433424.
Garza-Mayers AC, Kroshinsky D. Low-dose methotrexate for vitiligo. J Drugs Dermatol. 2017;16(7):705–6.
Ugurer E, Altunay IK, Erdem Y, Ozkur E, Tuncel D. Undesirable repigmentation in vitiligo patient receiving methotrexate therapy for the treatment of psoriasis: treatment or side effect? Dermatol Online J. 2022. https://doi.org/10.5070/D328157067.
Abdelmaksoud A, Dave DD, Lotti T, Vestita M. Topical methotrexate 1% gel for treatment of vitiligo: a case report and review of the literature. Dermatol Ther. 2019;32(5): e13013. https://doi.org/10.1111/dth.13013.
Song X, Xu A, Pan W, Wallin B, Kivlin R, Lu S, Cao C, Bi Z, Wan Y. Minocycline protects melanocytes against H2O2-induced cell death via JNK and p38 MAPK pathways. Int J Mol Med. 2008;22(1):9–16.
Parsad D, Kanwar A. Oral minocycline in the treatment of vitiligo: a preliminary study. Dermatol Ther. 2010;23(3):305–7. https://doi.org/10.1111/j.1529-8019.2010.01328.x.
Singh A, Kanwar AJ, Parsad D, Mahajan R. Randomized controlled study to evaluate the effectiveness of dexamethasone oral minipulse therapy versus oral minocycline in patients with active vitiligo vulgaris. Indian J Dermatol Venereol Leprol. 2014;80(1):29–35. https://doi.org/10.4103/0378-6323.125479.
Siadat AH, Zeinali N, Iraji F, Abtahi-Naeini B, Nilforoushzadeh MA, Jamshidi K, Khosravani P. Narrow-band ultraviolet B versus oral minocycline in treatment of unstable vitiligo: a prospective comparative trial. Dermatol Res Pract. 2014;2014: 240856. https://doi.org/10.1155/2014/240856.
Charoenpongpun N, Kamanamool N, Udompataikul M, Khunkhet S, Kanokrungsee S. A pilot study of combined oral minocycline and narrowband UVB phototherapy in vitiligo: a randomized, double-blind, placebo-controlled trial. Dermatol Ther. 2022;35(8): e15596. https://doi.org/10.1111/dth.15596.
Radmanesh M, Saedi K. The efficacy of combined PUVA and low-dose azathioprine for early and enhanced repigmentation in vitiligo patients. J Dermatolog Treat. 2006;17(3):151–3. https://doi.org/10.1080/09546630600791442.
Patra S, Khaitan BK, Sharma VK, Khanna N. A randomized comparative study of the effect of betamethasone oral mini-pulse therapy versus oral azathioprine in progressive nonsegmental vitiligo. J Am Acad Dermatol. 2021;85(3):728–9. https://doi.org/10.1016/j.jaad.2019.03.025.
Khondker L, Khan SI. Efficacy of levamisole for the treatment of slow spreading vitiligo. Mymensingh Med J. 2013;22(4):761–6.
Agarwal S, Ramam M, Sharma VK, Khandpur S, Pal H, Pandey RM. A randomized placebo-controlled double-blind study of levamisole in the treatment of limited and slowly spreading vitiligo. Br J Dermatol. 2005;153(1):163–6. https://doi.org/10.1111/j.1365-2133.2005.06556.x.
Pasricha JS, Khera V. Effect of prolonged treatment with levamisole on vitiligo with limited and slow-spreading disease. Int J Dermatol. 1994;33(8):584–7. https://doi.org/10.1111/j.1365-4362.1994.tb02903.x.
Majid I, Imran S, Batool S. Apremilast is effective in controlling the progression of adult vitiligo: a case series. Dermatol Ther. 2019;32(4): e12923. https://doi.org/10.1111/dth.12923.
Khemis A, Fontas E, Moulin S, Montaudié H, Lacour JP, Passeron T. Apremilast in combination with narrowband UVB in the treatment of vitiligo: a 52-week monocentric prospective randomized placebo-controlled study. J Investig Dermatol. 2020;140(8):1533-1537.e2. https://doi.org/10.1016/j.jid.2019.11.031.
Kim HJ, Singer GK, Del Duca E, Abittan BJ, Chima MA, Kimmel G, Bares J, Gagliotti M, Genece J, Chu J, Wilding G, Pavel AB, Guttman-Yassky E, Lebwohl MG. Combination of apremilast and narrowband ultraviolet B light in the treatment of generalized vitiligo in skin phototypes IV to VI: a randomized split-body pilot study. J Am Acad Dermatol. 2021;85(6):1657–60. https://doi.org/10.1016/j.jaad.2020.12.073.
Kim HJ, Del Duca E, Pavel AB, Singer GK, Abittan BJ, Chima MA, Kimmel G, Bares J, Baum D, Gagliotti M, Genece J, Chu J, Lebwohl MG, Guttman-Yassky E. Apremilast and narrowband ultraviolet B combination therapy suppresses Th17 axis and promotes melanogenesis in vitiligo skin: a randomized, split-body, pilot study in skin types IV–VI. Arch Dermatol Res. 2022. https://doi.org/10.1007/s00403-022-02343-1.
Dell’Anna ML, Mastrofrancesco A, Sala R, Venturini M, Ottaviani M, Vidolin AP, Leone G, Calzavara PG, Westerhof W, Picardo M. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32(6):631–6. https://doi.org/10.1111/j.1365-2230.2007.02514.x.
Jung HM, Jung YS, Lee JH, Kim GM, Bae JM. Antioxidant supplements in combination with phototherapy for vitiligo: a systematic review and metaanalysis of randomized controlled trials. J Am Acad Dermatol. 2021;85(2):506–8. https://doi.org/10.1016/j.jaad.2018.10.010.
Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28(3):285–7. https://doi.org/10.1046/j.1365-2230.2003.01207.x.
Szczurko O, Shear N, Taddio A, Boon H. Ginkgo biloba for the treatment of vitilgo vulgaris: an open label pilot clinical trial. BMC Complement Altern Med. 2011;11:21. https://doi.org/10.1186/1472-6882-11-21.
Gonzalez S, Gilaberte Y, Philips N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem Photobiol Sci. 2010;9(4):559–63. https://doi.org/10.1039/b9pp00156e.
Reyes E, Jaén P, de las Heras E, Carrión F, Alvarez-Mon M, de Eusebio E, Alvare M, Cuevas J, González S, Villarrubia VG. Systemic immunomodulatory effects of Polypodium leucotomos as an adjuvant to PUVA therapy in generalized vitiligo: a pilot study. J Dermatol Sci. 2006;41(3):213–6. https://doi.org/10.1016/j.jdermsci.2005.12.006.
Pacifico A, Damiani G, Iacovelli P, Conic RRZ, Young Dermatologists Italian Network (YDIN), Gonzalez S, Morrone A. NB-UVB plus oral Polypodium leucotomos extract display higher efficacy than NB-UVB alone in patients with vitiligo. Dermatol Ther. 2021;34(2): e14776. https://doi.org/10.1111/dth.14776.
Middelkamp-Hup MA, Bos JD, Rius-Diaz F, Gonzalez S, Westerhof W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007;21(7):942–50. https://doi.org/10.1111/j.1468-3083.2006.02132.x.
Phan K, Phan S, Shumack S, Gupta M. Repigmentation in vitiligo using janus kinase (JAK) inhibitors with phototherapy: systematic review and meta-analysis. J Dermatolog Treat. 2022;33(1):173–7. https://doi.org/10.1080/09546634.2020.1735615.
Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D, Sun K, Butler K, Ezzedine K. Efficacy and safety of ruxolitinib cream monotherapy for the treatment of vitiligo: results from two 52-week phase 3 studies. Presented at the American Academy of Dermatology Annual Meeting, 25–29 March 2022, Boston.
Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D, Sun K, Butler K, Ezzedine K, TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387(16):1445–55. https://doi.org/10.1056/NEJMoa2118828.
Rosmarin D, Pandya AG, Lebwohl M, Grimes P, Hamzavi I, Gottlieb AB, Butler K, Kuo F, Sun K, Ji T, Howell MD, Harris JE. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(10244):110–20. https://doi.org/10.1016/S0140-6736(20)30609-7.
Harris JE, Pandya AG, Lebwohl M, Hamzavi IH, Grimes P, Gottlieb AB, Sofen HL, Moore AY, Wang M, Kornacki D, Butler K, Rosmarin D. Safety and efficacy of ruxolitinib cream for the treatment of vitiligo: 156-week data from a phase 2 study. Presented at the British Association of Dermatologists Annual Meeting, 5–7 July 2022, Glasgow.
Rosmarin D, Ezzedine K, Desai SR, Seneschal J, Kornacki D, Sun K, Butler K, Passeron T. Efficacy and safety of Ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: pooled subgroup analysis from two randomized phase 3 studies. Presented at the American Academy of Dermatology Annual Meeting, 25–29 March 2022, Boston.
Rosmarin D, Seneschal J, Grimes P, Desai SR, Pandya AG, Kornacki D, Wang M, Butler K, Ezzedine K. Efficacy and safety of ruxolitinib cream in adolescent patients with vitiligo: pooled analyses of the 52-week TRuE-V phase 3 studies. Presented at the Society for Pediatric Dermatology Annual Meeting, 7–10 July 2022, Indianapolis.
Passeron T, Harris JE, Pandya AG, Seneschal J, Grimes P, Kornacki D, Wang M, Butler K, Ezzedine K, Rosmarin D. Effect of ruxolitinib cream on achievement of VASI50 by body region: week 52 pooled analysis of the TRuE-V phase 3 studies. Presented at the European Academy of Dermatology of Venereology (EADV) Congress, 7–10 September 2022, Milan.
Hamzavi I, Rosmarin D, Harris JE, Pandya AG, Lebwohl M, Gottlieb AB, Butler K, Kuo FI, Sun K, Grimes P. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86(6):1398–401. https://doi.org/10.1016/j.jaad.2021.05.047.
Rosmarin D, Pandya AG, Grimes P, Lebwohl M, Gottlieb AB, Hamzavi IH, Butler K, Wei S, Rumberger B, Harris JE. Maintenance of repigmentation after discontinuation of ruxolitinib cream in patients with vitiligo. Presented at the European Society for Dermatological Research Annual Meeting, 22–25 September 2021, Virtual.
Nicolaidou E, Antoniou C, Stratigos AJ, Stefanaki C, Katsambas AD. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. J Am Acad Dermatol. 2007;56(2):274–8. https://doi.org/10.1016/j.jaad.2006.09.004.
Joshipura D, Alomran A, Zancanaro P, Rosmarin D. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib: a 32-week open-label extension study with optional narrow-band ultraviolet B. J Am Acad Dermatol. 2018;78(6):1205-1207.e1. https://doi.org/10.1016/j.jaad.2018.02.023.
Olamiju B, Craiglow BG. Tofacitinib cream plus narrowband ultraviolet B phototherapy for segmental vitiligo in a child. Pediatr Dermatol. 2020;37(4):754–5. https://doi.org/10.1111/pde.14159.
Pandya AG, Harris JE, Lebwohl M, Hamzavi IH, Butler K, Kuo FI, Wei S, Rosmarin D. Addition of narrow-band UVB phototherapy to ruxolitinib cream in patients with vitiligo. J Investig Dermatol. 2022. https://doi.org/10.1016/j.jid.2022.05.1093.
Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110–2. https://doi.org/10.1001/jamadermatol.2015.1520.
Vu M, Heyes C, Robertson SJ, Varigos GA, Ross G. Oral tofacitinib: a promising treatment in atopic dermatitis, alopecia areata and vitiligo. Clin Exp Dermatol. 2017;42(8):942–4. https://doi.org/10.1111/ced.13290.
Komnitski M, Komnitski A, Komnitski Junior A, Silva de Castro CC. Partial repigmentation of vitiligo with tofacitinib, without exposure to ultraviolet radiation. An Bras Dermatol. 2020;95(4):473–6. https://doi.org/10.1016/j.abd.2019.08.032.
Moore AY, Cepica T, Maberry S. Amelioration of unstable vitiligo and normalization of thryroglobulin antibodies with oral tofacitinib. JAAD Case Rep. 2022;23:64–6. https://doi.org/10.1016/j.jdcr.2022.02.025.
Dong J, Huang X, Ma LP, Qi F, Wang SN, Zhang ZQ, Wei SN, Gao L, Liu F. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20(2): 15593258221105370. https://doi.org/10.1177/15593258221105370.
Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61(4):374–6. https://doi.org/10.1111/ajd.13348.
Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, Christiano AM, Mackay-Wiggan J. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74(2):370–1. https://doi.org/10.1016/j.jaad.2015.09.073.
Ezzedine, K, Peeva E, Yamaguchi Y, Cox LA, Banerjee A, Han G, Hamzavi I, Ganesan AK, Picardo M, Thaci D, Harris JE, Bae JM, Tsukamoto K, Sinclair R, Pandya AG, Sloan A, Yu D, Gandhi K, Vincent MS, King B. Efficacy and safety of the oral kanus kinase 3/TEC inhibitor ritlecitinib (PF-06651600) in adults with active non-segmental vitiligo: results from a phase 2b, randomized, dose-ranging study with an extension period. Presented at the European Academy of Dermatology and Venereology Congress, 29 September–2 October 2021.
Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77(4):675-682.e1. https://doi.org/10.1016/j.jaad.2017.05.043.
Kim SR, Heaton H, Liu LY, King BA. Rapid repigmentation of vitiligo using tofacitinib plus low-dose, narrowband UV-B phototherapy. JAMA Dermatol. 2018;154(3):370–1. https://doi.org/10.1001/jamadermatol.2017.5778.
Gianfaldoni S, Tchernev G, Wollina U, Roccia MG, Fioranelli M, Lotti J, Rovesti M, Satolli F, Valle Y, Goren A, Tirant M, Situm M, Kovacevic M, França K, Lotti T. Micro-focused phototherapy associated to janus kinase inhibitor: a promising valid therapeutic option for patients with localized vitiligo. Open Access Maced J Med Sci. 2018;6(1):46–8. https://doi.org/10.3889/oamjms.2018.042.
Tajalli M, Kabir S, Vance TM, Qureshi AA. Effective use of oral tofacitinib and phototherapy in a patient with concomitant alopecia areata, vitiligo, and plaque and inverse psoriasis. Clin Case Rep. 2020;8(5):819–22. https://doi.org/10.1002/ccr3.2759.
Elmariah SB, Smith JS, Merola JF. JAK in the [black] box: a dermatology perspective on systemic JAK inhibitor safety. Am J Clin Dermatol. 2022;23(4):427–31. https://doi.org/10.1007/s40257-022-00701-3.
Yang BJ, Fan SR, Zhang XF, Cai JY, Ruan T, Xiang ZR, Ren J, Hao XJ, Chen DZ. Design, synthesis and structure-activity relationship optimization of phenanthridine derivatives as new anti-vitiligo compounds. Bioorg Chem. 2022;119: 105582. https://doi.org/10.1016/j.bioorg.2021.105582.
Zou DP, Chen YM, Zhang LZ, Yuan XH, Zhang YJ, Inggawati A, Kieu Nguyet PT, Gao TW, Chen J. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/β-catenin signaling. Genes Dis. 2020;8(5):677–88. https://doi.org/10.1016/j.gendis.2020.06.003.
Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, Kumar P, Denman CJ, Lacek AT, Kohlhapp FJ, Alamiri A, Hughes T, Bines SD, Kaufman HL, Overbeck A, Mehrotra S, Hernandez C, Nishimura MI, Guevara-Patino JA, Le Poole IC. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5(174): 174ra28. https://doi.org/10.1126/scitranslmed.3005127.
Prajapati K, Perez C, Rojas LBP, Burke B, Guevara-Patino JA. Functions of NKG2D in CD8+ T cells: an opportunity for immunotherapy. Cell Mol Immunol. 2018;15(5):470–9. https://doi.org/10.1038/cmi.2017.161.
Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang HK, Kaur N, Naga OS, Murali A, Nishimura MI, Caroline Le Poole I, Mehrotra S. A quantitative increase in regulatory T cells controls development of vitiligo. J Investig Dermatol. 2014;134(5):1285–94. https://doi.org/10.1038/jid.2013.540.
Tulic MK, Cavazza E, Cheli Y, Jacquel A, Luci C, Cardot-Leccia N, Hadhiri-Bzioueche H, Abbe P, Gesson M, Sormani L, Regazzetti C, Beranger GE, Lereverend C, Pons C, Khemis A, Ballotti R, Bertolotto C, Rocchi S, Passeron T. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun. 2019;10(1):2178. https://doi.org/10.1038/s41467-019-09963-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kaitlynne N. Cunningham declares no conflicts of interest. David Rosmarin has received honoraria as a consultant for AbbVie, Abcuro, AltruBio, Arena, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Incyte, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio; has received research support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Dermira, Galderma, Incyte, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron Pharmaceuticals Inc; and has served as a paid speaker for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi.
Funding
Not applicable.
Ethics approval
Not applicable.
Patient consent to participate/publish
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
Kaitlynne N. Cunningham wrote the manuscript, completed the literature review, edited the manuscript, and read and approved the final version of the manuscript for publication. David Rosmarin supervised the work, critically reviewed and edited the manuscript, and read and approved the final version of the manuscript for publication.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cunningham, K.N., Rosmarin, D. Vitiligo Treatments: Review of Current Therapeutic Modalities and JAK Inhibitors. Am J Clin Dermatol 24, 165–186 (2023). https://doi.org/10.1007/s40257-022-00752-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00752-6