Abstract
Hypercholesterolemia is a leading cause of cardiovascular disease and mortality in men and women throughout the USA and abroad. The development of statins (HMG-CoA reductase inhibitors) to lower plasma atherogenic cholesterol levels and improve cardiovascular outcomes represents one of the greatest contributions to clinical science in the twentieth century, although residual risk remains even among statin-treated patients. Our understanding of lipid metabolism took a giant leap forward in 2003 with the discovery of proprotein convertase subtilsin/kexin type 9 (PCSK9), a low abundance circulating protein with an outsized effect on the regulation of plasma cholesterol levels. Evolocumab and alirocumab represent the first two US Food and Drug Administration-approved fully human monoclonal antibodies that target PCSK9, which not only lower low-density lipoprotein (LDL) cholesterol to unprecedented levels, but also further improve important cardiovascular outcomes. Small interfering RNA (siRNA) molecules now represent the next generation of drugs designed to antagonize PCSK9. Inclisiran is a siRNA specific for PCSK9 that prevents translation of PCSK9 messenger RNA, leading to decreased concentrations of the protein and lower concentrations of LDL cholesterol. The ORION clinical development program includes several completed and ongoing clinical trials designed to evaluate the safety and efficacy of inclisiran and test its ability to improve hard cardiovascular outcomes. This review discusses the mechanisms of action of inclisiran, examines the current evidence, and provides a comparison of similarities and differences relative to the PCSK9 inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The discovery of proprotein convertase subtilsin/kexin type 9 (PCSK9) and its ensuing development as a target of therapy has revolutionized the management of hypercholesterolemia. |
The therapeutic monoclonal antibodies to PCSK9 improve cardiovascular outcomes. |
Inclisiran is the first small interfering RNA molecule currently in clinical trials developed to help lower cholesterol by preventing production of PCSK9, with preliminary results showing improvements in important cardiovascular outcomes. |
1 Introduction
Hyperlipidemia is a public health epidemic, affecting almost 93 million US adults over age 20 years, and is a leading risk factor for the development of atherosclerotic cardiovascular disease (ASCVD) (coronary heart disease, cerebrovascular disease, or peripheral artery disease) [1]. In addition to the detrimental effects of hyperlipidemia on cardiovascular health, it also poses a substantial burden on life costs, accounting for an estimated 88.7 million disability-adjusted life-years [2]. Though there has been a decrease in the prevalence of hypercholesterolemia over the last decade, this likely reflects greater uptake of lipid-lowering medications rather than improvements in dietary patterns [3]. Given the difficulty in maintaining healthy lifestyle habits over time, lipid-lowering therapies are often necessary. Statins (HMG-CoA reductase inhibitors) remain the gold standard in lowering cholesterol and reducing ASCVD, though adherence is poor with discontinuation rates of approximately 50% after 1 year and 70% at 2 years [4, 5]. While there are many barriers to adherence, including cost, poor access to care, and concerns regarding polypharmacy, statin-associated muscle symptoms are responsible for discontinuation in a substantial number of patients that would otherwise greatly benefit from this therapy [6].
The seminal discovery of proprotein convertase subtilsin/kexin type 9 (PCSK9) in 2003 revolutionized our understanding of lipoprotein metabolism [7]. As it turns out, PCSK9 interferes with the natural recycling of the low-density lipoprotein (LDL) receptor by directing the protein towards the lysosome for degradation. Gain-of-function mutations in PCSK9 lead to severe hypercholesterolemia and represent the third locus for familial hypercholesterolemia (FH).
Given that loss-of-function mutations are far more common than gain-of-function mutations, Hobbs and colleagues [8] evaluated the impact of loss-of-function mutations in PCSK9 on the incidence of coronary heart disease in the ARIC (Atherosclerosis Risk in Communities) cohort. They identified 86 African American and 301 Caucasian participants with loss-of-function mutations in PCSK9, which were associated with modest reductions in LDL cholesterol (LDL-C) but larger than expected reductions in ASCVD (likely due to lifelong exposure to lower LDL-C). Based on these findings, the PCSK9 saga culminated in the rapid development of a new class of LDL-C-lowering drugs, known as PCSK9 inhibitors (PCSK9i). The two US Food and Drug Administration (FDA)-approved PCSK9i are fully humanized monoclonal antibodies (mAbs) that bind to PCSK9 in the circulation, effectively blocking its action. Binding of the therapeutic mAb to PCSK9 prevents the binding of PCSK9 to the LDL receptor (LDLR), thus enhancing its natural recycling loop (Fig. 1) [9]. This process ultimately leads to accelerated clearance of LDL particles, decreased plasma LDL-C [10], and even reductions in atheroma volume [11]. Both drugs lower LDL-C by ~ 50 to 60% and reduce major adverse cardiovascular events (MACE) by 15% in patients with stable vascular disease and recent acute coronary syndromes [12, 13]. Furthermore, given the swift accrual of events in a relatively short amount of time, one might expect even larger reductions in event rates had these trials continued with longer durations of follow-up [14].
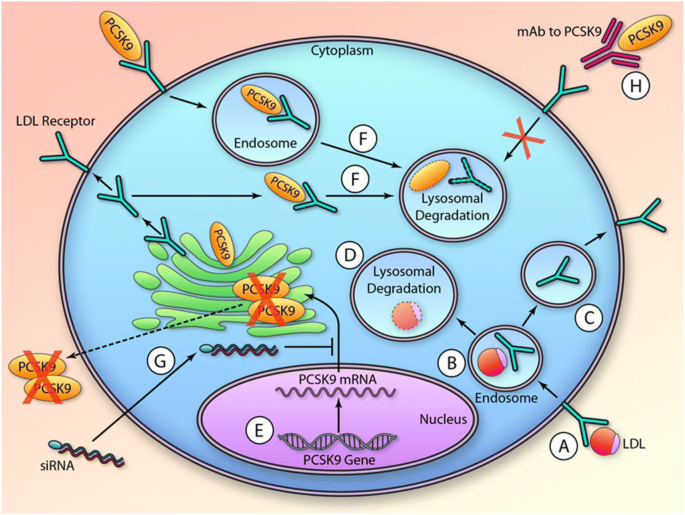
Mechanisms of action for proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and inclisiran (figure reproduced from Shapiro et al. [48], with permission from Circulation Research; illustration credit: Ben Smith). a The low-density lipoprotein (LDL) receptor (LDLR) binds LDL at the cell surface, and this complex is internalized within an endosome. As the endosome matures and the pH drops, the LDLR and LDL decouple (b). The LDLR recycles to the cell surface (c), whereas LDL ends up in the lysosome where it is fully catabolized (d). Transcription of the PCSK9 gene (e) is followed by transport of its messenger RNA (mRNA) to the cytoplasm where it is translated to protein. PCSK9 is secreted into the plasma compartment or can reside intracellularly. In either case, when PCSK9 binds to the LDLR, it is targeted for lysosomal degradation (f). Administration of silencing ribonucleic acid (siRNA) prevents translation of PCSK9 protein (g). Administration of monoclonal antibodies (mAbs) blocks PCSK9 binding to the LDLR on the cell surface (h). These therapeutic approaches seem to have similar LDL-C-lowering efficacy, as they both impede the biological effect of PCSK9 and cause unchecked LDLR recycling
2 The Proprotein Convertase Subtilsin/Kexin Type 9 (PCSK9) Revolution
While it had been established that mutations in the LDLR and APOB can lead to autosomal dominant hypercholesterolemia (ADH), Abifadel et al. [7] discovered a third locus for FH. They identified a French family with ADH and were able to demonstrate that a gain-of-function mutation in PCSK9 was the root cause, providing the first clue that PCSK9 plays an important role in lipid metabolism. Shortly thereafter, other scientists reported cases of FH inherited in a similar manner, bolstering the evidence that mutations in PCSK9 can lead to changes in cholesterol levels [15,16,17,18]. However, not all mutations led to high levels of cholesterol. Loss-of-function mutations were also identified, leading to lifelong reductions in total cholesterol and LDL-C [14, 19, 20]. This link was further explored in the ARIC study, which demonstrated that nonsense mutations in PCSK9 not only lead to decreases in LDL-C, but also robust reductions in the incidence of coronary heart disease (composite of myocardial infarction [MI], fatal coronary heart disease, or coronary revascularization) in both African Americans and Caucasians (88% and 47%, respectively) [8].
Although individuals with loss-of-function mutations seemed to have substantial protection against cardiovascular disease, there was concern that low LDL-C levels might be associated with adverse outcomes. However, even individuals found to be homozygous for loss-of-function mutations in PCSK9 with dramatically lower LDL-C (~ 15 mg/dL) enjoy normal health and reproductive capacity without evidence of neurological or cognitive dysfunction [21, 22]. These ‘human knockout’ observations with low exposure to LDL-C throughout their lifetime provided reassurance that PCSK9i would not only dramatically lower LDL-C but were likely to be safe in the long term.
3 Clinical Trials of Evolocumab and Alirocumab
The FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) and ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome with Alirocumab) trials were the first large-scale randomized cardiovascular outcomes trials with the two therapeutic mAbs evolocumab and alirocumab, respectively [12, 13]. Importantly, these drugs were approved by the US FDA on the basis of their LDL-C-lowering efficacy before the complete results of these trials were available.
FOURIER randomized individuals with established ASCVD on optimized statin therapy with LDL-C > 70 mg/dL to either evolocumab or placebo to determine if the addition of evolocumab to optimal medical therapy improved cardiovascular outcomes. The primary outcome consisted of a 5-point composite of MACE, defined as cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. The addition of evolocumab was associated with a 59% reduction in LDL at 48 weeks compared with placebo and resulted in a 1.5% absolute risk reduction in the primary outcome, driven largely by reductions in non-fatal MI, stroke, and revascularization. No overall or cardiovascular-specific mortality difference was observed between the two study arms at the time of study termination (median 2.2 years). Also of note, despite reaching a median LDL-C of 30 mg/dL in the evolocumab group, there were no key differences in the incidence of major adverse events, including new-onset diabetes mellitus or neurocognitive effects.
The ODYSSEY Outcomes trial enrolled subjects on high-intensity or maximally tolerated statin therapy with residual elevations in LDL-C ≥ 70 mg/dL with recent acute coronary syndrome (1–12 months prior to enrollment) and were randomized to alirocumab versus placebo [12]. Trial participants were followed for a median of 2.8 years to assess the primary 4-point composite MACE endpoint (death from coronary heart disease, non-fatal MI, fatal or non-fatal ischemic stroke, or unstable angina requiring hospitalization). At 48 weeks, the addition of alirocumab resulted in a reduction of LDL-C from the mean baseline of 92 mg/dL to 66 and 53 mg/dL in the intention-to-treat and on-treatment analyses, respectively.
This magnitude of LDL-C reduction resulted in an absolute risk reduction of 1.6% in the primary endpoint, with the greatest benefit noted in individuals with a baseline LDL-C > 100 mg/dL. Importantly, this trial demonstrated an all-cause mortality benefit in the alirocumab arm, though its interpretation is controversial as there was no significant difference in cardiovascular mortality. Other than a slightly higher incidence of injection-site reactions, the rates of major adverse events, including new-onset diabetes and neurocognitive effects, were similar between the two groups.
Several other large multicenter trials led by the SPIRE (Studies of PCSK9 Inhibition and Reduction of Vascular Events) investigators were conducted to evaluate the efficacy of bococizumab in reducing cardiovascular events in patients at high risk [23]. Though bococizumab is also a mAb targeted to PCSK9, it is not fully human, but rather humanized (retaining ~ 3% murine protein) [23]. While there was a significant reduction in LDL-C levels and reduction in MACE in those at highest risk, Pfizer ultimately discontinued development of bococizumab in November 2016. Unfortunately, administration of bococizumab was associated with the development of neutralizing antibodies in a significant number of trial participants, which led to attenuation in the LDL-C reduction over time and a higher rate of injection-site reactions [24].
4 Small Interfering RNA (siRNA)
Whereas therapeutic mAbs target plasma PCSK9, small interfering RNAs (siRNA) are ~ 20–30 nucleotide RNA molecules that prevent intracellular translation of PCSK9 messenger RNA (mRNA) to protein [25]. More specifically, these molecules selectively and catalytically silence translation of their complementary target mRNA in a sequence-specific manner through the formation of effector RNA-inducing silencing complexes (RISC) [26, 27]. While siRNAs act on the same pathway as the mAbs to reduce LDL-C, there are several important differences (Table 1).
5 Inclisiran
Inclisiran represents the first siRNA tested as a therapeutic class across the spectrum of medicine to undergo extensive testing in clinical trials to evaluate its ability to lower LDL-C and prevent cardiovascular events. It is a long-acting, synthetic siRNA directed against PCSK9 mRNA, conjugated to triantennary N-acetylgalactosamine carbohydrates (GalNAc) which bind liver expressed asialoglycoprotein receptors with high affinity. This formulation leads to efficient and targeted uptake of inclisiran by hepatocytes [28, 29]. Since the GalNAc platform allows precise targeting of the drug to the organ of interest, lower doses can be used to achieve the desired therapeutic effect, thereby decreasing the probability of adverse events that may be expected with higher doses.
5.1 Published Clinical Trial Data
Fitzgerald et al. [30] conducted a randomized, single-blind, placebo-controlled, phase I dose-escalation trial in healthy adults with LDL-C ≥ 3 mm/L (116 mg/dL). Subjects were randomized in a 3:1 fashion to receive one dose of ALN-PCSsc (an inclisiran precursor molecule), with doses ranging from 0.015 to 0.400 mg/kg versus placebo. Participants in the highest dose group experienced a mean reduction in LDL-C and PCSK9 by 40% and 70%, respectively (p < 0.001 for both comparisons), with no difference in adverse events between the inclisiran and placebo groups [30].
Three years later, Fitzgerald and colleagues [31] published another phase I clinical trial to assess the safety, adverse effect profile, and pharmacodynamic effects of ALN-PCSsc administered subcutaneously (SC) in single or multiple doses in healthy volunteers with an LDL-C of ≥ 100 mg/dL and in a small number of patients taking a stable dose of a statin. Volunteers were randomized in a 3:1 fashion to receive SC inclisiran or placebo in either a single ascending dose phase (25, 100, 300, 500, or 800 mg) or a multi-dose phase (125 mg weekly for four doses, 250 mg every other week for two doses, or 300 or 500 mg monthly for two doses, with or without concurrent statin therapy). There were no serious adverse events. In general, adverse events were mild or moderate in severity, including cough, musculoskeletal pain, nasopharyngitis, headache, back pain, and diarrhea. Doses of ≥ 300 mg reduced PCSK9 levels up to a least-squares mean reduction of 74.5% from baseline in the single-dose phase, and doses of ≥ 100 mg reduced LDL-C levels up to a least-squares mean reduction of 50.6% from baseline. Reductions in LDL-C and PCSK9 were maintained at day 180 for doses of ≥ 300 mg. PCSK9 levels were reduced by a least-squares mean of 83.8% from baseline and LDL-C was reduced by a least-squares mean of 59.7% from baseline in all multi-dose regimens. The efficacy of LDL-C lowering was similar in patients that received the 300 and 500 mg dose.
In a follow-up to this study, Ray et al. [32, 33] conducted the ORION-1 trial, a phase II multicenter, double-blind, placebo-controlled, multiple ascending dose trial of inclisiran in high-risk patients with LDL-C > 70 mg/dL in the presence of cardiovascular disease or LDL-C > 100 mg/dL in the absence of cardiovascular disease. Patients were randomized to receive a single dose of placebo or 200, 300, or 500 mg of inclisiran or two doses of placebo or 100, 200, or 300 mg of inclisiran at days 1 and 90. The primary endpoint was change from baseline LDL-C at 180 days. At enrollment, 73% and 35.5% of patients were on statin and ezetimibe therapy, respectively. The least-squares mean reductions in LDL levels were 27.9–41.9% in patients receiving a single dose of inclisiran and 35.5–52.6% in the patients receiving two doses (p < 0.001 for all comparisons) at day 180. Participants on the two-dose 300 mg regimen achieved the greatest reductions in LDL-C, with 48% of participants attaining an LDL-C < 50 mg/dL. Significant reduction in PCSK9 (69.1%) and high-sensitivity C-reactive protein (16.7%) were observed as well. Both LDL-C and PCSK9 levels remained significantly lower than baseline at day 240 regardless of dosing regimen. Adverse events included myalgia, headache, fatigue, nasopharyngitis, back pain, hypertension, diarrhea, and dizziness, though the incidences of these events did not differ significantly between the inclisiran and placebo groups. Serious adverse events occurred in 11% of participants receiving inclisiran and 8% of participants receiving placebo. As expected, injection-site reactions were more common among those that received two doses of inclisiran.
5.2 ORION Clinical Development Program
The ORION clinical development program includes several phase I, II, and III trials designed to evaluate the effects of inclisiran on plasma PCSK9 concentrations, LDL-C, and cardiovascular outcomes. These studies are in various stages of completion, with results from the ORION-11 trial recently presented at the 2019 European Society of Cardiology meeting [34]. A high-level summary of these trials is presented in Table 2.
5.2.1 Pivotal Trials: ORION-4, -5, -9, -10, and -11
ORION-4 is a phase III, double-blinded, randomized controlled trial assessing the effects of inclisiran on MACE among subjects with known ASCVD. This study will be conducted at approximately 150 sites across the UK and USA and will enroll approximately 15,000 subjects at least 55 years of age. Study participants will be randomized to inclisiran 300 mg SC at day 1, 3 months, and then every 6 months thereafter, versus placebo dosed in a similar fashion. Inclusion criteria will include history of MI, ischemic stroke, or peripheral artery disease. The primary composite endpoint will be the number of participants with MACE, defined as time to coronary heart disease death, MI, fatal or non-fatal ischemic stroke, or urgent coronary revascularization [35].
ORION-5 is a phase III, two-part, double-blind, placebo-controlled, randomized trial to evaluate the safety, tolerability, and efficacy of inclisiran in subjects with homozygous FH (HoFH). This study will have two sequential parts: part 1 will be a 6-month double-blinded period in which subjects will be randomized to receive inclisiran 300 mg SC or placebo at day 1 and day 90; part 2 will be an 18-month open-label follow-up period in which placebo-treated patients from part 1 will be transitioned to inclisiran 300 mg SC at day 180; and all subjects will participate in an open-label follow-up period of inclisiran only with repeated doses as days 270, 450, and 630. Inclusion criteria will include a genetic diagnosis of HoFH, clinical diagnosis of HoFH based on a history of untreated LDL-C > 500 mg/dL together with either xanthoma before age 10 years or evidence of heterozygous FH (HeFH) in both parents. Additionally, subjects must be on maximally tolerated statins or other lipid-lowering therapy with a fasting LDL-C ≥ 130 mg/dL, triglycerides < 400 mg/dL, and not on dialysis or planned for renal transplantation. The primary outcome measure will be percentage change in LDL-C from baseline to day 150 [36].
ORION-9 is a phase III, placebo-controlled, double-blind, multicenter randomized trial to evaluate the effect of inclisiran on LDL-C in subjects with HeFH. Subjects will be randomized to inclisiran 300 mg SC or placebo administered on days 1 and 90, and then every 6 months. Inclusion criteria will include male or female participants ≥ 18 years of age, history of HeFH with a genetic diagnosis and/or documented history of untreated LDL-C > 190 mg/dL, and a family history of FH, hypercholesterolemia, or premature ASCVD that may include FH, LDL-C ??100 mg/dL, or fasting triglycerides <?400 mg/dL, who are on maximally tolerated statin therapy. Additionally, if not on a statin, participants must have documented intolerance to all doses of at least two different statins. The primary outcome measures will include percentage change in LDL-C from baseline to day 510 and time-adjusted change in LDL-C from baseline after day 90 and up to day 540. This study will take place at multiple sites around the world [37]. Preliminary results from ORION-9 presented at the 2019 American Heart Association Scientific Sessions showed sustained LDL-C reductions of approximately 50% at day 510 in this population.
ORION-10 is a phase III, placebo-controlled, double-blinded, multicenter randomized trial in participants with ASCVD and elevated LDL-C despite maximally tolerated doses of LDL-C-lowering therapies to evaluate the safety, tolerability, and efficacy of SC inclisiran. Participants will receive either inclisiran 300 mg SC or placebo on days 1 and 90, and then every 6 months. Inclusion criteria will include participants who are ≥ 18 years of age with a history of ASCVD, serum LDL-C ≥ 70 mg/dL, fasting triglycerides < 400 mg/dL at screening, and on stable lipid-lowering therapy, including a maximally tolerated statin (if on a statin), of at least 30 days. If not on a statin, participants must have documented intolerance to all doses of at least two different statins. The primary outcome measures will be the same as ORION-9. This study will take place at sites in the USA only [38]. Notably, preliminary results from ORION-10 presented at the 2019 American Heart Association Scientific Sessions showed sustained LDL-C reductions of approximately 58% at day 510 in this population.
ORION-11 was a phase III, placebo-controlled, double-blind, multicenter randomized trial in participants with ASCVD or an ASCVD risk equivalent and elevated LDL-C despite maximum tolerated doses of LDL-lowering therapies to evaluate the safety, tolerability, and efficacy of SC inclisiran. Participants received either inclisiran 300 mg SC or placebo on days 1 and 90, and then every 6 months. Inclusion criteria were the same as ORION-10, and primary outcome measures were the same as ORION-9. This study took place at sites outside of the USA, primarily in Europe [39]. Results from ORION-11 were presented at the 2019 European Society of Cardiology meeting, demonstrating a significant and sustained reduction in LDL-C of 54% after 17 months. Additionally, the rate of adverse events was similar between the placebo and intervention arm, and an exploratory cardiovascular endpoint (cardiac death, any signs or symptoms of cardiac arrest, non-fatal MI or stroke) occurred in 7.8% of patients treated with inclisiran versus 10.3% of patients taking placebo [40].
5.2.2 Extension Trials: ORION-3 and -8
ORION-3 is a phase II open-label, non-randomized, active comparator (vs. evolocumab) extension trial of ORION-1 to evaluate the efficacy, safety, and tolerability of inclisiran in patients with clinical ASCVD, an ASCVD risk equivalent, or HeFH despite maximum tolerated LDL-C-lowering therapy. Subjects will be randomized to inclisiran 300 mg SC on day 1 and every 180 days thereafter for up to 4 years, or evolocumab 140 mg SC on day 1 and every 14 days thereafter until day 336. Participants in the evolocumab arm will then receive inclisiran 300 mg SC on day 360 and every 180 days thereafter for up to 4 years. Inclusion criteria will include completion of ORION-1 and no contraindication to receiving inclisiran or evolocumab. The primary outcome measure will be percentage change in LDL-C from day 1 to day 210 in the inclisiran group. The purpose of the second group will be to assess switching (from evolocumab to inclisiran at day 360), comparative efficacy, safety, and patient preference of inclisiran versus evolocumab [41]. Preliminary results from ORION-3 were presented at the 2019 National Lipid Association Scientific Sessions. Participants that received inclisiran twice a year demonstrated a 51% reduction in LDL-C from baseline at ~ 22 months, with minimal adverse effects.
ORION-8 is a phase III open-label long-term extension of ORION-5, -9, -10, and -11 designed to assess the effect of long-term dosing of inclisiran in subjects with high cardiovascular risk and elevated LDL-C. Subjects must have either established ASCVD, an ASCVD risk equivalent, HeFH, or HoFH and elevated LDL-C despite maximally tolerated LDL-lowering therapy. Subjects will receive inclisiran 300 mg SC on days 1 and 90, then every 180 days to day 990. Also of note, subjects that received placebo in the feeder study will receive blinded inclisiran, and vice versa, and subjects from the open-label ORION-5 study will not receive any injection of study drug or placebo on day 1. Inclusion criteria will include completion of one of the above-mentioned qualifying phase III lipid-lowering ORION feeder studies, meaning the subject received the last dose of study drug and completed the final study visit per protocol and is on stable doses of lipid-lowering therapy. The primary outcome measures will assess the proportion of subjects reaching an LDL-C of < 70 or < 100 mg/dL, considered goal LDL-C values based on their level of ASCVD risk, by day 1080 [42].
5.2.3 Supportive Trials: ORION-1 and -2
ORION-1 is discussed in Sect. 5.1. ORION-2 was a phase II open-label, single-arm, multicenter pilot study to evaluate the safety, tolerability, and efficacy of inclisiran in participants with HoFH. Subjects were randomized to standard of care plus inclisiran 300 mg SC on day 1 (PCSK9 levels not suppressed by > 70% at day 60 or day 90, as compared with baseline, received a second dose at day 90 or day 104, respectively) versus standard of care alone. Inclusion criteria included males and females ≥ 12 years of age with a genetic diagnosis of HoFH or clinical diagnosis of HoFH based on a history of untreated LDL-C > 500 mg/dL and either xanthoma before age 10 years or evidence of HeFH in both parents. Participants were on stable lipid-lowering therapy, with a fasting LDL-C > 130 mg/dL and body weight of ≥ 40 kg at screening. The primary outcome measures included percentage change in LDL-C from day 1 to day 90 and change in LDL-C from day 1 to day 180 (or final visit) [43]. Preliminary results presented at the 2019 European Atherosclerosis Society Congress showed LDL-C reductions of 11.7–33.1% at day 90 and 17.5–37.0% at day 180 in three patients, while one patient had an increase in LDL-C at days 90 and 180. Of note, that patient also had a minimal response to prior PCSK9i therapy [44].
5.2.4 Special Populations: ORION-6 and -7
ORION-6 is a phase I single-dose, open-label, parallel-group study to assess the pharmacokinetics, pharmacodynamics, and safety of inclisiran in subjects with hepatic impairment compared with subjects with normal hepatic function [34].
ORION-7 is a phase I single-dose, open-label, non-randomized trial to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single dose of SC inclisiran in participants with mild, moderate, and severe renal impairment compared with those with normal renal function. Subjects with normal (creatinine clearance [CrCl] ≥ 90 mL/min), mild (CrCl 60–89 mL/min), moderate (CrCl 30–59 mL/min), and severe (CrCl 15–29 mL/min) renal impairment will receive a single dose of inclisiran 300 mg SC on day 1 and will be followed prospectively to assess study endpoints. Outcome measures will be assessed at 30 min and 1, 2, 4, 6, 8, 12, 24, and 48 h post-injection, and on days 4, 7, 14, and 30 post-injection. Measures to be assessed will include maximum plasma concentration of inclisiran, time to reach maximum concentration, half-life, area under the curve of the plasma concentration, total clearance, volume of distribution, urine total excretion and fractional excretion, and renal clearance [45]. Preliminary results presented at the 2019 European Atherosclerosis Society Congress showed no difference in LDL-C reductions, PCSK9 reductions, and safety/tolerability between subjects with normal, mild, or moderate renal impairment [46].
5.2.5 Electrocardiographic Effects: ORION-12
ORION-12 is a randomized, placebo-controlled, double-blinded parallel-design study with an open-label and positive control to assess the electrocardiographic effects of inclisiran in healthy subjects [34].
6 Differences Between siRNA and Monoclonal Antibodies
Though siRNAs and PCSK9 mAbs both ultimately lower plasma LDL-C concentrations, there are several differences that should be noted. First, siRNAs have a substantially different pharmacodynamic profile to the mAbs, leading to an extended duration of action with sustained lowering of LDL-C over time [47]. Treatment with siRNAs can lead to reductions in PCSK9 and LDL-C, possibly enabling a twice-yearly dosing regimen, with little variation over the 6-month period after receipt of the first injection [31, 33]. Thus, inclisiran has the potential to provide effective LDL-C lowering with administration every 6 months compared with once or twice a month with the mAbs, which may facilitate adherence to therapy.
Second, whereas the mAbs bind to extracellular PCSK9 and prevent binding with the LDLR, siRNAs are targeted specifically to the liver and inhibit hepatic synthesis of PCSK9 [31]. Theoretically, this could lead to higher atheroma concentrations of PCSK9 in those treated with the siRNAs versus the mAbs [48], though the clinical implications of this scenario are unclear.
Third, siRNA-induced lowering of PCSK9 reflects true levels of reduced protein, as opposed to mAbs, which bind to PCSK9 protein and interfere with its ability to bind with the LDLR, thus increasing its concentration in the plasma [49]. Clinically, elevations in plasma PCSK9 levels can be used to confirm adherence to therapy or optimal injection technique in patients who do not show the expected LDL-C-lowering response to PCSK9i therapy, compared to decreases in plasma PCSK9 levels which would be expected in patients using siRNA therapy [48].
7 Conclusion
The PCSK9 adventure began in 2003 with the discovery of a protein that dramatically transformed our understanding of cholesterol metabolism from a process thought to be entirely regulated from within the cell to one that is largely controlled by a low abundance secreted plasma protein. The brief interval from discovery to commercialization of a drug that antagonizes PCSK9 to effectively lower LDL-C and improve cardiovascular outcomes is perhaps the best example of how genetic insights can be leveraged into intelligent, targeted drug discovery. The next chapter of this saga has been the entry of a new approach to antagonize PCSK9, namely silencing its production. Inclisiran, an siRNA complementary to PCSK9 mRNA, demonstrates sustained reductions in PCSK9 and LDL-C. Though inclisiran acts on the same pathway as the therapeutic mAbs, siRNA exhibits a more sustained duration of action requiring less frequent injections, which serve to overcome some of the known barriers to adherence. It is conceivable that patients with hypercholesterolemia and/or ASCVD treated with this therapy may only require one to two injections per year. The results from the ORION trials are eagerly awaited and will help to further establish the safety, tolerability, and efficacy of inclisiran in those at high risk for atherosclerotic events. We appear to be at the dawn of a new anti-PCSK9 therapy and the future is bright!
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528.
GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–724.
Ford ES, Capewell S. Trends in total and low-density lipoprotein cholesterol among U.S. adults: contributions of changes in dietary fat intake and use of cholesterol-lowering medications. PLoS One. 2013;8(5):e65228.
Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, et al. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2014;63(6):539–46.
Hirsh BJ, Smilowitz NR, Rosenson RS, Fuster V, Sperling LS. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015;66(2):184–92.
Laboy SM, Pulley MT. Statin-associated muscle symptoms: does the benefit outweigh the risk factor? Muscle Nerve. 2019;59(5):525–7.
Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
Shapiro MD, Fazio S. From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ Res. 2016;118(4):732–49.
Chaudhary R, Garg J, Shah N, Sumner A. PCSK9 inhibitors: a new era of lipid lowering therapy. World J Cardiol. 2017;9(2):76–91.
Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–84.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Ference BA, Graham I, Tokgozoglu L, Catapano AL. Reprint of: Impact of lipids on cardiovascular health: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(23 Pt B):2980–95.
Timms KM, Wagner S, Samuels ME, Forbey K, Goldfine H, Jammulapati S, et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum Genet. 2004;114(4):349–53.
Shioji K, Mannami T, Kokubo Y, Inamoto N, Takagi S, Goto Y, et al. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J Hum Genet. 2004;49(2):109–14.
Alves AC, Etxebarria A, Medeiros AM, Benito-Vicente A, Thedrez A, Passard M, et al. Characterization of the first PCSK9 gain of function homozygote. J Am Coll Cardiol. 2015;66(19):2152–4.
Iacocca MA, Wang J, Sarkar S, Dron JS, Lagace T, McIntyre AD, et al. Whole-gene duplication of PCSK9 as a novel genetic mechanism for severe familial hypercholesterolemia. Can J Cardiol. 2018;34(10):1316–24.
Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37(2):161–5.
Fasano T, Cefalu AB, Di Leo E, Noto D, Pollaccia D, Bocchi L, et al. A novel loss of function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2007;27(3):677–81.
Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79(3):514–23.
Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007;193(2):445–8.
Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376(16):1527–39.
Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–26.
Kosmas CE, Munoz Estrella A, Sourlas A, Silverio D, Hilario E, Montan PD, et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6(3):63.
Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55.
Bernards R. Exploring the uses of RNAi–gene knockdown and the Nobel Prize. N Engl J Med. 2006;355(23):2391–3.
Kosmas CE, DeJesus E, Morcelo R, Garcia F, Montan PD, Guzman E. Lipid-lowering interventions targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): an emerging chapter in lipid-lowering therapy. Drugs Context. 2017;6:212511.
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61.
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383(9911):60–8.
Fitzgerald K, Kallend D, Simon A. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(18):e38.
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–40.
Ray KK, Stoekenbroek RM, Kallend D, Nishikido T, Leiter LA, Landmesser U, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. Epub. 2019. https://doi.org/10.1001/jamacardio.2019.3502.
The Medicines Company. ORION clinical development program. Updated 12 March 2019. https://www.themedicinescompany.com/clinical-development/. Accessed 5 Nov 2019.
University of Oxford. A randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease [ClinicalTrials.gov identifier NCT03705234]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03705234. Accessed 5 Nov 2019.
The Medicines Company. A study of inclisiran in participants with homozygous familial hypercholesterolemia (HoFH) [ClinicalTrials.gov identifier NCT03851705]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03851705. Accessed 5 Nov 2019.
The Medicines Company. Trial to evaluate the effect of inclisiran treatment on low density lipoprotein cholesterol (LDL-C) in subjects with heterozygous familial hypercholesterolemia (HeFH) [ClinicalTrials.gov identifier NCT03397121]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03397121. Accessed 5 Nov 2019.
The Medicines Company. Inclisiran for participants with atherosclerotic cardiovascular disease and elevated low-density lipoprotein cholesterol [ClinicalTrials.gov identifier NCT03399370]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03399370. Accessed 5 Nov 2019.
The Medicines Company. Inclisiran for subjects With ACSVD or ACSVD-risk equivalents and elevated low-density lipoprotein cholesterol [ClinicalTrials.gov identifier NCT03400800]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03400800. Accessed 5 Nov 2019.
Ray K. The ORION-11 trial. European Society of Cardiology Congress 2019. 31 Aug–4 Sep 2019; Paris.
The Medicines Company. An extension trial of inclisiran compared to evolocumab in participants with cardiovascular disease and high cholesterol [ClinicalTrials.gov identifier NCT03060577]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03060577. Accessed 5 Nov 2019.
The Medicines Company. Trial to assess the effect of long term dosing of inclisiran in subjects with high CV risk and elevated LDL-C [ClinicalTrials.gov identifier NCT03814187]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03814187. Accessed 5 Nov 2019.
The Medicines Company. A study of ALN-PCSSC in participants with homozygous familial hypercholesterolemia (HoFH) [ClinicalTrials.gov identifier NCT02963311]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT02963311. Accessed 5 Nov 2019.
Raal F, Lepor N, Kallend D, Stoekenbroek R, Wijngaard P, Hovingh GK. Inclisiran durably lowers Ldl-C And Pcsk9 expression in subjects with homozygous familial hypercholesterolaemia: the Orion-2 pilot study. Atherosclerosis. 2019;287:e7.
The Medicines Company. A study of inclisiran in participants with renal impairment compared to participants with normal renal function (ORION-7) [ClinicalTrials.gov identifier NCT03159416]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03159416. Accessed 5 Nov 2019.
Kallend D, Collins M, Kastelein J, Landmesser U, Leiter L, Ray K, et al. Efficacy, safety and pharmacokinetics of inclisiran by renal function. Atherosclerosis. 2019;287:e38.
Henne KR, Ason B, Howard M, Wang W, Sun J, Higbee J, et al. Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement. J Pharmacol Exp Ther. 2015;353(1):119–31.
Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420–38.
Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Michael Shapiro is supported by NIH K12HD043488.
Conflict of interest
Michael Shapiro reports compensation for advisory activities from Esperion and consulting with Amarin. Charles German has no disclosures.
Rights and permissions
About this article
Cite this article
German, C.A., Shapiro, M.D. Small Interfering RNA Therapeutic Inclisiran: A New Approach to Targeting PCSK9. BioDrugs 34, 1–9 (2020). https://doi.org/10.1007/s40259-019-00399-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-019-00399-6