Abstract
Diuretic therapy is important in critically ill patients because fluid overload impairs organ function and increases mortality. Compared to intermittent administration, continuous infusion of loop diuretics is theoretically superior in terms of diuresis and electrolyte balance. However, the available evidence is susceptible to carryover diuretic effects and resistance in earlier crossover trials. Consequently, we conducted a systematic review and meta-analysis of parallel-group randomized controlled trials to compare these two strategies in adults with acute decompensated heart failure. We searched Medline, EMBASE, and the Cochrane Central Register of Controlled Trials from their inceptions to May 26, 2018. We pooled the data using a random effects model. Our primary outcomes were all-cause mortality, length of hospital stay, and body weight reduction. We analyzed 12 parallel-group randomized controlled trials involving 923 patients. Compared with intermittent administration, continuous infusion of furosemide was not associated with an improvement in all-cause mortality (risk ratio 1.19; 95% confidence interval [CI], 0.65 to 2.16), length of hospital stay (weighted mean difference [WMD] − 0.88 days; 95% CI, − 2.76 to 1.01), or 24-h urine output (WMD 489.17 mL; 95% CI, − 183.18 to 1161.51), but was significantly associated with a greater body weight reduction (WMD 0.63 kg; 95% CI, 0.23 to 1.02). No differences in hypokalemia, hyponatremia, increased serum creatinine level, and hypotension were noted. Continuous infusion of furosemide, compared to intermittent administration, is associated with a greater body weight reduction and potential increase in 24-h urine output. The limited available evidence suggests no difference in adverse events between both strategies. Trial registration: PROSPERO (CRD42017083878)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluid overload impairs organ function and increases mortality in critically ill patients [1,2,3,4]. A conservative fluid strategy improves outcomes in critically ill patients [5,6,7]. Fluid removal has thus become a key element of critical care, and diuretic therapy is one important mainstay treatment.
Although clinicians use intravenous loop diuretics in diverse ways [8], there are two main strategies, namely, continuous and intermittent administrations. Theoretically, the continuous infusion of loop diuretics is superior to intermittent administration in some aspects [9]: (1) consistent delivery of the drug to the nephron leads to more efficient diuresis by preventing rebound sodium retention and fluid reabsorption, that is, diuretic resistance [10,11,12]; (2) continuous diuretic infusion may decrease the fluctuation in intravascular volume [13]; and (3) continuous diuretic infusion allows the titration of diuretics depending on the patient’s condition.
Earlier studies that compared these two strategies included crossover trials [14,15,16,17,18], in which diuretic effect and resistance could have been carried over to the later phase due to the lack of adequate washout periods. The urine output measured in such trials was, therefore, affected by both the continuous and intermittent infusions of diuretics. Previous systematic reviews that compared two diuretic strategies included such crossover trials, which represented nearly half of the included studies [19,20,21]. Moreover, the populations of these studies were diverse, and the extent of diuretic resistance with respect to disease varied thereafter. Results from parallel-group trials in a homogenous disease population are reliable in precisely assessing the efficacy of a single diuretic strategy by eliminating the risk of diuretic effect and a variety of diuretic resistance.
Thus, we conducted a systematic review and meta-analysis of parallel-group randomized controlled trials that assessed two furosemide strategies. To eliminate the clinical heterogeneity, such as diuretic resistance, we evaluated these two strategies in adults with acute decompensated heart failure (ADHF).
Materials and methods
The conduct and reporting of this systematic review followed the Cochrane Collaboration methodology [22] and PRISMA statement [23], and the protocol is registered at PROSPERO (CRD42017083878).
Eligibility criteria
We considered parallel-group randomized controlled trials investigating adult patients with ADHF. The diagnosis of ADHF was based on the original study authors’ definition, and all etiologies and severities were considered.
The intervention and control groups had continuous and intermittent intravenous administrations of furosemide, respectively. We did not place restrictions on the dose of furosemide after randomization or whether patients received a loading dose before randomization. Given that the clinical management of heart failure may vary across settings, we allowed the concomitant use of other diuretics as long as they were administered to both groups.
We required that a trial used at least one of the following parameters as an outcome: mortality, length of stay, body weight loss, and urine output.
Search strategy
We searched Medline, EMBASE, and the Cochrane Central Register of Controlled Trials. We reviewed the references of the included trials. We also searched Google Scholar and Web of Science for relevant trials that prospectively cited eligible trials. No language or publication status restrictions were imposed. Our search strategy is outlined in Supplemental Table 1. We updated our search on May 26, 2018.
Study selection
Two authors (AK and SU) independently reviewed identified titles and abstracts and selected relevant articles after assessing the full text. Any disagreements were resolved through discussion.
Data extraction
The same authors extracted the data independently, using a pre-designed extraction form. We extracted the following information from each study: (1) patient demographics (age, sex, and left ventricular ejection fraction or New York Heart Association [NYHA] classification), (2) study characteristics (country), (3) information on interventions (dose and loading or furosemide, and duration of the interventions), and (4) outcomes of interest.
Risk of bias assessment
The same authors independently assessed the domain of bias with the Cochrane Risk of Bias assessment tool [24]. We assessed a trial to be at low risk of performance bias, when the study personnel were blinded to the type of interventions. We also examined industry sponsorship or conflicts of interest. Any inconsistency was resolved via consensus.
Statistical analysis
Our primary outcomes were (1) all-cause mortality, (2) length of hospital stay, and (3) reduction in body weight during the study period. Our secondary outcomes included (1) urine output, (2) hypokalemia, (3) hyponatremia, (4) increase in serum creatinine level, and (5) hypotension. For dichotomous and continuous outcomes, we calculated the risk ratio (RR) and weighted mean difference (WMD) with their corresponding 95% confidence intervals (CIs), respectively. When trials had zero events in either arm, continuity corrections were applied with the addition of 0.5 to each cell of the 2 × 2 tables from the trial [25]. Trial data available as median and interquartile range were converted to mean and standard deviation using the method proposed by Wan et al. [26]. Given that the studies were clinically diverse, we pooled data using the DerSimonian and Laird random effects model [27]. Statistical heterogeneity was assessed with I2 and Q statistics [28]. We tested for small-study effect or publication bias using Egger’s method [29] when there was a sufficient number of studies for an outcome.
We conducted a sensitivity analysis by excluding trials with a high or unclear risk of bias with regard to sequence generation, allocation concealment, blinding of personnel and outcome assessors, and sponsorship or conflicts of interest. We also conducted analyses limited to trials that used similar dosages between the groups. The threshold of statistical significance was set at p < 0.05. We conducted the analyses with Stata SE, version 15.1 (Stata Corp., College Station, TX).
Results
Overview of included studies
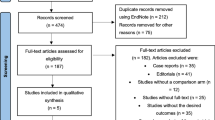
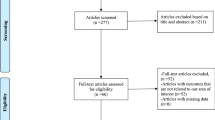
Our search produced 3221 articles (Supplemental Fig. 1). After applying the inclusion and exclusion criteria, we considered 12 parallel-group randomized controlled trials that compared the continuous and intermittent administrations of furosemide in 923 adults with ADHF (441, continuous; 482, intermittent) (Table 1) [30,31,32,33,34,35,36,37,38,39,40,41]. The reported mean age of participants ranged from 55.4 to 79.5 years, with the proportion of female patients ranging from 25 to 70.6%. The sample size ranged from 20 to 306. The daily amount of furosemide was similar between groups (range, 100 to 329 mg) in all trials, except for two trials. The first of these two trials compared two regimens of intermittent furosemide (20 mg every 6 and 8 h, respectively) with that of continuous furosemide infusion (10 mg per hour) [32]. The second trial used a protocol that allowed the titration of the furosemide dose in the group allocated to the continuous infusion of furosemide and administered furosemide of 62 and 157 mg in the continuous and intermittent infusion groups, respectively [36]. The daily dose of furosemide was fixed in five trials [32, 34, 37, 40, 41], three of which used the same accumulated dosages of furosemide per day between groups [34, 37, 40]; titration of the furosemide dose was allowed in the remaining seven trials. The durations of administrating furosemide varied across studies: 24 h (5 studies), 48 h (3 studies), 48 to 72 h (2 studies), and ≥ 100 h (2 studies). Primary outcomes varied across studies, and sample size calculation was performed in only four trials [31,32,33, 35]. All trials were published in full text. All trials were reported in English, except for one which was reported in Chinese [39]. Three trials were performed in India [34, 36, 37], two in the USA [30, 38], one in both Canada and the USA [31], and one each in China, Egypt, Israel, Italy, Spain, and Turkey [32, 33, 35, 39,40,41]. One trial author responded with data [34].
Risk of bias assessment
Overall, six trials (50%) had adequate sequence generation and four (33.3%) had adequately concealed allocations (Table 2). The study personnel and outcome assessors were judged to be adequately blinded in two (16.7%) and one (8.3%) trial(s), respectively. Seven (58.3%) and all studies were deemed to have a low possibility of incomplete outcome data and selective outcome reporting, respectively. Seven trials (58.3%) were free of industry sponsorship or conflicts of interest.
Primary outcomes
Five trials with 499 patients provided data on all-cause mortality. Two trials reported data at 1 month, another two reported data at 2 and 3 months, and the remaining one reported in-hospital mortality, respectively. The continuous infusion of furosemide was not associated with an improvement in all-cause mortality (RR 1.19; 95% CI, 0.65 to 2.16; p = 0.58; Q = 0.97; df = 4; I2 = 0.0%), compared with the intermittent infusion of furosemide (Fig. 1).
Eight trials involving 696 patients reported on the length of hospital stay. The continuous infusion of furosemide was not significantly associated with a reduction in length of hospital stay (WMD − 0.88 days; 95% CI, − 2.76 to 1.01; p = 0.36; Q = 54.04; df = 7; I2 = 87.0%), compared with the intermittent infusion of furosemide (Fig. 2). There was no evidence of publication bias (p = 0.91).
Seven trials with 626 patients reported on the reduction of body weight loss. The continuous infusion of furosemide was associated with a greater body weight reduction (WMD 0.63 kg; 95% CI, 0.23 to 1.02; p = 0.002; Q = 1.66; df = 6; I2 = 0.0%), compared to the intermittent infusion of furosemide (Fig. 3). There was no evidence of publication bias (p = 0.17).
Secondary outcomes
Nine (545 patients) and two (349 patients) trials reported on the amount of urinary output at 24 and 72 h, respectively. The continuous infusion of furosemide was not significantly associated with an increase in urine output at 24 h (WMD 489.17 mL; 95% CI, − 183.18 to 1161.51; p = 0.154; Q = 715.63; df = 8; I2 = 98.9%) (Supplemental Fig. 1) or at 72 h (WMD − 36.6 mL; 95% CI, − 335.9 to 386.9; p = 0.012; Q = 0.91; df = 1; I2 = 0.0%), compared to the intermittent infusion of furosemide. There was no evidence of publication bias for the outcome at 24 h (p = 0.41).
Seven trials screened for adverse effects, of which the most common were hypokalemia, hyponatremia, increased serum creatinine level, and hypotension. Compared with intermittent administration, the continuous infusion of furosemide was not associated with the incidence of hypokalemia (RR 1.41; 95% CI, 0.51 to 3.86; p = 0.51; Q = 6.12; df = 3; I2 = 51.0%), hyponatremia (RR 1.45; 95% CI, 0.75 to 2.80; p = 0.27; Q = 0.97; df = 2; I2 = 0.0%), increased serum creatinine level (RR 1.20; 95% CI, 0.85 to 1.69; p = 0.30; Q = 0.94; df = 3; I2 = 0.0%), or hypotension (RR 0.95; 95% CI, 0.48 to 1.88; p = 0.88; Q = 2.28; df = 1; I2 = 0.0%). Post-hoc analyses showed that the continuous infusion of furosemide was not associated with an increase in the change of serum creatinine level between before and after the intervention (Δ serum creatinine) (WMD 0.42 mg/dL; 95% CI, − 0.12 to 0.97; p = 0.129; Q = 372.30; df = 4; I2 = 98.9%) or in the absolute serum creatinine level at the end of the intervention (WMD, 0.18 mg/dL; 95% CI, − 0.06 to 0.41, p = 0.134; Q = 15.06; df = 3; I2 = 80.1%).
Sensitivity analyses
We conducted sensitivity analyses on all-cause mortality, length of hospital stay, body weight reduction, and 24-h urine output (Supplemental Table 2). A paucity of trials with adequate blinding of study personnel and outcome assessors precluded sensitivity analyses for these domains. Pre-planned sensitivity analyses otherwise yielded findings similar to the primary analyses.
Discussion
Our analysis suggested that the continuous administration of furosemide, compared to intermittent administration, was associated with a significant reduction in body weight and a tendency for increased 24-h urine output. There was no benefit in terms of all-cause mortality, length of hospital stay, and adverse events (hypokalemia, hyponatremia, increased serum creatinine level, and hypotension). Most sensitivity analyses were consistent with the primary analyses, thereby confirming the robustness of our findings.
There was no statistically significant difference in 24-h urine output between the two strategies; however, continuous administration was associated with a greater body weight reduction. There are some explanations for this inconsistency. First, three trials seemed to be outliers in terms of 24-h urine output. A post-hoc analysis excluding these trials tends to suggest that continuous infusion produced more 24-h urine output than intermittent administration, with reduced statistical heterogeneity (WMD 576.47 mL; 95% CI, 303.01 to 849.91; p < 0.001; Q = 8.09; df = 5; I2 = 38.2%). Second, trials with a higher risk of bias might have affected this outcome. Most sensitivity analyses with trials at lower risk of bias clearly showed that continuous infusion produced a significantly greater 24-h urine output than intermittent administration and reduced statistical heterogeneity. Thus, continuous infusion may generally produce an increased urine output compared to intermittent administration, which might lead to a great body weight reduction.
No significant benefits in all-cause mortality and length of hospital stay associated with continuous furosemide infusion were found in our analysis. Theoretically, a greater body weight reduction and urine output associated with continuous infusion of furosemide should help accelerate the reduction of congestive symptoms. However, ADHF is a multifactorial disorder [42]. While the continuous infusion of furosemide during the hospitalization may reduce the length of hospital stay and such a non-significant trend was confirmed in our analysis, it is reasonable that it does not directly affect mortality when the patients are discharged. Moreover, the sample size of each study was not calculated for these outcomes; thus, our meta-analysis may have been underpowered.
A theoretical merit associated with the continuous infusion of furosemide is the reduced possibility of electrolyte imbalance and hypotension. Our analysis, however, found no difference in hypokalemia, hyponatremia, and hypotension between the two strategies. This may have resulted from an underpowering due to the small number of trials included in the analyses, given that continuous infusion of furosemide was non-significantly associated with more frequent occurrence of adverse events.
An elevated serum creatinine level is a frequent and important adverse event associated with furosemide. Although statistically non-significant, our analyses suggest that the continuous infusion of furosemide can increase the incidence of increased serum creatinine level or the absolute serum creatinine level in comparison with the intermittent counterpart. Given that this tendency was consistent throughout the analyses, we may need to clinically recognize that the continuous infusion of furosemide is associated with an increased serum creatinine level in comparison with the intermittent furosemide administration.
Three previous systematic reviews have compared these two strategies of loop diuretics administration in heart failure: one focused on heart failure in general, including refractory chronic heart failure [21], another on hypervolemic status including ADHF [19], and the last on acute decompensated and chronic heart failure [20]. Nearly half of these included trials were small-sized crossover trials. As stated earlier, we focused on adults with ADHF in parallel-group randomized trials to investigate the exact efficacy of each furosemide strategy in a homogenous population in terms of diuretic resistance. A significant body weight reduction was consistently found in these reviews and ours. Unlike in our study, Salvador et al. found a significant reduction in the length of hospital stay and all-cause mortality with the continuous infusion of furosemide in refractory chronic heart failure or volume-overloaded status [20]. Two reviews reported a significant difference in 24-h urine output with the continuous infusion strategy; however, the participants were double-counted in some crossover trials [19, 20]. Such differences in patient and study designs might have resulted in clinical heterogeneity including diuretic resistance, thereby, leading to different findings.
Our study has some strengths. First, our search was comprehensive. We searched three large databases, supplemented by a manual search of two other platforms. This allowed us, compared to previous reviews that included both parallel-group and crossover trials, to review a larger number of participants as well as parallel-group trials. Second, we were able to examine clinically relevant, patient-oriented outcomes, such as mortality, length of hospital stay, and body weight reduction as our primary outcomes. Previous systematic reviews examined urine output as their primary outcomes, probably due to the limited number of parallel-group trials. Our study findings are more informative to clinicians who consider diuretic strategies. Third, we excluded crossover trials to eliminate the risk of a carryover diuretic effect and subsequent diuretic resistance. To our knowledge, this is the first systematic review of parallel-group randomized trials that purely compared two furosemide strategies in ADHF.
Our study also has limitations. First, the included trials differed in terms of diuretic protocols and the potential severity of heart failure. These might have led to high levels of statistical heterogeneity in the length of hospital stay and urine output. Lack of information on such variables precluded appropriate subgroup analysis. Second, most included studies were potentially at high risk of performance and attrition bias. Since six of our included studies allowed the titration of furosemide dosages at the treating physicians’ discretion, the study personnel should have been blinded from the type of interventions in order for a trial to be assessed as free of performance bias. A paucity of trials at low risk of such biases, however, precluded sensitivity analyses. Third, the durations of administrating furosemide in the included studies were relatively short and limited only to the acute phase of ADHF treatment; eight out of 12 studies used regimens with duration ≤ 72 h. Thus, our study failed to elucidate the impact on urine output and adverse events associated with either strategy of furosemide with a longer duration. Fourth, we did not discuss the adverse effects related to two furosemide strategies in detail. The CONSORT statement requires that trial investigators report “harms” associated with interventions [43]; however, randomized controlled trials in any fields generally under-report adverse events [44,45,46,47,48]. Only seven out of 12 trials partly reported on clinically important adverse events. Our study found no differences in hypokalemia, hyponatremia, serum creatinine level, and hypotension between the two strategies. Given that each of these outcomes was examined in a small number of studies, caution is still needed.
The continuous infusion of furosemide generally enhances body weight reduction, potentially increases urine output and reduces the length of stay in patients with ADHF. This evidence is derived from a relatively homogenous population and thus is reliable. Thus, clinicians need to select the continuous strategy, serially monitor patients, and try the intermittent one when there is inadequate urine response. Future studies need to select clinically homogeneous patients to reduce the variety of diuretic resistance, minimize the risk of performance and attrition bias, and report adverse events and patient-oriented outcomes.
Conclusions
Our study suggests that continuous infusion of furosemide leads to greater body weight reduction compared with intermittent administration. Limited available evidence suggests that there is no difference in adverse events between the two strategies. Thus, continuous infusion of furosemide should be considered for removing fluids in critically ill patients. However, the duration of the furosemide protocols examined in previous studies was mostly within 72 h, and thus their impact on urine output and adverse events in a longer duration remains to be elucidated.
Abbreviations
- ADHF :
-
Acute decompensated heart failure
- CI:
-
Confidence interval
- RR:
-
Risk ratio
- WMD:
-
Weighted mean difference
References
Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, Teboul JL, Monnet X (2013) Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med 41(2):472–480. https://doi.org/10.1097/CCM.0b013e31826ab377
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients I (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care (London, England) 12(3):R74. https://doi.org/10.1186/cc6916
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, Sepsis Occurrence in Acutely Ill Patients I (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34(2):344–353
Prowle JR, Kirwan CJ, Bellomo R (2014) Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 10(1):37–47. https://doi.org/10.1038/nrneph.2013.232
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, Blackwood B, Fan E (2017) Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 43(2):155–170. https://doi.org/10.1007/s00134-016-4573-3
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46(5):361–380. https://doi.org/10.5603/ait.2014.0060
National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354(24):2564–2575. https://doi.org/10.1056/NEJMoa062200
Jones SL, Martensson J, Glassford NJ, Eastwood GM, Bellomo R (2015) Loop diuretic therapy in the critically ill: a survey. Crit Care Resusc 17(3):223–226
Rocha BM, Menezes Falcao L (2016) Acute decompensated heart failure (ADHF): a comprehensive contemporary review on preventing early readmissions and postdischarge death. Int J Cardiol 223:1035–1044. https://doi.org/10.1016/j.ijcard.2016.07.259
Wakelkamp M, Alvan G, Gabrielsson J, Paintaud G (1996) Pharmacodynamic modeling of furosemide tolerance after multiple intravenous administration. Clin Pharmacol Ther 60(1):75–88. https://doi.org/10.1016/s0009-9236(96)90170-8
Hammarlund MM, Odlind B, Paalzow LK (1985) Acute tolerance to furosemide diuresis in humans. Pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther 233(2):447–453
Ellison DH (2001) Diuretic therapy and resistance in congestive heart failure. Cardiology 96(3–4):132–143
Copeland JG, Campbell DW, Plachetka JR, Salomon NW, Larson DF (1983) Diuresis with continuous infusion of furosemide after cardiac surgery. Am J Surg 146(6):796–799
Aaser E, Gullestad L, Tollofsrud S, Lundberg J, Hall C, Djoseland O, Kjekshus J, Forfang K (1997) Effect of bolus injection versus continuous infusion of furosemide on diuresis and neurohormonal activation in patients with severe congestive heart failure. Scand J Clin Lab Invest 57(4):361–367
Dormans TP, van Meyel JJ, Gerlag PG, Tan Y, Russel FG, Smits P (1996) Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion. J Am Coll Cardiol 28(2):376–382. https://doi.org/10.1016/0735-1097(96)00161-1
Kramer WG, Smith WB, Ferguson J, Serpas T, Grant AG 3rd, Black PK, Brater DC (1996) Pharmacodynamics of torsemide administered as an intravenous injection and as a continuous infusion to patients with congestive heart failure. J Clin Pharmacol 36(3):265–270
Lahav M, Regev A, Ra’anani P, Theodor E (1992) Intermittent administration of furosemide vs continuous infusion preceded by a loading dose for congestive heart failure. Chest 102(3):725–731
Pivac N, Rumboldt Z, Sardelic S, Bagatin J, Polic S, Ljutic D, Naranca M, Capkun V (1998) Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure. Int J Clin Pharmacol Res 18(3):121–128
Amer M, Adomaityte J, Qayyum R (2012) Continuous infusion versus intermittent bolus furosemide in ADHF: an updated meta-analysis of randomized control trials. J Hosp Med 7(3):270–275. https://doi.org/10.1002/jhm.991
Salvador DR, Rey NR, Ramos GC, Punzalan FE (2005) Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev (3):CD003178. https://doi.org/10.1002/14651858.CD003178.pub3
Wu MY, Chang NC, Su CL, Hsu YH, Chen TW, Lin YF, Wu CH, Tam KW (2014) Loop diuretic strategies in patients with acute decompensated heart failure: a meta-analysis of randomized controlled trials. J Crit Care 29(1):2–9. https://doi.org/10.1016/j.jcrc.2013.10.009
Higgins J, Green S (2008) Cochrane handbook for systematic reviews of interventions. Wiley cochrane series, 1st edn. Wiley, Chichester
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23(9):1351–1375. https://doi.org/10.1002/sim.1761
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Allen LA, Turer AT, Dewald T, Stough WG, Cotter G, O’Connor CM (2010) Continuous versus bolus dosing of furosemide for patients hospitalized for heart failure. Am J Cardiol 105(12):1794–1797. https://doi.org/10.1016/j.amjcard.2010.01.355
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM, Network NHFCR (2011) Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364(9):797–805. https://doi.org/10.1056/NEJMoa1005419
Llorens P, Miro O, Herrero P, Martin-Sanchez FJ, Jacob J, Valero A, Alonso H, Perez-Dura MJ, Noval A, Gil-Roman JJ, Zapater P, Llanos L, Gil V, Perello R (2014) Clinical effects and safety of different strategies for administering intravenous diuretics in acutely decompensated heart failure: a randomised clinical trial. Emerg Med J 31(9):706–713. https://doi.org/10.1136/emermed-2013-202526
Makhoul N, Riad T, Friedstrom S, Zveibil F (1997) Frusemide in pulmonary oedema: continuous versus intermittent. Clin Intensive Care 8(6):273–276
Malkiwodeyar PK, Hiregoudar N, Kabade D, Hasabi I (2017) Continuous versus bolus dosing of furosemide in the treatment of patients with acute decompensated heart failure. Sch J Applied Med Sci 5(9B):3551–3556. https://doi.org/10.21276/sjams.2017.5.9.17
Palazzuoli A, Pellegrini M, Ruocco G, Martini G, Franci B, Campagna MS, Gilleman M, Nuti R, McCullough PA, Ronco C (2014) Continuous versus bolus intermittent loop diuretic infusion in acutely decompensated heart failure: a prospective randomized trial. Crit Care (London, England) 18(3):R134. https://doi.org/10.1186/cc13952
Raghuraman M, Saravanavel A, Jayasingh K (2015) A comparative study of continuous infusion of frusemide vs intermittent bolus administration in congestive heart failure. Int J Health Sci Res 5(8):162–167
Shah RA, Subban V, Lakshmanan A, Narayanan S, Udhayakumaran K, Pakshirajan B, Krishnamoorthy J, Latchumanadhas K, Janakiraman E, Mullasari AS (2014) A prospective, randomized study to evaluate the efficacy of various diuretic strategies in acute decompensated heart failure. Indian Heart J 66(3):309–316. https://doi.org/10.1016/j.ihj.2014.03.006
Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB (2010) Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 16(3):188–193. https://doi.org/10.1016/j.cardfail.2009.11.005
Wang Y, Tian B, Xue L (2016) A comparative study of continuous and intermittent administration of furosemide in acute decompensated heart failure. J Clin Med 3(41):8154–8155. https://doi.org/10.16281/j.cnki.jocml.2016.41.044
Yayla C, Akyel A, Canpolat U, Gayretli Yayla K, Eyiol A, Akboga MK, Turkoglu S, Tavil Y, Boyaci B, Cengel A (2015) Comparison of three diuretic treatment strategies for patients with acute decompensated heart failure. Herz 40(8):1115–1120. https://doi.org/10.1007/s00059-015-4327-y
Ragab D, Taema KM, Farouk W, Saad M (2018) Continuous infusion of furosemide versus intermittent boluses in acute decompensated heart failure: effect on thoracic fluid content. Egypt Heart J 70(2):65–70. https://doi.org/10.1016/j.ehj.2017.12.005
Wencker D (2007) Acute cardio-renal syndrome: progression from congestive heart failure to congestive kidney failure. Curr Heart Fail Rep 4(3):134–138
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF (1996) Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 276(8):637–639
Kuriyama A, Umakoshi N, Sun R (2017) Prophylactic corticosteroids for prevention of postextubation stridor and reintubation in adults: a systematic review and meta-analysis. Chest 151(5):1002–1010. https://doi.org/10.1016/j.chest.2017.02.017
Kuriyama A, Endo K (2018) Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer 26(4):1051–1059. https://doi.org/10.1007/s00520-017-4028-6
Kuriyama A, Aga M, Maeda H (2018) Topical benzydamine hydrochloride for prevention of postoperative sore throat in adults undergoing tracheal intubation for elective surgery: a systematic review and meta-analysis. Anaesthesia 73(7):889–900. https://doi.org/10.1111/anae.14224
Kuriyama A, Maeda H, Sun R, Aga M (2018) Topical application of corticosteroids to tracheal tubes to prevent postoperative sore throat in adults undergoing tracheal intubation: a systematic review and meta-analysis. Anaesthesia. https://doi.org/10.1111/anae.14273
Kuriyama A, Katsura M, Urushidani S, Takada T (2018) Impact of polymyxin B hemoperfusion in the treatment of patients with sepsis and septic shock: a meta-analysis of randomized controlled trials. Ann Transl Med 6(11):206. https://doi.org/10.21037/atm.2018.05.41
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary materials
Supplemental Figure 1
Study selection. (PNG 241 kb)
Supplemental Figure 2
Urine output at 24 h (mL). (PNG 145 kb)
Supplementary Table 1
(DOC 27 kb)
Supplementary Table 2
(DOC 68 kb)
ESM 1
(DOC 63 kb)
Rights and permissions
About this article
Cite this article
Kuriyama, A., Urushidani, S. Continuous versus intermittent administration of furosemide in acute decompensated heart failure: a systematic review and meta-analysis. Heart Fail Rev 24, 31–39 (2019). https://doi.org/10.1007/s10741-018-9727-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9727-7