Abstract
Diabetes mellitus, a metabolic syndrome characterized by hyperglycemia and insulin dysfunction, often leads to serious complications such as neuropathy, nephropathy, retinopathy, and cardiovascular disease. Incretins, gut peptide hormones released post-nutrient intake, have shown promising therapeutic effects on these complications due to their wide-ranging biological impacts on various body systems. This review focuses on the role of incretin-based therapies, particularly Glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, in managing diabetes and its complications. We also discuss the potential of novel agents like semaglutide, a recently approved oral compound, and dual/triple agonists targeting GLP-1/GIP, GLP-1/glucagon, and GLP-1/GIP/glucagon receptors, which are currently under investigation. The review aims to provide a comprehensive understanding of the beneficial impacts of natural incretins and the therapeutic potential of incretin-based therapies in diabetes management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a metabolic syndrome disorder characterized by high blood glucose levels (hyperglycemia), defective action and/or secretion of insulin, impaired adipocyte’s secretory function, and multiple organ or tissue dysfunctions. Diabetes mellitus (DM), which is defined by hyperglycemia, describes a group of chronic metabolic disorders. DM complications, such as microvascular and neuropathic disorders may affect the lives of millions of people worldwide in the long term. It has been estimated that around 700 million people will live with DM by 2045 [1]. Apart from health problems, it will impose an economic burden of 776 billion USD on the health systems throughout the world [1]. The two primary forms of this disorder are type 1 (T1DM) and type 2 (T2DM). T1DM is an autoimmune disorder in which immune cells such as CD4 + and CD8 + T cells and macrophages invade the pancreatic islets and demolish the β cells, resulting in diminished insulin secretion [2]. However, the primary mechanism of T2DM is the dysfunction of synthesis, secretion, and response to insulin, which is known as insulin resistance [3]. Obesity and sedentary lifestyles are responsible for about 80% of T2DM [4]. Obese or non-obese T2DM patients develop insulin resistance due to alterations in cell receptors and post-receptor levels, respectively [5].
Long-term uncontrolled diabetes can lead to various complications, most importantly micro-and macrovascular, responsible for significant morbidity and mortality. In this regard, neuropathy, nephropathy, and retinopathy are categorized as microvascular complications, while macrovascular complications include cardiovascular, cerebrovascular, and peripheral artery diseases [6]. Besides, lung microvascular complications, nonalcoholic fatty liver disease (NAFLD), cancer, and atherosclerosis are the other nonclassical chronic complications of diabetes.
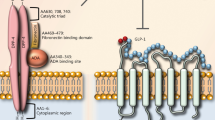
To date, various drugs and therapies have been used to control diabetic manifestations and complications, some of which have been relatively effective. Incretin-based therapy using the novel anti-diabetic agents, including injectable glucagon-like peptide-1 (GLP-1) agonists (Exenatide, Liraglutide, Albiglutide, dulaglutide, semaglutide (oral)) and oral dipeptidyl peptidase-4 (DPP-4) inhibitors (Alogliptin, Linagliptin, Saxagliptin, Sitagliptin, Vildagliptin) has shown promising impacts on diabetes and its complications [7, 8] (Table 1). Some of which, such as liraglutide, have had meaningful effects on obesity [9]. The newer agents, so-called twincretins and triple agonists such as tirzepatide, cotadutide (under development), and some molecules acting on GLP-1, GIP, and GLP-1/GIP/glucagon receptors respectively, have exerted substantial effects on glycemic control and bodyweight in various studies. The gut peptide hormones, incretins, are released after nutrient intake and provoke insulin secretion. GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) are known incretin hormones that exert an activity so-called the incretin effect, a phenomenon whereby oral glucose administration provokes further insulin release compared to intravenous injection at the same plasma glucose concentration [10]. Furthermore, GLP-1 is released in a lesser amount in the Central Nervous System (CNS) [11,12,13]. In addition to pancreatic β-cells, GLP-1 receptors are also expressed in the kidney, lung, heart, walls of arteries, and gastrointestinal tract [14], indicating the potential role of GLP-1R agonists in the therapy of diabetic complications. Moreover, there is evidence regarding the multiple biologic effects of incretin hormones on fat deposition, bones, cardiovascular system, nerve growth, appetite, obesity, blood glucose, and lipid metabolism [15,16,17,18,19,20,21,22], dysfunction of which is the leading cause of T2DM and its complications.
Hence, fat deposition, obesity, glucose, and lipid metabolism disorders are the leading causes of diabetes (T2DM), which in the long-term lead to multiple organ involvement, so-called diabetic complications. The present literature aims to review the beneficial impacts of natural incretins on human body systems, advantageous effects of incretin-based therapy on diabetes and its complications (Fig. 1), and finally, the novel incretin-based agents will be summarized.
Incretin hormones
The incretin hormones, GLP-1 and GIP are gut-derived peptide hormones, secreted by endocrine intestinal cells of L and K–types, respectively, [23, 24]. The K-cells are found in the duodenum and proximal jejunum, whereas the L–cells are expressed more in the ileum and less in the duodenum and even in the colon and rectum [25, 26]. Due to their crucial role in glucose homeostasis and the pathophysiology of T2DM, incretins have been broadly investigated so far [27].
Soon after nutrient intake, plasma concentrations of GIP and GLP-1 increase to reach their peak in almost one hour. Glucose and other carbohydrates, such as starch, sucrose, triglycerides, various amino acids and proteins provoke GIP and GLP-1 secretion, consequently stimulating insulin secretion [28, 29]. Incretins exert a phenomenon called the incretin effect, meaning that at the same plasma glucose concentration, oral glucose stimulates higher insulin secretion levels than glucose infusion [27, 30]. Besides their effect on insulin secretion, incretin hormones also affect glucagon release, so that while GLP-1 suppresses glucagon release, GIP stimulates it [31, 32]. Therefore, the insulinotropic effect, together with glucagon secretion inhibition of GLP-1, count as the prominent glucose-lowering mechanism of this hormone [33, 34]. However, it should be noticed that the function and activity of incretin hormones are not similar and completely equivalent in different tissues [24]. In contrast to GIP, which does not affect gastric emptying, GLP-1 slows it. On the other hand, GIP induces triglyceride storage, an activity that is unlikely to be done by GLP-1 [32, 35, 36]. As mentioned previously, the beneficial role of incretin receptors in improving diabetic complications can be postulated.
The beneficial effects of incretin-based therapy on diabetes and its complications. Incretins, the gut peptide hormones, are released after nutrient intake and provoke insulin secretion. In addition to pancreatic β-cells, incretin receptors are also expressed in the kidney, lung, heart, walls of arteries, CNS, and gastrointestinal tract which result in multiple biologic effects in various body tissues. The advantageous effects of incretins on diabetic complications have been demonstrated. The newer anti-diabetic agents (GLP-1 agonists and DPP-4 inhibitors) are supposed to improve diabetic complications
Incretin hormones in T2DM
Insulin resistance, inadequate insulin secretion, and hyperglucagonaemia are the main reasons for T2DM occurrence [37]. Regarding the role of incretin hormones in stimulating insulin secretion and the GLP-1 effect on glucagon release suppression, a review on the role of incretin hormones in T2DM would be rational. Although various studies and meta-analyses have not found significant differences in nutrient-induced incretin secretion between healthy individuals and T2DM subjects [38,39,40], different experiments indicated that GIP could not stimulate insulin secretion in patients with T2DM [31, 41, 42]. Nonetheless, the insulinotropic and glucagonostatic effects of GLP-1 have been well documented [31, 43,44,45]. Insulin secretory response to glucose decreases by 25% in patients with T2DM compared to healthy individuals [46]. This level of activity of GLP-1 in T2DM patients, however is adequate for a proportional reduction in plasma glucose [47]. Considering the prominent role of GIP in the incretin effect in healthy subjects, this phenomenon is decreased or even lost in T2DM patients [48, 49]. Despite the minimal impact of GIP on insulin secretion in patients with Type 2 Diabetes Mellitus (T2DM), and the hormone’s glucagonotropic effect, dual agonists that target both GLP-1 and GIP (such as tirzepatide) have demonstrated promising results in promoting weight loss. Besides, based on data indicating the effect of GIP on enhancing triglyceride deposition, GIP receptor antagonists have been suggested for the therapy of pre-diabetic subjects and metabolic disorders [50, 51]. On the contrary, as mentioned above, because GLP-1 sustains its effect on glucose metabolism, insulin secretion, and glucagon suppression in patients with T2DM, receptor agonist of this hormone has been widely used in the therapy of diabetes [27, 52].
Effect of incretins on appetite, caloric intake, and body weight
Approximately 80% of T2DM is linked to obesity and physical inactivity, as the crucial role of these factors in T2DM improvement is well-documented [4, 53]. Obesity causes excessive triacylglycerol (a fatty acid metabolite) and other fatty acid metabolites to deposit in the sarcoplasm of skeletal muscles [4], resulting in insulin signal inhibition [54, 55]. Moreover, insulin signaling suppression is associated with the level of circulated fatty acids [56]. Therefore, in obese patients with T2DM, insulin resistance impairs the ability of β-cells to compensate for decreased insulin sensitivity [57]. Besides, it has been documented that there is a profound relationship between physical activity and a reduction in T2DM incidence [4].
Incretins influence obesity through different mechanisms, such as fat storage promotion, gastric emptying slowness, appetite suppression, and satiety increase. In animal models, GIP enhanced fat storage in subcutaneous adipose tissue by lipoprotein lipase induction [17, 32, 36]. However, animals with a GIP receptor knockout did not get fat during a high-fat diet [17]. Furthermore, some studies have documented the hypersecretion of GIP in obesity to prevail over the metabolic disorders resultant from insulin resistance [32, 58]. Conversely, GLP-1 secretion is decreased in obese subjects for an unknown reason. Besides, GLP-1 injection increases satiety and decreases food intake and appetite [59], indicating this hormone’s potential role in the pathogenesis of obesity. Consequently, various studies have focused on the potential therapeutic effect of GLP-1 on obesity in diabetic and non-diabetic subjects [60,61,62,63,64,65]. Moreover, the subjects with obesity and T2DM receiving GLP-1 analogs exerted metabolic and appetite responses comparable to healthy individuals [16, 66,67,68,69]. Therefore, studies on GLP-1R agonists (GLP-1RAs) and DPP4 inhibitors were raised, eventually leading to the introduction of two groups of therapeutic agents used as incretin-based therapy [27, 70]. In addition to being broadly used as a blood sugar lowering agent, GLP-1RAs have recently been approved for weight management in the United States [71, 72]. In this regard dual agonists affecting on GLP-1/GIP (tirzepatide) have shown encouraging impacts on weight loss [73, 74]. Semaglutide, the only oral GLP-1R agonist (currently) has exerted inspiring effects on bodyweight reduction as well [75, 76]. Besides, various studies have shown that through some surgical methods, such as bariatric surgery and Roux-en-Y gastric bypass, GLP-1 secretion increases significantly, associated with further excess weight loss and blood glucose improvement [77,78,79]. However, as reviewed above, the potential therapeutic role of incretins in weight loss is not negligible. Differential effects of GLP-1 and GIP on various biological functions are summarized in Table 2.
Incretin effects on the peripheral and central nervous system
GLP-1R is also found in the central nervous system in the hypothalamus and brainstem, and in the peripheral nervous system (PNS), on afferent branches of the vagus nerve which originate from the vicinity of the intestinal L cells [80,81,82]. Since many diabetic patients suffer from neuronal or psychological complications of diabetes, pharmaceutical agents capable of ameliorating both hyperglycemia and neuronal complications of the disease may be of great importance. These agents might offer new treatment approaches for neurodegenerative diseases, such as Alzheimer’s disease, as well as debilities in cognition and memory [83]. Even some unrelated diabetes complications, such as obesity may be affected by the CNS which is partially regulated by GLP-1 receptors [82]. The benefits of incretin mimetics are also seen in non-diabetic patients, suggesting favorable central or peripheral neuronal roles which may be detached from their effect on plasma glucose levels [83, 84].
As mentioned above, GLP-1Rs are located on the vagus nerve and their stimulation by incretin ligands can hinder stomach emptying. This phenomenon, which is not merely insulin-dependent, can decrease postprandial glucose levels [85]. GLP-1 can ameliorate postprandial lipemia by diminishing chylomicron biosynthesis in the intestine [86]. It is mediated via the interaction of GLP-1 with melanocortin-4 receptors in the CNS in a brain-gut axis which may be a promising therapeutic strategy for hyperlipidemia and hyperchylomicronemia in diabetic patients [87]. Apart from its effect on gastric transit time, GLP-1 has an anti-appetite effect by stimulating its relevant receptors in the CNS. It seems that the vagus nerve mediates the transition of the satiety signals between the alimentary tract and the CNS, where signals are received by the solitary tract nucleus and are relayed to the hypothalamus to be sent back for food intake control [88].
Diabetic neuropathy spans a vast spectrum of sufferings from simple pain to death. Considering various groups of anti-diabetic agents, the incretin-based drugs (GLP-1 agonists and dipeptidyl peptidase-4 inhibitors) stand at the top of the anti-diabetic agents for managing peripheral neuropathy of diabetes [89]. Besides the incretins’ possible role in improving axonal regeneration and neural repair, hippocampal expression of GLP-1R and GIPR suggests their involvement in memory formation and synaptic plasticity, a concept highlighted by learning defects in GLP-1R knockout mice. Incretins also hinder the progression of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. These agents also impede associated memory and cognitive defects in animal models of the diseases, as mentioned earlier [89, 90]. In myelinated motor nerves and unmyelinated pain neurons, GLP-1R may have some roles in their function regardless of its glycemic controlling effects [90]. Some studies show that DPP-4 inhibitors can inhibit diabetic neuropathy in rodents [91].
The animal model findings are not confirmed unequivocally in human studies, maybe because of the small sample sizes and the paucity of clinical trials. For instance, concerns regarding the increased risk of pancreatitis due to GLP-1RAs observed in animal studies have not been consistently replicated in human trials, likely due to variations in study design and participant characteristics [92, 93]. In one clinical trial, exenatide did not improve diabetic peripheral neuropathy or the electrophysiologic profile of the nerves [94]. In another clinical trial, the liraglutide effect on improving polyneuropathy was discouraging [95]. It seems that further studies are required before concluding about the effect of GLP-1 and its receptor in human diabetic neuropathy [96].
Cardiovascular effects of incretin system
DM-associated cardiovascular disease (CVD) and stroke are the leading causes of morbidity and mortality in patients with diabetes [97]. Death due to coronary heart disease occurs much more frequently in diabetic patients than non-diabetic subjects (3 to 5-fold) [98, 99], especially those with other comorbidities such as hypertension and dyslipidemia who are at increased risk. Management of these risk factors may improve cardiovascular health. However, there is not adequate evidence regarding the favorable effect of conventional oral anti-diabetic drugs on cardiovascular disorders in diabetes [100]. Studies have shown the multiple effects of GLP-1 on the cardiovascular system [18, 19]. Various reports have presented the contradictory effects of GLP-1 and GLP-1RAs on endothelial function, atherosclerosis progression, and cardiac blood supply [18, 19]. The embryonic development of the cardiovascular system seems to be associated with GLP-1 activity. In a human study, treatment with GLP-1 decreased the ability of tumor necrosis factor-alpha (TNF-α) to induce gene and protein expression of plasminogen activator inhibitor type-1 (PAI-1), a prominent factor of endothelial cell dysfunction [101, 102]. Similarly, a randomized study indicated that GLP-1 administration in patients with T2DM increased the flow-mediated vasodilation, an effect absent in healthy individuals, suggesting the potential role of GLP-1 in improving endothelial dysfunction associated with atherosclerosis [103]. However, data on the effect of GLP-1 and/or GLP-1RAs on blood pressure have been controversial [104,105,106,107]. On the other hand, GLP-1RA agonists, like exenatide demonstrated cardioprotective effects by lowering apoptosis and oxidative stress [108]. The GLP-1 signaling pathway leads to a decrease in the proapoptotic markers such as caspase-3&9, Bax/Bcl-2, and p53 in cardiac Tissue (Fig. 2) [109]. Many human studies have been carried out to evaluate the cardioprotective effects of GLP-1 and GLP-1RAs. For example, GLP-1 infusion (4 weeks) in patients with severe heart failure improved left ventricular function, functional status, and Quality of Life (QoL) scores in subjects with and without diabetes. GLP-1 administration also improved left ventricular ejection fraction (LVEF) in patients with acute myocardial infarction [110, 111]. It is suggested that GLP-1 acts through two main pathways. The first one is through GLP-1R activation, which induces glucose uptake, ischemic preconditioning, and mild vasodilatory actions; and the second pathway is GLP-1R–independent, meaning that GLP-1 independently affects the cardiac post-ischemic recovery and vasodilation, probably via NOS-induced-cGMP formation [112]. In this regard, treatment with liraglutide in mice has improved the reduction of the endothelial nitric oxide synthase (eNOS), a crucial enzyme for vascular nitric oxide (NO) synthesis [113]. NO induces the production of cGMP, an intracellular second messenger that consequently stimulates phosphodiesterases (PDE) and cGMP-dependent protein kinases (PKGs) effectors. Hypertrophy inhibition, vaso-relaxation, and cellular proliferation are mediated by these effector molecules in the cardiovascular system [114]. Besides, the correlation between eNOS induction and reduction in TNF expression and NF-κB stimulation in cardiomyocytes has been documented (Fig. 2) [113]. However, these findings altogether indicate the cardiovascular protective effects of GLP-1R stimulation.
Effect of incretin-based therapy on cardiovascular activity in T2DM
Compared to non-diabetic individuals, diabetic subjects are at a much higher risk of cardiovascular (CV) events, so CVD accounts for almost 80% of the mortality in individuals with T2DM. Metabolic risk factors, such as insulin resistance, dyslipidemia, obesity, and hypertension contribute to CVD manifestation [115]. The beneficial cardiovascular effects of GLP-1RAs have been approved. In this regard, Nathanson et al. showed that two-day administration of exenatide in patients with T2DM and heart failure improved cardiac output and reduced pulmonary capillary pressure [116]. Notably, these promising effects of GLP-1RAs on the CV system are reported to be independent of glycemic control, likely through improvements in vascular risk factors and atherosclerosis [115, 117, 118].
Several significant trials have focused on the impact of GLP-1RAs on cardiovascular outcomes in patients with T2DM and elevated cardiovascular risk. These studies specifically examined the impact on cardiovascular mortality, non-fatal myocardial infarction (MI), and non-fatal stroke [119,120,121,122]. All trials confirmed the cardiovascular safety of GLP-1RAs, with several showing significant efficacy in reducing cardiovascular events. Noteworthy among these are the LEADER outcomes trial for liraglutide [117], the HARMONY trial for albiglutide [122], the REWIND trial for dulaglutide [121], and the SUSTAIN-6 trial for injectable semaglutide [118]. Liraglutide significantly lowered both cardiovascular and all-cause mortality, and both liraglutide and injectable semaglutide demonstrated improvements in kidney outcomes [117, 118]. Variations in trial outcomes may be due to the cardiovascular risk profiles of participants and the specific GLP-1RA class used. While A recent meta-analysis showed a 14% overall reduction in major adverse cardiovascular events another meta-analysis indicated greater benefits of GLP-1RAs in patients with established CVD compared to those without, highlighting the need for more studies on primary prevention [123]. Emerging evidence suggests GLP-1RAs could significantly reduce MI risk. Clinical studies have shown that GLP-1RAs, such as exenatide and liraglutide, administered during acute MI, can reduce infarct size and improve cardiac function [124, 125]. Furthermore, a prospective observational study with 17,868 diabetic patients discharged after their first MI event found that GLP-1RA use was associated with a reduced risk of stroke, heart failure, re-infarction, and cardiovascular death compared to standard diabetes care [126]. While GLP-1 RAs have demonstrated cardiovascular benefits, some evidence suggests that they may increase the risk of hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF). Therefore, until more randomized studies are conducted, it is advisable to avoid using GLP-1 RAs in patients with HFrEF [127].
Evidence regarding the impact of DPP4 inhibitors on cardiovascular events is paradoxical [164, 165]. Some studies have suggested that diabetic patients treated with DPP4 inhibitors experience fewer cardiovascular events compared to those on other anti-diabetic drugs [128, 129]. For instance, a meta-analysis reported that DPP4 inhibitors treatment is associated with a reduced risk of main adverse cardiovascular events [128]. Another one meta-analysis study on 40 trials suggested that long-term treatment of patients with T2DM with DPP-4 inhibitors and GLP-1As is associated with a lower risk of myocardial infarction compared to those receiving sulfonylurea drugs [130]. Despite this, several randomized controlled trials have been conducted to evaluate the cardiovascular outcomes of DPP4 inhibitors in diabetic patients, and none demonstrated significant cardiovascular benefits [131,132,133]. Notably, the SAVOR-TIMI 53 trial found that saxagliptin was linked to an increased risk of hospitalization for heart failure [134].
Anti-atherogenic role of incretin-based therapy
Numerous preclinical and experimental studies have highlighted the anti-atherogenic properties of GLP-1 by reducing aortic macrophage recruitment and atherosclerotic lesion formation [135]. Sudo et al. demonstrated that lixisenatide improves advanced atherosclerotic plaques in rabbits, increasing fibrotic areas and decreasing necrotic and calcified areas, without changing the overall plaque size [136]. Incretin treatment, specifically with GLP-1RAs, is linked to stable plaque characteristics, showing increased collagen and sirtuin-6, with reduced inflammation and oxidative stress in carotid plaques from diabetic patients [137]. The long-term effects of GLP-1RAs on atherosclerosis and during percutaneous coronary interventions remain unclear, with mixed evidence. Some studies suggest a link between GLP-1 levels and coronary artery disease progression [138], while others show GLP-1’s protective role after myocardial ischemia, such as exenatide reducing infarct size in both animal models and clinical settings [108].
DPP4 inhibitors have shown promising protective effects against atherosclerosis through multiple mechanisms. These inhibitors improve endothelial cell dysfunction, which is crucial for maintaining vascular health and preventing atherosclerosis [139, 140]. They regulate blood lipids by reducing LDL cholesterol and triglycerides and increasing HDL cholesterol, thereby mitigating atherosclerotic risk factors [141, 142]. Additionally, DPP4 inhibitors lower both systolic and diastolic blood pressure, contributing to a reduction in atherosclerotic progression [143, 144]. Inflammation and oxidative stress, key factors in the development of atherosclerosis, are significantly suppressed by DPP4 inhibitors [139, 143, 145]. They also affect mononuclear macrophages by reducing foam cell formation, which is essential in early atherosclerotic plaque development [146,147,148]. The inhibitors further inhibit the proliferation and migration of smooth muscle cells, thereby reducing intimal hyperplasia and stabilizing atherosclerotic plaques [149, 150]. By reducing the levels of MMP2 and MMP9, DPP4 inhibitors (Sitagliptin) enhance plaque stability, which lowers the risk of plaque rupture and subsequent cardiovascular events [151, 152]. Additionally, these inhibitors increase the levels of circulating endothelial progenitor cells (EPCs), aiding in vascular repair and maintenance [153, 154]. In conclusion, DPP4 inhibitors hold significant potential in preventing and treating atherosclerosis and related cardiovascular diseases through various mechanisms. However, more research is needed to fully understand their long-term effects and underlying mechanisms..
Renoprotective effect of incretin-based therapy in T2DM
Diabetic kidney disease (DKD) happens in 20-40% of patients with diabetes and is the main leading cause of the end-stage renal disease (ESRD) that requires dialysis treatment and affects their QoL [155, 156]. Moreover, DKD not only increases the risk of cardiovascular disease in patients, even in the early stages [157], but the negative linear relationship between the glomerular filtration rate (GFR) and mortality is reported [158]. Several pathogeneses have been described, such as hemodynamic alterations causing glomerular hypertension, oxidative stress [157], mitochondrial dysfunction [159], endoplasmic reticulum stress [160], activation of cytokines, profibrotic factors, inflammation, and growth factors [161, 162]. The current interventions to slow down the DKD development consist of controlling blood pressure and blood glucose level, decreasing urinary albumin excretion, and stopping smoking [157].
An experiment in rats has shown the GLP-1R mRNA expression in the proximal tubules and glomeruli (1). Moreover, a human study indicated that GLP-1R was expressed in tubules and glomeruli (2); however, another study stated that proximal tubules were the dominant location of GLP-1R expression (3). This discrepancy can be explained by the limitation of sensitivity and specificity of antibodies against these receptors (4). Studies on rats have shown the enhancement of DPP-4 activity in response to a high-fat diet or obese state (5) and downregulation of the expression of GLP-1R in the tubules of diabetic rats (6), demonstrating the potential role of incretin-based therapies against diabetic nephropathy. The probable mechanism underlying the beneficial effect of GLP-1RA and DPP-4 is mainly through the inhibition of sodium–hydrogen exchanger 3 (NHE3), which ultimately results in natriuresis as a result of inhibition of sodium reabsorption from the proximal tube. Moreover, tubuloglomerular feedback is activated because of enhanced sodium chloride delivery to distal tubules, leading to diminished glomerular hyperfiltration and pressure [163]. Furthermore, calcium, phosphate, chloride, and bicarbonate are excreted as well; however, the excretion of potassium is not influenced (Fig. 3) [164]. It has been shown that in the urine of individuals with T2DM, DPP-4 was found to correlate with albuminuria [165], which offers DPP-4 as a marker of tubular injury in diseases affecting the glomerulus, including DKD, lupus nephritis, and glomerulonephritis in this population [166].
Various studies in DKD models have demonstrated the beneficial effects of GLP-1RAs and DPP-4 inhibitors in lowering proteinuria and glomerular sclerosis by reducing oxidative stress and inflammation and protecting against endothelial injury [157]. The administration of DDP-4 inhibitors in rats with T1DM increased the serum concentration of active GLP-1, declined urinary albumin excretion, and improved diabetic nephropathy histology. They inhibited macrophage infiltration, inflammatory molecules, and downregulated nuclear factor NF-κB activity in the kidney [167]. Along with these effects, no significant change in glycemic profiles was observed, indicating anti-inflammatory and antifibrotic renoprotective impacts of DDP-4 inhibitors, such as sitagliptin, linagliptin, and vildagliptin through amplifying serum concentration of active GLP-1 [168]. Sitagliptin has decreased tubulointerstitial, glomerular, and vascular lesions in type 2 diabetic Zucker diabetic fatty (ZDF) rats [169]. Moreover, this drug attenuated the inflammatory cytokines and apoptosis of cells in the kidney, which diminished the glomerulosclerosis and tubulointerstitial fibrosis [170]. In addition, exenatide decreased the concentration of TGFβ (Transforming growth factor), a diabetic nephropathy-related cytokine [171].
In several clinical studies, the renoprotective effects of incretin-based therapies have been reported [172, 173]. For instance, in studies evaluating semaglutide [118] and liraglutide [117] effects on renal function, they lowered the rate of new-onset or deterioration of established nephropathy compared to the placebo group. Moreover, lixisenatide lowered the risk of new-onset macroalbuminuria even after adjustment for HbA1c concentration [174]. Importantly, liraglutide in the LEADER trial not only improved albuminuria, but reduced secondary kidney outcomes, such as the persistent doubling of the serum creatinine level, new-onset persistent macroalbuminuria, kidney failure, or death due to kidney disease, which was not observed by exenatide [175], albiglutide [176], linagliptin [132], dulaglutide [121], saxagliptin [177] and alogliptin [178] treatment in other studies. Linagliptin diminished albuminuria and UACR independent of HbA1c and systolic blood pressure (32). In two distinct studies about the effects of linagliptin and alogliptin, no beneficial effect was observed when the drugs were used as monotherapy. Nevertheless, adding these drugs to patients’ established treatment based on blocking the renin-angiotensin system remarkably diminished the albuminuria in patients with type 2 DM [179, 180]. It should be noted that though several studies failed to indicate the renoprotective impacts of DDP-4 inhibitors, they emphasized the safety and tolerability of these medications in patients with renal disorders [181,182,183].
The renoprotective effects of incretin-based therapies can be attributed to the modification of renal risk factors as well. In this regard, GLP1RA has been shown to reduce waist circumstance, body weight, visceral and trunk fat, and systolic blood pressure [184, 185]. This blood pressure reduction was significant when liraglutide and albiglutide were used. However, with exenatide and dulaglutide, this effect was insignificant compared to placebo [186]. On the other hand, the direct impact of GLP-1RA on the kidney is presumed to have a positive effect on albuminuria regardless of changes in hyperglycemia, blood pressure, and body weight [187]. It creates a consensus about the presence of GLP-1R in the kidney vessels [188]. Nonetheless, different trials failed to demonstrate the desirable effect of GLP-1Ras on renal hemodynamics, mainly on eGFR, reduction of which over time manifests a decline in intraglomerular pressure [187, 189,190,191,192,193,194,195,196]. Besides, sodium secretion resulting from inhibition of NHE3 was not persistent in the long term [187]. The central role of inflammation in DKD is demonstrated by several studies [197, 198], which motivated the scientists to seek the effect of incretin-based therapies on inflammation. In this regard, numerous studies on animals and humans showed the anti-inflammatory effect of GLP-1RA and DPP-4 inhibitors through attenuating inflammatory cytokines and markers, such as IL-1β, TNF-α, chemoattractant protein-1 (MCP1), and high-sensitivity C-reactive protein (hs-CRP) in DKD cases and models [170, 174, 199,200,201,202]. Moreover, these results strongly suggest that the renoprotective impact of GLP-1RAs might be exerted through inflammation suppression [203]. Furthermore, the mechanism behind the favorable effects of GLP-1RAs is speculated to be through reducing inflammation, improvement of renal substrate metabolism resulting in improved insulin sensitivity, production of valuable systemic metabolites through activation of GLP-1R agonism [204], or direct effect of GLP-1 on tubules of the kidney regardless of the mechanism mediated by the receptor [205]. Furthermore, GLP-1RAs show an antioxidant effect by lowering oxidative markers and improving oxidative damage, as reported by various investigations [200, 203, 206, 207]. For instance, exenatide is shown to exert an anti-inflammatory effect by directly inhibiting the generation of H2O2-induced free radical species, attenuating the lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), and malondialdehyde (MDA) levels [200, 207]. It is stated that the cAMP-dependent pathway could be responsible for the observed antioxidant impact [208]. Teneligliptin is shown to reduce free radical species generated by NADPH oxidase activity and act as an antioxidant [209]. According to the evidence, GLP-1 can regulate NO synthesis [210, 211], which can be beneficial for preventing renal failure progression in diabetes [212]. Moreover, NO synthesis modulation by exenatide lowered blood pressure [213]. Thomson et al. revealed that exenatide improved renal hemodynamics and GFR by NO synthesis-related mechanism [212]. In a study by Jensen and coworkers, hypertensive animals with problems in kidney functions GLP-1 receptors were reduced in the renal tissues, indicating the major role of GLP-1 in the homeostasis of the renal vasculature and renal function [214]. Other studies have confirmed the modulatory function of GLP-1 agonists on the renal renin-angiotensin system as well.
Effect of incretin-based therapy on diabetic retinopathy
Various clinical trials and studies have been carried out to scrutinize the effects of GLP-1RAs and DPP-4 inhibitors on diabetic retinopathy. It has been shown that subcutaneous injection of semaglutide, but not oral administration, in the SUSTAIN-6 study resulted in an increase in retinopathy in Type 2 DM patients [118, 120]. Nevertheless, a posthoc analysis of the SUSTAIN-6 trial did not demonstrate any difference in the incidence of diabetic retinopathy between the semaglutide and placebo groups in patients with no pre-existing diabetic retinopathy [215]. Moreover, liraglutide [117], albiglutide [216], and exenatide [217] in different studies showed progression of diabetic retinopathy. It should be noted that this increase in the liraglutide group was not statistically significant, and in follow-up of patients receiving exenatide the improvement or stability of diabetic retinopathy was reported. Besides, when GLP-1 RAs were compared to two or more oral glucose-lowering drugs in a study, the overall incidence of diabetic retinopathy was not enhanced. Nevertheless, the secondary analysis showed that GLP-1RA was associated with a transient 44% increased risk of diabetic retinopathy over 6–12 months, particularly in patients with arterial hypertension [218]. The possible explanations for these results seem to be due to a short period of diabetic retinopathy follow-up, lack of baseline diabetic retinopathy staging, a rapid drop in HbA1c, and retinal microvascular angiogenesis [219]. On the other hand, no association between GLP-1RA exposure and severe diabetic retinopathy has been reported by several studies [175, 215, 218, 220,221,222,223], even among patients with pre-existing ocular disease [224]. A meta-analysis revealed the safety of GLP-1RAs for diabetic retinopathy treatment [225]. Based on the FDA (Food and Drug Administration) report regarding ocular adverse events due to GLP-1RAs, such as vitreous hemorrhage, diabetic retinopathy, proliferative diabetic retinopathy, macular edema, or blindness, GLP-1RAs did not enhance the diabetic retinopathy development risk [226]. Hernandez and coworkers found that systemic administration of liraglutide in db/db mice inhibited retinal neurodegeneration by stimulating the AKT pathway, a vital pathway for retinal neurons to survive [227]. Moreover, they observed these desirable effects via administered liraglutide, native GLP-1, lixisenatide, or exenatide topically, suggesting that the neuroprotective effects are independent of the type of GLP-1 RA and blood glucose levels [227]. Besides, GLP-1RA therapy in diabetic rats improved B wave and OPs [228], the most sensitive index of electrophysiology of diabetic retinopathy declined in diabetic retinopathy. The differences in the structure of the medication could explain the observed discrepancy. It is essential to state that in the various studies examining GLP-1RA-related diabetic retinopathy, a standard AE report was used instead of a retinal image, except for one retrospective study in which a robust method was applied [229]. The results of a clinical trial (FOCUS), which will analyze semaglutide-related ocular events via retinal imaging, are expected to provide significant insights into this issue [135].
The detrimental effect of DDP-4 inhibitors on diabetic retinopathy has been reported recently. Lee and coworkers stated that DPP-4 inhibitors in their murine diabetic retinopathy model aggravated diabetic retinopathy after one week of treatment due to increased retinal vascular leakage [230].
On the other hand, Kolibabka et al. indicated the anti-angiogenic effects of linagliptin, a DPP-4 inhibitor [231]. Vildagliptin could inhibit the thrombogenic reactions and inflammation in the retina of Otsuka Long-Evans Tokushima Fatty rats (OLETF rats), models of obese type 2 diabetes [232]. Moreover, topical DPP-4 inhibitors have shown preventive effects on vascular leakage and neurodegeneration in the retinas of mice [233]. Besides, in a study by Kim et al., the overall risk of diabetic retinopathy did not increase with DPP-4 inhibitor treatment. At the same time, it was significantly raised within 12 months after treatment initiation and later decreased with no rational explanation.
It should be noted that the impact of undesirable glycemic control on diabetic retinopathy risk in the treatment group compared to the control one cannot be excluded [234]. Sitagliptin is shown to inhibit the breakdown of the blood-retina barrier in type 1 and 2 diabetes models through the alteration made in dispersing tight junction proteins, inflammatory cytokines, like IL-1β, and apoptosis-induced cell death. Moreover, it prevented the decline of endothelial progenitor cells (EPCs) adhesion to the retinal vessels [235, 236]. In a double-blind, placebo-controlled trial scrutinizing the six-week saxagliptin treatment effect on microvascular changes in retina in type 2 diabetes, it normalized the retina capillary flow (RCF) and ameliorated central hemodynamics [237]. These findings suggest that the beneficial effects of DPP-4 inhibitors are attributed to inhibiting retinal cell nitrosative stress, inflammation, and apoptosis; however, it should be tested to determine whether these effects are related to GLP-1 [90].
Novel incretin-based agents at a glance
Semaglutide
Semaglutide, initially designed as an injectable long-acting GLP-1RA, was approved in the United States in September 2019 as an oral compound [238]. With a slight change in the amino acid sequence of natural GLP-1, semaglutide has been developed to improve albumin binding, renal clearance, DPP-4 cleavage, and gastric mucosa absorption [239]. To protect the peptide-based construction of Semaglutide from proteolytic enzymes and gastric pH, the absorption enhancer sodium N-[8-(2-hydroxybenzoyl) amino] caprylate was added to the oral tablet [239, 240]. For predictable absorption, it should be given on an empty stomach with limited volumes of water (120 ml) [241]. The impacts of Semaglutide on glycemic control and body weight reduction have been demonstrated in different clinical trials [75, 76, 242]. The PIONEER clinical trial program consisted of eight Phase 3 trials designed to evaluate the efficacy and safety of oral semaglutide, a novel GLP-1 receptor agonist, in patients with type 2 diabetes [243]. In the PIONEER trials, oral semaglutide (14 mg) exerted a large reduction in HbA1c compared to placebo, empagliflozin, sitagliptin, liraglutide, and dulaglutide [75, 76, 242, 244, 245]. In addition, the efficacy of semaglutide in weight reduction across the PIONEER trials has been documented. Besides, 14 mg of oral semaglutide resulted in greater weight loss compared to the drugs mentioned above [75, 76, 242, 244, 245]. The cardiovascular safety of oral semaglutide was investigated in the PIONEER 6 trial. Compared to the placebo group (4.8%), the major adverse cardiovascular events were lower (3.8%) in the oral semaglutide group [120]. Given the oral dosage form of the drug compared to other GLP-1 analogues, its efficacy in glycemic control and weight loss, and its relatively safe cardiovascular profile, it can improve patient compliance.
Dual and triple agonists
Besides the GLP-1 receptor agonists with encouraging efficacy in glycemic control and weight loss, new agents acting on two or more different enteropancreatic receptors, including dual agonists acting on GLP-1/GIP or GLP-1/glucagon, and triple agonists stimulating GLP-1/GIP/glucagon receptors, are under development.
Dual agonists stimulating GLP-1 and GIP receptors (twincretins)
Tirzepaptide, a novel, once-weekly injectable agent recently approved for chronic weight management in adults with obesity or overweight and also to be used along with diet and exercise to help improve blood sugar (glucose) in adults with type 2, is a synthetic peptide with dual agonist activity on GLP-1 and GIP receptors [246, 247]. It has shown significant A1C and weight reductions in the SURPASS global clinical development program. The SURPASS trials (SURPASS-1-6) were a global series of Phase 3 clinical studies designed to evaluate the efficacy and safety of tirzepatide in various patient populations with type 2 diabetes. Across all SURPASS trials, tirzepatide consistently met its primary and key secondary endpoints for efficacy. Participants experienced sustained A1C reductions and progressive weight loss. The safety profile of tirzepatide was favorable, with gastrointestinal, nausea, diarrhoea and vomiting side effects being the most commonly reported adverse events [248, 249]. It decreases insulin resistance associated with lowering fasting insulin level and HOMA2-IR (an Insulin resistance index) that attenuates pancreatic beta cell stress to secrete insulin and improves insulin sensitivity [246]. The advantageous insulin-sensitizing action of tirzepatide seems to be mediated by weight reduction and activation of glucose, lipids and branched-chain amino acids oxidation [9]. Moreover, insulin-like growth factor-binding protein 1,2 (GFBP-1, 2) deficiency which is associated with insulin resistance and fatty liver respectively [250, 251] are increased after treatment with tirzepatide [246].
In T2DM, the conversion of proinsulin to insulin is impaired due to pancreatic beta cell dysfunction. However, tirazepatide dose-dependently attenuates proinsulin level, proinsulin/insulin and proinsulin/C-peptide ratios, indicative of ameliorated beta cell function [252, 253]. Different randomized clinical trials investigated the efficacy of various doses of tirzepatide in lowering HbA1c and body weight as monotherapy or add-on therapy to metformin in patients with T2DM when compared to dulaglutide, semaglutide or placebo [73, 254,255,256,257]. The results showed the superiority of tirzepatide in reducing body weight and glycated hemoglobin levels in a dose-dependent manner. The fasting hyperglucagonemia, commonly seen in diabetic patients that dysregulates hepatic glucose metabolism, was improved resulting in better HbA1c control in patients treated with the tirzepatide [258, 259]. Moreover, a meta-analysis study demonstrated a dose-dependent superiority of tirzepatide on glycaemic control and bodyweight reduction compared to placebo, GLP-1 RAs and basal insulin [74].
Furthermore, tirzepatide had favorable impacts on a range of cardiovascular risk factors, such as blood pressure and lipid profile. In this regard, a 26-week study demonstrated dose-dependently reduction of apoC- III, apoB levels, small low-density lipoprotein and large triglyceride-rich lipoprotein particles following treatment with tirzepatide. Besides, the effect of tirzepatide on lipid profile is mainly similar to that of semaglutide and dulaglutide, except for HDL-C level improvement which was significantly greater by tirzeatide than other drugs [260]. Moreover, several systemic inflammation and endothelial dysfunction biomarkers associated with atherosclerotic cardiovascular disease, including hsCRP, intercellular adhesion molecule-1and N-terminal-pro hormone B-type natriuretic peptide (NT-proBNP) have been shown to be suppressed after administration of tirzepatide [261].
Although the SURPASS-4 study found no tirzepatide-related cardiovascular adverse effects [262], increased heart rate without alteration in laboratory values, ECG or vital signs was reported by some studies as the serum concentration of the drug rose [255, 263]. However, in the phase 2 trial systolic and diastolic blood pressure as well as pulse rate were not significantly different in the tirzepatide treatment group compared to placebo or dulaglutide [256]. On the other hand, in the SURPASS-1 trial [257], tirzepatide in a dose of 10 mmHg lowered systolic blood pressure significantly when compared to placebo. A meta-analysis and systematic review of GLP-1RA- related cardiovascular outcomes reported a reduction of major cardiovascular side effects and all-cause mortality [264]. Moreover, its long-term cardiovascular safety is still being investigated in different RCTs (A Study of Tirzepatide (LY3298176) Compared with Dulaglutide on Major Cardiovascular Events in Participants with Type 2 Diabetes [249, 265, 266].
It has been shown that the serum markers such as keratin-18 (K-18) and adiponectin which are associated with Non-alcoholic fatty liver disease (NAFLD) (occurring in the most of diabetic patients) have been improved after tirzepatide therapy [267, 268]. The rodent models of obesity and human studies demonstrated the inverse association between adiponectin and insulin resistance [269].
Dual agonists stimulating GLP-1 and glucagon receptors
Dual agonists targeting GLP-1 and glucagon receptors (GLP-1R/GCGR) can decrease plasma glucose and bodyweight. Improvements in energy expenditure and reductions in food intake highlight the potential value of glucagon pharmacology as a treatment for metabolic syndrome. However, the hindrance posed by its hyperglycemic effects has rendered practical implementation non-existent. Data showed that glucagon receptor agonists enhance resting calorie consumption, which leads to greater bodyweight loss compared to GLP-1 receptor stimulation alone [270,271,272]. While it might appear that stimulating glucagon receptors could potentially elevate plasma glucose levels, simultaneous targeting of both receptors may likely act as a compensatory mechanism to mitigate this effect. Cotadutide (MEDI0382) was the first GLP-1R/GCGR dual agonist to enter clinical trials. [273]. Cotadutide revealed an adverse-effect profile similar to liraglutide in a phase I research on healthy participants, with dose-escalation associating with vomiting, nausea, dizziness, and small elevations in heart rate. There were dose-dependent effects on peak glucose levels and food intake, with the maximum dosage (300 g) showing a significant decrease in food intake but with a high rate of adverse effects [274]. In subsequent trials (Ib, IIa) involving individuals with T2DM and those who were overweight, Cotadutide demonstrated general safety and tolerability, as well as efficacy in reducing fasting blood glucose, post-prandial glucose flactuations, body weight, and liver fat [273]. The improvements in post-prandial glucose glycemic variation found with Cotadutide administration are related to increased insulin release and postponed stomach emptying. [275]. In a long-term investigation (54 weeks), both Cotadutide dosages (100 µg and 200 µg) reduced body weight to the same extent as the GLP-1R agonist liraglutide. Cotadutide, on the other hand, had a greater rate of adverse reactions resulting in study discontinuation. However, both doses of Cotadutide resulted in marginal enhancements in total cholesterol and triglyceride levels compared to liraglutide, but these improvements were not statistically significant [276].
However, AstraZeneca, the developer of cotadutide, has revealed plans to discontinue its clinical program for the daily GLP-1R/GCGR in favor of prioritizing the advancement of AZD9550, its once-weekly injectable GLP-1RA/GCGR. [277].
SAR425899, another dual agonist, has shown glycemic control, bodyweight reduction, and reduced gastric emptying effects in human studies. [278, 279]. Sanofi’s GLP-1R/GCGR dual-agonist SAR425899 has been evaluated in a phase I clinical study using single and multiple ascending subcutaneous doses daily. The experiment comprised healthy/overweight and overweight/obese type 2 diabetics. In healthy/overweight individuals and overweight/obese type 2 diabetes patients, SAR425899 reduced body weight by 5.32 kg and 5.46 kg, respectively, over 21–28 days. In the latter group, fasting plasma glucose and glycated hemoglobin decreased significantly. [278]. The research assessed the effect of SAR425899 on the functioning of β-cells in 36 obese individuals with type 2 diabetes. These patients were randomly assigned to receive either a placebo or different doses of SAR425899 (low or high) for a period of 28 days. The results demonstrated enhancements of 23% (placebo), 163%, and 95%, correspondingly. [279]. Despite the encouraging outcomes, SAR425899 was terminated during Phase II clinical trials owing to a significant prevalence of gastrointestinal side effects among patients.
Efinopegdutide also referred to as MK-6024, JNJ-64,565,111, and HM12525A, was investigated in a clinical study including individuals who were both obese and diagnosed with T2DM. During a 12-week treatment period, dosages of 5.0 mg, 7.4 mg, and 10 mg were administered once weekly, resulting in substantial decreases in body weight of -4.6%, -5.9%, and − 7.2% respectively, when compared to the placebo. Significantly, there were no considerable changes seen in HbA1C%, fasting insulin, or blood sugar levels [280]. Efinopegdutide (at 5.0, 7.4, and 10.0 mg) showed significant placebo-corrected body weight decreases of 6.7%, 8.1%, and 10.0% in non-diabetic obese individuals during 26 weeks, compared to 5.8% for Liraglutide (3 mg). For all Efinopegdutide dosages, side events caused 18–32% of patient discontinuation, whereas Liraglutide had 17% [281]. Efinopegdutide is being evaluated for non-alcoholic fatty liver disease after discontinuation for obesity and T2D.
Another dual agonist, once-weekly Mazdutide (IBI362 or LY3305677), showed therapeutic effectiveness in overweight and obese individuals with dose-escalation in a 12-week phase I clinical study. Body weight decreased by 4.8% for 3 mg, 6.4% for 4.5 mg, and 6.0% for 6 mg in the research. adverse events did not cause individuals to stop therapy. Gastrointestinal adverse effects were prevalent but insignificant. Three individuals had asymptomatic cardiac issues [282]. Both 4.5 mg and 6 mg Mazdutide provide comparable fasting glucose and HbA1c decreases to 1.5 mg dulaglutide over 12 weeks, but results in more weight loss [283].
BI 456,906, a Boehringer-Ingelheim GLP-1R/GcgR dual-agonist, reduced body weight in a repeated escalating dosage phase Ib clinical research. At maximum doses, decreases were − 5.8% in 6 weeks and − 13.8% in 16 weeks. Due to gastrointestinal, vascular, and cardiac side effects, 12.5% of patients discontinued at 6 weeks and 17.8% at 16 weeks. Plasma amino acid and glucagon decreases indicated GcgR and GLP-1R targeting, according to the research [284].
NNC9204–1777, a drug developed by Novo Nordisk, reduced obesity-related body weight by 12.6% in a 12-week multiple ascending dosage phase I clinical study. The research indicated safety concerns owing to dose-dependent heart rate increases, reticulocyte count decreases, higher markers of inflammation and liver abnormalities, and reduced glucose tolerance at high doses. The study consequently concluded that NNC9204–1777 had unacceptable safety risks. [285].
Triple agonists
Triple agonists, which activate GLP-1, GIP, and glucagon receptors, are primarily in the developmental stage. Three such triple-agonists, SAR441255, LY3437943 and, HM15211 have advanced to clinical trials. SAR441255, a triple-agonist utilizing the exendin-4 sequence, exhibits enhanced glycemic control and body weight reduction in phase Ia trials involving healthy humans and phase I trials with diabetic obese monkeys [286]. LY3437943, characterized by its C20-diacid acylated compound, demonstrates a harmonious interplay of activity between GLP-1R and GcgR in vitro. In a phase Ia trial, a single dose of LY3437943 induces body weight loss comparable to four weeks of Tirzepatide, with sustained effects beyond its six-day half-life exposure. A subsequent phase Ib trial validates significant reductions in blood glucose and body weight over 12 weeks [287, 288]. HM15211, incorporating a human Fc fragment for prolonged half-life, proves effective in reducing liver fat and body weight in obese subjects with non-alcoholic fatty liver disease during phase Ia and Ib studies. It is currently undergoing phase II trials for non-alcoholic steatohepatitis [289]. Despite their promise, these triple-agonists are in early development, requiring further clinical trials to address safety concerns and confirm their effectiveness in combating obesity and diabetes.
Conclusion
Diabetes and its complications are responsible for remarkable mortality all over the world. Conventional anti-diabetic agents have not been able to control these life-threatening complications efficiently. Incretins, the gut peptide hormones, along with insulin release stimulation, have some other effects on the cardiovascular system, appetite, obesity, lipid metabolism, and others. Incretin-based therapy relying on novel anti-diabetic agents like GLP-1 agonists and DPP-4 inhibitors acting on the incretin system has shown encouraging therapeutic effects on diabetic manifestations and complications. In this regard, semaglutide, initially designed as an injectable long-acting GLP-1RA, was recently approved in the United States as an oral compound. Dual and triple agonists targeting GLP-1, GIP, and glucagon receptors such as tirzepatide and cotadutide with inspiring impacts on glycemic control, bodyweight, live fatty acid, and plasma triglyceride levels are currently under investigation.
Data availability
Not applicable.
References
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49.
Phillips JM, Parish NM, Raine T, Bland C, Sawyer Y, De La Peña H, et al. Type 1 diabetes development requires both CD4 + and CD8 + T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Studies: RDS. 2009;6:97.
Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204:1.
Venables MC, Jeukendrup AE. Physical inactivity and obesity: links with insulin resistance and type 2 diabetes mellitus. Diab/Metab Res Rev. 2009;25:S18–23.
Kaul K, Tarr JM, Ahmad SI, Kohner EM, Chibber R. Introduction to diabetes mellitus. Diabetes 2013:1–11.
Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–64.
Gallwitz B. The evolving place of incretin-based therapies in type 2 diabetes. Pediatr Nephrol. 2010;25:1207–17.
Riedel MJ, Kieffer TJ. Treatment of diabetes with glucagon-like peptide-1 gene therapy. Expert Opin Biol Ther. 2010;10:1681–92.
Samms RJ, Christe ME, Collins KA, Pirro V, Droz BA, Holland AK et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J Clin Investig 2021;131.
Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metabolism. 2018;20:5–21.
Han V, Hynes M, Jin C, Towle A, Lauder J, Lund P. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16:97–107.
Larsen P, Tang-Christensen M, Holst J, Ørskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–70.
Bodnaruc AM, Prud’homme D, Blanchet R, Giroux I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review. Nutr Metabolism. 2016;13:1–16.
Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–90.
Turton M, O’shea D, Gunn I, Beak S, Edwards C, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72.
Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Investig. 1998;101:515–20.
Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–42.
Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–70.
Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metabol. 2016;24:15–30.
Mieczkowska A, Bouvard B, Chappard D, Mabilleau G. Glucose-dependent insulinotropic polypeptide (GIP) directly affects collagen fibril diameter and collagen cross-linking in osteoblast cultures. Bone. 2015;74:29–36.
Garg G, McGuigan FE, Kumar J, Luthman H, Lyssenko V, Akesson K. Glucose-dependent insulinotropic polypeptide (GIP) and GIP receptor (GIPR) genes: an association analysis of polymorphisms and bone in young and elderly women. Bone Rep. 2016;4:23–7.
Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol 2021:1040.
Holst JJ, Albrechtsen NJW, Rosenkilde MM, Deacon CF. Physiology of the Incretin Hormones, GIP and GLP-1—Regulation of release and posttranslational modifications. Compr Physiol. 2011;9:1339–81.
Holst JJ. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism. 2019;96:46–55.
Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold Re, et al. Glucagon-like peptide‐1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–91.
Jorsal T, Rhee NA, Pedersen J, Wahlgren CD, Mortensen B, Jepsen SL, et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61:284–94.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525–36.
Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Investig. 1996;97:92–103.
Madsbad S, Kehlet H, Hilsted J, Tronier B. Discrepancy between plasma C-peptide and insulin response to oral and intravenous glucose. Diabetes. 1983;32:436–8.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Investig. 1993;91:301–7.
Meier J, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt W, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiology-Endocrinology Metabolism. 2004;287:E199–206.
Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Ørskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiology-Endocrinology Metabolism. 1997;273:E981–8.
Wasada T, McCorkle K, Harris V, Kawai K, Howard B, Unger RH. Effect of gastric inhibitory polypeptide on plasma levels of chylomicron triglycerides in dogs. J Clin Investig. 1981;68:1106–7.
DeFronzo RA. From the triumvirate to the „ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Clin Diabetol. 2009;10:101–28.
Nauck M, Vardarli I, Deacon C, Holst J, Meier J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up. what is down? Diabetologia. 2011;54:10–8.
Calanna S, Christensen M, Holst J, Laferrere B, Gluud L, Vilsbøll T, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–72.
Calanna S, Christensen M, Holst JJ, Laferrère B, Gluud LL, Vilsbøll T, et al. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36:3346–52.
Mentis N, Vardarli I, Köthe LD, Holst JJ, Deacon CF, Theodorakis M, et al. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011;60:1270–6.
Vilsbøll T, Krarup T, Madsbad S, Holst J. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111–9.
Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59:1765–70.
Bagger JI, Grøndahl MF, Lund A, Holst JJ, Vilsbøll T, Knop FK. Glucagonostatic potency of GLP-1 in patients with type 2 diabetes, patients with type 1 diabetes, and healthy control subjects. Diabetes. 2021;70:1347–56.
Sharma D, Verma S, Vaidya S, Kalia K, Tiwari V. Recent updates on GLP-1 agonists: current advancements & challenges. Biomed Pharmacother. 2018;108:952–62.
Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–6.
Nauck M, Kleine N, Ørskov C, Holst J, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–4.
Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsbøll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metabolism. 2011;96:737–45.
Knop FK, Vilsbøll T, Højberg PV, Larsen S, Madsbad S, Vølund A, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56:1951–9.
Aaboe K, Akram S, Deacon CF, Holst JJ, Madsbad S, Krarup T. Restoration of the insulinotropic effect of glucose-dependent insulinotropic polypeptide contributes to the antidiabetic effect of dipeptidyl peptidase‐4 inhibitors. Diabetes Obes Metabolism. 2015;17:74–81.
Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, et al. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3) GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes. 2005;54:2436–46.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Diabetes Obes Metabolism. 2016;18:203–16.
Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;292:1188–94.
Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51:2005–11.
Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–6.
Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, et al. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005;54:1640–8.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
Roust L, Stesin M, Go V, O’Brien P, Rizza R, Service F. Role of gastric inhibitory polypeptide in postprandial hyperinsulinemia of obesity. Am J Physiology-Endocrinology Metabolism. 1988;254:E767–74.
Verdich C, Flint A, Gutzwiller J-P, Naslund E, Beglinger C, Hellstrom P, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metabolism. 2001;86:4382–9.
Fukase N, Igarashi M, Takahashi H, Manaka H, Yamatani K, Daimon M, et al. Hypersecretion of truncated glucagon-like peptide‐1 and gastric inhibitory polypeptide in obese patients. Diabet Med. 1993;10:44–9.
Fukase N, Manaka H, Sugiyama K, Takahashi H, Igarashi M, Daimon M, et al. Response of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide to glucose ingestion in non-insulin dependent diabetes mellitus. Acta Diabetol. 1995;32:165–9.
Ahrén B, Larsson H, Holst JJ. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol. 1997;137:127–31.
Lugari R, Dei Cas A, Ugolotti D, Barilli A, Camellini C, Ganzerla G, et al. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res. 2004;36:111–5.
Toft-Nielsen M-B, Madsbad S, Holst J. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metabolism. 2001;86:3853–60.
Toft-Nielsen M-B, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metabolism. 2001;86:3717–23.
Näslund E, Barkeling B, King N, Gutniak M, Blundell J, Holst J, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes. 1999;23:304–11.
Toft-Nielsen M-B, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–43.
Gutzwiller J-P, Drewe Jr, Göke B, Schmidt H, Rohrer B, Lareida Jr, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiology-Regulatory Integr Comp Physiol. 1999;276:R1541–4.
Aulinger BA, Vahl TP, Wilson-Pérez HE, Prigeon RL, D’Alessio DA. β-cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. J Clin Endocrinol Metabolism. 2015;100:2489–96.
Rizzo M, Nauck MA, Mantzoros CS. Incretin-based therapies in 2021–Current status and perspectives for the future. Metabolism-Clinical Experimental 2021;122.
Jensterle M, Rizzo M, Haluzík M, Janež A. Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: a narrative review. Adv Therapy. 2022;39:2452–67.
Food U, Administration D. FDA approves new drug treatment for chronic weight management, first since 2014. Washington, DC: Center for Drug Evaluation and Research; 2021.
Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once Weekly for the treatment of obesity. New England Journal of Medicine; 2022.
Karagiannis T, Avgerinos I, Liakos A, Del Prato S, Matthews DR, Tsapas A et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia 2022:1–11.
Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–32.
Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272–81.
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108.
Borg C, Le Roux C, Ghatei M, Bloom S, Patel A, Aylwin S. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. J Br Surg. 2006;93:210–5.
Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, et al. Gastric bypass reduces fat intake and preference. Am J Physiology-Regulatory Integr Comp Physiol. 2011;301:R1057–66.
Nauck MA, Quast DR, Wefers J, Pfeiffer AF. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metabolism. 2021;23:5–29.
Tilinca MC, Tiuca RA, Niculas C, Varga A, Tilea I. Future perspectives in diabesity treatment: Semaglutide, a glucagon–like peptide 1 receptor agonist. Experimental Therapeutic Med. 2021;22:1–11.
Wen S, Nguyen T, Gong M, Yuan X, Wang C, Jin J et al. An Overview of Similarities and Differences in Metabolic Actions and Effects of Central Nervous System Between Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) and Sodium Glucose Co-Transporter-2 Inhibitors (SGLT-2is). Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2021;14:2955.
Yaribeygi H, Ashrafizadeh M, Henney NC, Sathyapalan T, Jamialahmadi T, Sahebkar A. Neuromodulatory effects of anti-diabetes medications: a mechanistic review. Pharmacol Res. 2020;152:104611.
Seufert J, Gallwitz B. The extra-pancreatic effects of GLP‐1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metabolism. 2014;16:673–88.
Holst JJ. The physiology and pharmacology of incretins in type 2 diabetes mellitus. Diabetes Obes Metabolism. 2008;10:14–21.
Taher J, Baker C, Alvares D, Ijaz L, Hussain M, Adeli K. GLP-2 dysregulates hepatic lipoprotein metabolism, inducing fatty liver and vldl overproduction in male hamsters and mice. Endocrinology. 2018;159:3340–50.
Farr S, Baker C, Naples M, Taher J, Iqbal J, Hussain M, et al. Central nervous system regulation of intestinal lipoprotein metabolism by glucagon-like peptide-1 via a brain–gut axis. Arterioscler Thromb Vasc Biol. 2015;35:1092–100.
Joao A, Reis F, Fernandes R. The incretin system ABCs in obesity and diabetes–novel therapeutic strategies for weight loss and beyond. Obes Rev. 2016;17:553–72.
Yabe D, Seino Y. Incretin actions beyond the pancreas: lessons from knockout mice. Curr Opin Pharmacol. 2013;13:946–53.
Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon‐like peptide‐1: incretin actions beyond the pancreas. J Diabetes Invest. 2013;4:108–30.
Kawanami D, Matoba K, Sango K, Utsunomiya K. Incretin-based therapies for diabetic complications: basic mechanisms and clinical evidence. Int J Mol Sci. 2016;17:1223.
Ng E, Shaw JE, Wood A, Maple-Brown LJ, Hare MJ. Glucagon-like peptide-1 receptor agonist (GLP1-RA) therapy in type 2 diabetes. Australian J Gen Pract. 2022;51:513–8.
Smits MM, Van Raalte DH. Safety of semaglutide. Front Endocrinol. 2021;12:645563.
Jaiswal M, Martin CL, Brown MB, Callaghan B, Albers JW, Feldman EL, et al. Effects of Exenatide on measures of diabetic neuropathy in subjects with type 2 diabetes: results from an 18-month proof-of-concept open-label randomized study. J Diabetes Complicat. 2015;29:1287–94.
Brock C, Hansen CS, Karmisholt J, Møller HJ, Juhl A, Farmer AD, et al. Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol. 2019;85:2512–23.
El Mouhayyar C, Riachy R, Khalil AB, Eid A, Azar S. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol 2020;2020.
Grobbee DE. How to ADVANCE prevention of cardiovascular complications in type 2 diabetes. Metabolism. 2003;52:24–8.
Stamler J, Vaccaro O, Neaton JD, Wentworth D, Group MRFITR. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16:434–44.
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34.
Fisman EZ, Motro M, Tenenbaum A. Non-insulin antidiabetic therapy in cardiac patients: current problems and future prospects. Cardiovasc Diabetology: Clin Metabolic Inflamm Facets. 2008;45:154–70.
Liu H, Hu Y, Simpson RW, Dear AE. Glucagon-like peptide-1 attenuates tumour necrosis factor-alpha-mediated induction of plasminogen [corrected] activator inhibitor-1 expression. J Endocrinol. 2008;196:57–65.
Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009;201:59.
Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiology-Endocrinology Metabolism. 2004;287:E1209–15.
Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Investig. 2002;110:43–52.
Barragan J, Rodriguez R, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiology-Endocrinology Metabolism. 1994;266:E459–66.
Okerson T, Yan P, Stonehouse A, Brodows R. Effects of Exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334–9.
Gill A, Hoogwerf BJ, Burger J, Bruce S, MacConell L, Yan P, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:1–7.
Timmers L, Henriques JP, de Kleijn DP, DeVries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–10.
Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol 2018:672.
Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9.
Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5.
Ban K, Hui S, Drucker DJ, Husain M. Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin—based therapies. J Am Soc Hypertens. 2009;3:245–59.
Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li R-K, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85.
Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–38.
Yandrapalli S, Aronow WS. Cardiovascular benefits of the newer medications for treating type 2 diabetes mellitus. J Thorac Disease. 2017;9:2124.
Nathanson D, Ullman B, Löfström U, Hedman A, Frick M, Sjöholm Å, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55:926–35.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896–907.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29.
Sattar N, Lee MM, Kristensen SL, Branch KR, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–62.
Woo JS, Kim W, Ha SJ, Kim JB, Kim S-J, Kim W-S, et al. Cardioprotective effects of exenatide in patients with ST-segment–elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–60.
Chen W, Chen Y, Tian F, Yang N, Cheng L, Hu S, et al. Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2016;9:e005146.
Trevisan M, Fu EL, Szummer K, Norhammar A, Lundman P, Wanner C, et al. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction. Eur Heart Journal-Cardiovascular Pharmacotherapy. 2021;7:104–11.
Ferreira JP, Sharma A, Butler J, Packer M, Zannad F, Vasques-Nóvoa F, et al. Glucagon-like peptide-1 receptor agonists across the spectrum of heart failure. J Clin Endocrinol Metabolism. 2024;109:4–9.
Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27:57–64.
Gitt AK, Bramlage P, Binz C, Krekler M, Deeg E, Tschöpe D. Prognostic implications of DPP-4 inhibitor vs. sulfonylurea use on top of metformin in a real world setting - results of the 1 year follow-up of the prospective DiaRegis registry. Int J Clin Pract. 2013;67:1005–14.
Chou C-Y, Chang Y-T, Yang J-L, Wang J-Y, Lee T-E, Wang R-Y, et al. Effect of long-term incretin-based therapies on ischemic heart diseases in patients with type 2 diabetes mellitus: a network meta-analysis. Sci Rep. 2017;7:1–13.
Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155–66.
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
Liu Y, Jiang X, Chen X. Liraglutide and metformin alone or combined therapy for type 2 diabetes patients complicated with coronary artery disease. Lipids Health Dis. 2017;16:1–8.
Novo Nordisk A. S. A research study to look at how semaglutide compared to placebo affects diabetic eye disease in people with type 2 diabetes (FOCUS). Clinicaltrials gov [Internet] Bethesda, MD, National Library of Medicine; 2019.
Sudo M, Li Y, Hiro T, Takayama T, Mitsumata M, Shiomi M, et al. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 2017;265:283–91.
Balestrieri ML, Rizzo MR, Barbieri M, Paolisso P, D’Onofrio N, Giovane A, et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes. 2015;64:1395–406.
Piotrowski K, Becker M, Zugwurst J, Biller-Friedmann I, Spoettl G, Greif M, et al. Circulating concentrations of GLP-1 are associated with coronary atherosclerosis in humans. Cardiovasc Diabetol. 2013;12:1–5.
Salim HM, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Yagi S, et al. Dipeptidyl peptidase-4 inhibitor, linagliptin, ameliorates endothelial dysfunction and atherogenesis in normoglycemic apolipoprotein-E deficient mice. Vascul Pharmacol. 2016;79:16–23.
Tang S-T, Su H, Zhang Q, Tang H-Q, Wang C-J, Zhou Q, et al. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med. 2016;37:1558–66.
Cha S-A, Park Y-M, Yun J-S, Lim T-S, Song K-H, Yoo K-D, et al. A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes. Lipids Health Dis. 2017;16:1–8.
Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Therapy. 2012;29:14–25.
Hirano T, Yamashita S, Takahashi M, Hashimoto H, Mori Y, Goto M. Anagliptin, a dipeptidyl peptidase-4 inhibitor, decreases macrophage infiltration and suppresses atherosclerosis in aortic and coronary arteries in cholesterol-fed rabbits. Metabolism. 2016;65:893–903.
Yang C-J, Fan Z-X, Yang J, Yang J. DPP-4 inhibitors: a potential promising therapeutic target in prevention of atherosclerosis. Int J Cardiol. 2016;202:797–8.
Silva Júnior WSd G-M, AFd, Kraemer-Aguiar LG. Dipeptidyl peptidase 4: a new link between diabetes mellitus and atherosclerosis? BioMed research international 2015;2015:816164.
Dai Y, Wang X, Ding Z, Dai D, Mehta JL. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol. 2014;51:471–8.
Hwang H-J, Chung HS, Jung TW, Ryu JY, Hong HC, Seo JA, et al. The dipeptidyl peptidase-IV inhibitor inhibits the expression of vascular adhesion molecules and inflammatory cytokines in HUVECs via akt-and AMPK-dependent mechanisms. Mol Cell Endocrinol. 2015;405:25–34.
Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S-i. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013;12:1–9.
Choi SH, Park S, Oh CJ, Leem J, Park K-G, Lee I-K. Dipeptidyl peptidase-4 inhibition by gemigliptin prevents abnormal vascular remodeling via NF-E2-related factor 2 activation. Vascul Pharmacol. 2015;73:11–9.
Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, et al. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology. 2013;154:1260–70.
Lim S, Choi SH, Shin H, Cho BJ, Park HS, Ahn BY, et al. Effect of a dipeptidyl peptidase-IV inhibitor, Des-fluoro-sitagliptin, on neointimal formation after balloon injury in rats. PLoS ONE. 2012;7:e35007.
Vittone F, Liberman A, Vasic D, Ostertag R, Esser M, Walcher D, et al. Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in Apoe–/– mice. Diabetologia. 2012;55:2267–75.
Fadini GP, Avogaro A. Dipeptidyl peptidase-4 inhibition and vascular repair by mobilization of endogenous stem cells in diabetes and beyond. Atherosclerosis. 2013;229:23–9.
Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, De Kreutzenberg S, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1α. Diabetes Care. 2010;33:1607–9.
Pinelli NR, Moore CL, Tomasello S. Incretin-based therapy in chronic kidney disease. Adv Chronic Kidney Dis. 2010;17:439–49.
Esaki H, Tachi T, Goto C, Sugita I, Kanematsu Y, Yoshida A, et al. Renoprotective effect of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Front Pharmacol. 2017;8:835.
Tanaka T, Higashijima Y, Wada T, Nangaku M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014;86:701–11.
Muntner P, Bowling CB, Gao L, Rizk D, Judd S, Tanner RM, et al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011;6:2200–7.
Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–12.
Baban B, Liu JY, Mozaffari MS. Endoplasmic reticulum stress response and inflammatory cytokines in type 2 diabetic nephropathy: role of indoleamine 2, 3-dioxygenase and programmed death-1. Exp Mol Pathol. 2013;94:343–51.
Mu J, Pang Q, Guo Y-H, Chen J-G, Zeng W, Huang Y-J, et al. Functional implications of microRNA-215 in TGF-β1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1. PLoS ONE. 2013;8:e58622.
Deshpande SD, Putta S, Wang M, Lai JY, Bitzer M, Nelson RG, et al. Transforming growth factor-β–induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes. 2013;62:3151–62.
Przezak A, Bielka W, Pawlik A. Incretins in the Therapy of Diabetic kidney disease. Int J Mol Sci. 2021;22:12312.
Ivan P, Walter K. The privileged position of glp-1 in diabetic nephropathy. Endocrinol Metab Int J. 2018;6:233–9.
Sun A-l, Deng J-t, Guan G-j, Chen S-h, Liu Y-t, Cheng J, et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diabetes Vascular Disease Res. 2012;9:301–8.
Mitic B, Lazarevic G, Vlahovic P, Rajic M, Stefanovic V. Diagnostic value of the aminopeptidase N, N-acetyl-β-D-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren Fail. 2008;30:896–903.
Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, et al. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014;443:828–33.
Kang YM, Jung CH. Effects of incretin-based therapies on diabetic microvascular complications. Endocrinol Metabolism. 2017;32:316–25.
Mega C, Teixeira de Lemos E, Vala H, Fernandes R, Oliveira J, Mascarenhas-Melo F et al. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Experimental diabetes research 2011;2011.
Marques C, Mega C, Gonçalves A, Rodrigues-Santos P, Teixeira-Lemos E, Teixeira F et al. Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals. Mediators of inflammation 2014;2014.
Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579–89.
Bergenstal RM, Wysham C, MacConell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–9.
Muskiet MH, Tonneijck L, Huang Y, Liu M, Saremi A, Heerspink HJ, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. The lancet Diabetes & endocrinology 2018;6:859– 69.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2017;377:1228-39.
Hernandez AF, Green JB, Janmohamed S, D’Agostino Sr RB, Granger CB, Jones NP,et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. The Lancet 2018;392:1519-29.
Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes care 2017;40:69–76.
White W, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C, et al. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus.Diabetes, Obesity and Metabolism 2013;15:668– 73.
Groop P-H, Cooper ME, Perkovic V, Emser A, Woerle H-J, Von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes care 2013;36:3460-8.
Fujita H, Taniai H, Murayama H, Ohshiro H, Hayashi H, Sato S, et al. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1α in type 2 diabetic patients with incipient nephropathy. Endocrine journal 2013:EJ13-0305.
Groop PH, Del Prato S, Taskinen MR, Owens D, Gong Y, Crowe S, et al. Linagliptin treatment in subjects with type 2 diabetes with and without mild-to‐moderate renal impairment. Diabetes, Obesity and Metabolism 2014;16:560-8.
Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes, Obesity and Metabolism 2012;14:1032-9.
Chan J, Scott R, Arjona Ferreira J, Sheng D, Gonzalez E, Davies M, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency.Diabetes, Obesity and Metabolism 2008;10:545– 55.
Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734-7.
Tonneijck L, Muskiet MH, Blijdorp CJ, Smits MM, Twisk JW, Kramer MH, et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. American Journal of Physiology-Renal Physiology 2019;316:F231-F40.
Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes:a systematic review and network meta-analysis. Diabetes research and clinical practice 2015;110:26–37.
van Baar MJ, van Raalte DH. Renoprotection in diabetic kidney disease: can incretin-based therapies deliver? Current Opinion in Nephrology and Hypertension 2020;29:103– 11.
Muskiet MH, Tonneijck L, Smits MM, Van Baar MJ, Kramer MH, Hoorn EJ, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nature Reviews Nephrology 2017;13:605– 28.
Asmar A, Simonsen L, Asmar M, Madsbad S, Holst JJ, Frandsen E, et al. Renal extraction and acute effects of glucagon-like peptide-1 on central and renal hemodynamics in healthy men. American Journal of Physiology-Endocrinology and Metabolism 2015;308:E641-E9.
Asmar A, Simonsen L, Asmar M, Madsbad S, Holst JJ, Frandsen E, et al. Glucagon-like peptide-1 does not have acute effects on central or renal hemodynamics in patients with type 2 diabetes without nephropathy. American Journal of Physiology-Endocrinology and Metabolism 2016;310:E744-E53.
Muskiet MH, Tonneijck L, Smits MM, Kramer M, Diamant M, Joles J, et al. Acute renal haemodynamic effects of glucagon-like peptide‐1 receptor agonist exenatide in healthy overweight men. Diabetes, Obesity and Metabolism 2016;18:178– 85.
Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, et al. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. The Journal of Clinical Endocrinology & Metabolism 2013;98:E664-E71.
Skov J, Pedersen M, Holst J, Madsen B, Goetze J, Rittig S, et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes:a randomized clinical trial. Diabetes, Obesity and Metabolism 2016;18:581-9.
Tonneijck L, Muskiet MH, Smits MM, Hoekstra T, Kramer MH, Danser AJ, et al. Postprandial renal haemodynamic effect of lixisenatide vs once-daily insulin‐glulisine in patients with type 2 diabetes on insulin‐glargine: an 8‐week, randomised, open‐label trial.Diabetes, Obesity and Metabolism 2017;19:1669-80.
Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser A, et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia 2016;59:1412-21.
Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AJ, et al. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2016;39:2042-50.
Yaribeygi H, Atkin SL, Pirro M, Sahebkar A. A review of the anti-inflammatory properties of antidiabetic agents providing protective effects against vascular complications in diabetes. Journal of cellular physiology 2019;234:8286-94.
Yaribeygi H, Atkin SL, Sahebkar A. Interleukin-18 and diabetic nephropathy: A review. Journal of cellular physiology 2019;234:5674-82.
Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, et al.A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 2013;62:347– 51.
Wu J-d, Xu X-h, Zhu J, Ding B, Du T-x, Gao G, et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes technology& therapeutics 2011;13:143-8.
Cappetta D, Ciuffreda LP, Cozzolino A, Esposito G, Scavone C, Sapio L, et al.Dipeptidyl peptidase 4 inhibition ameliorates chronic kidney disease in a model of salt-dependent hypertension. Oxidative medicine and cellular longevity 2019;2019.
Wang X, Li Z, Huang X, Li F, Liu J, Li Z, et al. An experimental study of exenatide effects on renal injury in diabetic rats. Acta cirurgica brasileira 2019;34.
Yaribeygi H, Atkin SL, Montecucco F, Jamialahmadi T, Sahebkar A. Renoprotective effects of incretin-based therapy in diabetes mellitus. BioMed Research International 2021;2021.
Wang C, Li L, Liu S, Liao G, Li L, Chen Y, et al. GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis.PLoS One 2018;13:e0193473.
Bangshaab M, Gutierrez A, Huynh KD, Knudsen JS, Arcanjo DDR, Petersen AG, et al. Different mechanisms involved in liraglutide and glucagon-like peptide‐1 vasodilatation in rat mesenteric small arteries. British journal of pharmacology 2019;176:386– 99.
Bunck MC, Cornér A, Eliasson B, Heine RJ, Shaginian RM, Wu Y, et al. One-year treatment with exenatide vs. insulin glargine: effects on postprandial glycemia, lipid profiles, and oxidative stress. Atherosclerosis 2010;212:223-9.
Chang G, Zhang D, Yu H, Zhang P, Wang Y, Zheng A, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. International journal of molecular medicine 2013;32:1011-20.
Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes 2004;53:5–13.
Pujadas G, De Nigris V, Prattichizzo F, La Sala L, Testa R, Ceriello A. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine 2017;56:509– 20.
Barale C, Buracco S, Cavalot F, Frascaroli C, Guerrasio A, Russo I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation.Thrombosis and haemostasis 2017;117:1115-28.
Yamane S, Inagaki N. Regulation of glucagon-like peptide‐1 sensitivity by gut microbiota dysbiosis. Journal of Diabetes Investigation 2018;9:262-4.
Thomson SC, Kashkouli A, Liu ZZ, Singh P. Renal hemodynamic effects of glucagon-like peptide-1 agonist are mediated by nitric oxide but not prostaglandin. American Journal of Physiology-Renal Physiology 2017;313:F854-F8.
Sélley E, Kun S, Szijártó IA, Laczy B, Kovács T, Fülöp F, et al. Exenatide induces aortic vasodilation increasing hydrogen sulphide, carbon monoxide and nitric oxide production. Cardiovascular Diabetology 2014;13:1–9.
Jensen EP, Møller S, Hviid AV, Veedfald S, Holst JJ, Pedersen J, et al. GLP-1-induced renal vasodilation in rodents depends exclusively on the known GLP-1 receptor and is lost in prehypertensive rats. American Journal of Physiology-Renal Physiology 2020;318:F1409-F17.
Vilsbøll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simó R, et al. Semaglutide,reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes,Obesity and Metabolism 2018;20:889– 97.
Rendell MS. Albiglutide: a unique GLP-1 receptor agonist. Expert Opinion on Biological Therapy 2016;16:1557-69.
Varadhan L, Humphreys T, Hariman C, Walker AB, Varughese GI. GLP-1 agonist treatment:implications for diabetic retinopathy screening. Diabetes research and clinical practice 2011;94:e68-e71.
Gaborit B, Julla J-B, Besbes S, Proust M, Vincentelli C, Alos B, et al. Glucagon-like peptide 1 receptor agonists, diabetic retinopathy and angiogenesis: The AngioSafe type 2 diabetes study. The Journal of Clinical Endocrinology & Metabolism 2020;105:e1549-e60.
Simó R, Hernández C. GLP-1R as a target for the treatment of diabetic retinopathy:friend or foe? Diabetes 2017;66:1453-60.
Wang T, Hong J-L, Gower EW, Pate V, Garg S, Buse JB, et al. Incretin-based therapies and diabetic retinopathy: real-world evidence in older US adults. Diabetes Care 2018;41:1998–2009.
Tang H, Li G, Zhao Y, Wang F, Gower EW, Shi L, et al. Comparisons of diabetic retinopathy events associated with glucose-lowering drugs in patients with type 2 diabetes mellitus: A network meta‐analysis. Diabetes, Obesity and Metabolism 2018;20:1262-79.
Fadini G, Boscaro E. de KS, Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A, Avogaro A: Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 2010;33:1097– 102.
Dicembrini I, Nreu B, Scatena A, Andreozzi F, Sesti G, Mannucci E, et al. Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized controlled trials. Acta diabetologica 2017;54:933– 41.
Douros A, Filion KB, Yin H, Yu OH, Etminan M, Udell JA, et al. Glucagon-like peptide 1 receptor agonists and the risk of incident diabetic retinopathy. Diabetes Care 2018;41:2330-8.
Avgerinos I, Karagiannis T, Malandris K, Liakos A, Mainou M, Bekiari E, et al.Glucagon-like peptide‐1 receptor agonists and microvascular outcomes in type 2 diabetes:a systematic review and meta‐analysis. Diabetes, Obesity and Metabolism 2019;21:188– 93.
Wang T, Lu W, Tang H, Buse JB, Stürmer T, Gower EW. Assessing the association between GLP-1 receptor agonist use and diabetic retinopathy through the FDA adverse event reporting system. Diabetes Care 2019;42:e21-e3.
Hernández C, Bogdanov P, Corraliza L, García-Ramírez M, Solà-Adell C, Arranz JA, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016;65:172– 87.
Layton C, Safa R, Osborne N. Oscillatory potentials and the b-Wave: partial masking and interdependence in dark adaptation and diabetes in the rat. Graefe’s archive for clinical and experimental ophthalmology 2007;245:1335-45.
Varadhan L, Humphreys T, Walker AB, Varughese GI. The impact of improved glycaemic control with GLP-1 receptor agonist therapy on diabetic retinopathy. Diabetes Research and Clinical Practice 2014;103:e37-e9.
Lee C-S, Kim YG, Cho H-J, Park J, Jeong H, Lee S-E, et al. Dipeptidyl peptidase-4 inhibitor increases vascular leakage in retina through VE-cadherin phosphorylation.Scientific reports 2016;6:1–16.
Kolibabka M, Dietrich N, Klein T, Hammes H-P. Anti-angiogenic effects of the DPP-4 inhibitor linagliptin via inhibition of VEGFR signalling in the mouse model of oxygen-induced retinopathy. Diabetologia 2018;61:2412-21.
Maeda S, Yamagishi S-i, Matsui T, Nakashima S, Ojima A, Maeda S, et al. Beneficial effects of vildagliptin on retinal injury in obese type 2 diabetic rats. Ophthalmic Research 2013;50:221-6.
Hippisley-Cox J, Coupland C. Diabetes treatments and risk of amputation, blindness,severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. bmj 2016;352.
Kim N, Choi J, Choi K, Baik S, Lee J, Kim S. Dipeptidyl peptidase-4 inhibitor use and risk of diabetic retinopathy: a population-based study. Diabetes & Metabolism 2018;44:361-7.
Gonçalves A, Leal E, Paiva A, Teixeira Lemos E, Teixeira F, Ribeiro C, et al.Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood–retinal barrier in a type 2 diabetes animal model. Diabetes, Obesity and Metabolism 2012;14:454– 63.
Gonçalves A, Marques C, Leal E, Ribeiro CF, Reis F, Ambrósio AF, et al. Dipeptidyl peptidase-IV inhibition prevents blood–retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2014;1842:1454-63.
Ott C, Raff U, Schmidt S, Kistner I, Friedrich S, Bramlage P, et al. Effects of saxagliptin on early microvascular changes in patients with type 2 diabetes. Cardiovascular diabetology 2014;13:1–9.
Kim HS, Jung CH. Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes? International Journal of Molecular Sciences 2021;22:9936.
Davies M, Pieber TR, Hartoft-Nielsen M-L, Hansen OK, Jabbour S, Rosenstock J.Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. Jama 2017;318:1460-70.
Andersen A, Knop FK, Vilsbøll T. A pharmacological and clinical overview of oral semaglutide for the treatment of type 2 diabetes. Drugs 2021;81:1003-30.
Bækdal TA, Breitschaft A, Donsmark M, Maarbjerg SJ, Søndergaard FL, Borregaard J. Effect of Various Dosing Conditions on the Pharmacokinetics of Oral Semaglutide,a Human Glucagon-Like Peptide-1 Analogue in a Tablet Formulation. Diabetes Therapy 2021;12:1915-27.
Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea:the PIONEER 3 randomized clinical trial. Jama 2019;321:1466-80.
Singh AK, Singh R, Singh A, Misra A. Efficacy and Safety of Oral Semaglutide in Type 2 Diabetes: A Systematic Review of Real-World Evidence. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2024:103024.
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised,double-blind, phase 3a trial. The Lancet 2019;394:39–50.
Yabe D, Nakamura J, Kaneto H, Deenadayalan S, Navarria A, Gislum M, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial.The Lancet Diabetes & Endocrinology 2020;8:392–406.
Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism 2021;106:388– 96.
Abbasi J. FDA Green-Lights Tirzepatide, Marketed as Zepbound, for Chronic Weight Management. JAMA 2023.
Rosenstock J, Vázquez L, Del Prato S, Franco DR, Weerakkody G, Dai B, et al.Achieving Normoglycemia With Tirzepatide: Analysis of SURPASS 1–4 Trials. Diabetes Care 2023;46:1986-92.
Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist,in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Therapy 2021;12:143– 57.
Liew CF, Wise SD, Yeo KP, Lee KO. Insulin-like growth factor binding protein-1 is independently affected by ethnicity, insulin sensitivity, and leptin in healthy,glucose-tolerant young men. The Journal of Clinical Endocrinology & Metabolism 2005;90:1483-8.
Wittenbecher C, Ouni M, Kuxhaus O, Jähnert M, Gottmann P, Teichmann A, et al.Insulin-like growth factor binding protein 2 (IGFBP-2) and the risk of developing type 2 diabetes. Diabetes 2019;68:188– 97.
Hanley AJ, Zinman B, Sheridan P, Yusuf S, Gerstein HC, Ramipril DRAW, et al.Effect of rosiglitazone and ramipril on β-cell function in people with impaired glucose tolerance or impaired fasting glucose:the DREAM Trial. Diabetes Care 2010;33:608– 13.
Russo GT, Giorda CB, Cercone S, De Cosmo S, Nicolucci A, Cucinotta D, et al.Beta cell stress in a 4-year follow‐up of patients with type 2 diabetes: A longitudinal analysis of the BetaDecline Study. Diabetes/Metabolism Research and Reviews 2018;34:e3016.
Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes.New England Journal of Medicine 2021;385:503– 15.
Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon‐like peptide‐1 receptor agonist in patients with type 2 diabetes: A 12‐week, randomized,double‐blind, placebo‐controlled study to evaluate different dose‐escalation regimens.Diabetes, Obesity and Metabolism 2020;22:938– 46.
Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. The Lancet 2018;392:2180-93.
Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Landó LF, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. The Lancet 2021;398:143– 55.
Gilon P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. Journal of molecular biology 2020;432:1367-94.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nature reviews endocrinology 2017;13:572– 87.
Dutta D, Surana V, Singla R, Aggarwal S, Sharma M. Efficacy and safety of novel twincretin tirzepatide a dual GIP and GLP-1 receptor agonist in the management of type-2 diabetes: A Cochrane meta-analysis. Indian Journal of Endocrinology and Metabolism 2021;25:475.
Wilson JM, Nikooienejad A, Robins DA, Roell WC, Riesmeyer JS, Haupt A, et al.The dual glucose-dependent insulinotropic peptide and glucagon‐like peptide‐1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes, obesity and metabolism 2020;22:2451-9.
Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4):a randomised, open-label, parallel-group, multicentre, phase 3 trial. The Lancet 2021;398:1811-24.
Furihata K, Mimura H, Urva S, Oura T, Ohwaki K, Imaoka T. A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes. Diabetes,Obesity and Metabolism 2022;24:239– 46.
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular,mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials.The lancet Diabetes & endocrinology 2019;7:776– 85.
Company ELa, The Effect of Tirzepatide Versus Dulaglutide on Major Adverse Cardiovascular Events in Patients With Type 2 Diabetes (SURPASS-CVOT). The National Library of Medicine;2020 [updated 2022; cited]. Available from: https://clinicaltrials.gov/ct2/show/NCT04255433.
Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. Bmj 2021;372.
Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue-Valleskey JM, Hoogwerf BJ, et al.Non‐alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes, Obesity and Metabolism 2017;19:1630-4.
Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020;43:1352-5.
Putz DM, Goldner WS, Bar RS, Haynes WG, Sivitz WI. Adiponectin and C-reactive protein in obesity, type 2 diabetes, and monodrug therapy. Metabolism 2004;53:1454-61.
Holst JJ, Albrechtsen NJW, Gabe MBN, Rosenkilde MM. Oxyntomodulin: Actions and role in diabetes. Peptides 2018;100:48–53.
Liu Y, Ford H, Druce M, Minnion J, Field B, Shillito J, et al. Subcutaneous oxyntomodulin analogue administration reduces body weight in lean and obese rodents. International journal of obesity 2010;34:1715-25.
Scott R, Minnion J, Tan T, Bloom S. Oxyntomodulin analogue increases energy expenditure via the glucagon receptor. Peptides 2018;104:70– 7.
Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al.MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. The Lancet 2018;391:2607-18.
Ambery PD, Klammt S, Posch MG, Petrone M, Pu W, Rondinone C, et al. MEDI0382,a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single‐dose, healthy‐subject, randomized, phase 1 study. British journal of clinical pharmacology 2018;84:2325-35.
Parker VE, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT, et al. Efficacy,safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. The Journal of Clinical Endocrinology & Metabolism 2020;105:803– 20.
Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care 2021;44:1433-42.
Krauss Z, Hintz A, Fisk R. Tirzepatide: Clinical review of the “twincretin” injectable.American Journal of Health-System Pharmacy 2023;80:879– 88.
Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized,placebo‐controlled first‐in‐human and first‐in‐patient trials. Diabetes, Obesity and Metabolism 2019;21:120-8.
Visentin R, Schiavon M, Göbel B, Riz M, Cobelli C, Klabunde T, et al. Dual glucagon-like peptide‐1 receptor/glucagon receptor agonist SAR425899 improves beta‐cell function in type 2 diabetes. Diabetes, Obesity and Metabolism 2020;22:640-7.
Di Prospero NA, Yee J, Frustaci ME, Samtani MN, Alba M, Fleck P. Efficacy and safety of glucagon-like peptide‐1/glucagon receptor co‐agonist JNJ‐64565111 in individuals with type 2 diabetes mellitus and obesity: a randomized dose‐ranging study. Clinical Obesity 2021;11:e12433.
Alba M, Yee J, Frustaci ME, Samtani MN, Fleck P. Efficacy and safety of glucagon-like peptide‐1/glucagon receptor co‐agonist JNJ‐64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose‐ranging study. Clinical Obesity 2021;11:e12432.
Ji L, Jiang H, An P, Deng H, Liu M, Li L, et al. IBI362 (LY3305677), a weekly-dose GLP-1 and glucagon receptor dual agonist, in Chinese adults with overweight or obesity:a randomised, placebo-controlled, multiple ascending dose phase 1b study. EClinicalMedicine 2021;39.
Jiang H, Pang S, Zhang Y, Yu T, Liu M, Deng H, et al. A phase 1b randomised controlled trial of a glucagon-like peptide-1 and glucagon receptor dual agonist IBI362 (LY3305677)in Chinese patients with type 2 diabetes. Nature Communications 2022;13:3613.
Jungnik A, Arrubla Martinez J, Plum-Mörschel L, Kapitza C, Lamers D, Thamer C,et al. Phase I studies of the safety, tolerability, pharmacokinetics and pharmacodynamics of the dual glucagon receptor/glucagon‐like peptide‐1 receptor agonist BI 456906.Diabetes, Obesity and Metabolism 2023;25:1011-23.
Friedrichsen M, Endahl L, Kreiner FF, Goldwater R, Kankam M, Toubro S, et al.Glucagon/GLP-1 receptor co-agonist NNC9204-1177 reduced body weight in adults with overweight or obesity but was associated with safety issues. Medrxiv 2022:2022.06.02.22275920.
Bossart M, Wagner M, Elvert R, Evers A, Hübschle T, Kloeckener T, et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell metabolism 2022;34:59–74. e10.
Coskun T, Urva S, Roell WC, Qu H, Loghin C, Moyers JS, et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss:From discovery to clinical proof of concept. Cell Metabolism 2022;34:1234-47. e9.
Urva S, Coskun T, Loh MT, Du Y, Thomas MK, Gurbuz S, et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: a phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. The Lancet 2022;400:1869-81.
Lafferty RA, O’Harte FP, Irwin N, Gault VA, Flatt PR. Proglucagon-derived peptides as therapeutics. Frontiers in endocrinology 2021;12:689678.
Abdelmalek M, Choi J, Kim Y, Seo K, Hompesch M, Baek S. HM15211, a novel GLP-1/GIP/Glucagon triple-receptor co-agonist significantly reduces liver fat and body weight in obese subjects with non-alcoholic fatty liver disease: A Phase 1b/2a, multi-center, randomized,placebo-controlled trial. Journal of Hepatology 2020;73:S124.
Funding
No funding received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors have no conflict of interest to declare.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zarei, M., Sahebi Vaighan, N., Farjoo, M.H. et al. Incretin-based therapy: a new horizon in diabetes management. J Diabetes Metab Disord (2024). https://doi.org/10.1007/s40200-024-01479-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40200-024-01479-3