Abstract
Background and Objective
Management of non-valvular atrial fibrillation (NVAF) focuses on the use of anticoagulation to mitigate the risk of stroke. Until recently, vitamin K antagonist (VKA) treatment was considered the standard of care, with the emergence of non-VKA oral anticoagulants (NOACs) shifting treatment practice. The objective of this study was therefore to assess the use of warfarin and the NOACs for stroke prevention in patients with NVAF from the perspective of a Belgian healthcare payer using a cost-effectiveness analysis and the efficiency frontier approach.
Methods
A previously published Markov model was adapted to the Belgian healthcare setting. Clinical events modelled include ischaemic and haemorrhagic stroke, systemic embolism, intracranial haemorrhage, other major bleeding, clinically relevant non-major bleeding, myocardial infarction, cardiovascular hospitalisation and treatment discontinuations. Efficacy and bleeding data for warfarin and apixaban 5 mg twice daily were obtained from the ARISTOTLE trial, whilst those for other NOACs (rivaroxaban 20 mg once daily, dabigatran 110 mg twice daily, dabigatran 150 mg twice daily) were from published indirect comparisons. Acute medical costs were obtained from reimbursement payments made to Belgian hospitals, whilst long-term medical costs and utility data were derived from the literature. The efficiency frontier was calculated using total costs and quality-adjusted life-years (QALYs) as outcomes. Univariate and probabilistic sensitivity analyses were performed.
Results
Warfarin and apixaban were the two optimal treatment choices, as the other three treatment alternatives including dabigatran 110 mg, dabigatran 150 mg switching to dabigatran 110 mg at the age of 80 years and rivaroxaban were extendedly or strictly dominated on the efficiency frontier. Apixaban was a cost-effective alternative vs warfarin at an incremental cost-effectiveness ratio of €7,212/QALY gained.
Conclusions
Amongst NOACs, apixaban may be the most economically efficient alternative to warfarin in NVAF patients who are suitable for VKA treatment and eligible for stroke prevention in Belgium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The health economic implications of using non-vitamin K antagonist oral anticoagulants (OAC) and warfarin for the prevention of stroke and systemic embolism were assessed, using the efficiency frontier approach, as recommended by the Belgian Health Care Knowledge Centre. |
Our analysis shows apixaban and warfarin were the only two treatments that remained on the efficiency frontier while dabigatran and rivaroxaban were dominated. Compared with warfarin, apixaban provided additional life-years and quality-adjusted life-years (QALYs) with an increase in direct medical costs, leading to an incremental cost-effectiveness ratio of €7,212 per QALY. |
Apixaban appeared to be the most economically justifiable OAC offering additional health benefits over other OACs, at an acceptable cost for health payers according to current standards of willingness to pay. |
1 Introduction
Atrial fibrillation (AF) is a common cardiac disease affecting approximately 2.2 % of the screened population in Belgium [1]. AF is associated with a five-fold increase in the risk of stroke, a condition causing major disability, death and healthcare resource use [2, 3]. For example, the Belgian Sentinel Network found that approximately 39–50 % of stroke occurrences in Belgium have been fatal, estimating an annual stroke mortality rate of 88 per 100,000 inhabitants [4]. Further to the devastating humanistic consequences, stroke imposes a substantial financial burden with a hospital episode per patient cost in Belgium estimated to be €8,356 in 2010 [5].
Subsequently, the management of AF has focused on the prevention of stroke through the use of oral anticoagulant (OAC) treatment, traditionally vitamin K antagonists (VKAs) [3, 6]. The International Self-Monitoring Association of oral Anticoagulated Patients estimates that around 120,000 patients in Belgium are treated with OACs annually [7]. However, treatment with traditional VKAs may in some cases be problematic, as patients require frequent monitoring with blood tests, dose adjustments and diet; there are multiple drug–food and drug–drug interactions [3, 8]. Underuse or suboptimal VKA therapy in patients with AF has been reported and is thought to be linked to inconvenience associated with regular monitoring and the presence of contraindications [9, 10]. A cross-sectional study in Belgium reported that only 53 % of days of therapy were within a target international normalised ratio of 2–3 [10], suggesting that patients may receive inadequate anticoagulation leading to an increased risk of stroke or over-coagulation, thus increasing the chance of bleeding.
The introduction of non-VKA oral anticoagulants (NOACs) for stroke prevention in patients with AF, such as dabigatran, a direct thrombin inhibitor, rivaroxaban and apixaban, both oral factor Xa inhibitors, has led to an increased interest in the management of AF [3, 11]. These products are considered encouraging alternatives for stroke prevention in AF as they do not entail the inconveniences of VKA treatment and have been demonstrated to preserve and even improve the efficacy of traditional VKA treatment without increasing the risks of bleeding. Specifically, data from their three large multinational trials, RELY [12], ROCKET [13] and ARISTOTLE [14], demonstrated that dabigatran at a dose of 150 mg twice daily [12] and apixaban at a dose of 5 mg twice daily [14] were superior, whilst dabigatran at a dose of 110 mg twice daily [12] and rivaroxaban at a dose of 20 mg once daily [13] were non-inferior to dose-adjusted warfarin in preventing stroke and systemic embolism events. Importantly, these products offer similar benefits in reducing or maintaining the safety, compared with VKA treatment. For example, a significant reduction in major bleeding was observed amongst patients treated with dabigatran 110 mg [12] and apixaban [14] when compared with dose-adjusted warfarin. Dabigatran at a dose of 150 mg [12] and rivaroxaban [13] demonstrated non-inferiority in comparison to dose-adjusted warfarin in reducing the rates of major bleeding, whilst all three drugs significantly reduced the risk of intracranial haemorrhage compared with dose-adjusted warfarin [12–14].
In light of this evidence, the European Heart Rhythm Association updated anticoagulation guidelines in 2012 in response to the introduction of NOACs, suggesting use of one of the NOACs to be considered instead of adjusted-dose VKAs [3]. The guidelines suggest there is insufficient evidence to recommend one NOAC over the other; however, they note that cost may be an important consideration [3].
Assessing the cost effectiveness of new interventions is an important question, as new treatments are often associated with higher medication costs and may have uncertain effectiveness over standard of care. Several studies have conducted pairwise economic evaluations of an individual NOAC compared with warfarin [15–21] or comparisons of NOACs against each other [21–23]. Amongst those, two studies have compared an individual NOAC with VKA treatment from a Belgian perspective [20, 24]. Simultaneous assessment of the efficiency of the new interventions (i.e. efficiency frontier), presenting the trade-offs between costs and benefits and identifying OACs that provide most value for a given value of investment [25], has only been undertaken in two studies from a Canadian and French perspective [21, 26], but not previously from a Belgian perspective.
Decision makers are required to allocate a finite healthcare budget to maximise the health value obtained. Therefore, the objective of this study was to assess the health economic implications of using NOACs and warfarin for the prevention of stroke and systemic embolism from the perspective of the Belgian National Institute for Health and Disability Insurance by adopting the efficiency frontier approach [25, 27].
2 Methods
2.1 Model Design
A previously published Markov model using 6-week cycles was adapted [15, 23] to compare costs and outcomes for a cohort of patients with non-valvular AF (NVAF) in Belgium who were suitable for VKA treatment. In the model, five cohorts of patients initiated treatment with either: (a) dose-adjusted warfarin; (b) dabigatran 110 mg twice daily; (c) dabigatran 150 mg and switching to dabigatran 110 mg twice daily at the age of 80 years as per its European label [28] (referred to as dabigatran 150 mg hereafter); (d) rivaroxaban 20 mg once daily; or (e) apixaban 5 mg twice daily.
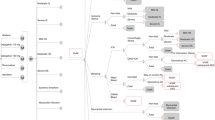
The health states, as depicted in Fig. 1, included in the model were NVAF, ischaemic stroke and haemorrhagic strokes (mild, moderate, severe and fatal), intracranial haemorrhage (ICH) other than haemorrhagic strokes (referred to as other ICH), systemic embolism, myocardial infarction (MI), other major bleeds (non-ICH major bleeds), clinically relevant non-major bleeding and NVAF with subsequent aspirin treatment or death. Details surrounding the model transitions, methodology around obtaining clinical inputs and assumptions have been previously described in detail [15, 23, 29], however, they are summarised in Tables 1 and 2 for completion.
2.2 Model Inputs
Clinical event rates for apixaban and warfarin were obtained from a within-trial analysis of ARISTOTLE [14, 15]. Comparative efficacy estimates between NOACs were obtained through means of a previously published network meta-analysis (NMA) [30] (Table 1). Though several meta-analyses have been conducted comparing NOACs [31–34], the particular publication [30] was chosen as the outcomes reported aligned with the definitions of the model (i.e. several analyses report on the composite of stroke and systemic embolism, however, for the purposes of the model, segregation between ischaemic stroke, ICH and systemic embolism is required). Increased mortality owing to the events modelled was incorporated through use of hazard ratios of mortality associated with the conditions vs the general population whilst utility data were obtained from a UK EuroQol-5 dimensions (EQ-5D) catalogue [35] as previously described [15].
The model was adapted through the use of Belgian age and sex-specific mortality [36], as well as local unit cost and resource use data. The analysis adopted the perspective of the Belgian National Institute for Health and Disability Insurance (RIZIV/INAMI) [37], applying direct healthcare costs at 2013 prices. Drug costs were obtained from RIZIV/INAMI tariffs [37], whilst event-related costs were based on reimbursement payments made by APR-DRG (All Patients Refined Diagnosis-Related Group) to Belgian hospitals [5], as detailed in Table 2. Long-term maintenance costs of stroke and MI were obtained from published estimates [38]. Health outcomes and costs were discounted at 1.5 and 3.0 % per annum, respectively [39].
3 Analyses
The total number of events, costs, life-years and quality-adjusted life years (QALYs) under each treatment strategy was estimated using a life-time horizon. Total QALYs produced by each intervention were then plotted on a vertical axis vs total costs on the horizontal axis to calculate the efficiency frontier [25], i.e. the “line on the cost-effectiveness plane connecting all non-dominated treatment alternatives” [27]. As described in the Belgian economic guidelines [39], all relevant OACs were then compared in a stepwise manner.
-
1.
Treatments were ordered by total QALYs gained in ascending order.
-
2.
Treatments that were strictly dominated by other interventions (i.e. producing lower total QALYs at higher total costs) were excluded.
-
3.
Treatments that were extendedly dominated (i.e. had an incremental cost-effectiveness ratio (ICER) higher than that of the next most effective treatment; therefore producing additional gains in effectiveness at incremental costs higher than those of the next most effective strategy) were excluded.
-
4.
For each remaining treatment, the ICERs were calculated compared with the next least effective treatment [25, 27, 40].
Univariate sensitivity analyses were conducted to examine the effect of variations of the following parameters: (1) discount rates, (i.e. 0 and 5 % for both health and cost outcomes, as recommended in Belgian guidelines [39]); (2) assumptions around treatment discontinuation; (3) individual utility estimates; (4) individual cost estimates; (5) relative efficacy estimates; and (6) baseline stroke risk as measured by congestive heart failure, hypertension, age ≥75 years, diabetes mellitus and prior stroke or transient ischaemic attack (CHADS2). Details around the parameters and assumptions tested are provided in the Electronic Supplementary material.
In addition to these analyses, probabilistic sensitivity analyses were conducted where the input parameters were varied according to statistical distributions over 2,000 iterations to assess how the uncertainty around the input values affected the model’s predictions. A beta distribution was used for transition probabilities and utilities, Dirichlet for event severity distribution, gamma for event risks and costs, and lognormal for efficacy hazard ratios, as detailed in the Electronic Supplementary material [41]. In each iteration, the ICERs were calculated to determine the proportion of iterations in which a technology was on the frontier. The results of the probabilistic analyses were used to generate a cost-effectiveness acceptability curve (CEAC), highlighting the probability that each OAC would be considered cost effective at different levels of willingness-to-pay thresholds.
4 Results
Among a cohort of 1,000 patients with AF treated with warfarin, the model predicted 317 occurrences of stroke or systemic embolism and 238 major bleed events. Treatment with dabigatran 110 mg, dabigatran 150 mg, rivaroxaban and apixaban was associated with a lower number of stroke and systemic embolism events (prevention of 3, 13, 4 and 17 stroke and systemic embolism events, respectively). The number of major bleed events was also reduced in patients treated with dabigatran 110 mg, dabigatran 150 mg and apixaban (55, 38 and 40 events avoided, respectively); however, it remained equal to warfarin in patients treated with rivaroxaban (i.e. 238 major bleeds in both arms).
Total discounted costs and QALYs varied from €12,600 to €13,992 and 6.763 to 6.956 QALYs, with apixaban being associated with the highest costs and QALYs (Table 3). Figure 2 presents the efficiency frontier. The deterministic analysis highlighted that dabigatran 110 mg was dominated (i.e. higher costs at lower QALYs) by dabigatran 150 mg and rivaroxaban. Treatment with apixaban extendedly dominated (i.e. higher QALYs and higher costs but lower ICER in comparison to the next most effective treatment) both dabigatran 150 mg and rivaroxaban in the incremental analysis. Thus, only apixaban and warfarin remained on the frontier with an ICER of €7,212 for apixaban vs warfarin.
Efficiency plot comparing oral anticoagulant strategies for stroke prevention in AF. Each marker denotes a specific intervention (total costs plotted on the x axis and total QALYs plotted on the y axis). The dotted line denotes the efficiency frontier. Any interventions plotted in the areas below and to the right of the frontier are considered inefficient “as they cost more and provide less value than existing ones or they are in a position where there is a lower price that would place them on the frontier” [25]. QALY quality-adjusted life-year
Results for all scenarios are presented in the Electronic Supplementary material. The ICER for apixaban varied between €5,971 and €24,233, with the most influential scenario being the variation of the stroke hazard ratio vs apixaban for rivaroxaban. In most scenarios, the comparative rankings of the treatment strategies in terms of total QALYs gained remained relatively unaltered; warfarin predicted to result in the lowest total QALYs gained and apixaban the highest. In several scenarios, specifically variations in the MI and ICH rates of dabigatran 150 mg and rivaroxaban as well as assumptions around treatment discontinuation (i.e. setting treatment discontinuation rates to be equal amongst NOACs or to be 0 beyond the trial period of 1.9 years), the relative ranking between dabigatran 150 mg and rivaroxaban varied. However, conclusions remained the same across scenarios (i.e. dabigatran 110 mg dominated by dabigatran 150 mg and rivaroxaban; both extendedly dominated by apixaban). The reference treatment strategy on the frontier (i.e. non-dominated strategy with the lowest number of total QALYs) was warfarin in all scenarios. From the 208 scenarios run, dabigatran 110 mg was dominated at all times, dabigatran 150 mg appeared on the frontier 17 times and rivaroxaban four times, whilst apixaban was permanently the most efficient strategy on the frontier.
The results of the probabilistic analysis are shown as the proportion of trials for which an intervention is not dominated (strictly or extendedly, thus, appearing on the frontier), as well as multi-way CEACs (Fig. 3). Each of warfarin, dabigatran 110 mg, dabigatran 150 mg, rivaroxaban and apixaban appeared on the frontier in 92, 5, 36, 29 and 92 % of simulations, respectively.The CEAC highlights that at thresholds above €10,000, apixaban had the highest probability of being the optimal treatment choice. At a commonly adopted Belgian threshold of €30,000 [Gross Domestic Product (GDP)/inhabitant] warfarin, dabigatran 110 mg, dabigatran 150 mg, rivaroxaban and apixaban have a probability of being the optimal treatment choice of 0, 1, 8, 9 and 82 %, respectively.
5 Discussion
Our study is the first published economic evaluation assessing all OACs simultaneously for stroke prevention in patients with NVAF from a Belgian payer’s perspective using the efficiency frontier approach. This methodology, as recommended by the Belgian Health Care Knowledge Centre, ensures that only interventions that are cost effective are analysed against each other, identifying the interventions that provide the most value for money. This avoids the situation of an intervention being compared with a treatment option that is not cost effective and, thereby, misguiding healthcare providers to make non-efficient decisions of limited resources. Whilst all four NOACs were found to be cost effective in comparison to warfarin (consistent with earlier findings [20, 24]), the incremental analysis highlighted that dabigatran 150 mg dominated dabigatran 110 mg and apixaban extendedly dominated both dabigatran 150 mg and rivaroxaban, although differences are small. Thus, the deterministic analysis would suggest that from available OACs on the Belgian market, apixaban and warfarin represent the “best that the system can do with available agents at current prices” [25].
Results from the univariate sensitivity analysis, however, suggest that depending on the assumptions used and uncertainty surrounding input parameters, rivaroxaban and dabigatran 150 mg may form part of the frontier. Nonetheless, in such instances, apixaban was considered incrementally cost effective vs the next less costly non-dominated alternative at ICERs that never exceeded €25,000. This was further strengthened by results of the probabilistic analysis, apixaban being the most efficient technology on the frontier in 82 % of simulations, considerably higher than that predicted for other technologies.
Our results were consistent with a previous study using the same model conducted from a French payer’s perspective that found apixaban to dominate or extendedly dominate all other NOACs [26]. Although there were some differences between our model and other published evidence, cost-effectiveness studies conducted in USA have also found apixaban to be the most optimal OAC, followed by dabigatran, rivaroxaban and warfarin, in the treatment of NVAF [19, 22]. Other studies, from Canada [21] and from a payer’s perspective or from Norway [42], found that depending on the assumptions of the model, apixaban or dabigatran 150 mg was the most cost-effective treatment choice compared with rivaroxaban and dabigatran 110 mg. In contrast to our study, earlier evaluations found rivaroxaban to be less efficient (in terms of QALYs gained) than dabigatran 150 mg. This inconsistency could be attributed to our study modelling dabigatran 150 mg as per its European label [28] and explicit modelling of treatment discontinuation, which was lower in patients treated with rivaroxaban in comparison to those treated with dabigatran 150 mg. The conclusions of the incremental analysis, however, remained constant to changes in these assumptions (Electronic Supplementary material). The modelling of treatment discontinuation is one of the key strengths of our model owing to (1) the inclusion of detailed treatment patterns on the occurrence of bleeds and other discontinuations, and (2) the use of event rates for patients treated with second-line aspirin based on patients who had demonstrated to be unsuitable for VKA treatment [43].
Other strengths distinguishing our study from other published cost-effectiveness studies include additional details around modelling the severity of stroke and bleeding events. Though we acknowledge there is considerable uncertainty around the differences in stroke and bleed severity between NOACs, this was included to assess the implications of the evidence suggesting the NOACs may result in less severe strokes [12–14]. The severity of stroke and bleeding events was set to be equal amongst all treatments in the scenario analysis; however, this had no impact on incremental results (Electronic Supplementary material).
Further differentiators include detailed modelling of mortality by incorporation of mortality rates found in the clinical trial for an initial period equivalent to the median duration of the ARISTOTLE (1.8 years), which was similar to the median duration in RELY and ROCKET trials (1.8 and 2.0 years, respectively) [14], and subsequent use of higher mortality rates than those found in the general population to account for this increased risk associated with NVAF. Our approach reduced the predicted number of additional QALYs gained from treatment and, thereby, was a more conservative estimate of the benefits of apixaban compared with other treatment options.
Several limitations and caveats apply to our analysis. First, the use of treatment outcomes from clinical trials where patients were closely monitored may have overestimated the clinical benefit of treatment compared with outcomes in real-world settings. Second, a key limitation of our analysis relates to the NMA of NOACs [30], which similar to other previously conducted studies [31, 32, 34] did not control for the differences in patient baseline characteristics, CHADS2 risk profile or time in therapeutic range between trials. For example, ROCKET-AF studied a higher risk population [13] compared with ARISTOTLE [14] and RELY [12]. The analysis also did not correct for differences between the designs of the trials, which was open label for RELY [12] and double blind for ROCKET-AF [13] and ARISTOTLE [14]. These differences were likely to favour other treatments than apixaban as the ARISTOTLE trial was double blinded and conducted in a less severe patient population. The latter is supported by the scenario analysis conducted increasing the baseline risk of stroke, reflecting a population closer to ROCKET-AF (Electronic Supplementary material). In this scenario, rivaroxaban was strictly dominated by other comparators. Thus, had comparative efficacy estimates been adjusted to reflect changes in baseline population characteristics, results would be anticipated to be less favourable for rivaroxaban. We therefore consider analysis using relative efficacy estimates adjusted by population characteristics to be an area of future research. Despite the limitations associated with the comparative efficacy estimates, we consider the use of NMA to be the best option to compare treatment options in the absence of head-to-head evidence. Furthermore, the results generated from the NMA used are consistent to earlier analyses conducted [31–34], with minor differences attributable to the use of odd ratios rather than hazard ratios, and discrepancies between papers (mainly owing to publication data) in use of the RELY data published in 2009 [12] and to the updated RELY data including some corrections published in 2010 [44]. Furthermore, evaluation of alternative relative efficacy estimates obtained through means of a pairwise indirect treatment comparison [23] resulted in the same conclusions in terms of the shape of the efficiency frontier consistently with the base case results (Electronic Supplementary material).
Third, given these trials were multinational trials, clinical and safety estimates were derived from multiple countries rather than Belgium or the European population specifically. In relation to the above, our analysis did not use Belgian-specific utilities as these were not identified. In accordance with Belgian guidelines encouraging consistency between the methodology used to derive utilities, particularly the EQ-5D, utilities were based on a UK EQ-5D catalogue [35] assuming that they would be similar for a Belgian population.
Though our model was improved from earlier Belgian evaluations [20] by inclusion of the long-term maintenance costs associated with stroke and MI, the costs used are based on the CAPRIE trial [38] and may be outdated with current practice. An earlier economic evaluation used alternative maintenance costs for stroke and no maintenance costs for MI [24], however, it was based on unpublished data. Subsequently data from the CAPRIE trial were maintained in the base case as the best currently available public evidence, but the unpublished data used in this earlier evaluation [24] were tested in sensitivity analysis. Use of these alternative costs resulted in negligible differences in results, with dabigatran 110 mg being dominated, both rivaroxaban and dabigatran 150 mg being extendedly dominated and apixaban resulting in an ICER of €7,312 vs warfarin per QALY gained (Electronic Supplementary material). Similarly, the definitions of mild, moderate and severe strokes used in the sources of cost estimates including the DRG costs applied in the acute period may not be consistent with those used in the clinical trials. Variation of these costs in the univariate sensitivity, however, had negligible impact on results. Given the consistency of these results with the base case, the limitation associated with the source data and assumptions around costs are likely to have had a negligible impact on the conclusions of our study.
6 Conclusion
The efficiency frontier approach adopted in this study provides a comparison of all currently available OACs in terms of their costs, QALYs and subsequent efficiency. Our study has shown that apixaban is a highly efficient alternative for patients with NVAF receiving care in Belgium, with the efficiency of rivaroxaban and dabigatran 150 mg being less clear. No scenario had the result that use of dabigatran 110 mg was of good economic value. In conclusion, apixaban appears to be the most economically justifiable OAC offering additional health benefits over other OACs, at an acceptable cost for health payers according to current standards of willingness to pay.
References
Claes N, Van Laethem C, Goethals M, Goethals P, Mairesse G, Schwagten B, et al. Prevalence of atrial fibrillation in adults participating in a large-scale voluntary screening programme in Belgium. Acta Cardiol. 2012;67(3):273–8.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8.
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47.
Devroey D, Van Casteren V, Buntinx F. Registration of stroke through the Belgian sentinel network and factors influencing stroke mortality. Cerebrovasc Dis. 2003;16(3):272–9.
National Databank Medische Diagnose [database on the Internet] 2009. https://tct.fgov.be/webetct/etct-web/. Accessed 11 Aug 2012.
Gailly J, Gerkens S, Van den Bruel A, Devriese S, Obyn C, Cleemput I. Utilisation des coagulomètres portables chez les patients sous anticoagulants oraux: health technology assessment. In: KCE report 117. 2009. https://kce.fgov.be/sites/default/files/page_documents/d20091027348.pdf. Accessed 11 Aug 2012.
ISMAAP. International self monitoring association of oral anticoagulated patients. http://www.ismaap.org/. Accessed 11 Aug 2012.
Maan A, Padmanabhan R, Shaikh AY, Mansour M, Ruskin JN, Heist EK. Newer anticoagulants in cardiovascular disease: a systematic review of the literature. Cardiol Rev. 2012;20(5):209–21.
Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–45 e4.
Neree C. Quality of oral anticoagulation in patients with atrial fibrillation: a cross-sectional study in general practice. Eur J Gen Pract. 2006;12(4):163–8.
Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15(5):625–51.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897–906.
Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110(6):845–51.
Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1–11.
Sorensen SV, Kansal AR, Connolly S, Peng S, Linnehan J, Bradley-Kennedy C, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost. 2011;105(5):908–19.
Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44(6):1676–81.
Wouters H, Thijs V, Annemans L. Cost-effectiveness of dabigatran etexilate in the prevention of stroke and systemic embolism in patients with atrial fibrillation in Belgium. J Med Econ. 2013;16(3):407–14.
Coyle D, Coyle K, Cameron C, Lee K, Kelly S, Steiner S, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health. 2013;16(4):498–506.
Pink J, Pirmohamed M, Hughes DA. Comparative effectiveness of dabigatran, rivaroxaban, apixaban, and warfarin in the management of patients with nonvalvular atrial fibrillation. Clin Pharmacol Ther. 2013;94(2):269–76.
Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014;36(2):192–210 e20.
Kleintjens J, Li X, Simoens S, Thijs V, Goethals M, Rietzschel ER, et al. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. Pharmacoeconomics. 2013;31(10):909–18.
Caro JJ, Nord E, Siebert U, McGuire A, McGregor M, Henry D, et al. The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ. 2010;19(10):1117–27.
Lanitis T, Cotte FE, Gaudin AF, Kachaner I, Kongnakorn T, Durand-Zaleski I. Stroke prevention in patients with atrial fibrillation in France: comparative cost-effectiveness of new oral anticoagulants (apixaban, dabigatran, and rivaroxaban), warfarin, and aspirin. J Med Econ. 2014;17(8):587–98.
Neyt M, Van Brabandt H. The importance of the comparator in economic evaluations: working on the efficiency frontier. Pharmacoeconomics. 2011;29(11):913–6.
European Medicines Agency (EMA). Pradaxa: EPAR-product information-EMEA/H/C/000829-PSU/0034. http://www.ema.europa.eu/. Accessed 11 Aug 2012.
Kongnakorn T, Lanitis T, Annemans L, Thijs V, Marbaix S. Cost-effectiveness of apixaban versus aspirin for stroke prevention in patients with non valvular atrial fibrillation in Belgium. Clin Drug Investig. 2014 (In press).
Mitchell SA, Simon TA, Raza S, Jakouloff D, Orme ME, Lockhart I, et al. The efficacy and safety of oral anticoagulants in warfarin-suitable patients with nonvalvular atrial fibrillation: systematic review and meta-analysis. Clin Appl Thromb Hemost. 2013;19(6):619–31.
Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5(5):711–9.
Lip GY, Larsen TB, Skjoth F, Rasmussen LH. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60(8):738–46.
Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012;108(3):476–84.
Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5(4):480–6.
Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–4.
Human Mortality Database, 2009 Belgian life tables.
National Institute for Health and Disability Insurance (RIZIV/INAMI) [database on the Internet]. http://www.inami.fgov.be. Accessed 11 Aug 2012.
Annemans L, Lamotte M, Levy E, Lenne X. Cost-effectiveness analysis of clopidogrel versus aspirin in patients with atherothrombosis based on the CAPRIE trial. J Med Econ. 2003;6:55–68.
Centre fédéral d’expertise des soins de santé. Recommandations pour les évaluations pharmacoéconomiques en Belgique. In: KCE reports 78B. 2008. http://kce.fgov.be/sites/default/files/page_documents/d20081027324.pdf. Accessed 11 Aug 2012.
Cleemput I, Neyt M, Van de Sande S, Thiry N. Belgian guidelines for economic evaluations and budget impact analyses: second edition. KCE reports 183C. 2012.
Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012;15(6):835–42.
Wisloff T, Hagen G, Klemp M. Economic evaluation of warfarin,dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. Pharmacoeconomics. 2014;32(6):601–12.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Randomized evaluation of long-term anticoagulation therapy investigators: newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–6.
Lamotte M, Annemans L, Kawalec P, Zoellner Y. A multi-country health economic evaluation of highly concentrated N-3 polyunsaturated fatty acids in secondary prevention after myocardial infarction. Pharmacoeconomics. 2006;24(8):783–95.
Acknowledgments
This study was funded by Pfizer and Bristol-Myers Squibb. We acknowledge the following physicians for their inputs on treatment pathways in Belgian settings (Advisory Board on 19 June 2012): Profs. H. Heidbuchel and V. Thijs (UZ Gasthuisberg, Leuven), Dr. G. Mairesse (Clinique Sud Luxembourg, Arlon), Dr. M. Goethals (HH Roeselare, Roeselare), Dr. A. Peeters (UCL, St-Luc, Brussels) and Dr. L. Pierard (Ulg, Sart Tilman, Lie`ge). The authors would like to thank Koo Wilson from Pfizer for her contribution in model development and preparation of the manuscript as well as David Jakouloff, ex-employee from Bristol-Myers Squibb, for his contribution in model development, and Ike Iheanacho from Evidera for editorial assistance.
Conflict of interest
Tereza Lanitis and Thitima Kongnakorn are employees of Evidera and were paid consultants to Pfizer in connection with the development of this manuscript and of the model. Profs. Lieven Annemans and Vincent Thijs received an honorarium from Pfizer for advice in connection with the inputs of the health economic model. Sophie Marbaix is an employee of Pfizer. Profs. Wautrecht and Goethals have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kongnakorn, T., Lanitis, T., Annemans, L. et al. Stroke and Systemic Embolism Prevention in Patients with Atrial Fibrillation in Belgium: Comparative Cost Effectiveness of New Oral Anticoagulants and Warfarin. Clin Drug Investig 35, 109–119 (2015). https://doi.org/10.1007/s40261-014-0253-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0253-7