Abstract
Introduction
Acute decompensated heart failure (ADHF), with an incidence of 1–2%, is a clinical syndrome with significant morbidity and mortality despite therapeutic advancements and ongoing clinical trials. A recent therapeutic approach to patients with ADHF includes combination therapy with hypertonic saline solution (HSS) and furosemide, based on the hypothesis that resistance to loop diuretics occurs because of achievement of plateau in water and sodium excretion in patients receiving long-term loop diuretic therapy.
Objective
Our aim was to conduct a meta-analysis to evaluate the efficiency of combination HSS plus furosemide therapy in patients with ADHF in terms of mortality, readmissions, length of hospital stay, kidney function, urine output, body weight, and B-type natriuretic peptide (BNP).
Methods
A total of 14 studies—four observational and ten randomized studies (total 3398 patients)—were included in the meta-analysis.
Results
Our results demonstrate the superiority of combination HSS plus furosemide therapy over furosemide alone in terms of kidney function preservation (mean creatinine difference − 0.33 mg/dL; P < 0.00001), improved diuresis (mean difference [MD] 581.94 mL/24 h; P < 0.00001) and natriuresis (MD 57.19; P < 0.00001), weight loss (MD 0.99 kg; P < 0.00001), duration of hospital stay (MD − 2.72 days; P < 0.00001), readmissions (relative risk 0.63; P = 0.01), and mortality (relative risk 0.55; P < 0.00001). However, no difference in BNP levels was detected (MD 19.88 pg/mL; P = 0.50).
Conclusion
Despite the heterogeneity and possible risk of bias among the studies, results appear promising on multiple aspects. A clear need exists for future randomized controlled trials investigating the role of combination HSS plus furosemide therapy to clarify these effects and their possible mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hypertonic saline with furosemide therapy might be a promising therapy in heart failure. |
Combination of hypertonic saline with furosemide decreases mortality and length of hospital stay. |
1 Introduction
The European Society of Cardiology defines congestive heart failure (CHF) as a clinical syndrome characterized by signs and symptoms of pulmonary and systemic congestion, including dyspnea, orthopnea, pretibial edema, hepatomegaly, and jugular venous distention, caused by cardiac dysfunction [1]. The prevalence rate of CHF is 1–2% and increases considerably with age, and the World Bank estimates that annual medical spending for treatment of CHF is $US108 billion [2, 3]. Patients with decompensated heart failure (HF) are more likely to have comorbid conditions, with valvular heart diseases (44%), atrial flutter or fibrillation (30%), diabetes mellitus (40%), and renal diseases (30%) being the most commonly encountered [4,5,6,7]. In-hospital and 1-year post-discharge mortality rates of hospitalized patients with decompensated HF are high at 4–11% and 20–36%, respectively, in large-scale studies [8,9,10]. Intravenous loop diuretics and vasodilators are the most common therapeutic approach, whereas inotropic agents or vasopressors may be preferred in cases with low systolic blood pressure or features of cardiogenic shock [11]. Given the ongoing high mortality rates, novel therapeutic approaches, including levosimendan (a calcium-sensitizing agent), nesiritide (a recombinant human brain natriuretic peptide [BNP]), and istaroxime (stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a), have been proposed as potential therapies [12]. Another novel approach is the combination of hypertonic saline solution (HSS) with furosemide. This is based on the hypothesis that resistance to loop diuretic occurs because of achievement of plateau in water and sodium excretion in patients receiving long-term loop diuretic therapy, which is referred as “chronic braking” therapy [13]. Another mechanism of diuretic resistance is the functional adaptation of the distal tubule regarding transporters or prevention of intravascular volume depletion [14,15,16]. Potential beneficial effects with HSS in clinical trials include improved cardiac biomarkers, weight loss, symptom resolution, increased urine output, and improved kidney function. However, results vary significantly among studies, and there exists a clear need to re-evaluate the evidence, taking into account the potential impacts on clinical practice.
In this meta-analysis, we aim to evaluate the efficiency of HSS plus furosemide therapy in patients with decompensated HF in terms of mortality, readmissions, length of hospital stay, kidney function, urine output, body weight, and BNP levels.
2 Methods
We conducted a literature review and meta-analysis according to the methods specified by the Cochrane Collaboration and Quality of Reporting of Meta-Analyses (QUOROM) [17]. We used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to guide reporting of this study [18].
2.1 Literature Search
The literature search for this meta-analysis was performed up to 25 May 2020 and included three databases: Embase (Elsevier), the Cochrane Central Register of Controlled Trials (Wiley), and PubMed/MEDLINE Web of Science. The following terms and their combinations were used: acute heart failure, heart failure, decompensated heart failure, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction, systolic heart failure, diastolic heart failure, pulmonary congestion, furosemide, hypertonic saline, saline, saline infusion, hypertonic saline infusion, loop diuretics, diuretics, treatment, and therapy. The titles and abstracts of each study were independently evaluated by two authors (S.C. and B.A.), with consensus reached after detailed examination of the study and discussion of conflicts with the third author. In addition, we hand searched journals, conference proceedings, and current awareness alerts without applying language restrictions.
After preliminary elimination of the studies by evaluation of the titles and abstracts, full texts were independently assessed by each author. Selected studies and references of the included studies were further evaluated.
2.2 Inclusion and Exclusion Criteria
All randomized controlled trials (RCTs) and observational studies with retrospective or prospective designs investigating the efficiency of HSS with furosemide infusion in patients with acute decompensated HF and published in a peer-reviewed journal in English before June 2020 were included in this meta-analysis. We excluded studies that were not considered original articles (i.e., systematic reviews, meta-analyses, editorials, and commentaries), studies with missing data or inadequate descriptions of outcomes, study types not listed in the inclusion criteria, studies lacking clear methodology (i.e., case reports, case series), and unpublished data.
Outcome measures in the meta-analyses included mortality; readmissions; length of hospital stay; kidney function, measured as serum creatinine value, urine output, and natriuresis; body weight, and BNP levels. Figure 1 shows the details of the study selection procedure.
2.3 Quality Assessment
The Newcastle–Ottawa Scale was utilized for the observational studies included in this meta-analyses. This scale includes three main criteria for evaluation: selection of study groups, comparability of groups, and assessment of outcomes. Nine stars indicates the highest-quality research. For RCTs, we assessed the risk of bias in the included studies by standard domains of the risk of bias tool developed by the Cochrane Collaboration [17]. Quality assessment of each study was mediated via consensus decision of the authors (S.C. and B.A.).
2.4 Statistical Analysis
We used a random-effects model for meta-analysis and expressed treatment effects as relative risks (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes (readmissions, long-term mortality, in-hospital death) and as mean differences (MDs) for continuous outcomes with 95% CIs (kidney function, diuresis, and urinary sodium, BNP, body weight loss, length of hospital stay). We converted median and interquartile ranges to means and standard deviations and converted standard errors to standard deviations using standard formulas [19,20,21].
We used the I2 statistic to assess for inconsistency across individual studies [22]. An I2 > 50% indicated a large heterogeneity that was not explained by chance. If a sufficient number of studies were identified, subgroup analysis was used to explore possible sources of heterogeneity. All statistical analyses were performed using Review Manager version 5.3 (The Cochrane Collaboration 2012) [23].
3 Results
We included 14 studies (four observational [24,25,26,27] and ten randomized [28,29,30,31,32,33,34,35,36,37]) in our final analysis, with a total of 3398 included patients (minimum 32 [29], maximum 1927 [35]). The treatment arm consisted of HSS plus furosemide. The concentrations of the administered HSS were reported as follows: 1.4–4.6% [26, 30, 33,34,35], 1.7% [27, 31], 1.95% [36], 2.4% [28], 2.8% [37], 3% [24, 25, 32], and 7.5% [29]. None of the included studies reported the mean dosage of HSS. However, one study [25] reported a mean of 5.1 ± 2 doses of HSS, and another [24] reported a median of three doses of HSS. Only one study used carperitide for the control arm [27]; all other studies used furosemide alone. In addition, only one study [25] reported the administration of seven doses of metolazone during standard and experimental treatment. Four studies used high doses of furosemide [24, 26, 30, 33], with all other studies using conventional doses.
Only two of the included studies reported outcomes as changes per day of treatment [24, 25]; all others reported the outcomes as MDs between groups or between baseline and post-intervention values measured at different times across the study (24 h [31], 4 days [29], 5 days [28], 6 days [32], 8 days [26], and discharge [27, 30, 33,34,35,36,37]). When necessary, we calculated the MD between the pre- and post-intervention groups.
3.1 Outcome Measures Reporting
3.1.1 Kidney Function
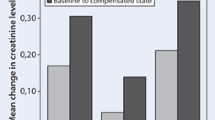
Eleven studies (nine randomized [28,29,30,31,32,33,34,35,36] and two observational [24, 25]) evaluated the effect on renal function of adding HSS to furosemide. As shown in Fig. 2, the combined therapy preserved renal function, leading to an overall decrease of serum creatinine in the HSS plus furosemide arm from admission to discharge (MD − 0.33 mg/dL [95% CI − 0.42 to − 0.23]; P < 0.00001).
Given the increased heterogeneity (χ2 = 291.17; I2 = 97%; P < 0.00001), we also separately analyzed the effect of HSS treatment on renal function without the two studies reporting daily changes in serum creatinine. There was a trend for a further decrease in serum creatinine levels in the HSS + furosemide arm (MD − 0.45 mg/dL [95% CI − 0.51 to − 0.39]; P < 0000.1) (Fig. 1 in the electronic supplementary material). We also performed a separate analysis after removing studies that used high doses of furosemide [24, 30, 33]. The beneficial effect of the administration of HSS plus furosemide was slightly attenuated (MD − 0.25 mg/dL [95% CI − − 0.48 to − 0.03]; P = 0.003).
3.1.2 Diuresis and Urinary Sodium
To assess the efficacy of adding HSS to furosemide, we evaluated two outcomes: diuresis (mL/24 h) and urinary sodium (mEq/24 h). Seven RCTs [30,31,32,33,34,35, 37] and one observational study [24] reported daily diuresis in both arms, allowing us to calculate the MD between them. In both groups (1436 subjects treated with HSS plus furosemide and 1465 treated with furosemide), an increase in daily diuresis was observed, with 581 mL per 24 h higher volumes in the intervention group (MD 581.94 mL/24 h [95% CI 495.94–667.94]; P < 0.00001). When analyzing separately the studies that used conventional doses of furosemide, a further increase in daily diuresis was observed (MD 620.82 mL/24 h [95% CI 510.79–730.86]; P < 0.00001). Urinary sodium variation with treatment was reported in five randomized studies [30,31,32,33, 35]. HSS administration led to a significant increase in natriuresis (MD 57.19 [95% CI 47.56–66.82]; P < 0.00001) (Fig. 3). After removing the two studies that used high doses of furosemide [30, 33], a trend towards a smaller increase in natriuresis was observed (MD 46.9 [95% CI 41.14–52.66]; P < 0.00001).
3.1.3 B-Type Natriuretic Peptide
Variations in BNP levels were reported in four RCTs [29, 34, 35, 37] and one observational study [26] that included 1347 subjects treated with HSS and furosemide and 1276 subjects treated with furosemide alone. Four studies [26, 34, 35, 37] reported BNP values in pg/mL and showed a reduction in BNP levels in the HSS plus furosemide group. One study [29] did not mention the unit used to measure BNP and showed no change in BNP levels between the two groups. Overall, the meta-analysis did not find a significant difference between the two groups (MD 19.88 pg/mL [95% CI − 37.93 to 77.68]; P = 0.50) (Fig. 4).
3.1.4 Body Weight Loss
In total, 12 studies (nine RCTs [28,29,30,31,32,33,34,35,36] and three observational [24, 25, 27]) reported data for change in body weight. Overall, treatment with HSS led to more substantial weight loss than control (MD 0.99 kg [95% CI 0.59–1.39]; P < 0.00001) (Fig. 5). After excluding studies that used high doses of furosemide [24, 30, 33], there was a trend towards lower body weight loss (MD 0.96 kg [95% CI 0.32–1.6]; P = 0.003).
3.1.5 Length of Hospital Stay
The length of hospital stay was reported in ten studies (eight RCTs [28, 30, 32,33,34,35,36,37] and two observational [26, 27]) as MDs between the HSS (n = 1579) and the control group (n = 1580). In two studies [26, 28], the length of hospitalization was higher in the HSS group (MD 0.12 days [95% CI − 2.15 to 2.39] and 0.36 days [95% CI − 0.52 to 1.24], respectively). Overall, treatment with HSS significantly reduced the length of hospital stay, by approximately 3 days (MD −2.72 days [95% CI − 3.59 to − 1.86]; P < 0.00001) (Fig. 6). After excluding studies that used high doses of furosemide [26, 30, 33], the length of hospital stay was further reduced in the HSS arm (MD − 3.13 days [95% CI − 4.18 to − 2.08]; P < 0.00001).
3.1.6 Readmissions
The number of readmissions was reported in four studies (three RCTs [30, 33, 35] and one observational study [27]). Notably, there were 210 events in the HSS-treated group (20.5%) and 400 events in the control group (37.03%). Thus, the use of HSS was associated with a reduction in the risk of readmission of 37% compared with the control arm (RR 0.63 [95% CI 0.44–0.9]; P = 0.01) (Fig. 7).
Excluding the study by Licata et al. [30] led to a loss of statistical significance (RR 0.62 [95% CI 0.32–1.18]; P = 0.14), as did excluding the study by Paterna et al. [35] (RR 0.65 [95% CI 0.33–1.25]; P = 0.20). Moreover, excluding studies that used high doses of furosemide [30, 33] resulted in no significant statistical difference between the two arms (RR 0.7 [95% CI 0.4–1.25]; P = 0.23).
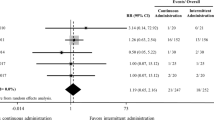
3.1.7 Mortality
Five studies (four randomized [30, 33, 35, 37] and one observational [27]) reported long-term mortality, and two studies (one RCT [29] and one observational [27]) reported in-hospital death. Overall, we performed the analysis in 2338 patients and found 174 deaths in the HSS group and 322 in the control group (15.19 vs. 26.99%, respectively). The risk of long-term mortality was 45% lower in patients treated with HSS than in controls (RR 0.55 [95% CI 0.47–0.65]; P < 0.00001). On the other hand, the risk of in-hospital death was 46% higher in the HSS arm, but data were available for only 207 patients (RR 1.46 [95% CI 0.70–3.07]; P = 0.32) (Fig. 8). The risk of long-term mortality was 40% lower in patients who were treated with HSS and conventional doses of furosemide (RR 0.60 [95% CI 0.43–0.89]; P = 0.002).
3.1.8 Risks of Bias
All trials had serious limitations because of a risk of bias in most of the domains evaluated. Most were at high risk of selection bias, and outcome assessors were not blinded in 80% of studies. Outcome reporting seemed to be selective in almost 50% of the included studies.
4 Discussion
Our meta-analysis, which included 14 studies and 3398 patients, indicated that treatment with HSS plus furosemide in patients with decompensated HF had positive effects on mortality, mean hospital stay, renal function, and readmissions.
Decompensated HF is a clinical condition with high morbidity and mortality that may develop in patients with or without preexisting cardiovascular comorbidities. Intravenous loop diuretics, including furosemide, are the most commonly administered medication in such cases. However, mortality rates remain relatively high. In this meta-analysis, we demonstrated that intravenous administration of HSS with furosemide in patients with acute decompensated HF led to shorter mean hospital stays, lower mortality rates, fewer readmissions, and significant improvements in serum creatinine levels, 24-h urine output, and weight loss compared with intravenous furosemide therapy alone. Although the exact mechanism of action of HSS is unclear, a few hypotheses have been generated. Furosemide reaches the intraluminal site of nephrons, where it exerts its function via active secretion from proximal tubules. Most patients with decompensated HF develop hypovolemia and decreased renal blood flow (RBF), which impairs the active secretion process [38]. Administration of HSS increases intraluminal furosemide concentrations, 24-h diuresis, urinary sodium levels, and urinary osmolarity [39]. Another aspect of reduced RBF is the over-activation of the tubuloglomerular feedback mechanism, which may be defined as vasomotor response to tubular osmolarity and sodium concentrations detected by macula densa cells [40]. For correction of such compensatory feedback mechanisms, HSS treatment as well as many other drugs that may attract extravascular volume towards intravascular compartments, such as mannitol and dextran, have been proposed [41, 42].
Additionally, reduced RBF and load of tubular volume and solute may cause a shift in renal plasma flow, which may be reversible via administration of HSS [43, 44]. The importance of that shift depends upon the presence of deep medullary nephrons with well-developed loop of Henle in medullary in contrast to cortical nephrons. Moreover, HSS caused a decrease in plasma renin activity and atrial natriuretic peptide levels [45].
Increased myocardial contractility with HSS may be another possible reason for the observed outcomes. Indeed, HSS improved myocardial contractility in experimental models [46]. It has also been shown that HSS improved cardiac contractile function during sepsis by preserving calcium handling [47].
HSS may also have anti-inflammatory actions, as evidenced by inhibition of neutrophil activation and infiltration in lungs [48]. Furthermore, HSS can ameliorate organ dysfunction in severe sepsis caused by cecal ligation and puncture, and this is mediated via its antioxidant and anti-inflammatory effects [49]. Anti-apoptotic actions of HSS have also been demonstrated [50]. Lastly, it has been hypothesized that HSS with furosemide attenuates the possible harmful effects of neuro-hormonal excitation that occurs in HF [15].
The results of this meta-analyses offer the potential for changes to management guidelines for decompensated HF. In addition to improvements in clinical outcomes, such as readmissions and mean length of hospital stay, HSS offers potential improvements in renal function, which is one of the primary poor predictive factors for adverse outcomes [51]. Baseline urine urea nitrogen/creatinine ratio, a prognostic factor in patients with HF, has been shown to be the strongest predictor of HSS treatment-related diuretic response [52]. A retrospective analysis of 58 diuretic therapy-refractory patients with decompensated HF demonstrated that administration of HSS improved serum creatinine levels, total urinary output, and body weight loss, a change that was statistically significant, without any significant pulmonary or neurological adverse effects [53]. This study is crucial as it provides further clinical evidence for the use of HSS. Additionally, HSS administration reduces serum levels of many proinflammatory cytokines, including tumor necrosis factor-α and interleukin (IL)-6 and IL-1β, providing evidence that HSS therapy may reverse the inflammatory response that develops in response to congestion, edema, and tissue injury [54]. An increasing level of scientific evidence favors the use of HSS in the management of decompensated HF. Nevertheless, comprehensive multicenter large-scale clinical trials are required to reach a definitive conclusion. Also, the possible role of confounding factors, including comorbidities frequently present in patients with HF, such as diabetes mellitus, renal diseases, and arrhythmia, should not be overlooked in study groups (Tables 1, 2).
We included 14 studies in this meta-analyses, with ten RCTs, which is considerably more than in previous meta-analyses. We also included more clinical and laboratory parameters in the qualitative analysis [40, 55]. Limitations of our study include the exclusion of severe renal disorders, which is a common comorbidity in patients with long-standing HF, possible variations in baseline serum electrolyte levels among participants, and possible bias associated with the high number of studies performed by the same research group. It should be noted that two of the included studies [30, 35] reported different long-term sodium intake regimens for the HSS and furosemide group and the control group (120 and 80 mmoL/day, respectively). It could be concluded that the long-term effects could be solely due to differences in sodium restriction. Other important limitations of our analysis include the use of different protocols for the administration of hypertonic saline solutions, the limited number of patients included, and the increased heterogeneity of the studies.
In conclusion, the intravenous administration of HSS with furosemide in patients with acute decompensated HF may result in shorter mean hospital stays, lower mortality rates, fewer readmissions, and significant improvements in serum creatinine levels, 24-h urine output, and weight loss compared with intravenous furosemide therapy alone.
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975.
Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368–76.
Cowie M, Mosterd A, Wood D, Deckers J, Poole-Wilson P, Sutton G, et al. The epidemiology of heart failure. Eur Heart J. 1997;18(2):208–25.
Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V-P, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27(22):2725–36.
Afsar B, Rossignol P, van Heerebeek L, Paulus WJ, Damman K, Heymans S, et al. Heart failure with preserved ejection fraction: a nephrologist-directed primer. Heart Fail Rev. 2017;22(6):765–73.
Afsar B, Ortiz A, Covic A, Solak Y, Goldsmith D, Kanbay M. Focus on renal congestion in heart failure. Clin Kidney J. 2016;9(1):39–47.
Yerlikaya A, Bulbul MC, Afsar B, Dagel T, Aslan G, Voroneanu L, et al. Iron in kidney and heart failure: from theory to practice. Int Urol Nephrol. 2018;50(3):481–93.
Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46(1):57–64.
Ahmed A, Allman RM, Fonarow GC, Love TE, Zannad F, Dell’Italia LJ, et al. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Cardiac Fail. 2008;14(3):211–8.
Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–16.
Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505–35.
Shah P, Pellicori P, Cuthbert J, Clark AL. Pharmacological and non-pharmacological treatment for decompensated heart failure: what is new? Curr Heart Fail Rep. 2017;14(3):147–57.
Krämer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106(1):90–6.
Dormans TP, Gerlag PG, Russel FG, Smits P. Combination diuretic therapy in severe congestive heart failure. Drugs. 1998;55(2):165–72.
De Vecchis R, Ciccarelli A, Ariano C, Pucciarelli A, Cioppa C, Giasi A, et al. Renoprotective effect of small volumes of hypertonic saline solution in chronic heart failure patients with marked fluid retention: results of a case-control study. Herz. 2011;36(1):12–7.
Nistor I, Bararu I, Apavaloaie MC, Voroneanu L, Donciu MD, Kanbay M, et al. Vasopressin receptor antagonists for the treatment of heart failure: a systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol. 2015;47(2):335–44.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Oxford: Wiley; 2019.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Griffin M, Soufer A, Goljo E, Colna M, Rao VS, Jeon S, et al. Real world use of hypertonic saline in refractory acute decompensated heart failure: a U.S. center’s experience. JACC Heart Fail. 2020;8(3):199–208.
Lafrenière G, Béliveau P, Bégin JY, Simonyan D, Côté S, Gaudreault V, et al. Effects of hypertonic saline solution on body weight and serum creatinine in patients with acute decompensated heart failure. World J Cardiol. 2017;9(8):685–92.
Tuttolomondo A, Pinto A, Di Raimondo D, Corrao S, Di Sciacca R, Scaglione R, et al. Changes in natriuretic peptide and cytokine plasma levels in patients with heart failure, after treatment with high dose of furosemide plus hypertonic saline solution (HSS) and after a saline loading. NMCD. 2011;21(5):372–9.
Okuhara Y, Hirotani S, Ando T, Nishimura K, Orihara Y, Komamura K, et al. Comparison of salt with low-dose furosemide and carperitide for treating acute decompensated heart failure: a single-center retrospective cohort study. Heart Vessels. 2017;32(4):419–27.
Engelmeier RS, Le TT, Kamalay SE, Utecht KN, Nikstad TP, Kaliebe JW, et al. Randomized trial of high dose furosemide-hypertonic saline in acute decompensated heart failure with advanced renal disease. J Am Coll Cardiol. 2012;59(13 Supplement):E958.
Issa VS, Andrade L, Ayub-Ferreira SM, Bacal F, de Bragança AC, Guimarães GV, et al. Hypertonic saline solution for prevention of renal dysfunction in patients with decompensated heart failure. Int J Cardiol. 2013;167(1):34–40.
Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145(3):459–66.
Okuhara Y, Hirotani S, Naito Y, Nakabo A, Iwasaku T, Eguchi A, et al. Intravenous salt supplementation with low-dose furosemide for treatment of acute decompensated heart failure. J Card Fail. 2014;20(5):295–301.
Parrinello G, Paterna S, Di Pasquale P, Torres D, Mezzero M, Cardillo M, et al. Changes in estimating echocardiography pulmonary capillary wedge pressure after hypersaline plus furosemide versus furosemide alone in decompensated heart failure. J Card Fail. 2011;17(4):331–9.
Paterna S, Di Pasquale P, Parrinello G, Amato P, Cardinale A, Follone G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail. 2000;2(3):305–13.
Parrinello G, Di Pasquale P, Torres D, Cardillo M, Schimmenti C, Lupo U, et al. Troponin I release after intravenous treatment with high furosemide doses plus hypertonic saline solution in decompensated heart failure trial (Tra-HSS-Fur). Am Heart J. 2012;164(3):351–7.
Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci. 2011;342(1):27–37.
Yayla Ç, Akyel A, Canpolat U, Gayretli Yayla K, Eyiol A, Akboğa MK, et al. Comparison of three diuretic treatment strategies for patients with acute decompensated heart failure. Herz. 2015;40(8):1115–20.
Wan Y, Li L, Niu H, Ma X, Yang J, Yuan C, et al. Impact of compound hypertonic saline solution on decompensated heart failure. Int Heart J. 2017;58(4):601–7.
Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19(7):821–36.
Paterna S, Di Gaudio F, La Rocca V, Balistreri F, Greco M, Torres D, et al. Hypertonic saline in conjunction with high-dose furosemide improves dose-response curves in worsening refractory congestive heart failure. Adv Ther. 2015;32(10):971–82.
De Vecchis R, Esposito C, Ariano C, Cantatrione S. Hypertonic saline plus iv furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure. Herz. 2015;40(3):423–35.
Maningas PA, Mattox KL, Pepe PE, Jones RL, Feliciano DV, Burch JM. Hypertonic saline-dextran solutions for the prehospital management of traumatic hypotension. Am J Surg. 1989;157(5):528–33.
Redfors B, Swärd K, Sellgren J, Ricksten S-E. Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption, blood flow and glomerular filtration after cardiac surgery. Intensive Care Med. 2009;35(1):115–22.
Riegel JA. Comparative physiology of renal excretion. New York: Hafner Publisher; 1972.
Sjöquist M, Göransson A, Hansell P, Isaksson B, Ulfendahl H. Redistribution of glomerular filtration and renal plasma flow in CNS-induced natriuresis. Acta Physiol Scand. 1986;127(4):491–7.
Kimura T, Abe K, Ota K, Omata K, Shoji M, Kudo K, et al. Effects of acute water load, hypertonic saline infusion, and furosemide administration on atrial natriuretic peptide and vasopressin release in humans. J Clin Endocrinol Metabol. 1986;62(5):1003–10.
Kien ND, Reitan JA, White DA, Wu CH, Eisele JH. Cardiac contractility and blood flow distribution following resuscitation with 7.5% hypertonic saline in anesthetized dogs. Circ Shock. 1991;35(2):109–16.
Wang YL, Lam KK, Cheng PY, Kung CW, Chen SY, Chao CC, et al. The cardioprotective effect of hypertonic saline is associated with inhibitory effect on macrophage migration inhibitory factor in sepsis. Biomed Res Int. 2013;2013:201614.
Pascual JL, Khwaja KA, Ferri LE, Giannias B, Evans DC, Razek T, et al. Hypertonic saline resuscitation attenuates neutrophil lung sequestration and transmigration by diminishing leukocyte-endothelial interactions in a two-hit model of hemorrhagic shock and infection. J Trauma. 2003;54(1):121–30 (discussion 30–2).
Shih CC, Chen SJ, Chen A, Wu JY, Liaw WJ, Wu CC. Therapeutic effects of hypertonic saline on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. Crit Care Med. 2008;36(6):1864–72.
Hogue B, Chagnon F, Lesur O. Resuscitation fluids and endotoxin-induced myocardial dysfunction: is selection a load-independent differential issue? Shock. 2012;38(3):307–13.
Bielecka-Dabrowa A, Godoy B, Schefold JC, Koziolek M, Banach M, von Haehling S. Decompensated heart failure and renal failure: what is the current evidence? Curr Heart Fail Rep. 2018;15(4):224–38.
Ando T, Okuhara Y, Orihara Y, Nishimura K, Yamamoto K, Masuyama T, et al. Urinary composition predicts diuretic efficiency of hypertonic saline solution with furosemide therapy and heart failure prognosis. Heart Vessels. 2018;33(9):1029–36.
Griffin M, Soufer A, Goljo E, Colna M, Rao VS, Jeon S, et al. Real world use of hypertonic saline in refractory acute decompensated heart failure: a US center’s experience. JACC Heart Fail. 2020;8(3):199–208.
Tuttolomondo A, Pinto A, Di Raimondo D, Corrao S, Di Sciacca R, Scaglione R, et al. Changes in natriuretic peptide and cytokine plasma levels in patients with heart failure, after treatment with high dose of furosemide plus hypertonic saline solution (HSS) and after a saline loading. Nutr Metab Cardiovasc Dis. 2011;21(5):372–9.
Gandhi S, Mosleh W, Myers RB. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: a systematic review and meta-analysis. Int J Cardiol. 2014;173(2):139–45.
Acknowledgements
MK gratefully acknowledges use of the services and facilities of the Koc University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of manuscript.
Conflicts of interest
Adrian Covic, Sidar Copur, Laura Tapoi, Baris Afsar, Carina Ureche, Dimitrie Siriopol, Ionut Nistor, and Mehmet Kanbay have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Adrian Covic, Sidar Copur, Carina Ureche, Dimitrie Siriopol, Laura Tapoi, and Mehmet Kanbay contributed substantially to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work. Baris Afsar, Ionut Nistor, Adrian Covic, and Mehmet Kanbay drafted the work or revised it critically for important intellectual content. Mehmet Kanbay, Adrian Covic, and Ionut Nistor approved the final version to be published.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Covic, A., Copur, S., Tapoi, L. et al. Efficiency of Hypertonic Saline in the Management of Decompensated Heart Failure: A Systematic Review and Meta-Analysis of Clinical Studies. Am J Cardiovasc Drugs 21, 331–347 (2021). https://doi.org/10.1007/s40256-020-00453-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-020-00453-7