Abstract
Purpose of Review
The purpose of this article is to review the literature relevant to point of care ultrasound (POCUS) with an emphasis on the application of ultrasound in preoperative assessment. It addresses the use of cardiac, lung, gastric, and vascular ultrasonography.
Recent Findings
The use of POCUS as an adjunct to the physical examination is gaining traction as high-quality equipment becomes increasingly portable and dramatically more affordable. While the literature on preoperative ultrasound by anesthesiologists is limited, there is growing evidence that it is not only feasible, but also improves patient morbidity and mortality.
Summary
Anesthesiologists frequently encounter patients with signs and symptoms of heart failure, significant cardiac murmurs, hemodynamic instability, unexplained dyspnea, and unknown gastric contents. Studies to date show that POCUS can change management of these patients and improve morbidity without delaying care. Randomized trials are needed to validate these findings. Professional societies in anesthesiology need to define standards for POCUS training, as well as establish mechanisms for certification and maintenance of proficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fundamental concept of point of care ultrasound (POCUS) is that it is bedside ultrasound performed by the treating physician and interpreted in real time, assessing for limited but clinically relevant findings that can immediately influence treatment [1••]. In the past two decades, the use of POCUS as an extension of the physical examination has grown exponentially. POCUS is increasingly applied across diverse specialties including anesthesiology, internal medicine, trauma surgery, emergency medicine, and critical care. This growth has been spurred by the dramatic decrease in the size of affordable, high-quality ultrasound equipment, as well as by the evolving understanding of the limitations of the history and physical examination that can be overcome with bedside ultrasound [2, 3]. The change has occurred so rapidly that even the latest American Society of Anesthesiologists’ (ASA) Practice Advisory for Preanesthesia Evaluation makes no mention of bedside ultrasound and only discusses the use of history and physical examination along with more traditional diagnostic studies [4].
Anesthesiologists are ideally positioned to utilize POCUS in the preoperative setting, as they are already trained in using ultrasound to establish vascular access, to perform peripheral nerve blocks and to perform basic transesophageal echocardiography (TEE) [5]. The performance and interpretation of basic bedside ultrasound (for regional anesthesia and rescue TEE) is now considered a fundamental skill of a qualified anesthesiologist and is being included in the Objective Structured Clinical Examination administered by the American Board of Anesthesiology [6]. Extending the use of preoperative ultrasound, the anesthesiologist can gain information about the presence and severity of a variety of pathologies that impact perioperative patient care. As the use of POCUS spreads, formal processes for training, evaluation of competence, and maintenance of competence will need to be developed.
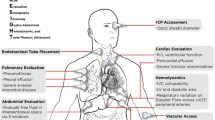
This review will discuss the value of bedside ultrasound by anesthesiologists in the preoperative assessment and management of patients. It will cover cardiac, lung, gastric, and vascular ultrasound for deep venous thrombosis (DVT.) While it will not neglect any of the classic references, every attempt will be made to focus on recent literature and that which applies best to the practice of anesthesiology. As perioperative POCUS is a new field, there is still a paucity of literature, however, and much of what does exist was generated by ultrasound enthusiasts in other fields.
Cardiac Ultrasound
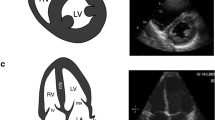
For decades, cardiac ultrasound was considered the exclusive domain of the cardiologist. This was due in part to the prohibitive cost of ultrasound platforms as well as the lack of training of other providers in the appropriate techniques. The reduction in cost and increase in training that has taken place in recent years has begun to change the landscape of cardiac ultrasound in dramatic ways. Among the applications of POCUS, the evaluation of the cardiovascular system is both the most common and the most well studied. Many bedside ultrasound protocols have been created that include a limited cardiac evaluation, but all can be grouped together under the concept of focused cardiovascular ultrasound (FOCUS). FOCUS is defined by the American Society of Echocardiography (ASE) as “a focused examination of the cardiovascular system performed by a physician using ultrasound as an adjunct to the physical examination to recognize specific ultrasonic signs that represent a narrow list of potential diagnoses in specific clinical settings” [1••].
While the details of performing a FOCUS exam are beyond the scope of this review, the requisite characteristics of the exam are that it can be rapidly acquired with basic 2-dimensional ultrasound equipment, is qualitative rather than quantitative, goal directed, problem oriented, and limited in scope [1••, 7•, 8]. FOCUS is appropriate when signs or symptoms are identified that suggest underlying cardiovascular pathology. Some of the common indications in the preoperative arena include evidence of heart failure, heart murmur with concern for significant valvular disease, hemodynamic instability, and findings concerning for pericardial effusion or tamponade. It is important to note that FOCUS is distinct from limited transthoracic echocardiography (TTE) and is intended only to identify the presence or absence of a limited set of critical findings. The information is then utilized immediately to affect management or to trigger a complete echocardiogram.
Several studies have shown that bedside ultrasound is superior to the physical examination in identifying left ventricular (LV) systolic dysfunction [9,10,11,12,13]. One study of medical students and residents showed that with only 2 h of ultrasound training, the ability to diagnose LV systolic dysfunction improved dramatically. The history, physical examination, and electrocardiogram (ECG) had a sensitivity and specificity of 26 and 85% for establishing LV dysfunction. FOCUS improved the sensitivity and specificity to 74 and 94%, respectively.
Another study compared the diagnostic accuracy of first-year medical students using FOCUS to attending cardiologists evaluating patients with physical examination. When evaluating patients with valvular lesions, the students’ sensitivity was 89% compared with that of the cardiologists at 50%. Both groups had specificities of 90%. The study concluded that for both valvular and non-valvular cardiac abnormalities, “the diagnostic accuracy of first-year medical students using bedside cardiac ultrasound examinations was significantly superior to that of board-certified cardiologists performing cardiac physical examinations” [14••]. This study encapsulates the concept that, no matter the skill of the evaluating physician, “If you don’t look, you don’t know!”
While literature on the application of preoperative FOCUS performed by anesthesiologists is limited, it has been shown to be both feasible and capable of changing management. An evaluation of five anesthesiology residents showed that they were able to identify significant aortic stenosis after only 2 hours of training [15•]. A study of 50 consecutive preoperative patients showed that major findings from anesthesiologist-performed FOCUS correlated with cardiology-based TTE in 87% of cases [16]. Prospective observational studies have shown that selective preoperative FOCUS prior to noncardiac surgery changed management in 34–82% of cases [17, 18]. The observed changes included step up in treatment, step down in treatment, level of postoperative care, and cancelation of surgery.
A small retrospective study found the use of FOCUS in patients undergoing hip fracture surgery with a high risk of cardiac complications was associated with lower 30-day mortality (4.7 vs. 15.2%) and lower 1-year mortality (17.1 vs. 33.3%) without delaying entry into the operating room [19•]. The most common abnormality found was hypovolemia, which was present in 34% of the study group. Other findings that were not suggested by physical examination included heart failure (20%), aortic stenosis (14%), and pulmonary hypertension (11%).
In a study of 112 unselected patients undergoing emergency surgery, the application of preoperative FOCUS changed the clinical management in 12% of cases. While this still represents a valuable intervention, it also suggests that a selective application of the techniques may be more appropriate [20].
While there have been a number of studies showing that bedside ultrasound can be used to assess volume status by evaluating the inferior vena cava (IVC), a recent study of anesthesiologists-performed FOCUS showed that the preoperative size and collapsibility of the IVC accurately predicted hypotension after induction of general anesthesia [21,22,23,24,25].
With the ability to quickly evaluate for abnormalities of global biventricular systolic function, valvular abnormalities, and prediction and etiology of hemodynamic instability with minimal cost or delay, it is likely that the application of preoperative FOCUS will follow basic TEE as a core skill of anesthesiologists in the future.
Lung Ultrasound
For years, the lungs have been seen only as a barrier to the application of ultrasound, creating artifact and inhibiting visualization of cardiac structures during TTE. Due largely to the pioneering work of Dr. Daniel Lichtenstein, the use of ultrasound to diagnose a variety of pulmonary conditions has become common in medical and surgical critical care practices, and is now spreading to other specialties [26••]. As both the indications and the conditions identified are common in the preoperative setting, the value of these techniques to anesthesiologists should be apparent even if the literature has focused largely on applications in critical care settings.
Indications for lung ultrasound include dyspnea, pleuritic chest pain, hypoxia, and abnormal breath sounds. The identification of pneumothorax (PTX), pleural effusion, and increased lung water (from pulmonary edema, pneumonia, or pulmonary contusion) is the primary goal of preoperative bedside lung ultrasound. When compared with chest radiography (CXR) or computed tomography (CT), lung ultrasound has been shown to have similar or better sensitivity and specificity with much lower cost, faster turn-around, no requirement for transport of the patient, and no exposure to radiation.
In one influential study, lung ultrasound was compared to CXR in the identification of traumatic PTX using chest CT as the gold standard. While CXR identified only 13 of 25 PTX (sensitivity 52%, specificity 100%), lung ultrasound was able to identify 23 of 25 PTX with one false positive (sensitivity 92%, specificity 99%). This suggested that not only was ultrasound superior to CXR in the identification of PTX but that its diagnostic yield approached that of CT [27]. In a separate study, it was shown that a high degree of sensitivity (86%) and specificity (100%) can be obtained by physicians after only 2 hours of training in lung ultrasound [28].
In comparing ultrasound to CXR in patients with ARDS, ultrasound had a much better diagnostic accuracy in the detection of pleural effusion. Specifically, ultrasound had a sensitivity and specificity of 92 and 93% compared with 39 and 85% with CXR [29]. Similarly, ultrasound was able to identify alveolar-interstitial syndrome with a sensitivity of 98% compared with 60% with CXR [29].
A recent prospective study of preoperative combined cardiopulmonary ultrasound in patients undergoing emergency surgery found a 27% incidence of unexpected abnormal findings which resulted in a change in clinical management in 43% of cases and was associated with a significant increase in hospital mortality (13 vs. 0%) and 30-day mortality (17 vs. 1%.) The most common abnormality identified was unexpected pleural effusion, leading to changes in ventilator management as well as pleural drainage [20].
While there is a paucity of literature on the impact of preoperative lung ultrasound and no randomized controlled trials exist, the feasibility, diagnostic accuracy, and ability to rapidly identify common and important pathologies make it a natural extension of the anesthesiologist’s preoperative evaluation. An excellent recent review has provided a detailed description of the performance and interpretation of a lung ultrasound examination [30•].
Gastric Ultrasound
In a field relatively bereft of robust guidelines, by far, the most commonly applied must be the ASA’s practice guideline on preoperative fasting [31]. Patient fasting prior to anesthesia aims to minimize the risk and consequences of pulmonary aspiration. In an attempt to achieve that goal, consensus-based recommendations are made on the duration of fasting that will likely result in minimal gastric contents in the majority of patients. The guidelines are unable to make specific recommendations for modifications in circumstances where there may be abnormal gastric emptying, such as with conditions such as diabetes, gastroparesis, trauma, or end-stage renal disease. These patients may well have full stomachs well beyond the recommended fasting periods, yet other patients may have empty stomachs well before the suggested delay. While gastric ultrasound has been practiced for decades, its application by anesthesiologists in the preoperative period has only recently been accelerating. Nonetheless, it may serve to answer the important question “What is in my patients’ stomachs [32]?”
In a study designed to assess the learning curve for gastric ultrasound, it was found that an anesthesiologist had to perform an average of 33 examinations to achieve a 95% success rate in distinguishing the stomach as empty, having liquid content or solid contents [33]. In an attempt to quantify the volume of gastric contents, a recent study evaluated gastric ultrasound findings in 108 patients undergoing upper endoscopy who had their gastric contents suctioned and measured directly. A formula was created that accurately identified gastric volumes between 0 and 500 mL, which can help identify patients with fluid in their stomachs that represent normal gastric residual volumes [34].
In a study of 38 preoperative patients, the information obtained from gastric ultrasound resulted in a change in anesthetic timing or technique in 71% of cases with a trend toward shorter surgical delay [35]. While it is hard to imagine a randomized controlled trial evaluating the outcome of gastric ultrasound on pulmonary aspiration in violation of current fasting guidelines, it is also reasonable to assume that knowing what is in a patient’s stomach is better than guessing. A recent review described the techniques of performing and interpreting bedside gastric ultrasound [36•].
Deep Vein Thrombosis Determination
Traditionally, recognized risk factors for deep venous thrombosis (DVT) include advanced age, trauma, immobility, cancer, hypercoagulable states, as well as a history of DVT [37]. These risk factors are commonly present in patients presenting for surgery and acute pulmonary embolus is a recognized and feared cause of perioperative morbidity and mortality. The imaging test of choice for diagnosing DVT is comprehensive lower extremity ultrasonography performed by a vascular sonographer and interpreted off-line by a physician [38].
A more limited evaluation can be performed by clinicians at the bedside using two-dimensional compression ultrasonography alone. This is a binary test that can detect the presence or absence of thrombus in the popliteal or femoral venous systems. While not designed to identify distal or iliac thrombus, the majority of clinically relevant DVTs will be identified in a fraction of the time of a formal study. These techniques have been best studied by emergency medicine physicians. A meta-analysis shows sensitivity of 96% and specificity of 96% when compared with either color flow duplex sonography or venogram in patients where there was clinical suspicion of DVT [39•].
While there are no studies evaluating preoperative investigation of DVT by anesthesiologists, there is at least a theoretical benefit to identifying an existing DVT prior to elective surgery, with the potential to avoid pulmonary embolism either by treatment with anticoagulation, delay of surgery, or placement of an inferior vena cava filter.
Training and Competence
There is no standardized approach to training in POCUS and no detailed guidelines for either trainees or anesthesiologists in practice to acquire the skills needed to perform the studies described in this review [40]. The Society of Critical Care Anesthesiologists has published specific learning goals for POCUS for critical care trainees, but those may exceed the scope necessary for basic preoperative POCUS [41].
In the case of FOCUS, the ASE notes that novice users developed “acceptable” proficiency in performing and interpreting studies after 20–30 studies if the scope of the studies were limited [1••]. However, the ability to make formal training recommendations is limited by the heterogeneity of the studies investigating training in FOCUS. The ASE does specify that training should include a combination of didactic education, hands-on image acquisition, and experience interpreting images, but is not more specific than that.
Maintenance of proficiency is an area that needs further investigation in order to develop meaningful guidelines. One study of 30 non-cardiologists found that skills in FOCUS were notably diminished within 2 years of nonuse [42]. The ASE only notes that a minimum number of studies performed annually needs to be determined, and continuing education courses should be included. Absent more robust guidance regarding acquisition, demonstration, and maintenance of competence, each institution will need to establish internal processes to allow for the credentialing of skilled providers in the techniques of bedside ultrasound.
Conclusions
Ultrasound is a powerful tool that augments the anesthesiologist’s preoperative assessment. Assessment of the heart, lungs, stomach, and vasculature does not delay care and can change management and improve perioperative mortality. The studies performed to date show benefit from use of preoperative ultrasound, but randomized studies are needed. As use of preoperative ultrasound grows, anesthesiologists need their professional societies to define standards for training and develop pathways for certification and maintenance of proficiency.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567–58. Society recommendations for standardizing the concept of FOCUS, including a general approach to training and the important distinction between FOCUS and limited TTE.
Di Bello V, La Carrubba S, Conte L, et al. Incremental value of pocket-sized echocardiography in addition to physical examination during inpatient cardiology evaluation: a multicenter Italian study (SIEC). Echocardiography. 2015;32:1463–70.
Kobal SL, Atar S, Siegel RJ. Hand-carried ultrasound improves the bedside cardiovascular examination. Chest. 2004;126(3):693–701. https://doi.org/10.1378/chest.126.3.693.
Committee on Standards and Practice Parameters. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522–38.
Coker BJ, Zimmerman JM. Why anesthesiologists must incorporate focused cardiac ultrasound into daily practice. Anesth Analg. 2017;124(3):761–5. https://doi.org/10.1213/ANE.0000000000001854.
Anesthesiology TABO Applied Examination-Objective Structured Clinical Examination Content Outline. http://www.theaba.org/PDFs/APPLIED-Exam/APPLIED-OSCE-ContentOutline. 2016;1–5 (Accessed 16 Sept 2017).
• Zimmerman JM, Coker BJ. The nuts and bolts of performing focused cardiovascular ultrasound (FoCUS). Anesth Analg. 2017;124(3):753–60. A detailed description of the techniques of performing FOCUS with numerous images and an accompanying video walking the student through the exam. https://doi.org/10.1213/ANE.0000000000001861.
Via G, Hussain A, Wells M, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27:683.e1–683.e33.
Galderisi M, Santoro A, Versiero M, et al. Improved cardiovascular diagnostic accuracy by pocket size imaging device in non-cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study. J Cardiovasc Ultrasound. 2010;8:51.
Kimura BJ, Amundson SA, Willis CL, Gilpin EA, DeMaria AN. Usefulness of a hand-held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–9. https://doi.org/10.1016/S0002-9149(02)02699-1.
DeCara JM, Kirkpatrick JN, Spencer KT, Ward RP, Kasza K, Furlong K, et al. Use of hand-carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses. J Am Soc Echocardiogr. 2005;18(3):257–63. https://doi.org/10.1016/j.echo.2004.11.015.
Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319–24. https://doi.org/10.1016/j.echo.2011.07.013.
Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92–9. https://doi.org/10.1002/jcu.22272.
•• Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96:1002–6. Compelling study showing that first-year medical students using hand-carried ultrasound had superior diagnostic ability than attending cardiologists not using ultrasound.
• Cowie B, Kluger R. Evaluation of systolic murmurs using transthoracic echocardiography by anaesthetic trainees. Anaesthesia. 2011;66(9):785–90. A limited teaching intervention allowed trainees in anesthesiology to identify significant aortic stenosis. https://doi.org/10.1111/j.1365-2044.2011.06786.x.
Cowie B. Focused cardiovascular ultrasound performed by anesthesiologists in the perioperative period: feasible and alters patient management. J Cardiothorac Vasc Anesth. 2009;23(4):450–6. https://doi.org/10.1053/j.jvca.2009.01.018.
Canty DJ, Royse CF. Audit of anaesthetist-performed echocardiography on perioperative management decisions for non-cardiac surgery. Br J Anaesth. 2009;103(3):352–8. https://doi.org/10.1093/bja/aep165.
Canty DJ, Royse CF, Kilpatrick D, Bowman L, Royse AG. The impact of focused transthoracic echocardiography in the pre-operative clinic. Anaesthesia. 2012;67(6):618–25. https://doi.org/10.1111/j.1365-2044.2012.07074.x.
• Canty DJ, Royse CF, Kilpatrick D, et al. The impact on cardiac diagnosis and mortality of focused transthoracic echocardiography in hip fracture surgery patients with increased risk of cardiac disease: a retrospective cohort study. Anaesthesia. 2012;67:1202–9. Anesthesiologist-performed FoCUS improved mortality of elderly hip fracture patients and did not delay their entry into the operating room.
Botker MT, Vang ML, Grofte T, et al. Routine pre-operative focused ultrasonography by anesthesiologists in patients undergoing urgent surgical procedures. Acta Anaesthesiol Scand. 2014;58(7):807–14. https://doi.org/10.1111/aas.12343.
Zhang J, Critchley LAH. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124(3):580–9. https://doi.org/10.1097/ALN.0000000000001002.
Feissel M, Michard F, Faller JP, Teboul J-L. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834–7. https://doi.org/10.1007/s00134-004-2233-5.
Dipti A, Soucy Z, Surana A, Chandra S. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. Am J Emerg Med. 2012;30(8):1414–1419.e1. https://doi.org/10.1016/j.ajem.2011.10.017.
Lee C, Kory P, Arntfield R. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care. 2016;31(1):96–100. https://doi.org/10.1016/j.jcrc.2015.09.016.
Khandwalla R, Birkeland K, Zimmer R, et al. Usefulness of serial measurements of inferior vena cava diameter by vscantm to identify patients with heart failure at high risk of hospitalization. Am J Cardiol. 2017;119:1631–6.
•• Lichtenstein D. Novel approaches to ultrasonography of the lung and pleural space: where are we now? Breathe (Sheff). 2017;13(2):100–11. Excellent review and update by the leader in critical care lung ultrasound. https://doi.org/10.1183/20734735.004717.
Soldati G, Testa A, Sher S, et al. Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest. 2008;133:204–11.
Abbasi S, Farsi D, Hafezimoghadam P, et al. Accuracy of emergency physician-performed ultrasound in detecting traumatic pneumothorax after a 2-h training course. Eur J Emerg Med. 2013;20:173–7.
Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15.
• Kruisselbrink R, Chan V, Cibinel GA, et al. I-AIM (Indication, Acquisition, Interpretation, Medical decision-making) framework for point of care lung ultrasound. Anesthesiology. 2017;127:568–82. Review of the approach to lung ultrasound by anesthesiologists.
Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376–93. https://doi.org/10.1097/ALN.0000000000001452.
Holt S, McDicken WN, Anderson T, Stewart IC, Heading RC. Dynamic imaging of the stomach by real-time ultrasound—a method for the study of gastric motility. Gut. 1980;21(7):597–601. https://doi.org/10.1136/gut.21.7.597.
Arzola C, Carvalho JCA, Cubillos J, Ye XY, Perlas A. Anesthesiologists’ learning curves for bedside qualitative ultrasound assessment of gastric content: a cohort study. Can J Anesth. 2013;60(8):771–9. https://doi.org/10.1007/s12630-013-9974-y.
Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–63.
Alakkad H, Kruisselbrink R, Chin KJ, et al. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: a prospective case series. Can J Anesth. 2015;62:1188–95.
• Perlas A, Van de Putte P, Van Houwe P, Chan VWS. I-AIM framework for point-of-care gastric ultrasound. Br J Anaesth. 2016;116(1):7–11. Review of the approach to bedside assessment of gastric contents with ultrasound. https://doi.org/10.1093/bja/aev113.
Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28(3):370–2. https://doi.org/10.1161/ATVBAHA.108.162545.
Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European society of cardiology working groups of aorta and peripheral circulation and pulmonary circulation and right ventricular function. Eur Heart J. 2017;00:1–14.
• Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician-performed ultrasonography in the diagnosis of deep-vein thrombosis: a systematic review and meta-analysis. Thromb Haemost. 2013;109:137–45. Meta-analysis showing that emergency physicians can diagnose DVT accurately with bedside ultrasound.
Ramsingh D, Rinehart J, Kain Z, et al. Impact assessment of perioperative point-of-care ultrasound training on anesthesiology residents. Anesthesiology. 2015;123:670–82.
Fagley RE, Haney MF, Beraud A-S, Comfere T, Kohl BA, Merkel MJ, et al. Critical care basic ultrasound learning goals for american anesthesiology critical care trainees: recommendations from an expert group. Anesth Analg. 2015;120(5):1041–53. https://doi.org/10.1213/ANE.0000000000000652.
Kimura BJ, Sliman SM, Waalen J, Amundson SA, Shaw DJ. Retention of ultrasound skills and training in “point-of-care” cardiac ultrasound. J Am Soc Echocardiogr. 2016;29(10):992–7. https://doi.org/10.1016/j.echo.2016.05.013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jen Chang and Josh Zimmerman declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Preoperative Evaluation
Rights and permissions
About this article
Cite this article
Chang, J., Zimmerman, J. Preoperative Ultrasound: If You Don’t Look, You Don’t Know. Curr Anesthesiol Rep 8, 32–37 (2018). https://doi.org/10.1007/s40140-018-0249-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-018-0249-6