Opinion statement
Historically, type B aortic dissection was managed as a medical condition with limited surgical intervention unless aortic rupture occurred. Today, however, evidence is building that highlights the importance of strict medical management, timely surveillance, and windows of opportunity for surgical intervention to address both early and late aortic-based morbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute type B aortic dissection (TBAD) was previously thought to be a rare problem, with estimated incidence of 2.9–3.5 per 100,000 person per year [1]. However, studies identify the prevalence is five times higher at 15 per 100,000 with 20-year follow-up [2]. This incidence has increased, partly, as the computed axial tomography (CT) scans are utilized more frequently in patients with the chief complaint of chest or abdominal pain. While an early report documented 62 % patients died within the first week and 93 % at the first year [3], today, the mortality of TBAD has been reduced and was reported to be as low as 10.6 % in-hospital mortality with medical and surgical treatment [2, 4].
In this article, we will discuss contemporary management of aortic dissection. We will limit the analysis to the descending thoracic and abdominal aortas. We will briefly review the pathophysiology as this often affects the treatment options. The majority of the review will focus on the important foundation of medical management as well as the facets of surgical treatment, especially the growing role for endovascular repair.
Pathophysiology
Aortic wall
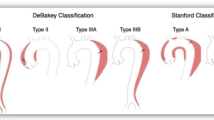
Acute aortic dissection occurs when there is a tear in the aortic intima. This tear is typically transverse [5], rarely circumferential but travels longitudinally, cleaving the intima and medial layers of the aortic wall in a slow spiral. In approximately 60 % of patients, this tear will originate in the ascending aorta within the sinotubular junction and extend into the thoracoabdominal aorta [6•]; these are classified as a Stanford type A dissection. Another 20–25 % of entry tears will begin near the origin of the left subclavian artery, known as Stanford type B dissections [6•]. Although many variations have been described, the tear most commonly extends posterolaterally into the thoracoabdominal aorta. This usually results in the mesenteric vessels and the right renal artery arising from the true lumen, the left renal from the false lumen [5]. The tear may terminate anywhere along the aorta and frequently travels all the way to, and involving, the common iliac arteries (Fig. 1). An “acute dissection” is defined as an aortic dissection occurring within 14 days of the symptoms onset. It is considered “chronic” after 3 months and “sub-acute” as the interim. Type A dissections are regarded as surgical emergencies, and their management is not referenced further in this review. For the remainder of the chapter, we will focus on acute type B aortic dissections (TBADs).
Malperfusion
The relationship of the true and false lumens is dynamic. Initially, the true lumen collapses because of the loss of transmural pressure across the dissection flap and the subsequent elastic recoil of the medial smooth muscle [7]. Alternatively, the false lumen expands immediately because of reduced elastic recoil, and percentage of the wall circumference involved [7]. Malperfusion syndrome occurs when there is end-organ ischemia due to aortic branch compromise. Any of the aortic branches may suffer some degree of compromise because of false lumen expansion, compression of the true lumen, and in situ thrombosis [8]. Dynamic obstruction occurs when the septum of the dissected intima prolapses into the ostia of a branch, usually during systole, thereby not allowing adequate flow to perfuse the vessel [9]. The ostia itself remains anatomically undamaged. When the dissection tear extends into the vessel proper and narrows or thromboses the artery, static obstruction occurs [9]. Static obstruction is unlikely to resolve with restoration of more normal flow to the true lumen and may result in stroke, renal failure, leg ischemia, etc. Approximately 20–30 % of patients with type B dissections will have malperfusion syndrome, and this strongly correlates with early mortality [10].
Risk factors
The most common predisposing factor for TBAD is hypertension. A history hypertension is noted in 70–80 % of patients [4, 6•, 10]. Cocaine use is also suspected to be a risk factor, although the actual frequency is debated. In an inner-city hospital, as many as 37 % of patients admitted with TBAD reported prior cocaine use [11]. However, the International Registry of Acute Aortic Dissection (IRAD) study found only 0.5 % of their enrolled patients were associated with cocaine abuse [10]. It has been suggested that adventitial mast cells in cocaine users may potentiate atherosclerosis and vasospasm [12]. Atherosclerosis is present in 40 % of TBAD [10]. Previous cardiac surgery or prior aortic injury has also been associated. Connective tissue disease can be related to structural weakness of the aortic wall and therefore also lead to TBAD. While uncommon as a whole, the four relatively common disorders are Marfan’s, Ehlers Danlos, Loeys Dietz, and Turner syndromes. TBAD in pregnancy is rare, but it is hypothesized that the physiologic changes of pregnancy can accelerate the development of pathologic changes in the arterial wall. Half of the aortic dissections in women less than 40 years of age are in association with pregnancy [13]. Finally, high speed or extreme impact trauma is linked with acute blunt aortic dissection. Various mechanisms explaining the relative fixation of the aorta by the ligamentum arteriosum, entrapment between the anterior thoracic bony structures with the vertebral column, and sudden flow obstruction of a non-compressible fluid column have been proposed.
Management
The goal for treatment of an acute TBAD should be to decrease the shear force on the aortic wall and reduce the force of the left ventricular ejection (dP/dT) while maintaining perfusion to all vascular beds [14]. Indeed, control of hemodynamics is critical to maximize true lumen perfusion and reduce the likelihood of malperfusion and/or rupture.
Medical therapy
In uncomplicated TBAD, class I evidence supports medical therapy as the primary therapy [6•, 15–21]. These patients should be admitted to an intensive care unit, with an objective to maintain the systolic blood pressure between 100–120 mmHg and decrease the heart rate to less than 60 beats per minute [16]. First-line choice, if no contraindication, is beta-blockade (class IIa evidence) [22, 23]. Short-acting infusions such as esmolol are favored as they can be easily titrated. In our practice, we often employ labetolol infusion for the combined anti alpha and beta effect. Acceptable alternatives to beta-blockers are calcium channel antagonists [22, 23]. Additionally, renin-angiotensin inhibitors, alpha-1 adrenergic agents, and sodium nitroprusside have been used. It should be noted that angiotensin II receptor blockers have been shown to decrease aortic diameter growth in Marfan patients but are generally not used in the acute setting of aortic dissection [24–26].
Adequate pain control is essential since pain can drive an elevated blood pressure. Persistent or increasing pain, or new territory of pain may also indicate progression of the dissection [19]. Foley catheter for hourly urine output is requisite to identify the often insidious onset of renal malperfusion. Once the blood pressure and pain are appropriately controlled, the patient may be transitioned to oral medications and plans for mandatory follow-up imaging and aggressive outpatient antihypertensive management.
Surgery
Open repair
Surgical treatment was previously reserved for patients with complicated TBAD. This included any patient with threatened or actual rupture as well as anyone with malperfusion syndrome. Historically, open repair was associated with substantial surgical morbidity. Mortality rates for open repair of an acute TBAD ranged widely, from 6 to 69 % in several large series [27–30]. The anatomic goal of open repair is to obliterate the false lumen flow by reconstruction the layers of the wall at the distal anastomosis. This repair is limited to the proximal descending thoracic aorta to avoid increased risk of paraplegia. However, up to half of patients may still have persistent flow in the false lumen, and central aortic grafting may fail to correct distal malperfusion despite surgery [31, 32]. Therefore, because of the variable success of open repair as well as the equivalent results of medical therapy, open surgery for acute TBAD has been nearly abandoned.
Endovascular repair
In 1999, endovascular repair of acute TBAD was reported in two sentinel papers [33•, 34•]. Its minimally invasive access was attractive, obviating the considerable morbidity of an open thoracic procedure. The intent of a stent-graft repair is to cover the entry tear of the acute dissection which reduces the flow and pressure in the false lumen. This is thought to lessen the incidence and severity of malperfusion as well as minimize aneurysmal degeneration of the outer wall by inducing false lumen thrombosis.
The technical considerations of endovascular repair in acute dissection are many. The operator must have firm knowledge of three-dimensional aortic topography provided by the pre-operative CT angiogram (CTA) as pre-procedural planning is critical (Fig. 2). The 1–3 mm slices of the CTA should include not only the major arch vessels and thoracic aorta but also the iliofemoral arteries where many will obtain access for device delivery. As thoracic endografts can be much larger than abdominal aortic endografts, it is vital that adequate iliac diameter access is ensured. It is often necessary to identify the true lumen with intravascular ultrasound (IVUS) during the case to avoid inadvertent deployment of the graft into the false lumen. If there is difficulty tracking the endograft up the thoracic aorta due to tortuosity or underlying atherosclerotic disease, one may access the right brachial artery and employ “through and through” access with intravascular snares. In acute TBAD, unlike chronic thoracic aortic aneurysms, there is no oversizing of the graft. In fact, many operators decline to post-dilate the proximal neck in fear of further aortic disruption in the region of the entry tear of the acute dissection (Fig. 3).
Uncomplicated TBAD
To further explore the evolving role of stent grafting in acute TBAD, the Investigation of Stent-grafts in Aortic Dissection (INSTEAD) trial was designed [35•]. It was a European prospective randomized multicenter trial which compared stent grafting to maximal medical treatment for chronic uncomplicated TBAD. Patients were randomized at 2 weeks after the onset of symptoms, and the investigators found no difference in 30-day mortality between the two groups. The 1 year mortality was higher in the thoracic endovascular aortic repair (TEVAR) group (8.6 vs 3 %) likely a consequence of periprocedural mortality. Notably, they found that over 90 % of patients in the TEVAR group had complete thrombosis of their false lumen at 1 year, and at 2 years there was a significant improvement in overall aortic remodeling (91.3 vs 19.4 %, p < 0.0001). More recently, the extended follow-up showed that those undergoing TEVAR had significantly lower aorta-related mortality (6.9 vs 19.3 %, p = 0.04) [36•]. The International Registry of Acute Aortic Dissection (IRAD) also reported decreased 5-year mortality in TEVAR patients compared to medical therapy alone [37]. This suggests that those undergoing medical therapy will continue to degenerate their aortas after 2 years and will eventually reach size criteria for repair of a chronic TAA. Obviating the late need for aortic intervention after TBAD remains an area of intense study to the merits of early TEVAR.
Complicated TBAD
Endovascular management of complicated TBAD, or TBAD with the presence of malperfusion syndrome, is multifaceted. The entry tear, most commonly in the thoracic aorta, should still be covered with an endograft. Distally, each malperfused artery must be evaluated individually. A dynamic obstruction can be managed with a balloon fenestration of the intimal flap thus increasing the outflow of the false lumen and releasing the prolapsing intima of the arterial ostia [38]. Conversely, a static obstruction will require accessing the true lumen of the vessel and placing a stent [39] from the target vessel into the aortic lumen.
In principle, self-expanding stents in a compromised branch vessel should precede aortic fenestration as the latter may alter aortic blood flow and make it particularly difficult to regain access to the affected branch [40]. There are several technique operators used to create an aortic fenestration. Reentry fenestration is usually made from the smaller (true) lumen into the larger (false) lumen using various endovascular needles close to the branch itself. Confirmation is then performed by contrast injection, and a large angioplasty balloon (12–15 mm) is expanded to create a fenestration tear of 20–40 mm. Similarly, a “snare” fenestration can be utilized after delivering the wire between lumens and snaring the wire from the contralateral femoral artery. The “scissors technique” uses two stiff guide wires in each lumen from a single femoral access while a single long sheath is advanced over the wires thereby creating a fenestration [41]. The ideal result is a clean vertical tear not a circumferential separation of the flap from the aortic wall. The goal is to equalize the peak systolic pressures between the two lumens of the aorta and thereby decompressing the false lumen. Because overaggressive fenestration can result in dramatic and unpredictable flow dynamic or complete intimal dehiscence and obstruction, some limit fenestration to the distal aorta [42].
Because of the acute and emergent need for surgical intervention, there are no randomized control trials of complicated TBAD. A prospective multicenter European registry of 50 patients with complicated TBAD reported a 30-day mortality of 8 % [43]. There are currently three meta-analyses detailing the short- and mid-term results of complicated TBAD. Technical success was high, 95–99 %, while hospital mortality and neurological complications low, 2.6–9.8 and 0.6–3.1 %, respectively [44–46]. These results compare favorably with surgical historical controls. As such, there is class IIa recommendation that endovascular repair should be considered as a first interventional option for complicated acute TBAD [37, 43–55].
Complications
TEVAR in acute TBAD is clearly advantageous in terms of mortality when compared to open repair. The related complications, however, can be devastating, and an open procedure may be the only method of correcting the situation in rare instances.
Device related
Any migration, mal-deployment, or increased angulation can cause “bird-beaking” of the proximal edge of the endograft. This occurs as the edge of the stent pulls away from the inner curvature of the aortic arch and can lead to complete collapse of the entire endograft. This may require a second stent to reinforce the collapsed stent and, in rare instances, can present with complete cardiovascular collapse [56]. If the patient has underlying atherosclerotic disease and iliac stenosis, rupture of the iliac system can occur, during sheath insertion, leading to massive hemorrhage or an ischemic leg. Retrograde type A dissection after stent deployment can also develop, and although less than 2 % of patients, the risk is thought to be increased with proximal balloon dilatation, rigid non-compliant endografts, or lengthy bare metal stents [57].
Stroke
Because manipulation of the aortic arch with catheters and sheaths is necessary to accurately deploy a thoracic endograft, there is a risk of embolic stroke. Stroke is reported in 3–10 % of cases and is naturally higher in those patients with atherosclerosis in the aortic arch [58].
Spinal ischemia
The most dreaded complication of aortic surgery is spinal cord ischemia (SCI). Historically, especially with open repair, incidence of SCI was as high as 20 % [59]. Predictors of SCI are many and include the following: age, clamp time, extent of aortic reconstruction, and emergency surgery [60]. As such, many techniques to protect spinal cord perfusion have been developed. Somatosensory-evoked potentials or motor evoked potentials can be utilized intraoperatively to directly monitor spinal cord function in thoracic aortic surgery [61, 62]. The use of distal aortic perfusion either by left heart bypass or extracorporeal circulation is protective of SCI in open repair, and class IIa evidence shows a reduction of SCI from 11.2 to 4.5 % [63]. Another advantage of full circulatory bypass is the permission of systemic hypothermia which is also an effective method to reduce SCI [64, 65].
In endovascular repair, SCI for acute TBAD is related to the extent of aortic coverage, history of previous aortic surgery, occlusion of left subclavian artery or hypogastric arteries, and malperfusion as a result of hypotension at presentation [66–68]. SCI is reported in approximately 2–8 % of patients undergoing TEVAR [43]. Cerebral spinal fluid (CSF) drainage has a role in decreasing the risk of SCI in both open repair and TEVAR. Randomized controlled trials and meta-analyses show a risk reduction of up to 75 % [59, 69]. Therefore, CSF drainage should be considered in TEVAR for patients at high risk for SCI and planned extensive coverage of the thoracic aorta (class IIa recommendation) [67, 68]. Additionally, a recent study described further benefit when CSF drainage is combined with intrathecal papaverine or intrathecal hypothermia [70]. Potential complications of CSF drainage include as follows: meningitis, epidural hematoma, subdural hematoma, and CSF leakage syndrome [71].
Special considerations
Age
Age has been recently shown to be independent risk factor for mortality in complicated acute TBAD. In-hospital mortality for patients undergoing endovascular repair older than 70 years was 30.0 % compared to 10.1 % for those younger than 70 years [72]. This trend is similar when comparing the different age groups for open treatment as well as medical treatment.
Pregnancy
Resultant hypertension from preeclampsia is most commonly the etiology of aortic dissection during pregnancy; however, various connective tissue disorders can also put pregnant women at high risk. A dilated aortic root of 40 mm is the best predictor of dissection in a pregnant Marfan’s patient [73]. Those with Marfan’s syndrome should be closely managed medically, unless rapid degeneration of the aorta, malperfusion, or rupture occur [74]. The timing of delivery will depend on the fetal viability, although many recommend cesarean section before aortic repair, if possible [75].
Summary
Type B aortic dissections are more common than previously thought and should be evaluated for interventional consideration in a clear stepwise fashion (Fig. 4). If the TBAD is complicated, emergent TEVAR is the first-line choice of treatment. Aortic branch malperfusion should be evaluated and stented if possible. In the setting of an uncomplicated TBAD, medical management should be employed first with target blood pressure of <120 mmHg systolic and HR < 60 bpm. If these can be achieved, and the patient remains asymptomatic, conservative management may be applied and surveillance performed with CTA or MRA imaging at 1 month, 3 months, 6 months, 12 months, and annually thereafter. Those patients with persistent pain or rapid aortic enlargement despite medical management should be offered a TEVAR if anatomically suitable. Open surgery for acute TBAD should not be performed unless rupture is present or as a means to repair endovascular-related complications.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Clouse WD, Hallett Jr JW, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–80.
Landenhed M, Engstrom G, Gottsater A, Caulfield MP, Hedblad B, Newton-Cheh C, et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc. 2015;21:4(1).
Hirst Jr AE, Johns Jr VJ, Kime Jr SW. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine. 1958;37:217–79.
Tolenaar JL, Froehlich W, Jonker FH, Upchurch Jr GR, Rampoli V, Tsai TT, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 2014;120(11 Suppl 1):S45–50.
Crawford ES. The diagnosis and management of aortic dissection. JAMA. 1990;264(19):2537–41.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897–903. IRAD is a consortium comprised of 11 countries and 30 large referral centers participating in a registry to assess multiple factors and outcomes of acute aortic dissection. This is the first publication of many; this evaluates risk factors and short term outcomes.
Williams DM, LePage MA, Lee DY. The dissected aorta: part I. Early anatomic changes in an in vitro model. Radiology. 1997;203:23–31.
Oderich GS, Panneton JM, Bower TC, Ricotta 2nd JJ, Sundt 3rd TM, Cha S, et al. Aortic dissection with aortic side branch compromise: impact of malperfusion on patient outcome. Perspect Vasc Surg Endovasc Ther. 2008;20(2):190–200.
Williams DM, Lee DY, Hamilton BH, Marx MV, Narasimham DL, Kazamjian SN, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology. 1997;203(1):37–44.
Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg. 2009;37(2):149–59.
Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation. 2002;105(1):1592–5.
Kolodgie FD, Virmani R, Cornhil JF, Herderick EE, Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated coronary vasospasm and thrombosis. J Am Coll Cardiol. 1991;17(7):1553–60.
Williams GM, Gott VL, Brawley RK, Schauble JF, Labs JD. Aortic disease associated with pregnancy. J Vasc Surg. 1988;8(4):470–5.
Isselbacher EM. Diseases of the aorta. Braunwald E heart disease. Philadelphia: WB Saunders; 2002. p. 1422–55.
Mackenzie KS, LeGuillan MP, Steinmetz OK, Montreuil B. Management trends and early mortality rates for acute type B aortic dissection: a 10-year single-institution experience. Ann Vasc Surg. 2004;18:158–66.
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey Jr DE, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369.
Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al. IRAD. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108 Suppl 1:II312–7.
Estrera AL, Miller CC, Goodrick J, Porat EE, Achouh PE, Dhareshwar J, et al. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg. 2007;83:S842–5.
Trimarchi S, Eagle KA, Nienaber CA, Pyeritz RE, Jonker FH, Suzuki T, et al. IRAD. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2010;122:1283–9.
Trimarchi S, Tolenaar JL, Tsai TT, Froehlich J, Pegorer M, Upchurch GR, et al. Influence of clinical presentation on the outcome of acute B aortic dissection: evidences from IRAD. J Cardiovasc Surg (Torino). 2012;53:161–8.
Suzuki T, Isselbacher EM, Nienaber CA, Pyeritz RE, Eagle KA, Tsai TT, et al. IRAD. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol. 2012;109:122–7.
Mochizuki S, Dahlof B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–9.
Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet. 2000;356:1955–64.
Chiu HH, Wu MH, Wang JK, Lu CW, Chiu SN, Chen CA, et al. Losartan added to beta-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc. 2013;88(3):271–6.
Pees C, Laccone F, Hagl M, Debrauwer V, Moser E, Michel-Behnke I. Usefulness of losartan on the size of the ascending aorta in an unselected cohort of children, adolescents, and young adults with Marfan syndrome. Am J Cardiol. 2013;112(9):1477–83.
Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013;34(45):3491–500.
Coselli JS. Thoracoabdominal aortic aneurysms: experience with 372 patients. J Card Surg. 1994;9(6):638–47.
Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Dissection of the aorta and dissecting aortic aneurysms. Improving early and long-term surgical results. Circulation. 1990;82(5 Suppl):IV24–38.
Verdant A, Cossette R, Page A, Baillot R, Dontigny L, Page P. Aneurysms of the descending thoracic aorta: three hundred sixty six consecutive cases resected without paraplegia. J Vasc Surg. 1995;21(3):385–90. discussion 390-1.
Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Bossone E, et al. IRAD. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114(1 Suppl):I357–64.
Sasaki S, Yasuda K, Kunihara T, Shiiya N, Murashita T, Matsui Y, et al. Surgical results of Stanford type B aortic dissection. Comparisons between partial and subtotal replacement of the dissected aorta. J Cardiovasc Surg (Torino). 2000;41(2):227–32.
Lansman SL, Hagl C, Fink D, Galla JD, Spielvogel D, Ergin MA, et al. Acute type B aortic dissection: surgical therapy. Ann Thorac Surg. 2002;74(5):S1833–5. discussion.
Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340(20):1539–45. One of the two sentinel papers describing stent-graft placement for acute aortic dissection.
Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340(20):1546–52. One of the two sentinel papers describing stent-graft placement for acute aortic dissection.
Neinaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, et al. Randomized comparison of strategies for type B aortic dissection: the Investigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120(25):2519–28. First randomized comparison of endovascular repair vs maximal medical therapy.
Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection: long term results of the randomized investigation of stent grafts in aortic dissection trial (INSTEAD-XL). Circ Cardiovasc Interv. 2013;6:407–16. Two-year follow up of INSTEAD trial showing excellent aortic remodeling in the TEVAR group but no improvement in 2-year survival.
Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv. 2013;6(8):876–82.
Deeb GM, Patel HJ, Williams DM. Treatment for malperfusion syndrome in acute type A and B aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S98–100. discussion S142-S146.
Midulla M, Renaud A, Martinelli T, Koussa M, Mounier-Vehier C, Prat A, et al. Endovascular fenestration in aortic dissection with acute malperfusion syndrome: immediate and late follow-up. J Thorac Cardiovasc Surg. 2011;142(1):66–72.
Clair DG. Aortic dissection with branch vessel occlusion: percutaneous treatment with fenestration and stenting. Semin Vasc Surg. 2002;15(2):116–21.
Beregi JP, Prat A, Gaxotte V, Delomez M, McFadden EP. Endovascular treatment for dissection of the descending aorta. Lancet. 2000;356(9228):482–3.
Slonim SM, Miller DC, Mitchell RS, Semba CP, Razavi MK, Dake MD. Percutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissection. J Thorac Cardiovasc Surg. 1999;117(6):1118–26.
Heijmen R, Fattori R, Thompson M, Eggebrecht H, Degriecke I, Nienaber C, et al. Virtue Registry Investigators. The VIRTUE Registry of type B thoracic dissections—study design and early results. Eur J Vasc Endovasc Surg. 2011;41:159–66.
Eggebrecht H, Nienaber CA, Neuhauser M, Baumgart D, Kische S, Schmermund A, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489–98.
Parker JD, Golledge J. Outcome of endovascular treatment of acute type B aortic dissection. Ann Thorac Surg. 2008;86:1707–12.
Xiong J, Jiang B, Guo W, Wang SM, Tong XY. Endovascular stent graft placement in patients with type B aortic dissection: a meta-analysis in China. J Thorac Cardiovasc Surg. 2009;138:865–72.
Lombardi JV, Cambria RP, Nienaber CA, Chiesa R, Teebken O, Lee A, et al. STABLE. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55:629–40.
Tsai TT, Fattori R, Trimarchi S, Isselbacher E, Myrmel T, Evangelista A, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114:2226–31.
Cambria RP, Crawford RS, Cho JS, Bavaria J, Farber M, Lee WA, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255–64.
Zeeshan A, Woo EY, Bavaria JE, Fairman RM, Desai ND, Pochettino A, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J Thorac Cardiovasc Surg. 2010;140(Suppl):S109–15.
Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85:S1–41.
Verhoye JP, Miller DC, Sze D, Dake MD, Mitchell RS. Complicated acute type B aortic dissection: midterm results of emergency endovascular stentgrafting. J Thorac Cardiovasc Surg. 2008;136:424–30.
Steuer J, Eriksson MO, Nyman R, Bjorck M, Wanhainen A. Early and long term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;41:318–23.
Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option? A report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv. 2008;1:395–402.
Khoynezhad A, Donayre CE, Omari BO, Kopchok GE, Walot I, White RA. Midterm results of endovascular treatment of complicated acute type B aortic dissection. J Thorac Cardiovasc Surg. 2009;138:625–31.
Kasirajan K, Dake MD, Lumsden A, Bavaria J, Makaroun MS. Incidence and outcomes after infolding or collapse of thoracic stent grafts. J Vasc Surg. 2012;55:652–8.
Eggebrecht H, Thompson M, Rousseau H, Czerny M, Lonn L, Mehta RH, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation. 2009;120(Suppl):S276–81.
Buth J, Harris PL, Hobo R, van Eps R, Cuypers P, Duijm L, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007;46:1103–10.
Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–9.
Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–68.
Jacobs MJ, Mess W, Mochtar B, Nijenhuis RJ, Statius van Eps RG, Schurink GW. The value of motor evoked potentials in reducing paraplegia during thoracoabdominal aneurysm repair. J Vasc Surg. 2006;43:239–46.
Nijenhuis RJ, Jacobs MJ, Schurink GW, Kessels AGH, Van Engelshoven JMA, Backes WH. Comparison of magnetic resonance with computed tomography angiography for preoperative localization of the Adamkiewicz artery in thoracoabdominal aortic aneurysm patients. J Vasc Surg. 2007;45:677–85.
Coselli JS. The use of left heart bypass in the repair of thoracoabdominal aortic aneurysms: current techniques and results. Semin Thorac Cardiovasc Surg. 2003;15:326–32.
Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:953–60.
Svensson LG, Khitin L, Nadolny EM, Kimmel WA. Systemic temperature and paralysis after thoracoabdominal and descending aortic operations. Arch Surg. 2003;138:175–9.
Schlösser FJ, Verhagen HJ, Lin PH, Verhoeven EL, van Herwaarden JA, Moll FL, et al. TEVAR following prior abdominal aortic aneurysm surgery: increased risk of neurological deficit. J Vasc Surg. 2009;49:308–14.
Khoynezhad A, Donayre CE, Bui H, Kopchok GE, Walot I, White RA. Risk factors of neurologic deficit after thoracic aortic endografting. Ann Thorac Surg. 2007;83:S882–9.
Amabile P, Grisoli D, Giorgi R, Bartoli JM, Piquet P. Incidence and determinants of spinal cord ischemia in stent-graft repair of the thoracic aorta. Eur J Vasc Endovasc Surg. 2008;35:455–61.
Khan SN, Stansby GP. Cerebrospinal fluid drainage for thoracic and thoracoabdominal aortic aneurysm surgery. Cochrane Database Syst Rev. 2012. doi:10.1002/14651858.CD003635.pub3.
Lima B, Nowicki ER, Blackstone EH, Williams SJ, Roselli EE, Sabik 3rd JF, et al. Spinal cord protective strategies during descending and thoracoabdominal aortic aneurysm repair in the modern era: the role of intrathecal papaverine. J Thorac Cardiovasc Surg. 2012;143:945–52.
Wynn MM, Mell MW, Tefera G, Hoch JR, Acher CW. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg. 2009;49:29–34.
Jonker FH, Trimarchi S, Muhs BE, Rampoldi V, Montgomery DG, Froehlich JB, et al. The role of age in complicated acute type B aortic dissection. Ann Thorac Surg. 2013;96(6):2129–34.
Elkayam U, Ostrzega E, Shotan A, Mehra A. Cardiovascular problems in pregnant women with the Marfan syndrome. Ann Intern Med. 1995;123(2):117–22.
Zeebregts CJ, Schepens MA, Hameeteman TM, Morshuis WJ, de la Riviere AB. Acute aortic dissection complicating pregnancy. Ann Thorac Surg. 1997;64(5):1345–8.
Task Force on the Management of Cardiovascular Diseases During Pregnancy of the European Society of Cardiology. Expert consensus document on management of cardiovascular diseases during pregnancy. Eur Heart J. 2003;24(8):761–81.
Compliance with Ethics Guidelines
Conflict of Interest
Kristine C. Orion and James H. Black III each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Vascular Disease
Rights and permissions
About this article
Cite this article
Orion, K.C., Black, J.H. Management of Aortic Dissection: Medical Therapy and Intervention. Is There a Growing Role for Endovascular Techniques?. Curr Treat Options Cardio Med 17, 24 (2015). https://doi.org/10.1007/s11936-015-0386-x
Published:
DOI: https://doi.org/10.1007/s11936-015-0386-x