Abstract
Background and Objectives
The aim of this study was to evaluate the cost effectiveness of novel oral anticoagulants (NOACs) for stroke prevention among atrial fibrillation (AF) patients by incorporating Taiwanese demographic information derived from a population-based database, the National Health Insurance Research Database (NHIRD), into cost-effectiveness analysis.
Methods
From 1 January to 31 December 2012, 98,213 AF patients were selected from the NHIRD database. A Markov model was constructed that combined published secondary data with the Taiwan NHIRD to compare the cost and incremental cost effectiveness of apixaban 5 mg twice daily, dabigatran 110 or 150 mg twice daily, rivaroxaban 20 mg once daily, and warfarin.
Results
The lifetime costs of warfarin, dabigatran 110 mg, dabigatran 150 mg, rivaroxaban 20 mg, and apixaban 5 mg were US$10,660, US$13,693, US$13,426, US$13,455, US$15,965, respectively. Apixaban resulted in an incremental cost effectiveness of US$39,351, US$27,039, US$41,298, and US$48,896 per quality-adjusted life-year (QALY) compared with warfarin, dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban 20 mg, respectively. In Monte-Carlo analyses, apixaban 5 mg, rivaroxaban 20 mg, warfarin, and dabigatran 110 mg were cost effective in 83, 10.4, 7, and 0.8%, respectively, of the simulations using a willingness-to-pay (WTP) threshold of US$50,000 per QALY.
Conclusions
Apixaban was more cost effective than warfarin, dabigatran, and rivaroxaban for stroke prevention in patients with AF. Among the anticoagulant therapies, the WTP threshold of apixaban was about US$50,000 per QALY gained. These cost-effectiveness estimations provide useful information to aid clinical decision making in stroke prevention for AF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In comparison with the published literature, the sex distribution, mean age for males and females, prevalence rate, incidence rate and distributions of CHADS2 (congestive heart failure history, hypertension history, age ≥ 75 years, diabetes mellitus, ischemic stroke or TIA history [double]) scores, and some cost information for this study were calculated using the population-based National Health Insurance Research Database in Taiwan. |
This cost-effectiveness analysis provided evidence that apixaban 5 mg was a more cost-effective alternative than warfarin, dabigatran 110 or 150 mg, and rivaroxaban 20 mg for stroke prevention in patients with atrial fibrillation (AF). |
This study incorporated the demographic information into the cost-effectiveness analysis of novel oral anticoagulants (NOACs) in Taiwan, and thus its findings can provide a more precise estimation for the clinical decision making of the NOACs uses in Taiwan. |
1 Introduction
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disturbance, which is highly prevalent with advancing age, associated with a substantial morbidity, and has an increased risk of all-cause mortality [1–5]. A nationwide study in Taiwan involving hospitalized patients reported a mean annual frequency of 127 hospitalization-based AF cases per 100,000 persons and a 1.65-fold increase in frequency from 1997 to 2002 [6]. A community-based cohort study estimated the prevalence of AF to be 1.4% in men and 0.7% in women, consistent with data in Caucasians [1, 4, 7]. Incidence rates of AF were 1.68 per 1000 person-years for men and 0.76 per 1000 person-years for women [1]. However, data from both studies were collected before 2002, and the updated true prevalence and incidence of AF from a nationwide population-based in Taiwan are currently unavailable.
A noticeable risk factor for ischemic stroke, AF is independently associated with a two- to seven-fold increase in the risk of ischemic stroke occurrence [8, 9]. In Taiwan, the prevalence of ischemic stroke was reported to be 21,000 per 100,000 persons among those aged 60–85 years and the mortality rate was 2300 per 100,000 among individuals aged 50–90 years in 2005 [10]. In 2007 alone, the total medical cost for stroke in Taiwan was up to US$375 million. Vitamin K antagonists such as warfarin are superior to antiplatelet agents for secondary stroke prevention in AF, but warfarin has significant limitations that result in its underuse [11]. A Taiwanese study showed that while 89% of the AF patients were considered to be at the highest or high risk for thromboembolism, only 24.7% of the total number of AF patients received appropriate antithrombotic therapy [12]. A cost-effectiveness analysis, using hypothetical data assumption, has compared the use of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in AF in the USA and showed that apixaban, dabigatran, and rivaroxaban were all cost-effective alternatives to warfarin [13–17]. However, a cost-effectiveness analysis was considered to use the local countries’ demographic information. It would be more convincing if the important parameters, including the sex distribution, mean age for males and females, prevalence rate, incidence rate and distributions of CHADS2 (congestive heart failure, hypertension, age = 75 years, diabetes mellitus, stroke [doubled]) score, and some cost information were estimated using a population-based database. Therefore, our study aimed to incorporate these same important parameters, estimated using a population-representative database, the Taiwan National Health Insurance Research Database (NHIRD) database, into the cost-effectiveness analysis in Taiwan, and to compare the costs and incremental cost effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in AF patients in Taiwan.
2 Methods
2.1 Data
This study used two types of data resources. The demographic data were retrieved from the NHIRD in Taiwan. Taiwan launched the NHI program in 1995 and now enrolls more than 99.6% of the population in Taiwan [18]. The database is one of the largest and most comprehensive population-based databases in the world, and has been confirmed by the National Health Insurance Administration (NHIA) to be representative of the Taiwan population [18]. The database used in this study consisted of de-identified secondary data released for research purposes; this principle complies with the Personal Information Protection Act in Taiwan, and this study was exempt from full review by the National Taiwan University Hospital Research Ethics Committee. The data in this study were retrieved from the NHIRD for patients who had a primary or secondary diagnosis of AF based on the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes (ICD-9-CM 427.13) between January 2005 and December 2012 and did not have AF in 2004. Patients were included if they were aged 18 years or over, continuously enrolled in the NHI program, and had two or more diagnoses of AF in outpatient visits or one or more inpatient admission with AF. Patients who had evidence of congenital cardiac diseases were excluded.
Among the three novel anticoagulants (NOACs) in this study, dabigatran was approved by the NHIA on 13 July 2011, rivaroxaban on 1 January 2012, and apixaban on 14 August 2013. Due to the 2-year time lag of database release, and the 1–2 years required to be able to observe stable use of the newly approved drug in Taiwan, complete effectiveness data cannot be obtained from the NHIRD; therefore, we still needed to use related information taken from published studies. The adverse events and clinical data were taken from three trials: (1) the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial for apixaban; (2) the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial for dabigatran 110 and 150 mg; and (3) ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism) for rivaroxaban. The adverse events and clinical data for warfarin were pooled from ARISTOTLE, RE-LY, and ROCKET-AF. Data on transition probabilities and increases in death risk were taken from other published economic evaluations. Multiple classifications of ischemic stroke, intracranial hemorrhage, and myocardial infarction (MI) were used, with probabilities derived from the literature.
2.2 Decision Model
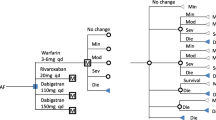
A Markov model was constructed to estimate the state-transition probabilities of disease states and pathways among patients after non-valvular AF (NVAF), including NVAF, mild ischemic stroke (mild stroke), moderate ischemic stroke (moderate stroke), severe ischemic stroke (severe stroke), mild hemorrhagic stroke (HS), moderate HS, severe HS, MI, systemic embolism, NVAF without original anticoagulation, and death, which is shown in Fig. 1. After taking into account the estimated state-transition probabilities of the above-mentioned disease states and pathways, we estimated the expected cost-related values of five anticoagulation treatment strategies, including apixaban 5 mg twice daily, dabigatran 110 mg twice daily, dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, and adjusted-dose warfarin with a target international normalized ratio (INR) of 2–3. The base-case patient population was derived from demographic information from the Taiwan NHIRD in 2012. The base case included the following health states: well, MI, major or minor intracranial hemorrhage (ICH), major or minor ischemic stroke, and death, which are shown in the Markov model diagram in Fig. 1.
Our model adopted the payer’s perspective and utilized a lifetime time horizon. Outcomes were modeled as the transition from one health state to another using 6-week cycles, and costs and utilities were applied to each outcome over the expected duration. Costs and benefits were discounted at 3.5% per year starting from the first year, and reported in 2012 US dollars. Cost effectiveness was determined as the cost per quality-adjusted life-year (QALY) gained and all analyses were performed using TreeAge Pro Suite 2012 (TreeAge Software, Williamstown, MA, USA) [19].
2.3 Probability of Adverse Outcomes in the Decision Model
The risks of adverse events were based on data from four clinical trials: the ARISTOTLE and AVERROES (Apixaban versus Acetylsalicylic Acid to Prevent Strokes in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trials for apixaban 5 mg twice daily; the RE-LY trial for dabigatran 110 and 150 mg twice daily; and ROCKET-AF for rivaroxaban 20 mg once daily [20–23]. Because apixaban had not been approved by the Taiwan Department of Health at the time of study initiation, and dabigatran and rivaroxaban were approved in 2011 and 2012, the study database did not include data for apixaban, dabigatran, or rivaroxaban. However, the outcome variables for warfarin in this study were derived from the NHIRD.
We classified ischemic stroke into one of four categories—no residual neurological sequelae, minor, major, or fatal [24]—and assumed 28% of ischemic neurologic events would be transient ischemic attacks (TIAs) [25–27]. Additionally, we assumed that the risk of stroke would increase by 1.4-fold per 10 years of life [28], and that the risk of mortality increased by 3.7-fold after an ischemic stroke or ICH [29]. Hemorrhages were classified into four categories: minor, gastrointestinal, myocardial, or fatal [13, 18, 21, 28]. ICHs were further classified as minor, major, or fatal [24]. We assumed patients who had a major hemorrhage would discontinue anticoagulation therapy and switch to aspirin therapy alone. The risk of MI was increased by 1.3-fold per 10 years of life, compounded monthly [30]. Mortality rates were assumed to increase by 1.05-fold after an MI [7], and were adjusted for age (beginning at age 70 years), presence of AF and prior stroke or TIA, and type of antithrombotic therapy. We assumed that event rates for other conditions not included in our model were similar across all treatments.
2.4 Quality-Adjusted Life-Years Estimates
QALYs were calculated by multiplying the time spent in each health state by corresponding utility (quality-of-life) estimates derived from the medical literature [5, 31–34]. The measurement of quality of life used EQ-5D and was estimated based on a large-scale survey data from Taiwan [35]. The baseline utility value was adjusted for age, AF, and anticoagulation therapy [31].
2.5 Costs
In this study, costs were projected over 30 years or until death, and future costs and life-years (LYs) were discounted at 3.5% per year. Because apixaban, dabigatran, and rivaroxaban were not available through the NHI before 2010, the medication costs of dabigatran and rivaroxaban were estimated based on those previously reported by Harrington and colleagues [13]. The warfarin medication cost was based on the dose and cost reimbursed by the Taiwan NHI. The one-time event cost was estimated from costs published by the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project under the relevant diagnosis-related group (DRG) [36]. Long-term costs for adverse events and warfarin- and non-warfarin-associated costs were calculated from the median value of published studies or the US Medicare reimbursement rates for the appropriate DRG.
2.6 Sensitivity Analyses
One-way sensitivity analyses of all variables included in the decision model were performed over their plausible ranges to identify influential model variables. Secondly, a probabilistic sensitivity analysis was performed using a first-order Monte-Carlo simulation for 2000 iterations. For each iteration, the values for all input variables were randomly sampled from their respective distributions. A normal distribution was assumed for continuous-type data, a log-normal distribution for hazard rates, beta distribution for stroke onset probabilities, and gamma distribution for costs.
All the parameters that were used for the base case for Markov model analysis, cost-effectiveness analysis, and sensitivity analysis are shown in the Electronic Supplementary Material.
3 Results
3.1 Prevalence and Incidence of Atrial Fibrillation
Using data from the nationally representative database (NHIRD), the prevalence rates of AF showed an increasing trend from 0.28% in 2005 to 0.43% in 2012. The incidence rates of AF also showed a slightly increasing trend from 1.54 per 1000 person-years in 2005 to 1.80 per 1000 person-years in 2012 (Fig. 2).
3.2 Base-Case Analysis
In this study, the incidence-based demographic information for AF patients in the 2012 study population were applied to the Markov model as the base-case patient population, including 55.6% male and 44.4% female, mean age 75.1 (male) and 77.1 (female) years, and mean CHADS2 score of 1.4 (Table 1).
Under base-case conditions, apixaban 5 mg was associated with higher QALYs and LYs than warfarin, dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban 20 mg (Table 2). The lifetime costs were US$10,660, US$13,693, US$13,426, US$13,455, and US$15,965 for warfarin, dabigatran 110 mg, dabigatran 150 mg, rivaroxaban 20 mg, and apixaban 5 mg, respectively. Apixaban resulted in incremental cost-effectiveness ratios (ICERs) of US$39,351, US$27,039, US$41,298, and US$58,991 per QALY gained compared with warfarin, dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban 20 mg, respectively. The ICERs for apixaban per LY gained were US$46,830 versus warfarin, US$24,548 versus dabigatran 110 mg, US$37,732 versus dabigatran 150 mg, and US$48,896 versus rivaroxaban 20 mg.
3.3 Probabilistic Sensitivity Analysis
Key variables with the most influence in total costs included the daily cost of apixaban, risk of treatment discontinuation of apixaban, cardiovascular risks of apixaban and warfarin, age, and the risk of stroke associated with apixaban (Fig. 3). The daily cost of apixaban had the greatest effect on incremental costs. Risk of treatment discontinuation associated with apixaban and cardiovascular risks associated with apixaban and warfarin were among the risks with most effect on incremental costs. Using a willingness-to-pay (WTP) threshold of US$50,000 per QALY, apixaban 5 mg, warfarin, rivaroxaban 20 mg, dabigatran 110 mg, and dabigatran 150 mg were cost effective in 85, 5, 9, 1.1, and 0.2% of the Monte-Carlo simulations, respectively (Fig. 4). By taking into account two willingness-to-pay thresholds of US$40,000 and US$50,000, the probabilistic sensitivity analyses of apixaban 5 mg in comparison with warfarin, dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban 20 mg is shown in Table 3.
4 Discussion
This study incorporated demographic information derived from a population-based database in Taiwan into the cost-effectiveness study to investigate the cost effectiveness of apixaban for stroke prevention among AF patients. The data showed increasing trends in both prevalence and incidence rates of AF from 2005 to 2012. The prevalence rates in this study were lower than estimates from a community-based cohort study in Taiwan, which reported 0.7% prevalence in men and 1.4% in women [1]. Data from a cross-sectional survey in the USA also showed a higher overall prevalence of 0.95%, estimated based on age-specific prevalence from 1996 to 1997 [4]. Other Asian studies reported prevalence rates of 0.61% among a randomly recruited Chinese sample of 2000 adults and 0.64% in a Japanese study [32, 37]. The incidence of AF reported in this study were consistent with the estimates of 1.68 per 1000 person-years for men and 0.76 per 1000 person-years for women from Chien et al. [1], but were lower than the report of 2–3 cases per 1000 person-years among adults aged 55–64 years in the Framingham Heart Study [5].
The results indicated that apixaban was more cost effective than warfarin, dabigatran 100 or 150 mg, and rivaroxaban 20 mg for stroke prevention in patients with AF. The base-case analysis estimated an incremental cost of US$39,351 per QALY gained for apixaban 5 mg compared with warfarin, which was well within the range considered to be cost effective [32]. Apixaban 5 mg was also associated with ICERs of US$39,351, US$27,039, US$41,298, and US$58,991 per QALY gained compared with warfarin, dabigatran 110 mg, dabigatran 150 mg, and rivaroxaban 20 mg, respectively. Based on the simulation results, 83% of the simulations would accept the WTP threshold of US$50,000 per QALY; and the WTP was estimated to be US$50,000, was a percentage of acceptance of almost over 90%. In comparison with published studies, this was similar to the USA (US$50,000) [13] and Japan (US$50,000) [38] but less than Germany (€50,000 ≈ US$54,415) [39]. In Taiwan, once a new drug is approved by the NHIA, the NHI program will fully or partly reimburse the drug costs. Therefore, the WTP threshold of US$50,000 is not only similar to that of a near Asian country (Japan), but is also acceptable by Taiwanese people due to coverage in the NHI program.
In another published study, a model established by Dorian et al. compared apixaban 5 mg, dabigatran 150 mg, and rivaroxaban 20 mg with warfarin from the US perspective and showed that all three oral anticoagulants were cost-effective alternatives to warfarin [15]. In agreement with our findings, apixaban was considered the optimal anticoagulant with an ICER of US$15,026 compared with warfarin and it was cost effective in 45% of the Monte-Carlo simulations. Another model comparing new oral anticoagulants with warfarin showed that apixaban or dabigatran 150 mg were optimal for different patient characteristics, while dabigatran 110 mg and rivaroxaban were reported to be unlikely to be cost effective [16].
Of the models that directly compared apixaban 5 mg with warfarin, Kamel et al. estimated an ICER of US$11,400 per QALY for apixaban compared with warfarin based on a subgroup of patients with AF and prior stroke or TIA from the ARISTOTLE trial [36]. In the Monte-Carlo analysis, apixaban was cost effective in 62% of simulations using a threshold of US$50,000 per QALY. A similar study by Lee et al. [21] also demonstrated apixaban to be a dominant economic strategy compared with warfarin and the Monte-Carlo analysis deemed apixaban to be cost effective in 98% of the simulations.
The demographic parameters used are a strength of this study; for example,the gender distribution, mean age of males and females, prevalence rate, incidence rate and distribution of CHADS2 scores, and some cost information were estimated using the population-based database, NHIRD. In addition, the Markov model was created using a base-case population derived from the NHIRD to avoid heterogeneity of patient populations from clinical trials and ensure relevance to the Taiwan setting. However, there are limitations to our study that should be considered when interpreting the results. First, this study adapted the ICD-9-CM codes to identify AF diagnoses from the NHIRD and the coding accuracy of the relevant ICD-9-CM codes has not been properly evaluated. Second, the costs of dabigatran and rivaroxaban were estimated using US costs and apixaban was estimated to be 125% of the dabigatran 150 mg cost, since apixaban and rivaroxaban were not available before 2012 for inclusion in the cost-effectiveness analysis. The results of this study might be different if they were based on the costs of the same novel oral anticoagulants in different countries. The conclusions of our analyses may be interpreted differently if decision makers do not prescribe based on the US$50,000 per QALY threshold.
5 Conclusion
By incorporating demographic information from a population-based database into the cost-effectiveness analysis, apixaban 5 mg was found to be a cost-effective alternative to warfarin, dabigatran 110 and 150 mg, and rivaroxaban 20 mg for stroke prevention in patients with AF. Apixaban 5 mg was the more cost-effective treatment of the three new oral anticoagulants and the most likely to be cost effective at a WTP threshold above US$50,000 per QALY gained. These cost-effectiveness estimations provide useful information to aid clinical decision making in stroke prevention for AF patients.
References
Chien KL, Su TC, Hsu HC, et al. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 2010;139:173–80.
Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52.
Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84.
Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5.
Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–62.
Lee CH, Liu PY, Tsai LM, et al. Characteristics of hospitalized patients with atrial fibrillation in Taiwan: a nationwide observation. Am J Med. 2007;120:819.e1–7.
Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17–40, ix.
Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–367.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267.
Chen CY, Huang YB, Tzu-Chi Lee C. Epidemiology and disease burden of ischemic stroke in Taiwan. Int J Neurosci. 2013;123:724–31.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Lin LJ, Cheng MH, Lee CH, et al. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation–a nationwide descriptive study in Taiwan. Clin Ther. 2008;30:1726–36.
Harrington AR, Armstrong EP, Nolan PE Jr, et al. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–81.
Lip GYH, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban against other novel oral anticoagulants (NOACs) for stroke prevention in atrial fibrillation patients [abstract no. P563]. Eur Heart J. 2012;33:54.
Dorian P, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35:1897–906.
Fernandez Avila Y, Garcia KC, Garrido Lecca S, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants and warfarin for stroke prevention in atrial fibrillation in Venezuela [abstract no. PCV14]. Value Health. 2015;18:A829.
Tanaka S, Preto MC, Bernardino G, et al. Cost-effectiveness of apixaban versus other noacs and warfarin, during hospitalization in the private Brazilian health system [abstract no. PCV17]. Value Health. 2015;18:A830.
Yeh MJ, Chang HH. National health insurance in Taiwan [letter]. Health Aff (Millwood). 2015;34:1067.
Rubrichi S, Rognoni C, Sacchi L, et al. Graphical representation of life paths to better convey results of decision models to patients. Med Decis Making. 2015;35:398–402.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Lee S, Monz BU, Clemens A, et al. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: a cross-sectional analysis using the General Practice Research Database. BMJ Open. 2012;2:e001768.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875–6.
Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–36.
Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–20.
Pignone M, Earnshaw S, Pletcher MJ, et al. Aspirin for the primary prevention of cardiovascular disease in women: a cost-utility analysis. Arch Intern Med. 2007;167:290–5.
Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43:736–49.
Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21:191–200.
National Health Insurance Administration. Taiwan diagnosis-related group payment system. Ministry of Health and Welfare; Taipei City, Taiwan, R.O.C. 2015.
Zhou ZQ, Hu DY, Chen J, et al. An epidemiological survey of atrial fibrillation in China [in Chinese]. Zhonghua Nei Ke Za Zhi. 2004;43:491–4.
Ohsawa M, Okayama A, Okamura T, et al. Mortality risk attributable to atrial fibrillation in middle-aged and elderly people in the Japanese general population: nineteen-year follow-up in NIPPON DATA80. Circ J. 2007;71:814–9.
Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N.
Coyle D, Coyle K, Cameron C, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health. 2013;16:498–506.
Yu ST, Chang HY, Yao KP, Lin YH, Hurng BS. The reliability and validity of EQ-5D in Taiwan: results from 2009 National Health Interview Survey. Zhu-Nan, Taiwan, R.O.C. 2010.
Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428–34.
Tanahashi N, Hori M, Matsumoto M, et al. Rivaroxaban versus warfarin in Japanese patients with nonvalvular atrial fibrillation for the secondary prevention of stroke: a subgroup analysis of J-ROCKET AF. J Stroke Cerebrovasc Dis. 2013;22:1317–25.
Kamae I, Hashimoto Y, Koretsune Y, et al. Cost-effectiveness analysis of apixaban against warfarin for stroke prevention in patients with nonvalvular atrial fibrillation in Japan. Clin Ther. 2015;37:2837–51.
Krejczy M, Harenberg J, Marx S, et al. Comparison of cost-effectiveness of anticoagulation with dabigatran, rivaroxaban and apixaban in patients with non-valvular atrial fibrillation across countries. J Thromb Thrombolysis. 2014;37:507–23.
Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716–7.
Acknowledgements
The authors thank the National Health Research Institutes (NHRI) for providing the National Health Insurance Research Database (NHIRD). The study data were partly retrieved from the NHIRD, which is managed by the National Health Insurance Administration (NHIA), Ministry of Health and Welfare, and maintained by the NHRI in Taiwan. The results, interpretation, and conclusions of this paper do not represent the opinions of the NHIA, Ministry of Health and Welfare, or the NHRI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Pfizer (Taiwan), grant number DN-PC-100-015.
Conflict of interest
Neither of the authors is an employee of Pfizer (Taiwan). Chieh-Yu Liu is a full-time associate professor in the Department of Midwifery and Women Health Care, National Taipei University of Nursing and Health Sciences, and has received research grants from Pfizer Taiwan Inc. Hui-Chun Chen is a graduate student in the Department of Nursing, National Taipei University of Nursing and Health Sciences.
Ethical approval
Due to the nature of secondary data analysis and using previously collected and anonymous data, all data analysis procedures conducted in this paper were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and this study was classified as being in the Exempt Review category.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, CY., Chen, HC. Cost-Effectiveness Analysis of Apixaban, Dabigatran, Rivaroxaban, and Warfarin for Stroke Prevention in Atrial Fibrillation in Taiwan. Clin Drug Investig 37, 285–293 (2017). https://doi.org/10.1007/s40261-016-0487-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0487-7