Abstract
The association between low-density cholesterol (LDL-C) and cardiovascular disease (CVD) is well-established, with an emphasis on lowering LDL-C levels to reduce cardiovascular events. Statin therapy has been the traditional treatment for LDL-C reduction, in addition to lifestyle modifications, but studies have shown that a substantial proportion of patients does not reach target LDL-C goals despite receiving maximally tolerated statin medications. Additionally, statin therapy is associated with a few shortcomings as many patients initiated on these medications discontinue treatment within 1 year because of lack of tolerability. Furthermore, guidelines from both the American College of Cardiology and the American Heart Association highlight the importance of obtaining LDL-C goals because of the residual atherosclerotic CVD risk that remains in high-risk populations. That the residual cardiovascular risk remains despite statin therapy highlights the importance of evaluating therapeutic approaches that possess effective lipid lowering that can be used adjunctively with statins. Much focus has been directed towards the proprotein convertase subtilisin/kexin type 9 (PCSK9) pathway, leading to the development of evolocumab and alirocumab, two human monoclonal antibodies directed against PCSK9. These agents have been shown to markedly decrease LDL-C levels and significantly reduce cardiovascular risk, but the need for biweekly or monthly subcutaneous injections has generated concerns for patient compliance. A new pathway is being studied in which a synthetic small interfering ribonucleic acid (siRNA) targets the PCSK9 gene expressed in hepatocytes to prevent PCSK9 production. The siRNA, inclisiran sodium, significantly reduces hepatic production of PCSK9, causing a marked reduction in LDL-C levels, and exhibits sustained pharmacodynamic effects when dosed subcutaneously every 6 months. This review presents and discusses the current clinical and scientific evidence pertaining to inclisiran sodium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Phase III pivotal trials (Orion-9, -10, and -11) have demonstrated inclisiran’s significant low-density lipoprotein cholesterol-lowering effects in patient populations with very high cardiovascular risk, specifically patients with atherosclerotic cardiovascular disease (ASCVD) and heterozygous familial hyperlipidemia. |
Whether inclisiran can improve cardiovascular outcomes in patients with cardiovascular disease is not known but is being evaluated in the ORION-4 study, a long-term cardiovascular outcomes trial that will include approximately 15,000 subjects aged ≥ 55 years with established ASCVD and an elevated total cholesterol (>155 mg/dL). |
Inclisiran is the first small interfering ribonucleic acid being evaluated for dyslipidemia and has the potential to be used for the primary and secondary prevention of cardiovascular disease. The main benefit of inclisiran, compared with proprotein convertase subtilisin/kexin type 9 inhibitor monoclonal antibodies, is the less frequent dosing. |
1 Background
Cardiovascular disease (CVD) is the leading cause of death worldwide, affecting over 120 million adults in the USA alone [1]. The relationship between clinical atherosclerotic CVD (ASCVD) and prolonged elevated low-density lipoprotein cholesterol (LDL-C) levels is established and corroborated by the existence of familial hypercholesterolemia (FH), an autosomal dominant genetic disorder characterized by very high levels of LDL-C and early ASCVD [1, 2]. Elevated LDL-C levels significantly increase the risk of major adverse cardiovascular events (MACE) and contribute to premature morbidity and mortality in patients with FH, emphasizing the importance of initiating cholesterol-lowering agents [1, 2]. Reducing LDL-C levels is the most effective intervention to change the course of ASCVD in FH, yet residual cardiovascular risk remains despite aggressive treatment with statins and other oral lipid-lowering agents [1].
1.1 Approaches in the Treatment of Hypercholesterolemia
The gold standard of treatment for hypercholesteremia are statin medications [1, 3]. Statins are first-line agents for both primary and secondary ASCVD prevention, and their use is recommended for four patient population groups: established clinical ASCVD, LDL ≥ 190 mg/dL, diabetes (age 40–75 years) with LDL-C 70–189 mg/dL, and primary prevention (age 40–75 years, no clinical ASCVD, nondiabetic, LDL 70–189 mg/dL with a 10-year ASCVD risk ≥ 7.5%) [1, 3]. The American College of Cardiology (ACC) and American Heart Association as well as the European Atherosclerotic Society and the European Society of Cardiology all recommend specific statin intensity therapy and LDL-C goals for very high-risk populations (clinical ASCVD and LDL ≥ 190 mg/dL), which have shown benefit in reducing ASCVD risk and mortality [1, 4]. One large meta-analysis that included 26 randomized controlled trials (RCTs) showed an average reduction of LDL-C of 39 mg/dL, which reduced the risk of all-cause mortality by 10%, risk of cardiovascular mortality by 20%, and risk of coronary revascularization by 19% [5]. However, these robust benefits have not been optimized in clinical practice, where data have shown that over 70% of patients with established ASCVD do not reach an LDL-C < 70 mg/dL [3, 6]. In addition, treatment with statin therapy is associated with adverse effects (AEs), most commonly myalgias and myopathies, and many patients initiated on statins discontinue treatment within 1 year because of these real or perceived AEs. Poor adherence to currently available therapies is an important contributing factor and has been shown to be associated with an increased risk of CVD [3, 5, 7].
1.2 Role of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Low-Density Lipoprotein Cholesterol Metabolism
As lipid disorders increased in prevalence and statin therapy failed to meet the goals for some, new approaches to lipid-lowering therapy (LLT) were investigated. One pathway that was discovered and intensively studied involves proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is a protein that plays a key role in lipid metabolism, particularly in the internalization of the LDL receptor (LDLR) from the cell surface into the hepatocyte and chaperones LDLRs into lysosomes for degradation [8]. This process is upregulated in hypercholesteremia, which leads to a decrease in LDLR and an increase in circulating LDL-C. In FH, PCSK9 genetic mutations have been identified, with the gain-of-function PCSK9 mutation contributing to the phenotype of extremely elevated LDL-C with an elevated ASCVD risk [8, 9].
1.3 Currently Approved PCKS9-Targeting Agents
Soon after the role of PCSK9 in lipid metabolism was discovered, there was a surge to develop therapies to target its pathway as a treatment for dyslipidemia. Subsequently, the PCSK9 inhibitors evolocumab and alirocumab were approved for use as an adjunctive therapy to standard of care for patients with established CVD and/or FH [1, 8]. Evolocumab and alirocumab are monoclonal antibodies that target PCSK9 with high specificity, preventing degradation of the LDLRs, and studies showed that both agents reduced cardiovascular risk in high-risk patients [10, 11]. A phase III cardiovascular outcomes study, FOURIER, evaluated the efficacy of evolocumab compared with placebo in 27,564 patients with CVD [10]. After 48 weeks of therapy, patients on evolocumab showed a reduction in median LDL-C from 92 to 30 mg/dL and an absolute risk reduction of 1.5% in the incidence of composite MACE compared with intensive statin therapy (p < 0.001) in patients with FH [10]. In addition, the ODYSSEY study demonstrated similar LDL-C lowering (> 50% LDL-C reduction) and cardiovascular outcome results with alirocumab [11]. Both studies supported PCSK9 inhibitor (PCSK9i) placement as an adjunctive strategy for the treatment of dyslipidemia in the ACC consensus decision pathway, which recommends their use for patients with established ASCVD and LDL-C ≥ 90 mg/dL despite the use of maximal tolerated statin therapy and/or ezetimibe [1].
Despite the topline lipid-lowering data for PCSK9Is and their ability to be dosed biweekly or monthly, a risk of low compliance remains. A small retrospective analysis conducted by Gragnano et al. [12] evaluated evolocumab use in a real-world setting and showed that the level of full adherence was, overall, higher than that for statins (79.4 vs. 30.9%, respectively); however, it also revealed that 11.8% of patients taking evolocumab were only partially adherent and 8.8% were nonadherent [13]. Given this, it was also shown that treatment with evolocumab presents an initial therapy denial by insurance companies as high as 80% even when the US FDA-approved indications for therapy have been met by the patient [13]. Furthermore, it appears that the major barrier to compliance for these agents are due to cost and duration of action [14].
1.4 Inclisiran Molecule and Properties
Inclisiran sodium (inclisiran) is a short-chain, synthetic, small interfering ribonucleic acid (siRNA) that binds to PCSK9 messenger ribonucleic acid (mRNA) in hepatocytes, blocking PCSK9’s translation and production [15]. Promising data from the ORION-1 phase II study, which demonstrated LDL-C lowering of > 50% with additional sustained pharmacodynamic effect and an ideal safety profile, led to the development of inclisiran’s phase III ORION program [16, 17]. The sustained pharmacodynamic effect allows for dosing once every 6 months, providing benefits for patient convenience and compliance [10]. Confirmation of the efficacy of inclisiran in the long-term reduction of LDL-C, along with its tolerability, has been evaluated in the ORION-9, ORION-10, and ORION-11 studies (Fig. 1).
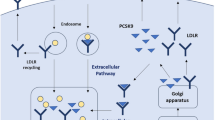
Mechanism of action of inclisiran (reproduced with permission from [36]). GalNAc N-acetylgalactosamine, LDL-C low-density lipoprotein cholesterol, mRNA messenger ribonucleic acid, PCSK9 proprotein convertase subtilisin/kexin type 9, RISC ribonucleic acid-induced silencing complex
2 Mechanism of Action
PCSK9 is a serine protease involved in the regulation of hepatic apolipoprotein B (ApoB) uptake and cholesterol metabolism via the LDLR. The LDLR pathway is the primary pathway in which plasma LDL-C is cleared from the circulation [15]. LDL-C binds to LDLRs, triggering endocytosis, whereby the LDL-C is degraded in the lysosome and the LDLR is recycled back to the cell membrane surface. PCSK9 interferes with this process by binding to the LDLR and mediating LDLR integration and degradation in the lysosome with LDL-C, preventing LDLR recycling to the cell membrane and subsequently decreasing LDL-C clearance [15].
Inclisiran’s unique mechanism inhibits the synthesis of PCSK9 within hepatocytes, as opposed to extracellular inhibition by the currently approved PCSK9i monoclonal antibodies. Inclisiran is a double-stranded siRNA that harnesses the intrinsic process of RNA interference and blocks hepatocyte production of PCSK9 [15, 18]. Inclisiran is composed of 21–23 nucleotide sequences that have been modified for durability and low immunogenicity and is highly distributed to the liver through N-acetylgalactosamine (GalNAc) conjugation [12]. Inclisiran binds specifically to the mRNA precursor of PCSK9, preventing its translation and the production of PCSK9 [15, 18]. The reduction of PCSK9 proteins promotes the recycling of LDLRs, increasing the uptake and degradation of plasma LDL-C and lowering plasma LDL-C levels (Tables 1, 2).
3 Clinical Evaluation of Inclisiran
3.1 Phase I Clinical Studies
A phase I study reported by Fitzgerald et al. [19] evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of inclisiran in healthy participants with elevated LDL-C in both a single-dose and a multiple-dose study. The primary outcome studied was the incidence of AEs and severe AEs (SAEs) in each group, and secondary outcomes included area under the plasma concentration–time curve (AUC), plasma concentration, and change from baseline in fasting plasma PCSK9 and serum LDL-C [19].
The single-dose study used varying doses of inclisiran (25–800 mg) and included participants aged 18–60 years who had an LDL-C of ≥ 100 mg/dL and fasting triglycerides <400 mg/dL. Participants were randomized 3:1 to either inclisiran or placebo for a total of six cohorts with four participants each [19]. Participants were excluded if they had a history of CVD, cerebrovascular disease, or diabetes mellitus; with the exception of those taking statins, such patients could be permitted if they had non-insulin-dependent diabetes or controlled hypertension.
All AEs were mild to moderate in severity, and none resulted in the discontinuation of study treatment. The most common AEs were cough, musculoskeletal pain, and nasopharyngitis, and these occurred more in participants in the inclisiran group than in those in the placebo group, with 11.1% (n = 2) reporting each of cough, musculoskeletal pain, and nasopharyngitis in the inclisiran group compared with no AEs in the placebo group. Inclisiran 800 mg had the highest incidence of treatment-emergent AEs (TEAEs): 66.7% compared with 33.3% for all other inclisiran doses [19].
In the single-dose phase, inclisiran ≥ 300 mg was associated with significant reductions in PCSK9 levels from baseline compared with placebo at day 84 (p < 0.001) [19]. The extent of PCSK9 reduction was similar across the dose range of 300–800 mg (mean change 69.9–74.5%), with the largest reduction of 74.5% occurring with inclisiran 300 mg. At day 180, PCSK9 levels returned to baseline values for participants who received inclisiran 25 and 100 mg. This was not observed for the cohorts that received a single dose of inclisiran ≥ 300 mg, where PCSK9 levels remained reduced at day 180 compared with baseline. LDL-C reductions from baseline were significant compared with placebo at day 84 for inclisiran ≥ 100 mg (p < 0.05), with mean LDL-C level reductions ranging from 36.7 to 50.6%. The largest reduction observed, 50.6%, occurred in the inclisiran 500 mg cohort, whereas the 800 mg cohort exhibited a reduction of 43.4%. Similar to the PCSK9 observations, LDL-C levels returned to baseline values for recipients of inclisiran 25 and 100 mg at day 180, whereas the levels remained reduced, as compared with baseline, for recipients of inclisiran ≥ 300 mg. Results from the single-dose study determined the most optimal fixed dose for a range of inclisiran and demonstrated that inclisiran exposure increased in a dose-dependent manner [19].
The multiple-dose study included four doses of inclisiran (125 mg weekly for 4 weeks, 250 mg every other week for 4 weeks, and 300 or 500 mg monthly for 2 months) and participants aged 18–75 years who met the same criteria as in the single-dose study described earlier [19]. The study consisted of eight cohorts, where participants were randomized into groups and then further grouped based on statin use status for the inclisiran 300 and 500 mg and placebo groups.
A total of 45 participants were included, all of whom completed the study and were included in the safety and full analyses. Safety analysis revealed that inclisiran was generally well-tolerated, and AEs were consistent amongst all groups. The most common AEs were headache (18%), back pain (5%), diarrhea (5%), and nasopharyngitis (12%) and were deemed mild to moderate in severity [19]. Inclisiran 300 mg monthly exhibited the lowest incidence of TEAEs compared with all other doses and among participants not receiving statin therapy; more TEAEs were reported for inclisiran 125 mg weekly and 250 mg biweekly (83.3% for both) than for all other nonstatin groups. Overall, no systematic differences in the proportions of participants reporting AEs were observed between treatment groups.
In the multiple-dose study, all inclisiran regimens demonstrated significant reductions from baseline in the PCSK9 level as compared with placebo at day 84 after administering the first dose (p < 0.001). The magnitude of PCSK9 lowering was similar across all inclisiran groups, with mean reduction ranging from 71.8 to 83.8%. Furthermore, PCSK9 levels remained reduced for all inclisiran cohorts when compared with baseline at day 196. At day 84, after receiving the first dose, LDL-C change from baseline was significant when compared with placebo (p < 0.05) for all inclisiran regimens except for the cohort receiving 125 mg weekly for 4 weeks (ranging from 45.1 to 59.7%). The largest LDL-C percentage reduction (59.7%) was produced with inclisiran 300 mg dosed monthly. In addition, reduced LDL-C levels persisted when compared with baseline for all inclisiran cohorts at day 196 [19].
Findings from both the single-dose and multiple-dose studies provide further clinical evidence that supports the ability of inclisiran to inhibit the synthesis of hepatic-derived target proteins [19].
The phase I study, ORION-7, assessed the pharmacokinetics, safety, and tolerability of inclisiran in participants with renal impairment [20, 21]. A total of 31 participants were randomized 1:1:1:1 according to their renal function (normal ≥ 90 mL/min, mild impairment 60–89 mL/min, moderate impairment 30–59 mL/min, severe impairment 15–29 mL/min) to receive a single subcutaneous dose of inclisiran 300 mg. Participants were observed until day 60 with an extended observation period at day 180. The primary pharmacokinetic endpoints included the relationship between the degree of renal impairment and maximum plasma drug concentration (Cmax) of inclisiran, AUC0–infinity, and plasma half-life. Percent change of LDL-C from baseline was evaluated at 4 and 48 h and on days 4, 7, 30, 60, 120, and 180.
It was observed that the AUC0–infinity for inclisiran increased with worsening renal function, with a mean AUC0–infinity of 7890 h·ng/mL for normal renal function compared with 18,800 h·ng/mL for severe renal impairment. Cmax also increased with worsening renal function, with a fourfold increase of inclisiran levels in severe renal impairment when compared with normal renal function [20, 21]. It should be noted that, at 48 h after drug administration, inclisiran was not detected in plasma in any of the groups. Mean PCSK9 levels were significantly reduced at day 60 in all groups (normal − 68.1%, mild − 74.2%, moderate − 79.8%, and severe − 67.9%), and this observed lowering was not statistically different in normal function compared with all other renal function groups (p = 0.24). LDL-C level reductions were also similar across all groups (p = 0.17), with an observed mean LDL-C lowering of − 57.6% for normal, − 35.1% for mild, − 53.1% for moderate, and − 49.2% for severe renal impairment at day 60. The reductions for PCSK9 and LDL-C persisted to the end of the study [20].
All participants completed the study and were included in the safety data analysis, with the most common AEs being consistent with results from other phase I studies: nasopharyngitis, headache, cough, nausea, and back pain [19, 21]. Inclisiran systemic clearance was reduced with progressed renal disease but, despite elevated plasma levels, did not show any novel AEs or result in study discontinuation in the renally impaired groups [21].
3.2 Phase II Clinical Studies
A dose-finding phase II study by Leiter et al., ORION-1, evaluated inclisiran (100–500 mg) compared with placebo in participants with elevated LDL-C despite LLT. A total of 501 participants with or without ASCVD were randomized to either inclisiran or matching placebo using two different dosing regimens (one dose vs. two dose: day 1 and day 90) [16].
At day 180, inclisiran 300 mg reduced the average baseline LDL-C of 131.3 mg/dL by 52.6% after two doses compared with an increase of 1.8% with placebo (95% confidence interval [CI] − 57.1 to − 48.1; p < 0.001), and this was consistently significant for all dosing regimens of inclisiran [16]. The two-dose inclisiran 300 mg regimen produced the greatest reduction in LDL-C, and 48% of participants within this group achieved an LDL-C level < 50 mg/dL. Furthermore, PCSK9 and LDL-C levels remained significantly lower than baseline at day 210 with all inclisiran regimens (p < 0.001). Inclisiran appeared to have a relatively benign AE profile, with an incidence of SAEs of 11% among participants who received inclisiran and 8% among participants who received placebo. Injection-site reactions were notable for only inclisiran and occurred in 5% of participants [16].
Thus, in this phase II study, inclisiran produced significant reductions in LDL-C and PCSK9 levels with an acceptable AE profile, as compared with placebo, and showcased a sustained effect of 6 months. However, given the relatively small number of participants and the short duration of the study, no definitive conclusions could be drawn regarding the long-term side effect profile of inclisiran.
To address these limitations, the extension study, ORION-3, assessed the long-term efficacy and tolerability of inclisiran compared with evolocumab [22]. Participants who previously received inclisiran in ORION-1 continued inclisiran treatment, and participants previously treated with placebo received 1 year of treatment with evolocumab followed by 3 years with inclisiran dosed every 6 months. Interim analysis results of ORION-3 revealed the primary endpoint was met and inclisiran demonstrated an LDL-C reduction of 51% through day 210 (p < 0.001) and a time-average absolute LDL-C reduction of 59.4 mg/dL (p < 0.001) [22, 23]. In addition, no change in the overall safety profile was observed. These results provide validation of the dose–pharmacodynamic response model observed in its parent study, ORION-1. Follow-up is ongoing, and completion is projected to occur in 2022. These anticipated results may provide the conclusive evidence needed to support the comparative efficacy of > 50% LDL-C lowering shown from both agents.
3.3 Phase III Clinical Studies
The global inclisiran phase III program, ORION, consists of four phase III pivotal studies and has enrolled 3660 participants with hypercholesterolemia as of October 2020. The pivotal ORION-9, -10, and -11 studies are complete, and the currently enrolling study, ORION-4, is anticipated to conclude in late 2024.
3.3.1 ORION-9
ORION-9, a randomized, double-blind, placebo-controlled study, evaluated the safety, tolerability, and efficacy of inclisiran when added to maximally tolerated statin therapy with or without ezetimibe in patients with heterozygous FH (HeFH) compared with placebo [24]. Participants who were included had an LDL-C ≥ 100 mg/dL and were on a stable dose of statin and/or ezetimibe therapy for at least 30 days. A total of 482 participants were randomized (1:1) to receive subcutaneous injections of inclisiran 300 mg or matching placebo, administered on day 1 and day 90 and then every 6 months. The median age was 56 years, and the mean baseline LDL-C level was 153 mg/dL. Among all participants, 90% were receiving statin therapy at baseline, of whom 75% were taking high-intensity statin therapy and > 50% were on ezetimibe. The coprimary endpoints of this study were percent change in LDL-C from baseline to day 510 and time-adjusted percent change from baseline LDL-C between day 90 and day 540.
At day 510, the mean percent change in LDL-C from baseline was superior for inclisiran (− 39.7% vs. + 8.2% for placebo), for a between-group difference of − 47.9% (95% CI − 53.5 to − 42.3; p < 0.001). Time-average percent change from baseline LDL-C between day 90 and day 540 was statistically significant, with a decrease of 38.1% for inclisiran and an increase of 6.2% for placebo (p < 0.001) [24]. In addition, inclisiran resulted in a superior reduction in LDL-C levels at day 510 (mean change − 59.0 mg/dL) compared with placebo (mean change + 9.9 mg/dL; p < 0.001). The LDL-C-lowering effect was exhibited at day 90 and day 540, with an observed LDL-C difference of − 56.9 mg/dL (time average) for inclisiran and + 5.8 mg/dL (time average) for placebo (p < 0.001). A subanalysis of HeFH genotype LDLR variants revealed consistent LDL-C reduction among all genotypes with inclisiran; within the LDLR pathogenic subset, an LDL-C change of − 58.4 mg/dL and 11.2 mg/dL was shown for inclisiran and placebo, respectively. Furthermore, participants randomized to inclisiran showed lower levels of total cholesterol (TC), ApoB, triglycerides, lipoprotein(a) [Lp(a)] and non-high-density lipoprotein (HDL-C) when compared with placebo. Specifically, inclisiran reduced Lp(a) levels by 13% compared with a baseline of 121 nmol/L [25]. The percentage of participants achieving their lipid targets was substantially higher in the inclisiran group, where 64% of participants with established ASCVD had an LDL-C level < 70 mg/dL at day 510 [24].
Regarding the side effect profile, the occurrence of AEs was slightly higher with inclisiran (77%) than with placebo (72%), but all AEs (nasopharyngitis, influenza, upper respiratory tract infection, back pain, and gastroenteritis and, most commonly, injection-site reactions) were mild to moderate in severity [24]. Three participants receiving inclisiran discontinued study treatment early because of AEs. The proportion of participants that experienced SAEs was similar between groups, with one death occurring in each group, neither of which were related to the study interventions.
The findings of ORION-9 support the addition of inclisiran as an effective adjunctive treatment strategy for patients with HeFH who do not reach or maintain LDL-C targets despite maximum tolerated LLT [24].
3.3.2 ORION-10
ORION-10, a 78-week phase III study, evaluated the safety and efficacy of inclisiran when added to standard-of-care statin therapy with or without ezetimibe [26]. This randomized, placebo-controlled, parallel-group study included 1561 participants with ASCVD, aged 56–76 years, and with elevated LDL-C despite receiving a maximum tolerated dose of LLT. Participants were randomized 1:1 to subcutaneous injections of inclisiran 300 mg or matching placebo administered on day 1 and day 90 and then subsequently every 6 months over a period of 540 days. Participants were included if their LDL-C levels were ≥ 70 mg/dL and excluded if they were treated with a PCSK9 monoclonal antibody within 90 days of screening. The study population mean LDL-C at baseline was 104.7 mg/dL; 89% of participants were receiving statin therapy and 10% were on ezetimibe. Among the participants treated with statin therapy, 68% were on high-intensity statin therapy (i.e., at least 20 mg rosuvastatin, 40 mg atorvastatin, or 40 mg simvastatin daily).
Participants receiving inclisiran achieved an LDL-C reduction of 51.3% at day 510 compared with + 1% for participants receiving placebo (p < 0.001). Additionally, inclisiran lowered LDL-C by 56.2 mg/dL compared with 2.1 mg/dL with placebo, with a between-group difference of − 54.1 mg/dL (95% CI − 57.4 to − 50.9; p < 0.001). Further, the time-adjusted percent change was highest for participants randomized to inclisiran, with a 51.3% reduction (p < 0.001) by day 540 compared with a 2.5% increase with placebo, demonstrating sustained LDL-C lowering with inclisiran [26]. By week 72, a higher proportion of participants on inclisiran achieved an LDL-C therapeutic target of a 50% reduction in LDL-C level, with 73 and 2.6% (odds ratio [OR] 67.1; 95% CI 41.8–107.6) for inclisiran and placebo, respectively. Inclisiran resulted in improvements in other key secondary endpoints at day 510, including lower levels of ApoB, TC, Lp(a), and non-HDL-C. At 72 weeks, inclisiran reduced the average baseline Lp(a) of 122 nmol/L by 22% [25]. The prespecified exploratory cardiovascular endpoint (incidence of cardiovascular death, any signs or symptoms of cardiac arrest, nonfatal myocardial infarction [MI], or stroke) showed a lower incidence for participants receiving inclisiran (7.4%) than for those receiving placebo (10.2%) (p < 0.001).
The occurrence of AEs, regardless of causality, was similar between participants receiving inclisiran and placebo, at 73.5 and 74.8%, respectively (p < 0.001) [26]. The most frequently reported AEs were injection-site reactions, which were more common with inclisiran but deemed as mild to moderate in severity. SAEs were similar between groups, with 12 deaths in the inclisiran group and 11 deaths in the placebo group, all deemed unrelated to study treatment. Overall, among participants with ASCVD on maximum tolerated LLT, twice-yearly inclisiran resulted in a significant reduction in LDL-C and was considered safe and well-tolerated [26].
3.3.3 ORION-11
The safety and efficacy of inclisiran in patients with ASCVD and LDL-C ≥ 70 mg/dL receiving standard-of-care therapy was evaluated in the ORION-11 study over 18 months. ORION-11 had a similar study design to ORION-10 but included participants with either CVD or risk-equivalent disease (type 2 diabetes, FH, or a 10-year risk of cardiovascular event of ≥ 20% as assessed by the Framingham Risk Score for CVD or equivalent) [26]. A total of 1617 participants were randomized 1:1 to receive either inclisiran 300 mg or matching placebo administered subcutaneously on day 1 and day 90 and then every 6 months for a total of four doses. Baseline characteristics were well-balanced, and diabetes was the most prominent of the CVD risk-equivalent factors. The use of stable statin treatment was high (94.7%), with 78.6% of participants receiving high-intensity statins.
From a mean baseline LDL-C of 105.5 mg/dL, participants treated with inclisiran experienced statistically significant reductions in LDL-C (− 45.8%) compared with an increase of 4% with placebo at the primary efficacy timepoint (day 510) (p < 0.001). In addition, inclisiran demonstrated superiority, with a time-averaged LDL-C reduction of 45.8% compared with an increase of 3.4% in the placebo group over the 18-month study (p < 0.001). Furthermore, inclisiran achieved a statistically significant greater reduction in LDL-C, with a difference of − 48.6 mg/dL (p < 0.001) at 3 months compared with placebo (+ 0.3 mg/dL). This trend continued after 510 days, with participants experiencing significantly greater reductions in LDL-C and a between-group difference of − 51.9 mg/dL (p < 0.0001) [26]. Similar to ORION-10, inclisiran showed improved levels of TC, non-HDL-C, Lp(a), and ApoB compared with placebo. Specifically, Lp(a) levels were lowered by 19% from the average baseline of 107 nmol/L [25]. The prespecified exploratory cardiovascular endpoint (including cardiovascular death, signs or symptoms of cardiac arrest, MI, or stroke) was lower in those receiving inclisiran (7.8%) than in those receiving placebo (10.3%).
Injection-site reactions were more common with inclisiran (4.7 vs. 0.5% placebo; p < 0.001) but were considered mild to moderate in severity. Premature discontinuation of treatment occurred in 36 participants because of AEs, and the number of participants was slightly higher with inclisiran than with placebo (19 and 17, respectively). SAEs were similar between groups and occurred in 181 patients for both groups (relative risk 1.0; 95% CI 0.8–1.2) [26]. These included death from cardiovascular causes and cancer-related deaths, with 14 deaths recorded in the inclisiran group and 15 deaths in the placebo group; all deaths were deemed unrelated to study intervention.
In conclusion, inclisiran was superior at reducing LDL-C when compared with placebo and exhibited a sustained LDL-C lowering effect over 540 days. In addition, the safety profile for inclisiran in the ORION-11 study was consistent with that in previous studies. These findings support the addition of inclisiran as an effective LLT for patients with CVD or at elevated risk for ASCVD who do not achieve or maintain LDL-C goals with maximally tolerated LLT [26].
4 Discussion
Medications targeting PCSK9 have emerged as a promising therapeutic strategy for the treatment of hyperlipidemia. By blocking the expression of PCSK9, significant reductions in plasma LDL-C levels have been obtained that may lead to a reduction of cardiovascular risk [8, 21, 27]. In all three phase III ORION studies (i.e., ORION-9, -10, and -11), inclisiran reduced plasma PCSK9 levels by approximately 80% without any indication that this reduction attenuated over the duration of the studies [24, 26]. Thus, the observed durability enables an infrequent dosing regimen (i.e., twice yearly), which may contribute to higher adherence.
The phase III pivotal studies (ORION-9, -10, and -11) discussed have demonstrated inclisiran’s significant LDL-C-lowering effects in patient populations with very high cardiovascular risk, specifically patients with ASCVD and HeFH, contributing to inclisiran’s recent December 2020 approval for use in the EU. It should be acknowledged that the phase III ORION-5 study is ongoing and is evaluating inclisiran’s effects on LDL-C in participants with homozygous FH (HoFH). As of January 2021, a total of 56 participants with HoFH were actively enrolled and randomized (1:1) to receive two doses (on day 1 and day 90) of either inclisiran 300 mg or matching placebo for a duration of 150 days [28]. ORION-5 is anticipated to complete in mid-to-late 2021.
As noted, two monoclonal antibody PCSK9i (evolocumab and alirocumab) have already scored in large cardiovascular outcome studies and have been shown to lower cardiovascular risk significantly [10, 11]. Inclisiran displayed comparable LDL-C lowering (> 50%) to its PCSK9-targeting counterparts evolocumab and alirocumab; however, whether inclisiran can improve cardiovascular outcomes in patients with CVD is not known [10, 24, 26]. This is currently being evaluated in the ORION-4 study, a long-term cardiovascular outcomes study that will include approximately 15,000 participants aged ≥ 55 years with established ASCVD and elevated TC (> 155 mg/dL) [29]. This study’s primary objective is the time to occurrence of MACE, defined as coronary heart disease death, MI, fatal or nonfatal ischemic stroke, or urgent coronary revascularization. The results generated from this study will provide essential evidence to determine whether the marked LDL-C reductions attained with inclisiran are translatable into a reduction of cardiovascular risk and MACE. It may also bring clarity to help resolve clinical inertia if the use of intensive LDL-C-lowering strategies in patients with CVD near their therapeutic target is found to provide further benefit versus a more conservative lipid-lowering approach.
Furthermore, as more attention is directed towards understanding the role of Lp(a) in cardiovascular risk, results from the phase III pivotal ORION studies and the FOURIER study have provided insights on this undefined relationship. FOURIER revealed that evolocumab lowered Lp(a) levels by 27% from baseline and reduced the risk of coronary artery disease (CAD) death, MI, or urgent revascularization by 23% in patients with a baseline Lp(a) > 37 nmol/L (median baseline 37 nmol/L) [24, 26, 30]. When compared with evolocumab, inclisiran demonstrated a similar Lp(a) lowering of 26% from baseline; however, it is important to note that the median baseline levels of Lp(a) were greater in ORION-9, -10, -11 (57, 57, and 47 nmol/L, respectively) [24,25,26].
Although assumptions can be made about the potential benefits of inclisiran, its long-term safety remains ill defined. To further evaluate the long-term effects and safety of inclisiran, ORION-8, an extension study, is currently ongoing and will include up to 3700 participants from ORION-5, -9, -10, and -11 for a duration of 4 years [31, 32]. The primary endpoints of this study are the proportion of participants who attain LDL-C targets of < 70 mg/dL for ASCVD and < 100 mg/dL for ASCVD risk-equivalent disease. The significant LDL-C reductions produced by inclisiran are substantial and similar to those of high-intensity statins (> 50% LDL-C reduction), and combining these therapies should contribute to a substantial increase in the percentage of patients achieving their recommended LDL-C goals [6, 17, 27]. These data should provide the necessary information to conclude the clinical utility of inclisiran in patients at high cardiovascular risk. In addition, the extended duration of this study may provide insight into whether extreme lowering of LDL is better and whether obtaining considerably lower LDL-C levels (< 30 mg/dL) for a prolonged duration provides any further benefit or, conversely, incurs any unfavorable events.
The collective clinical data demonstrated from the phase III pivotal studies contributed to the EU’s December 2020 approval of inclisiran under the brand Legvio® as a treatment for hypercholesterolemia. In the EU, inclisiran is labeled for use as adjunctive therapy to maximally tolerated statins in patients at high cardiovascular risk or established ASCVD [25]. US approval is currently stalled because of plant inspection issues but, given the available clinical data, the US label is likely to be similar to the EU label [33].
While inclisiran’s price is not yet known, a recent incremental cost-effectiveness ratio (ICER) report recommended that the health-benefit price range for inclisiran for the population of likely eligible patients be $US3600–6000 per year to be cost effective [34, 35]. This benchmark price range falls in line with the current list prices of the existing PCKS9 agents of approximately $US5400–5850 for evolocumab and alirocumab [34]. With a similar price range to the current PCKS9 agents, one can speculate payer pushback and slow uptake; however, inclisiran’s twice-yearly dosing can be viewed as an advantage over the biweekly or monthly dosing for current PCSK9, which could increase adherence and treatment compliance.
In summary, the recent publication of three RCTs investigating inclisiran reaffirm the efficacy of this compound in reducing LDL-C and show that its long therapeutic half-life comes with a favorable AE profile. The data strengthen the conviction that inclisiran addresses two critical unmet needs (additional LDL-C lowering and poor adherence) that must be resolved to get many more patients to goal.
5 Conclusion
Inclisiran has demonstrated substantial efficacy and safety when compared with placebo as an LDL-C-lowering agent [24, 26]. Inclisiran is the first siRNA being evaluated for dyslipidemia and has the potential to be used for the primary and secondary prevention of CVD. The main benefit of inclisiran, compared with PCSK9i monoclonal antibodies, is the less frequent dosing. Inclisiran has the potential to improve the ability of patients with hypercholesterolemia to reach and/or maintain their LDL-C goals when used in addition to maximally tolerated doses of LLTs. Finally, the highly targeted PCSK9i technology of inclisiran is expected to produce additive benefit for these high-risk populations (ASCVD and FH) for the prevention of MACE [15, 24].
References
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Am Coll Cardiol. 2019;74:177–232.
Ogura MMP. PCSK9 inhibition in the management of familial hypercholesterolemia. J Cardiol. 2018;71:1–7.
Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol. 2018;15:757–69.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–8.
Collaboration CTT. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Perrerault S, Dragomir A, Blais L, et al. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Pharmacoepidemiolo Prescr. 2009;65:1013–24.
Wang N, Fulcher J, Abeysuriya N, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327,037 participants. Lancet Diabetes Endocrinol. 2020;8(1):36–49.
Lin XL, Xiao L, Tang ZH, Jiang ZS, Liu MH. Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed Pharmacother. 2018;104:36–44.
Singh S, Bittner V. Familial hypercholesterolemia—epidemiology, diagnosis, and screening. Curr Atheroscler Rep. 2015;17:1–8.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Gragnano F, Concilio C, Cesaro A, et al. P1513 Adherence to PCSK9 inhibitors in high cardiovascular risk patients in real-world setting: results from a single-center experience and comparison with statin therapy. Eur Heart J. 2017;38(1):314.
Kosmas CE, Silverio D, Ovalle J, Montan PD, Guzman E. Patient adherence, compliance, and perspectives on evolocumab for the management of resistant hypercholesterolemia. Patient Prefer Adherence. 2018;2018(12):2263–6.
Thompson G. Limitations of cholesterol lowering with PCSK9 inhibitors. Lancet Diabetes Endocrinol. 2017;5(4):241–3.
Dyrbus KM, Gasior MM, Penson PM, Ray KKM, Banach MM. Inclisiran—new hope in the management of lipid disorders? J Clin Lipidol. 2020;14:16–27.
Leiter LA, Teoh H, Kallend D, et al. Inclisiran lowers LDL-C and PCSK9 irrespective of diabetes status: the ORION-1 randomized clinical trial. Diabetes Care. 2019;42:173–6.
Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. 2019;4(11):1067–75.
Cupido AJ, Kastelein JJP. Inclisiran for the treatment of hypercholesterolaemia: implications and unanswered questions from the ORION trials. Eur Soc Cardiol. 2020;116:136–9.
Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51.
A Study of Inclisiran in Participants With Renal Impairment Compared to Participants With Normal Renal Function (ORION-7). ClinicalTrials.gov. March 24, 2018. https://clinicaltrials.gov/ct2/show/study/NCT03159416?intr=inclisiran&draw=3&rank=1.
Wright RS, Collins MG, Stoekenbroek RM, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clinc Proc. 2020;95:77–89.
An Extension Trial of Inclisiran Compared to Evolocumab in Participants With Cardiovascular Disease and High Cholesterol (ORION-3). NIH U.S National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03060577. Accessed Oct 2020.
New Long-Term Data Show that Twice-a-Year Dosing with Inclisiran Results in Persistent Lowering of LDL Cholesterol with No Material Safety Observations Out to Three Years. The Medicines Company. May 18, 2019. https://www.themedicinescompany.com/investor/pr/3820269/. Accessed 4 Nov 2020.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30.
European Medicines Agency. Human medicine European public assessment report (EPAR): Leqvio. September 12, 2020. http://www.ema.europa.eu. Accessed 21 Mar 2021.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–19.
Stock J. Targeting LDL cholesterol: early treatment is key to population health. Eur Atheroscler Soc. 2020;300:37–8.
A Study of Inclisiran in Participants With Homozygous Familial Hypercholesterolemia (HoFH) (ORION-5). NIH U.S National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03851705. Accessed Oct 2020.
A randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease (ORION-4). NIH U.S National Library of Medicine ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03705234. Accessed Oct 2020.
O’Donoghue ML, Fazio S, Guigliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Insights from the FOURIER Trial. Circulation. 2018;139(12):1483–92.
Trial to assess the effect of long term dosing of inclisiran in subjects with high CV risk and elevated LDL-C (ORION-8). NIH U.S National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03814187. Accessed Oct 2020.
The Medicine Company. August 2019. https://www.themedicinescompany.com/media/2019MediaKit_FactSheet_ORION-8_82119.pdf. Accessed 29 Oct 2020.
Liu A. Novartis set to answer inclisiran's FDA rebuff by Q3. But what about an approval timeline? Fierce Pharma. January 26, 2021. https://www.fiercepharma.com. Accessed 21 Mar 2021.
Lui A. Inclisiran now has its cost-effectiveness report from ICER. Should Novartis be concerned? Fierce Pharma. January 29, 2021. https://fiercepharma.com. Accessed 21 Mar 2021.
Lin G, Kazi D, Jih J, Agboola F, Chapman R, Pearson S. Inclisiran and bempedoic acid for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value; evidence report: Institute for Clinical and Economic Review; 2021
Sinning D, Landmesser U. Low-density lipoprotein-cholesterol lowering strategies for prevention of athersclerotic cardiovascular disease: focus on siRNA treatment targeting PCSK9 (Inclisiran). Curr Cardiol Rep. 2020;22(12):1–7.
A study of ALN-PCSSC in participants with homozygous familial hypercholesterolemia (HoFH) - Study results - ClinicalTrials.gov. clinicaltrials.gov.. https://clinicaltrials.gov/ct2/show/results/NCT02963311?term=Inclisiran&draw=2&rank=18. Accessed 23 Apr 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Jennifer Hardy, PharmD; Stephanie Niman, PharmD; Edward Pereira, MD; Todd Lewis, MD; Jessica Reid, PharmD; Rushab Choksi, PharmD; and Rebecca F. Goldfaden, PharmD, have no conflicts of interest that might be relevant to this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors' contributions
All authors contributed to the review. Rebecca Goldfaden had the idea for the article, Stephanie Niman and Jessica Reid performed the literature search and data analysis, and all authors drafted and/or critically revised the review. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Hardy, J., Niman, S., Pereira, E. et al. A Critical Review of the Efficacy and Safety of Inclisiran. Am J Cardiovasc Drugs 21, 629–642 (2021). https://doi.org/10.1007/s40256-021-00477-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00477-7