Abstract
Objective
The effects of exercise training on suppression of inflammation have been proposed as a therapeutic approach in recent years to modify the obesity-induced inflammatory status and immunometabolic disorders. The present study aimed to assess the impacts of an all-extremity combined high-intensity interval training (HIIT) on inflammatory state and glycolipid metabolism in young sedentary overweight and obese females.
Method
This was an quasi-experimental study which was applied by comparing two groups. The participants were allocated to two active (AG, n = 15) and inactive (IG, n = 15) groups. The serum level of adiponectin, interleukin (IL)-10, pentraxin 3 (PTX3), and tumor-necrosis factor α (TNFα) was measured in all subjects. Also, glycolipid metabolism was assessed by measuring the fasting lipid profile parameters, glucose, and insulin levels and calculating the homeostasis model assessment of insulin resistance (HOMA2-IR).
Results
Following a 10-week combined all-extremity HIIT in the active subjects, the TNFα, PTX3/IL-10, and TNFα/adiponectin were significantly reduced. However, the absolute levels of adiponectin, IL-10, and PTX3 remained unchanged. Additionally, a significant decrease was found in insulin, LDL, and HOMA2-IR, while insulin sensitivity and HDL levels showed a significant increase in the active group compared to the inactive group.
Conclusions
Our 10-week time-efficient combined all-extremity HIIT promoted an anti-inflammatory state and glycolipid metabolism improvement, suggesting this protocol as a practical therapeutic approach in sedentary obese females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is accompanied by adipose tissue (AT) homeostasis disturbances as a result of adipocyte hypertrophy. A relation between excess nutrients and chronic low-grade systemic inflammation (CLSI) persistency implicates the immune cell dysfunction and/or an imbalanced cytokine production during obesity [1].
Accordingly, the assessment of cytokines as the key orchestrators of the immune system could be useful for finding out the genesis, progression, and pathogenesis of these disturbances. Protein 3 (PTX3) is a critical mediator that links obesity, CLSI, and innate immunity [2, 3], since it is produced by the inflammatory pathways-induced tumor necrosis factor (TNF) α and interleukin (IL) 1β [4]. Several human studies, though not all [5], have demonstrated the increased PTX3 plasma levels in patients with low-grade inflammation such as non-alcoholic fatty liver (NAFL) [6], metabolic syndrome (MetS), [7] and obesity [8]. However, recent evidence has indicated a counter-regulatory function for PTX3 through the increase of IL-10 production [9], suggesting a double-edged role of PTX3 in immune regulation. The plasma level of IL-10, a suppressor of inflammation, reduces during obesity [10, 11]. However, there has been resistance to this anti-inflammatory function in reducing the inflammation in obese (OB) state [12]. Adiponectin is another potent regulator of innate immune system which its downregulation induces inflammation progression in obesity and related metabolic diseases [13]. It promotes the production of the anti-inflammatory macrophage (M2) markers such as IL-10 [13]. In contrast, the activation of the nuclear factor kappa-B (NF-κB) signaling and increase of TNFα production have been indicated in adiponectin-treated macrophages [14]. Accordingly, a complex interaction of anti- and pro-inflammatory cytokines is involved in obesity-related meta-inflammation initiation and progression, which needs to be further investigated.
Recent microarray analysis has revealed the combined exercise training overlaps with different immune-associated pathways, including toll-like receptors (TLRs), B and T cells-related signaling, and leukocytes’ migration [15]. These pathways promote the inflammatory cytokines’ production, such as TNFα and PTX3 [15]. Evidence has indicated that obesity-induced endogenous ligands, such as lipopolysaccharide and free fatty-acids, constantly stimulate the inflammatory pathways such as TLR4 [16]. Therefore, assessing the effects of an exercise intervention on reducing inflammatory status has arisen.
Concerning the imbalanced work-life time and lack of time in modern society, the high-intensity interval training (HIIT) is mostly recommended as a time-efficient protocol to solve the worldwide problem of the low rate of participating in exercise programs [17]. The high-intensity combined training is the most effective approach in reducing inflammation [11]. Considering that the high-intensity protocols induce the anti-inflammatory [18, 19], pro-inflammatory [20], or even immunosuppressive [10] status, the precise immune responses to these time-efficient training largely remain unclear. Therefore, as the act and/or interact of the anti- and pro-inflammatory cytokines arrange the network of inflammatory status during obesity, apparently involved in glycolipid metabolism, this study aimed to assess the influence of a time-efficient combined HIIT on this network and inflammatory status. We measured the anti- and pro-inflammatory cytokines, lipid profile and glucose metabolism parameters, and assessed their ratio changes and correlations following this protocol.
Materials and methods
Study design
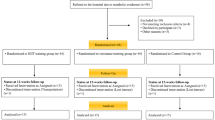
The present investigation was a quasi-experimental study. In this sub-study, we assessed the TLR4-related immunometabolic alterations and its negative regulator in response to different combined all-extremity HIIT protocols, and we suggested a new practical preventive and therapeutic approach for obesity-induced cardiometabolic diseases (the data not yet published). A schematic design of the present study is shown in Fig. 1. This experiment was a controlled exercise training intervention trial approved by the Office of Research Affairs, Ethics Research Committee of the University of Isfahan, Iran (Ethics NO. IR.UI.REC.1396.039). The inflammatory profile, lipid panel, and glucose metabolism were assessed following a 10-week combined all-extremity HIIT. The training sessions were fully supervised and conducted four days per week. The blood samples were taken once one week before the training intervention and then at the end of the ten weeks (~ 48 h after the last training session), Fig. 1(b). A trained person who was blinded to the group allocation collected the samples and coded the subject’s data to concern blinding in outcome assessments.
Schematic design of the study. (a) A total of 86 sedentary females (18–25 years) with OW/OB interested to participate in this study. 15 out of 86 were excluded because of not meeting the age and BMI range. Following a verbal meeting, another 41 subjects were excluded as a result of meeting exclusion criteria including consuming specific medication, being on a diet, time conflict, any diagnosed diseases, participating in any training at least 6 months before this study, and smoking. The remaining 30 eligible individuals were allocated into two inactive (IG: n = 15, continued their regular diet and physical activities) and active (AG: n = 15, participating in the proposed combined HIIT) groups. Four subjects who uncompleted the training sessions or post-intervention procedures were omitted from the final statistical analysis. (b) Both groups underwent two blood sampling collection days. The pre-intervention sampling was conducted one week prior to the first training session and the post-intervention sampling approximately 48 h after the last training session. (c) The newly proposed all-extremity combined HIIT consisted of 4*4 min intervals separated by a 3-minutes active rest (lower-body cycling). Every four minutes’ interval consisted of a 45-sec all-extremity cycling followed by a 45-sec a lower-body or an upper-body resistance training alternatively. The intensity and volume of the training increased progressively based on the HRmax and 1RM percentage
Participants
All interested participants were invited to the Laboratory of the Sports Science Faculty at the University of Isfahan, Iran. Thirty females (BMI = 25–35 kg/m2, age = 18–25 years, sedentary who not meet the exclusion criteria (Fig. 1(a)) were recruited in the study. They were asked about their physical activity range, including leisure-time, job, and house- related physical activities. The eligible subjects were randomly allocated in two inactive (IG, n = 15) and active (AG, n = 15) groups. The coin tossing was used to determine randomization performing by an independent researcher who was unaware of the control and intervention groups. The participants involved in both groups were notified about the experiment procedures and possible risks before signing a written consent. They were instructed not to change their regular physical activities and diet. The IG continued their routine inactive lifestyle, whereas the AG was involved in the proposed exercise training.
Exercise training protocol
According to Fig. 1 (c), this newly proposed combined all-extremity HIIT consisted of four intervals separated by the 3-min active rest (lower-body cycling). Each interval consisted of 4*45 sec. During every 4-min interval, the participants performed two aerobic and two resistance exercises alternatively. The RT involved the leg press, chest press, leg flexion, and lat pulldown (Vectra fitness on-line 4800; USA). The leg press and chest press were performed in the first and third interval, and the leg flexion and lat pulldown were performed in the second and the last interval. The AT, which involved the all-extremity cycling (Monark Ergomedic 839 E and 831 E; Monark, Sweden), were performed after a 45-sec of every RT in each interval. During the intervals, subjects were instructed to move the next exercise in a 15-sec. The AT intensity was monitored in all intervals by Polar watch (F4 Electro, Oy, Kempele, Finland), and increased progressively based on age-predicted maximum heart rate (HRmax) percentage; Fig. 1(c). The RT load was gradually increased according to one maximum repetition (1RM) test; Fig. 1(c). The AG was familiarized with training protocol procedures and intensity during two familiarization sessions. They performed two or three intervals in each familiarization session.
Maximum strength calculation
The maximum strength of each resistance exercise was assessed by 1RM prediction through a 1-6RM equation [21]. The AG was invited to the lab five days before the pre-intervention blood sampling collection day. They were instructed on how to perform all resistance exercises and allowed to practice the lifting techniques. The next day, the testing procedure was explained once, and then the 1-6RM test was assessed for each resistance exercise.
Blood analysis
Blood collection and serum preparation
Following overnight fasting (approximately 12 h), 10 ml of venous blood samples were taken from the antecubital vein. To prevent the circadian status effects, both pre- and post-intervention sampling was taken between 07:30 to 9 a.m. The serum was separated by centrifuging and stored in 150 µl aliquots at -80 °C for analysis.
Biochemical markers assay
The serum biochemical markers concentrations, including high-density lipoprotein (HDL), triglyceride (TG) [23], cholesterol [24], fasting blood glucose (FBG), and insulin (FBI) were measured by the photometric method (Autoanalyzer, Alpha-Classic, Tehran, Iran). The low-density lipoprotein (LDL) values were calculated by the Friedewald formula [24]. The homeostasis model assessment of insulin resistance (HOMA2-IR) and HOMA2- β cell function (HOMA-β) were calculated by the University of Oxford Diabetes Trial Unit software (www.dtu.ox.ac.uk/homacalculator).
Quantitative detection of cytokines
The serum concentrations of cytokines were assessed using an ELISA kit to examine the activity levels of the biological pathway. The concentration of PTX3 (MyBioSource, Inc., USA), TNFα (BioLegend, Inc., USA), IL-10 (BioLegend, Inc., USA) and adiponectin (BioLegend, Inc., USA) were analyzed by microplate reader (Hiperion, MPR 4+, Germany) and the absorbance was adjusted according to manufacturer’s protocol of kits.
Statistical analysis
The Q-Q plots and Shapiro-Wilks tests were performed to assess the data distribution. The non-normal data were analyzed in a transformed logarithm (log10). Levene’s test was run to analyze the homogeneity of variances. Assessing the intervention effects on dependent variables was run by ANCOVA analysis, while the pre-testing values were inputted as the covariate factor. The values of ∆% were calculated to assess the magnitude of intervention impacts. The Spearman’s Correlation Coefficient was used to investigate the association of serum cytokine level changes with the lipid profile and glucose metabolism indices in response to exercise. The sensitivity test was run to assess the omitted outlier effects on the reported results, demonstrating the non-significant changes. The significant level was adjusted at p < 0.05. All data were reported in mean ± SD. All results were analyzed by IBM SPSS Statistics 22 and GraphPad Prism 8.0.1.
Results
According to Fig. 1(a), the two out of fifteen subjects of each group were omitted, who not completed the training or testing procedure, so the data were analyzed from twenty-six out of thirty subjects test results. There were no significant differences at baseline in two groups (P > 0.05) (Table 1).
Serum cytokine concentrations
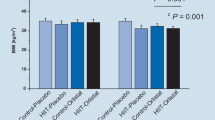
The serum concentration changes of TNFα after 10-week exercise indicated the significant reduction in both within (p = 0.001, ∆% = -71.84) and between group (p = 0.001) comparisons. The decreased level of PTX3 (∆% = -5.82) and increased values of IL-10 (∆% = 11.59) and adiponectin (∆% = 5.51) were not significant (p = 0.3, p = 0.12 and p = 0.22, respectively) (Table 2). As we shown in Fig. 2, the total status of inflammatory profile of AG demonstrated an anti-inflammatory state following exercise as the AG showed the significant reduction in the levels of PTX3/IL-10 (p < 0.05) compared to IG. Also, the significant reduction of TNFα/IL-10 and TNFα/adiponectin compared to the pre-intervention (p < 0.01, p < 0.05) and IG (p < 0.01, p < 0.01) was found, respectively. The changes of PTX3/adiponectin were not statistically significant (p > 0.05).
Pro-inflammatory to anti-inflammatory balance before and after of 10-week CHIIT in both control (IG) and exercise (AG) subjects. All data presented as mean ± SEM.* p < 0.05 and * * p < 0.01
Abbreviations: IG, inactive group; AG, active group; PTX3, pentraxin 3; TNFα, tumor-necrosis factor α; IL-10, interleukin 10
Glucose and lipid profile parameters
Overall a significant improvement was observed in glucose metabolism after exercise intervention (Table 2). The FBG and FBI values in AG were significantly reduced in comparison with IG (p = 0.004). Also, the within group comparison showed a significant reduction in FBI levels (p < 0.01, ∆% = -31.67) in AG, where this comparison was not significant for FBS (p > 0.05). The AG within group comparison indicated a significant reduction in HOMA2-B% (p < 0.01, ∆% = -28.52) and HOMA2-IR (p < 0.01, ∆% = -36.92). Also, the HOMA2-B%, HOMA2-IR and HOMA2.S% values significantly decreased in AG compared to IG (p = 0.01, p = 0.002 and p = 0.01, respectively), Fig. 3. The level of TG and TC did not change in either within or between group comparison (p = 0.74, p = 0.61, respectively), Fig. 4. In contrast, the HDL levels significantly increased in AG following exercise in both within (p < 0.05, ∆% = -36.92) and between (p = 0.04) group comparisons. Although the LDL levels significantly decreased in AG and IG compared to their pre-intervention states (p < 0.05, ∆% = -1.51 and p < 0.05, ∆% = -10.99, respectively), there was no significant changes in AG in comparison with IG (p = 0.33).
Glucose metabolism state. The HOMA2-β% and – S% demonstrating the β-cell function and insulin sensitivity, respectively, were shown before and after of 10-week CHIIT in both control (IG) and exercise (AG) subjects. All data presented as mean ± SEM. * p < 0.05 and * * p < 0.01. Abbreviations: IG, inactive group; AG, active group; HOMA2, homeostasis model assessment 2
Lipid profile changes before and after of 10-week CHIIT in both control (IG) and exercise (AG) subjects. All data presented as mean ± SEM. Abbreviations: IG, inactive group; AG, active group; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol. * p < 0.05 and * * p < 0.01
Correlation analysis
The correlation analysis indicated the statistical significance relations between TNFα level changes and HDL (r = -0.62, p < 0.01), HOMA2.B% (r = -0.49, p < 0.05) and HOMA2.IR (r = -0.4, p < 0.05) following exercise training (Table 3). The adiponectin concentrations showed the significant correlations with FBI levels (r = -0.47, p < 0.05) and HOMA.B% changes (r = 0.63, p < 0.01). The IL-10 levels significantly correlated with HDL (r = 0.53, p < 0.01), TG (r = -0.74, p < 0.01) and FBG (r = -0.42, p < 0.05) changes. Also, the PTX3 level showed the significant relations with LDL (r = 0.65, p < 0.01) and TC (r = 0.58, p < 0.01) levels after a 10-week combined all-extremity HIIT.
Discussion
This study assessed the effects of a new all-extremity combined HIIT on the inflammatory status and glycolipid metabolism among sedentary overweight/obese females. We found that a 10-week combined all-extremity HIIT improves the inflammatory profile (pro- and anti-inflammatory factors), cardiometabolic risk factors (lipid profile parameters), and glucose metabolism (insulin and glucose homeostasis).
Regarding CLSI improvement by the exercise-induced anti-inflammatory environment [10, 16], we found a slight increase in adiponectin levels which was supported by Christiansen et al. [25]. Adiponectin is involved in M2 polarization through suppressing the TLRs pathway and promoting signal transducer and activator of transcription (STAT) 6 and IL-4 related pathway [13], suggesting a potential role of this exercise in the maintenance of the anti-inflammatory state. Another immunomodulatory act of adiponectin is related to increase the anti-inflammatory cytokines’ production such as IL-10 [13]. Although the IL-10 levels have increased following a 12-week HIIT [27], the 11% increase in IL-10 levels were not statistically significant in our study. As high glucose level is a common condition in the obese state, it has been reported that IL-10 production rates are impaired during high glucose exposure [28]. Neither insulin level reduction nor HOMA-IR improvement could affect IL-10 production levels in this study, indicating the complex of factors determining stimulation or inhibition of an anti-inflammatory response after exercise in obesity.
A potential mechanism describing the immunoregulatory ability of the exercise training is associated with reducing the TLR4 activities [10, 16]. In this context, the PTX3 and TNFα expression are controlled by NF-κB pathway when TLR-induced inflammation [29]. Our findings revealed a significant reduction in TNFα, however, the PTX3 decreased was not statistically significant. The insignificant PTX3 changes were in agreement with other’s findings following both acute [30] and long-term exercises [19, 31]. In contrast, either an unchanged [12, 32, 33] or increased TNFα levels [20] have been revealed following different exercise interventions. The contracting skeletal muscle-released IL-6 and irisin might be a reason for a decrease in TNFα level in this study, since these myokines interfere the TLR4/NF-κB pathway activities [11, 34]. Although the PTX3 basal levels in obese subjects were not significantly different from normal weight individuals, a continuous aerobic exercise session reduced PBMCs-stimulated production of PTX3 in both the normal weight and obese groups [18]. The role of PTX3 in adipocyte differentiation has revealed its potential power in lipid accumulation and, consequently, increase the meta-inflammatory state during obesity [35]. Therefore, these double-edge responses of PTX3 either in obesity or following the exercise training could be partially related to the different action/interaction of various circulating immune cells in producing PTX3, metabolic dysfunction rates, and severity of meta-inflammatory state.
An imbalanced cytokine network induces multifactorial diseases because of neutralizing and/or antagonizing influences of the anti-inflammatory cytokines on pro-inflammatory ones, indicating the importance of evaluating the pro- to anti-inflammatory cytokines ratios. These ratios are the general immunoreactivity indicators which may be more informative than a cytokine’s absolute level. Accordingly, the helper T cells type 1 to type 2 (Th1/Th2)-produced cytokines’ ratio, like TNFα/IL-10, has been reported to be an indicator of increased risk of coronary heart diseases [36], a metabolic dysfunction-mediated disorder. In this regard, despite the insignificant increase in absolute levels of evaluated cytokines in this study, the immunoreactivity assessments revealed an overall anti-inflammatory status because of a significant reduction in TNFα/IL-10, TNFα/adiponectin, and PTX3/IL-10 values. The reduction in the immunoreactivity assessments has been indicated following HIIT protocol [37]. In contrast, the increased IL-6/IL-10 values in response to lipopolysaccharide have been shown after the maximal exercises [38, 39]. These discrepancies may be related to the difference in the healthiness and sex of participants and/or duration of protocols in two studies.
Hyperlipidemia partially induces by the failed attempts of AT-resident macrophages to maintain the tissue homeostasis [40], indicating that the improvement in lipid profile parameter could reflect improved AT macrophages function. In this context, although the TG and TC reduction was not significant in response to combined all-extremity HIIT in our study, increased HDL and decreased LDL levels revealed overall improvement in lipid profile. Although these results are in accordance with some studies [27, 33, 41, 42], others showed a significant reduction in TG and TC levels [19, 33, 42]. According to correlation analyses here, the increased HDL levels showed either a positive relation with IL-10 changes or a negative association with TNFα and TG changes. Moreover, PTX3 changes were positively associated with TC and LDL reduction. Accordingly, as dyslipidemia influences on the innate immune system through TLR4 pathway [43], it could be reflected the TNFα reduction here.
Hyperglycemia is an essential factor in neural damage, cognitive failure, liver cancer, and NAFL [44, 45]. An acute aerobic training with sever intensity has reduced the glucose levels more than the same with lower intensities [39], suggesting the effectiveness of exercise protocols with higher intensities in modifying glucose metabolism. In this context, we found a decrease in the FBI, HOMA2-IR, and -B% values and an increase in HOMA2-S% levels. These results were supported by others evaluating the effects of a circuit RT [42]. The significantly increased FBG values in the AG in comparison with the IG could be related to the pre-intervention significant differences between two groups in glucose levels at baseline. Liu et al. have reported a reduction in FBG and FBI levels without significant changes in HOMA-IR values in T2D patients [19]. In contrast, the FBG and FBI levels have not been significantly altered following 12 weeks among dysglycaemia males and sedentary females [15, 32]. Concerning the negative impact of TNFα on insulin signaling [11], we found a negative correlation between the decreased TNFα levels and HOMA2-IR, HOMA2-B%, and HDL changes. The adiponectin changes were negatively correlated with values of the FBI and HOMA2-B%, suggesting the possible mechanism in which adiponectin enhances the glucose metabolism, since it is known as an insulin sensitizer [13]. Also, Ptx3 knockout mice have shown an increase in insulin plasma concentration [46], indicating the PTX3 role in IR development [40]. Accordingly, the improvement of insulin sensitivity and glucose metabolism in our study confirm the inverse correlation findings.
Limitations
Although this study revealed the impressive results, it should be noted the limitations. The small sample size could be affected by some insignificant results. This was related to, first, the limited volunteers during the participant recruitment stage and then, the precise inclusion/exclusion criteria to provide consistency in all subjects, providing more reliable results. Moreover, limited funding forced the authors to limit the evaluated cytokines network.
Suggestions for future studies
As a cytokine is produced by the specific immune cells related to innate or adaptive immunity, it is suggested that the production of a cytokine and its receptor is evaluated in a specific immune cell, simultaneously. Furthermore, it is more informative that future exercise immunology studies evaluate the anti-inflammatory cytokine function in response to the different exercise protocols, such as assessment the adiponectin or IL-10 ability to suppress the lipopolysaccharide-induced PTX3 or TNFα production through whole blood cultures.
Conclusions
In conclusion, according to Fig. 5, our proposed combined all-extremity HIIT induced positive anti-inflammatory adaptation in sedentary obese females, indicating either a failure in mechanisms-mediated the anti-inflammatory cytokines’ production or an insufficient time, duration, and intensity of this intervention to stimulate their pathways. Furthermore, the impaired function in anti-inflammatory cytokine production might be associated with obesity-induced impaired glycolipid metabolism. However, more investigations are needed to achieve a better understanding of cytokine crosstalk (Th1/Th2) in meta-inflammatory status in responses to exercise. However, the glycolipid metabolism improvement and inflammatory status reduction indicated that this time-efficient practical exercise training could be proposed either as a therapeutic approach among sedentary overweight/obese individuals or a solution promoting exercise program participating in modern societies with time-complicated-induced metabolic disorders.
The immunoreactivity of a 10-week CHIIT. The hyper-immune system activities induced in an obese state promote an imbalanced cytokines network relating to the pathology of the obesity-associated metabolic diseases. On the contrary, exercise training promotes a balanced state through increasing the anti-inflammatory cytokines production, suggesting neutralizing and/or antagonizing the inflammatory cytokines. In this study, the 10 weeks CHIIT promoted the increased levels of inflammatory cytokines (PTX3 and TNFα) and glycolipid metabolism parameters (FBG, FBI, LDL, TG and TC) (a) towards an anti-inflammatory balanced state through decreasing the values of FBG, FBI, TNFα, PTX3/IL-10, TNFα/adiponectin and increasing the HOMA-IR and HDL levels (b)
Abbreviations: CHIIT, combined high-intensity interval training; adipo, adiponectin; PTX3, pentraxin 3; TNFα, tumor-necrosis factor α; IL-10, interleukin 10; TG, triglyceride; HDL, high-density lipoprotein; FBG, fasting blood glucose; FBI, fasting blood insulin; LDL, low-density lipoprotein; TC, total cholesterol; HOMA2-IR, homeostasis model assessment of insulin resistance
References
Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–85.
Bonacina F, Moregola A, Porte R, Baragetti A, Bonavita E, Salatin A, et al. Pentraxin 3 deficiency protects from the metabolic inflammation associated to diet-induced obesity. Cardiovasc Res. 2019.
Alberti L, Gilardini L, Zulian A, Micheletto G, Peri G, Doni A, et al. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis. 2009;202(2):455–60.
Abderrahim-Ferkoune A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, et al. Characterization of the long pentraxin PTX3 as a TNFα-induced secreted protein of adipose cells. J Lipid Res. 2003;44(5):994–1000.
Ogawa T, Kawano Y, Imamura T, Kawakita K, Sagara M, Matsuo T, et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity. 2010;18(9):1871–4.
Gurel H, Genc H, Celebi G, Sertoglu E, Cicek A, Kayadibi H, et al. Plasma pentraxin-3 is associated with endothelial dysfunction in non-alcoholic fatty liver disease. Eur Rev Med Pharmacol Sci. 2016;20(20):4305–12.
Zanetti M, Bosutti A, Ferreira C, Vinci P, Biolo G, Fonda M, et al. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: evidence for association with atherogenic lipid profile. Clin Exp Med. 2009;9(3):243–8.
Miyaki A, Maeda S, Yoshizawa M, Misono M, Sasai H, Shimojo N, et al. Is pentraxin 3 involved in obesity-induced decrease in arterial distensibility? J Atheroscler Thromb. 2010:1002040175-.
Slusher AL, Mischo AB, Acevedo EO. Pentraxin 3 is an anti-inflammatory protein associated with lipid-induced interleukin 10 in vitro. Cytokine. 2016;86:36–40.
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop N, et al. Position statement part one: immune function and exercise. 2011.
Nimmo M, Leggate M, Viana J, King J. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. 2013;15(s3):51–60.
Barry JC, Simtchouk S, Durrer C, Jung ME, Mui AL, Little JP. Short-term exercise training reduces anti-inflammatory action of interleukin-10 in adults with obesity. Cytokine. 2018;111:460–9.
Luo Y, Liu M. Adiponectin: a versatile player of innate immunity. J Mol Cell Biol. 2016;8(2):120–8.
Park P-h, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264. 7 macrophages with adiponectin increases tumor necrosis factor-α (TNF-α) expression via ERK1/2 activation and Egr-1 expression role of TNF-α in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282(30):21695–703.
Lee S, Norheim F, Langleite TM, Gulseth HL, Birkeland KI, Drevon CA. Effects of long-term exercise on plasma adipokine levels and inflammation-related gene expression in subcutaneous adipose tissue in sedentary dysglycaemic, overweight men and sedentary normoglycaemic men of healthy weight. Diabetologia. 2019;62(6):1048–64.
Soltani N, Marandi SM, Kazemi M, Esmaeil N. The exercise training modulatory effects on the obesity-induced immunometabolic dysfunctions. Diabetes Metab Syndr Obes. 2020;13:785–810. https://doi.org/10.2147/DMSO.S234992.
Gibala MJ, McGee SL. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exercise and sport sciences reviews. 2008;36(2):58–63.
Slusher AL, Shibata Y, Whitehurst M, Maharaj A, Quiles JM, Huang C-J. Exercise reduced pentraxin 3 levels produced by endotoxin-stimulated human peripheral blood mononuclear cells in obese individuals. Exp Biol Med. 2017;242(12):1279–86.
Liu Y, Liu S-x, Cai Y, Xie K-l, Zhang W-l, Zheng F. Effects of combined aerobic and resistance training on the glycolipid metabolism and inflammation levels in type 2 diabetes mellitus. J Phys Ther Sci. 2015;27(7):2365–71.
Zwetsloot KA, John CS, Lawrence MM, Battista RA, Shanely RA. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J Inflamm Res. 2014;7:9.
Nutter J. Weight training adds up. Strategies. 1995;8(7):15–8.
Kaspersen KA, Dinh KM, Erikstrup LT, Burgdorf KS, Pedersen OB, Sørensen E, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the danish blood donor study (DBDS). PloS one. 2016;11(10):e0164220.
Kim H-K, Hwang C-L, Yoo J-K, Hwang M-H, Handberg EM, Petersen JW, et al. All-extremity exercise training improves arterial stiffness in older adults. Med Sci sports Exerc. 2017;49(7):1404.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298(4):E824–31.
Liu Y, Sweeney G. Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab. 2014;28(1):33–41.
Steckling FM, Farinha JB, Figueiredo FdC, Santos DLD, Bresciani G, Kretzmann NA, et al. High-intensity interval training improves inflammatory and adipokine profiles in postmenopausal women with metabolic syndrome. Arch Physiol Biochem. 2019;125(1):85–91.
Barry JC, Shakibakho S, Durrer C, Simtchouk S, Jawanda KK, Cheung ST, et al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep. 2016;6:21244.
Doni A, Stravalaci M, Inforzato A, Magrini E, Mantovani A, Garlanda C, et al. The long pentraxin PTX3 as a link between innate immunity, tissue remodeling, and cancer. Front Immunol. 2019;10(712). https://doi.org/10.3389/fimmu.2019.00712.
Slusher AL, Mock JT, Whitehurst M, Maharaj A, Huang C-J. The impact of obesity on pentraxin 3 and inflammatory milieu to acute aerobic exercise. Metabolism. 2015;64(2):323–9.
Hovsepian V, Marandi SM, Esfarjani F, Zavar R, Sadeghi M. The Effect of all-extremity high-intensity interval training on plasma pentraxin 3 in young overweight and obese women. Asian J Sports Med. 2019;10(4).
Markofski MM, Flynn MG, Carrillo AE, Armstrong CL, Campbell WW, Sedlock DA. Resistance exercise training-induced decrease in circulating inflammatory CD14 + CD16 + monocyte percentage without weight loss in older adults. Eur J Appl Physiol. 2014;114(8):1737–48.
Nunes PRP, Barcelos LC, Oliveira AA, Júnior RF, Martins FM, Orsatti CL, et al. Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women: controlled and randomized clinical trial of efficacy of training volume. Age. 2016;38(2):40.
Mazur-Bialy AI, Pocheć E, Zarawski M. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 2017;18(4):701.
Shin M-K, Choi B, Kim E-Y, Park J-E, Hwang ES, Lee HJ, et al. Elevated pentraxin 3 in obese adipose tissue promotes adipogenic differentiation by activating neuropeptide Y signaling. Front Immunol. 2018;9(1790). https://doi.org/10.3389/fimmu.2018.01790.
Goswami B, Rajappa M, Mallika V, Shukla DK, Kumar S. TNF-α/IL-10 ratio and C-reactive protein as markers of the inflammatory response in CAD-prone North Indian patients with acute myocardial infarction. Clin Chim Acta. 2009;408(1–2):14–8.
de Souza DC, Matos VA, dos Santos VO, Medeiros IF, Marinho CS, Nascimento PR, et al. Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front Physiol. 2018;9.
Slusher AL, Zúñiga TM, Acevedo EO. Maximal exercise alters the inflammatory phenotype and response of mononuclear cells. Med Sci Sports Exerc. 2018;50(4):675–83.
Antunes BM, Campos EZ, dos Santos RVT, Rosa-Neto JC, Franchini E, Bishop NC, et al. Anti‐inflammatory response to acute exercise is related with intensity and physical fitness. J Cell Biochem. 2019;120(4):5333–42.
Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–94.
Park S-M, Kwak Y-S, Ji J-G. The effects of combined exercise on health-related fitness, endotoxin, and immune function of postmenopausal women with abdominal obesity. J Immunol Res. 2015;2015.
Kolahdouzi S, Baghadam M, Kani-Golzar FA, Saeidi A, Jabbour G, Ayadi A, et al. Progressive circuit resistance training improves inflammatory biomarkers and insulin resistance in obese men. Physiol Behav. 2019;205:15–21.
Chen S, Lin G, Lei L, You X, Wu C, Xu W, et al. Hyperlipidemia modifies innate immune responses to lipopolysaccharide via the TLR-NF-κB signaling pathway. Inflammation. 2013;36(4):968–76.
Treviño S, Aguilar-Alonso P, Flores Hernandez JA, Brambila E, Guevara J, Flores G, et al. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 2015;69(9):421–33.
Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68(4):1308–18.
Guo H, Qiu X, Deis J, Lin T-Y, Chen X. Pentraxin 3 deficiency exacerbates lipopolysaccharide-induced inflammation in adipose tissue. Int J Obes. 2019:1.
Acknowledgements
The authors extended their appreciation to Dr. Hossein Khanahmad for his valuable advice. The support of Mrs. Mohadeseh Toghyani and Mrs. Fahimeh Hosseininasab involved in the Immunology Department of Isfahan University of Medical Science Staff is appreciated as well.
Funding
This study was funded by University of Isfahan, Isfahan, Iran (NO. IR.UI.REC.1396.039).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. N.S., S.M.M., and N.E. designed the study and performed the statistical analysis. N.S, N.E., and M.K. run the laboratory experiments and analyzes. N.S. drafted the initial manuscript and N.E. edited the final manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
There are no competing conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soltani, N., Marandi, S.M., Kazemi, M. et al. Meta-inflammatory state and insulin resistance can improve after 10 weeks of combined all-extremity high-intensity interval training in sedentary overweight/obese females: a quasi-experimental study. J Diabetes Metab Disord 19, 717–726 (2020). https://doi.org/10.1007/s40200-020-00550-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00550-z