Abstract
Purpose of Review
Hemorrhagic shock remains the leading cause of potentially preventable death following traumatic injuries. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) has demonstrated promising results in the control of non-compressible torso hemorrhage. The purpose of this review is to summarize the indications, procedural techniques, and complications of REBOA in order to help define practice management guidelines.
Recent Findings
Recent studies suggest that REBOA is advantageous in aiding hemodynamic stabilization as a bridge to definitive hemorrhage control. REBOA is a feasible option for non-compressible torso hemorrhage as well as traumatic cardiac arrest and has the potential to improve morbidity and mortality in select patient populations.
Summary
Despite favorable emerging data, varying results in the literature indicate the need for further high-quality reproducible data and multicenter trials to identify the ideal clinical scenarios for REBOA. This review discusses special considerations and future directions for next generation REBOA use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemorrhage remains the leading cause of potentially preventable death in trauma, yet the majority of these injuries result in death prior to definitive hemorrhage control [1,2,3,4,5,6,7,8]. Non-compressible torso hemorrhage (NCTH) accounts for approximately 60–70% of deaths from exsanguination [4, 7, 9,10,11,12]. In patients with NCTH, mortality rates approach 50% in the civilian trauma setting and nearly 80% in the military setting. Even with short transportation times, only 25% of NCTH injuries are survivable [13,14,15].
Non-compressible torso hemorrhage was defined by Morrison et al. as either vascular disruption to the axial torso vessels, grade 4 solid organ injury (liver, kidney, spleen), intrathoracic injury (massive hemothorax, pulmonary vascular injury) or pelvic fracture with ring disruption, accompanied by shock with systolic blood pressure (SBP) less than 90 mmHg [2, 5, 12, 16,17,18]. A variety of bleeding control adjuncts for NCTH injuries have been described, including pelvic binders, preperitoneal packing, junctional tourniquets, resuscitative thoracotomy with aortic cross-clamping, endovascular angioembolization, and resuscitative endovascular balloon occlusion of the aorta (REBOA) [3, 18, 19].
More recently, REBOA has emerged as a technique used to stabilize patients with hemorrhagic shock by temporarily occluding the aorta. REBOA was first described in 1954 by military surgeon Carl Hughes during the Korean War. Hughes used an intravascular balloon for two patients as a bridge to laparotomy for intra-abdominal hemorrhage. Although neither patient survived, his method was effective in temporarily restoring blood pressure, which gave rise to the field of aortic occlusion to augment myocardial and cerebral perfusion [3, 7, 9, 16, 19,20,21]. This technology ultimately led to the intra-aortic balloon pump (IABP), which was pioneered by cardiac surgeons who described improved coronary perfusion during cardiogenic shock [22].
Limited reports on aortic occlusion were seen in the trauma literature until 1986, when several case series were reported [7, 9]. In 1989, Gupta et al. published a multicenter experience utilizing aortic balloon occlusion for penetrating abdominal trauma in 21 patients, where authors described significant morbidity including paraplegia and access site thrombosis [20]. The technique was largely abandoned until the late 2000s, with reports of successful REBOA deployment by the military in Iraq and Afghanistan for temporary hemorrhage control [19, 23].
Over the last 20 years, the implementation of REBOA has been rapidly expanding worldwide. Advancements in endovascular therapy as well as military and civilian collaboration led to its resurgence and use at the bedside [4, 7, 20, 23]. In 2014, a Joint Trauma System Clinical Practice Guideline was published on REBOA, indicating that REBOA may be considered an alternative to resuscitative thoracotomy in the setting of extrathoracic blunt or penetrating injury for severe shock including pre-hospital cardiac arrest [19, 24]. In 2017, the Food and Drug Administration (FDA) approved the REBOA device as an alternative to emergent resuscitative thoracotomy [1].
Comparison to Resuscitative Thoracotomy
Traditional management of uncontrollable sub-diaphragmatic hemorrhage or traumatic cardiac arrest is by resuscitative thoracotomy and aortic cross-clamping [17]. REBOA is a minimally invasive method that uses a balloon catheter to temporarily occlude the descending thoracic aorta using Seldinger technique.
In 2013, the American Association for the Surgery of Trauma (AAST) Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry was created as a means to capture contemporary methods of aortic occlusion [25]. Results from this multi-institutional study demonstrated no significant difference in adjusted mortality between REBOA and resuscitative thoracotomy [25]. Additionally, there was no significant difference in the time from admission or initiation of REBOA to successful aortic occlusion between the two groups [26]. The AAST AORTA 2 study group then removed penetrating thoracic injury as a cause of hemorrhage or cardiac arrest and only enrolled patients who received Zone 1 REBOA. Results showed a potential overall survival benefit compared to resuscitative thoracotomy [25].
A meta-analysis by Manzano et al. evaluated the comparative effectiveness of REBOA versus resuscitative thoracotomy among trauma patients with NCTH and found the odds of mortality did not significantly differ between the groups [27]. However, of the patients reviewed, REBOA patients underwent endovascular angioembolization more often, which supports the concept that in patients with NCTH, early REBOA deployment may aid in hemodynamic stabilization as a bridge to endovascular bleeding control [27]. A retrospective review by Romagnoli et al. showed that once arterial access is achieved, time to aortic occlusion is quicker with REBOA and provides similar stabilization in hemodynamic parameters [15, 20]. With the advancement of REBOA technology, time to aortic occlusion is expected to continue to decline [15].
Bradley et al. demonstrated in both blunt and penetrating trauma patients, there was no significant improvement in return of spontaneous circulation (ROSC) or end tidal carbon dioxide (EtCO2) with either open chest cardiac massage as opposed to closed chest compressions [28]. Unlike thoracotomy, REBOA use allows for continuous closed chest compressions during the procedure [29]. Therefore, REBOA in combination with closed chest compressions may be a safer, less invasive, and equally efficacious way of obtaining ROSC and hemorrhage control as compared to resuscitative thoracotomy with open chest cardiac massage [28, 30]. Thoracotomy is still preferred in penetrating thoracic trauma, as REBOA does not offer definitive hemorrhage control and may exacerbate proximal hemorrhage [17, 19, 29].
Clinical Outcomes
There is ongoing debate on the benefit of managing patients with REBOA. White et al. was the first to describe the benefits of aortic balloon occlusion in animal models as a possible alternative to open aortic occlusion for non-compressible torso hemorrhage [7]. In 2013, Brenner et al. published the first clinical series on REBOA at a large civilian trauma center, with 4 out of 6 survivors [7, 31]. This study reported a mean increase in SBP of 55 mmHg and established REBOA as a clinically viable option in trauma centers [7, 31].
The AAST AORTA multicenter study analyzed data from 114 patients and found comparable outcomes between REBOA and open aortic occlusion [25]. REBOA demonstrated improvement in hemodynamics following aortic occlusion in 62.3% (71 out of 114) [25]. While the mean initial post-aortic occlusion SBP was 74.4 mmHg, only 36% achieved initial hemodynamic stability with SBP greater than 90 mmHg for at least 5 min [26]. This study revealed 50% of arterial access was obtained via femoral cutdown and the average time to balloon occlusion was 6.6 min using REBOA [7]. Overall survival rate was 21.1%, which was higher than prior case reports [26].
In 2016, the AAST AORTA 2 study demonstrated a potential overall survival benefit for patients without penetrating thoracic injury who receive Zone 1 REBOA compared to resuscitative thoracotomy, particularly those who do not require cardiopulmonary resuscitation (CPR) before aortic occlusion [25]. In 2019, a propensity score-matched analysis in Japan by Yamamoto et al. showed REBOA was independently associated with improved in-hospital survival in trauma patients [32]. Furthermore, a study by Northern et al. demonstrated that REBOA allows surgical teams to rapidly stabilize severely injury combat causalities, expand capability and provide enhanced damage control resuscitation while minimizing personnel, resources and blood product utilization [32, 33].
While the use of REBOA for non-compressible torso hemorrhage seems promising, there are several conflicting reports regarding overall survival benefit. In 2015, Norii et al. reviewed the Japan Trauma Data Bank in a propensity score-matched investigation and showed REBOA treatment was associated with higher mortality compared with similarly ill trauma patients not treated with REBOA (hazard ratio = 0.52) [34]. This analysis was limited to blunt trauma patients and the authors suggest that REBOA might have been used as a “last ditch effort” [34]. In 2019, the ACE study in Australia evaluated 13 patients who met criteria for REBOA placement and evidence failed to support improved survival [35].
Similarly, Joseph et al. analyzed the American College of Surgeons (ACS) Trauma Quality Improvement Program (TQIP) data in a propensity-matched analysis [36]. Results demonstrated a higher mortality rate for patients who underwent REBOA (35.7%) compared to those who did not [36]. Patients who underwent REBOA were more likely to develop acute kidney injury and require lower-extremity amputations, but there was no significant difference in requirement of blood products at 4 and 24 h after injury [36]. These varying reports necessitate the need for further prospective studies.
Indications and Contraindications
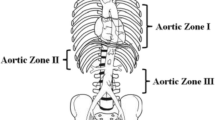
In a joint statement from the ACS Committee on Trauma (COT) and the American College of Emergency Physicians (ACEP), indications for REBOA include traumatic life-threatening hemorrhage below the diaphragm in patients with hemorrhagic shock who are unresponsive or transiently responsive to resuscitation, as well as patients arriving in arrest from injury due to a presumed life-threatening hemorrhage below the diagram [1, 30]. Two anatomical locations are described for REBOA placement (Fig. 1a) [37]. Zone 1 is the descending thoracic aorta distal to the left subclavian origin and proximal to the celiac axis, whereas Zone 3 is the infra-renal abdominal aorta distal to the renal arteries and proximal to the aortic bifurcation [1, 4, 17, 20, 38,39,40,41].
REBOA overview and selected complications. a Anatomic depiction the zones of aortic occlusion. b Peel-pack supply list for ER-REBOA ™ catheter device, includes balloon catheter, 5-Fr micropuncture sheath, 7-Fr sheath, catheter lock and syringes. c Overinflation injury resulting from balloon rupture. d Inflation of Zone 2 REBOA at the level of renal arteries. e Migration of the balloon into the right iliac artery due to poor catheter-securing technique. Images provided courtesy of Johnathan J. Morrison MD PHD, R Adams Cowley Shock Trauma Center, University of Maryland Medical Center
Zone 1 occlusion is indicated for control of severe intra-abdominal or retroperitoneal hemorrhage as well as those with traumatic arrest or undifferentiated extrathoracic hemorrhagic [30, 42]. Zone 3 REBOA is indicated for severe pelvic, junctional or proximal lower-extremity hemorrhage, and may be used as an adjunct in the management of pelvic fractures including angioembolization, pelvic packing and fracture stabilization [19, 30, 42,43,44]. It is recommended that 30 min for Zone 1 and 60 min for Zone 3 be considered the absolute limit of safe occlusion, as REBOA times greater than 60 min have been associated with increased metabolic derangement and mortality [4, 45, 46]. These occlusion times are largely theoretical and should not delay brisk definitive hemorrhage control.
Practice management guidelines and treatment algorithms for NCTH have been proposed. Patients in shock should undergo proper clinical assessment including chest and pelvic radiographs, as well as focused assessment with sonography in trauma (FAST) to guide zone selection if REBOA is indicated [19]. Many experts advocate for a step-up approach, emphasizing early common femoral artery access for REBOA if needed, as opposed to waiting for loss of palpable pulses or hemodynamic collapse, as arterial access is the rate-limiting step in obtaining aortic occlusion. This facilitates arterial access during an interval when cannulation is less challenging [8, 17, 24, 47].
High-quality, closed chest compressions coupled with REBOA may generate physiologic perfusion pressures that increase the rate of ROSC in traumatic arrest and improve mortality [48, 49]. Additionally, the role of REBOA in non-traumatic cardiac arrest has also been shown to significantly increase coronary artery flow and cerebral perfusion pressures in animal models [22, 50]. However, a recent report found that while REBOA improved diastolic pressures with Zone 1 occlusion, there was no significant improvement in systolic blood pressure, carotid flow or change in time to achieve ROSC in patients with ventricular fibrillation cardiac arrest [51]. Further clinical trials are required to elucidate indications for REBOA in non-traumatic cardiac arrest.
Experts agree that any signs of proximal great vessel injury preclude the use of REBOA, as distal occlusion could exacerbate proximal injuries [20]. Current recommendations from the ACS COT and ACEP state that REBOA is contraindicated in the setting of major thoracic hemorrhage or pericardial tamponade [3, 42]. This includes patients with a widened mediastinum on imaging concerning for blunt thoracic injury or patients with penetrating thoracic injury [45]. However, more recent studies have shown potential improvement in outcomes when surgical capability is unavailable for thoracic bleeding [52]. Furthermore, experts suggest patients with traumatic brain injury should be approached with caution as elevated proximal pressures may exacerbate intracranial hemorrhage, yet patients may benefit from restored cerebral perfusion in the setting of shock [45].
REBOA may provide a survival benefit for patients with major abdominal hemorrhage, ruptured abdominal aortic aneurysm and other causes of significant blood loss including post-partum hemorrhage, gastrointestinal bleeding, hemorrhagic necrotizing pancreatitis or exsanguination during pelvic surgery [5, 16, 23, 53,54,55]. Reports of successful REBOA use have been described for truncal hemorrhage from iliac artery blowout, iatrogenic intraoperative hemorrhage and ruptured visceral artery aneurysm [14, 21]. A systematic review of 8 cases found that REBOA is a viable, safe and therapeutic option for hemorrhage control in cases of abnormal placentation [56]. Prophylactic placement of endovascular balloon occlusion catheters has become integrated into surgical planning as an alternative to open laparotomy for temporary hemorrhage control during robotic and vascular surgery [56,57,58].
REBOA Devices
Aortic occlusion catheter devices have evolved significantly over the years. There are currently 29 different catheters from 10 manufacturers including the Coda Balloon Catheter® (Cook Medical) and ER-REBOA ™ (Prytime Medical Devices) [59]. Earlier REBOA devices including the Coda Balloon Catheter® utilized guidewires and 12-Fr introducer sheaths that necessitated open repair at the time of sheath removal [45, 60]. The newer and more commonly used ER-REBOA™ uses a 7-Fr introducer sheath and does not require guidewires (Fig. 1b). The newly designed catheter possesses an atraumatic tip to prevent migration into branch vessels and has external markings on the catheter that facilitate placement without imaging guidance [1, 45, 47, 60]. This device also provides proximal arterial pressure transduction that allows for accurate measurements of arterial response to occlusion [45, 47, 60].
Scott et al. provided the first translational work in animals that helped establish the technology of the first fluoroscopy-free REBOA device that could be placed through a 7-Fr sheath and allowed for a broader application of REBOA without requiring vascular repair [7]. This device can significantly elevate systolic blood pressure with minimal access-related complications and may remain in place during post-procedure resuscitation without further sequelae [60, 61]. Moore et al. published the first prospective, observational, multicenter evaluation of ER-REBOA ™ [62]. Results showed this device can be used safely and effectively for Zone 1 and Zone 3 occlusion in patients with varied patterns and severity of injury, including those receiving CPR [62]. This study demonstrated that catheter placement takes less than 10 min, while achieving successful aortic occlusion in 97% of cases with overall mortality of 52% [62].
Hemodynamics of Aortic Occlusion
Aortic occlusion has been shown to preserve cerebral and myocardial perfusion, improve brain oxygenation and increase carotid arterial blood flow [1, 2]. REBOA in conjunction with closed chest compressions may provide central perfusion to critical organs that translates to higher rates of EtCO2 levels, achieving ROSC and overall survival [60].
Aortic occlusion immediately increases blood pressure, with effects of supra-celiac occlusion being greater than infra-renal occlusion [47]. Animal models demonstrate a more robust augmentation of blood pressure for Zone 1 occlusion when compared to Zone 3 occlusion, but also leads to increased ischemia–reperfusion injury [39]. Similar results were shown in the AAST AORTA study when evaluating Zone 1 versus Zone 3 REBOA placement [4].
A study by Hoehn et al. evaluated the hemodynamics of aortic occlusion in an animal model and demonstrated that REBOA significantly increases systolic blood pressures, base deficit and lactic acidosis [63]. While acidosis worsened and became statistically significant after 120 min, heart rate and potassium levels remained unchanged [63]. Carotid artery flow increased by 223% with Zone 1 occlusion [63]. Additionally, there was a significant reduction in blood flow of the distal branch vessels causing severe perfusion deficits to visceral organs, especially the kidneys [63].
Balloon deflation can result in dramatic hemodynamic changes and potential drop in blood pressure more than 50 mmHg [47]. Balloon deflation causes an abrupt loss of afterload, resulting in profound vasodilation with a bolus of ischemic metabolites returning to the circulation that may cause cardiovascular collapse [20]. Despite achieving normotension or even hypertension with aortic occlusion, patients may still be under resuscitated and unable to tolerate the normalization of blood volume distribution after balloon deflation, which has been seen with both Zone 1 and Zone 3 occlusion [47].
Procedures and Techniques
The following technique for REBOA placement is categorized into specific steps originally described in the landmark study by Stannard et al. [5, 17, 20, 37, 64].
Arterial Access and Sheath Selection
Arterial access is the rate-limiting step in performing REBOA and is often the most technically challenging [15]. Access is obtained in the common femoral artery (CFA) overlying the femoral head below the inguinal ligament. Strategies to improve successful arterial cannulation include cutdown and ultrasound [15]. Some experts recommend first accessing the artery with a 4-Fr to 5-Fr micropuncture catheter, which can be upsized to a 7-Fr sheath via the Seldinger technique. Others recommend initial cannulation with a 7-Fr sheath in order to avoid time-consuming catheter exchange [15]. Currently there is no consensus as to when arterial access should be obtained.
Balloon Positioning
Balloon positioning can be obtained by radiographic visualization or external measurement when using the ER-REBOA™. If resources are available, once the balloon is in the desired location, confirmation of the catheter position under fluoroscopy is preferred prior to balloon inflation [15]. Selecting the most appropriate anatomic location for balloon inflation is based on aortic zones of occlusion for corresponding injuries.
For proper Zone 1 placement, the tip of the catheter should be placed at the sternal notch [45]. The balloon should appear between the levels T2 and T12, as the celiac trunk emerges at or below T12 in 95% of the population [1]. For Zone 3 placement, the catheter tip should lie at the level of the xiphoid and radiographic landmarks are between the level L2 and L4 above the aortic bifurcation, as 98% of patients have renal arteries above L2 [1, 45].
A retrospective review from Japan found that the accuracy of REBOA placement averaged 71.1% with blind external measurements alone, with 86.7% accuracy for Zone 1 placement and 12.5% for Zone 3 [65]. Eliason et al. showed using CT morphometry, a fixed REBOA catheter insertion length of 48 cm for Zone 1 and 28 cm for Zone 3 is optimal for average-height individuals with improved accuracy [66]. Regardless of the measurement technique used, once the proper positioning is achieved, the device is advanced through a peel-away cover sheath [45].
Balloon Inflation
Once the balloon is confirmed in the correct position, it must be manually inflated until aortic flow is halted [15]. The balloon should be inflated in a controlled fashion until resistance is met and the arterial waveform is observed for response distal to the balloon [45]. Ideally the balloon is inflated with a mixture of contrast and saline to visualize occlusion, with a recommended 8 cc for Zone 1 occlusion and 3 cc for Zone 3 occlusion when using the ER-REBOA™ to prevent overinflation injuries (Fig. 1c) [15, 41].
Management
Rapid definitive care should be provided once aortic occlusion is achieved such as further diagnostic imaging, angioembolization or laparotomy [15]. Mechanisms to reduce occlusion time and restore distal perfusion include partial REBOA and repositioning of the balloon from Zone 1 to Zone 3 if indicated [15]. Given the inherent thromboembolic risk of arterial sheaths, regular side port flushes should be utilized to prevent the development of thrombus around the sheath [24]. If possible, dedicating a team member to the management of REBOA is helpful to appropriate independent focus on this important intervention.
Balloon Deflation
Balloon deflation is the final step and can lead to cardiovascular collapse. Guidelines from the Basic Endovascular Skills for Trauma course recommends a slow, graded balloon deflation over a period of approximately 5 min [15]. Overall, deflation requires a team approach to resuscitation. Some experts advocate for the use of vasoactive medications as a bridge for blood pressure support, EtCO2 to guide reperfusion, and calcium administration to reduce the cardiac effects of hyperkalemia as reperfusion may exacerbate coagulopathy [15].
Sheath Removal and Post-Resuscitative Care
The catheter can be removed if aortic occlusion is no longer required, while the arterial sheath can be kept longer if needed; however, the sheath should be removed as soon as possible to mitigate thromboembolic complications [15]. The sheath should be removed with manual compression held for 15–30 min, followed by the application of a compression dressing to prevent the formation of hematoma or pseudoaneurysm [1, 15]. Arterial duplex ultrasound or angiogram is recommended after sheath removal to rule out arterial occlusion [15]. A vigilant assessment of lower-extremity perfusion is required before, during and after aortic occlusion including neurovascular checks of the ipsilateral extremity for at least 24 h following sheath removal [24, 41, 42].
Challenges and Complications
Despite advancements in technology, REBOA is associated with risks due to complications from access and ischemia–reperfusion. The inherent morbidity and mortality of aortic occlusion from REBOA is compounded by coexisting injury, shock and higher injury severity scores [20]. These complications are further exacerbated by providers with limited endovascular experience and resources [20]. Inherent risks associated with blind balloon inflation can be minimized through education, increased utilization of imaging and improvements in catheter design [67].
Arterial Access
The technical challenges of obtaining arterial access are aggravated by a chaotic environment, with need for rapid intervention [20]. Hemorrhagic shock, arterial vasospasm and the catecholamine surge of trauma cause vasospasm and makes cannulation more difficult [20]. Furthermore, the lethal triad of hypothermia, acidosis and coagulopathy may increase risks of bleeding from access sites [20].
The technical barriers associated with advancing the REBOA catheter include arterial thrombosis, placement into branch vessels, variant anatomy, arterial tortuosity, aneurysm, dissection flap or atherosclerotic lesions [20, 41]. Cannulating the superficial femoral artery results in a higher rate of thromboembolic events [3]. Studies from Russia and Japan report increased rate of lower-extremity amputation following REBOA access with larger arterial sheaths [20]. These femoral artery access complications may require patch repair, complex arterial reconstruction, bypass or even amputation [30]. Accidental proximal puncture of the external iliac artery may cause non-compressible retroperitoneal hemorrhage. Inadvertent IVC cannulation by inexperienced providers has also been described [20].
Balloon Positioning
Malpositioning of the balloon catheter within the aortic arch or heart can induce myocardial damage either directly by creating excessive ventricular afterload or cerebral ischemia by occlusion of the arch vessels [20]. Balloon inflation of the visceral aorta (Zone 2) can increase blood flow through the celiac axis, worsening upper abdominal hemorrhage while inducing renal ischemia (Fig. 1d) [20]. Zone 1 aortic occlusion has a greater risk of end organ ischemia, while Zone 3 aortic occlusion provides less support to myocardial and cerebral perfusion [20]. Complications of balloon positioning including exiting through an aortic injury, balloon migration and balloon rupture have been described [3].
Balloon Overinflation and Deflation
The risk of overinflation of the aorta increases with large diameter balloons and small diameter aortas, with a circumferential stretch ratio greater than 1:8 that may result in aortic rupture [67]. Balloon elongation is a radiographic feature of overinflation of the balloon and may indicate exceeding the landing zone or risking aortic rupture [67]. The Joint Trauma guidelines recommend incremental adjustments in conjunction with arterial monitoring, as overinflation of the balloon may lead to aortic overpressure injury, arterial dissection or intimal injury that can lead to long-term vascular complications or even aortic rupture [41, 67]. After inflation, the device must be properly secured in place to prevent migration of the balloon (Fig. 1e). The inherent complications of balloon deflation result from the rapid release of ischemic metabolites such as nitric oxide and pro-inflammatory mediators that result in vasodilation and refractory hypotension leading to hemodynamic collapse [41].
Systemic Complications
The supraphysiologic pressures created by REBOA may result in systemic complications such as myocardial dysfunction, pulmonary edema, intracranial hypertension, exacerbation of intracranial hemorrhage, heart failure, acute respiratory distress syndrome, acute kidney injury requiring hemodialysis, rhabdomyolysis, non-survivable traumatic brain injury, spinal cord ischemia and paraplegia, sepsis or cerebrovascular events [3, 30, 39, 41, 42, 60, 68]. These complications have been replicated in both animal and human observational studies. Animal models have shown that the supranormal increase in myocardial work and strain with balloon occlusion can lead to type II myocardial ischemic insult with prolonged occlusion times [69]. Thromboembolic events, pseudoaneurysm, compartment syndrome, dissection flap or femoral artery injury may all lead to critical limb ischemia or amputation [3, 42]. Although multifactorial, patients with hemorrhagic shock following trauma are predisposed to acute kidney injury regardless of aortic occlusion, which can be potentiated by ischemic-reperfusion injury following REBOA [26, 40, 60].
Ischemic-Reperfusion Injury
The pathophysiology of ischemic-reperfusion injury relates to microvascular dysfunction characterized by capillary dilation, reduced tissue perfusion and organ failure [68]. Multiple studies have shown a greater risk of ischemic-reperfusion injury burden that manifests as organ failure with increasing periods of aortic occlusion [9, 68]. Following balloon deflation, the circulating blood includes lactic acid, potassium and pro-inflammatory cytokines that immediately reduce inotropy (acidemia and hypocalcemia), induce dysthymias (hyperkalemia) and reduce cardiac output [18].
Partial Aortic Occlusion
The concept of partial aortic occlusion was first proposed by Johnson et al. as a technique to help ameliorate ischemic-reperfusion injury [70]. By partially deflating the REBOA balloon, a proportion of aortic flow is allowed distally, which permits sufficient oxygen delivery to effected tissues in order to offset the ischemic burden without raising pressures that disrupt forming clot or exacerbate bleeding [5, 64, 70, 71]. Allowing blood flow beyond the balloon may alleviate supraphysiologic proximal pressures while maintaining distal organ perfusion and extending the golden hour of resuscitation [72,73,74].
Partial REBOA (p-REBOA) has the potential to maintain the benefits of preserved perfusion above the level of occlusion, while creating permissive regional hypoperfusion to areas of uncontrolled hemorrhage distal to the balloon [64]. Compared to complete aortic occlusion, several studies have demonstrated a decreased resuscitation requirement, lower risk of coagulopathy and improved survival in appropriately selected patients [45].
Experts suggest maintaining an initial period of complete aortic occlusion to allow for the assessment of hemodynamics, initial resuscitation and distal clot formation; however, optimal timing is not yet established [45, 64]. Once hemodynamic stability has been achieved, the provider may consider reintroducing distal blood flow by removing 0.5 mL of saline at a time using a large-volume syringe, until a pulsatile waveform is observed [64]. A period of observation of proximal hemodynamics should be allowed to determine if any further distal perfusion will be tolerated by the current physiologic state [64].
Animal models have demonstrated higher blood pH, lower blood lactate concentrations and decreased hyperkalemia during reperfusion [71, 75, 76]. Furthermore, measured cytokines were decreased in p-REBOA groups, suggesting there is less of an inflammatory response, although statistical significance is lacking [71]. A recent clinical study by Madurska et al. found that p-REBOA was associated with fewer ventilator days, need for dialysis and significantly less vasopressor requirements when compared to complete REBOA [77].
Parameters used to define the degree of aortic occlusion have been investigated. Reva et al. found femoral artery mean atrial pressure and femoral/carotid flow gradient had the strongest correlation with the proportion of aortic occlusion, which may be useful in defining p-REBOA during Zone 1 occlusion [70]. Sadeghi et al. suggested that monitoring EtCO2 may provide a good estimate of the degree of partial aortic occlusion, distal organ metabolism and perhaps end organ damage [71]. Other techniques including intermittent REBOA with cyclical balloon inflation and deflation as well as automated endovascular variable aortic occlusion (EVAC) have been described; however, further investigation is warranted [5, 78, 79].
Special Considerations
REBOA in Children
Currently there is limited data on REBOA use in children. A study by Norii et al. concluded that both young children and adolescents who underwent REBOA had high injury severity scores and an equivalent survival rate of 43% when compared to reported survival rates from studies in adults [9, 80]. However, most data only include children between the ages of 16 to 18 and further large-scale studies are required [9, 80]. The AAST AORTA registry was reviewed for patients ages 16–17 years old, which revealed a significant hemodynamic improvement and 30% survival to hospital discharge [81].
Review of the Japan Trauma Data Bank demonstrated that 53.3% of young children and 38.5% of adolescents survived to discharge after undergoing REBOA [80]. There are several challenges to implementing REBOA in children. The CFA varies with age, making proper selection size of the introducer sheath challenging [80]. Similarly, aortic length and balloon deployment zones have been widely studied in adults, but data is lacking in children. No REBOA devices smaller than 7-Fr are currently available for use in children [9, 80].
REBOA Training and Credentialing
All physicians involved in REBOA placement should receive formal training, such as the ACS COT BEST Course [30, 42]. Didactic training should include proper patient selection, anatomy and physiology of REBOA, complications and their management, limb assessment, sheath management and establishing an appropriate system of care to support REBOA [42]. The critical skills training should include ultrasound-guidance access of the CFA as well as surgical cutdown if needed, sheath and device management, positioning of the catheter, management of inflation volumes and avoiding catheter migration [42].
One study showed that knowledge and skills from REBOA training courses persists up to six months, therefore competency courses should be considered at six-month intervals in the absence of clinical use [82]. Anatomically correct models are critical to support training for obtaining CFA access, which remains the rate-limiting step in deployment, and perfused cadavers are currently the best option for this requirement [42].
Guidelines for REBOA Implementation
A multi-disciplinary team-based approach is required for the development of REBOA protocols [30, 42]. Additionally, there should be a Quality Assurance program at each institution to evaluate REBOA on appropriate patient selection, complications and timelines to definitive hemorrhage control in order to improve patient outcomes [42]. Studies show that successful aortic occlusion with REBOA is increased at high to mid volume REBOA hospitals compared to low volume REBOA hospitals [83]. Furthermore, all REBOA procedures should be coded according to the ICD-10 hospital procedures and all institutions performing REBOA should enroll patients in the AAST multi-institutional AORTA trial to further research [30].
Pre-Hospital REBOA
The use of pre-hospital REBOA has been described in rural civilian settings and austere military environments [13]. Due to limited data, pre-hospital placement of REBOA is not currently recommended [42]. An autopsy study by Henry et al. found that less than 10% of patients who developed pre-hospital cardiac arrest sustained NCTH without associated severe head or thoracic injuries and may have potentially benefitted from REBOA use in the pre-hospital setting [13]. However, challenges of pre-hospital REBOA placement include time away to perform advanced airway procedures, the lack of access to diagnostic adjuncts and obtaining vascular access without compromising expedient transfer to the nearest trauma facility [13].
A study from London reported Zone 3 REBOA placement as a pre-hospital resuscitation strategy in patients with exsanguinating pelvic hemorrhage was associated with lower rates of cardiac arrest and death due to blood loss in a physician-led pre-hospital setting [84]. Ultimately, pre-hospital REBOA benefits must outweigh the opportunity cost of providing other life-saving procedures and should not be used when prolonged transport time is expected and distal ischemia may be irreversible [24, 85].
Future Directions
Prospective randomized trials are difficult in trauma, especially when related to unpredictable, life-saving procedures with possible ethical dilemmas [3]. Continued review of the AAST AORTA and European ABOTrauma registries is essential [3].
REBOA technology continues to evolve. There is ongoing research on partial and intermittent REBOA, with industry backing in the development of a “smart balloon” for endovascular variable aortic control [5, 7, 78, 79]. Barron et al. described using infrared and mobile thermal technology to confirm placement of REBOA in animal models, which may facilitate use in the field [86]. The use of transesophageal echography to assess hemodynamic changes during REBOA has also been studied in animal models, which may be useful for confirming REBOA positioning in austere environments [87]. Furthermore, Morrison et al. described the delivery of a specialized endovascular trauma service at a large trauma center, which reduces the time to endovascular intervention and increases case volume in a trauma hybrid operating room [18, 88].
Selective aortic arch perfusion (SAAP) is a novel resuscitation technique that utilizes an endovascular balloon catheter to provide aortic occlusion similar to REBOA, as well as to deliver oxygenated resuscitation fluids and drugs directly into the aorta to increase afterload and limit sub-diaphragmatic hemorrhage in the case of exsanguination [89,90,91]. In large animal models, SAAP has been shown to effectively deliver adequate cerebral and coronary flow during cardiac arrest resulting in improved survival and rate of achieving ROSC [89,90,91]. A study by Barnard et al. showed that SAAP demonstrated a significant survival advantage over Zone 1 REBOA in a large swine model [89]. Hoops et al. demonstrated that SAAP is effective in eliciting ROSC after traumatic cardiac arrest, using either fresh whole blood or a hemoglobin-based oxygen carrier, with associated improvement in overall survival and lactic acidosis in a swine model [90]. SAAP is still in the preclinical phases and no SAAP-specific catheter has yet been approved for clinical use [91].
There is also emerging data on the use of endovascular resuscitative balloon technology in occlusion of the inferior vena cava (IVC), also known as REBOVC [5]. Retro-hepatic IVC injuries have a high mortality rate due to difficulty in achieving total vascular occlusion [5]. A study by Reynolds et al. demonstrated superior hemorrhage control and prolonged time to death with REBOVC as opposed to total hepatic vascular isolation [92]. There is some concern that balloon occlusion in venous injuries occludes aortic outflow and may lead to increased pulmonary and central venous pressures that could potentially lead to increased bleeding, therefore further large-scale studies are indicated [55].
Conclusions
REBOA has emerged as a promising technique to stabilize patients with hemorrhagic shock and is an evolving tool in the armamentarium of the trauma and acute care surgeon as an adjunct to the management of non-compressible torso hemorrhage and traumatic cardiac arrest as a viable alternative to resuscitative thoracotomy in select patients.
References
Papers of particular interest, published recently (last 3 years), have been highlighted as: • Of importance •• Of major importance
Arndt L, Mir D, Nguyen J, et al. The resuscitative endovascular balloon occlusion of aorta (REBOA) device—what radiologists need to know. Emerg Radiol. 2019;26(6):691–4. https://doi.org/10.1007/s10140-019-01724-w.
Barnard EBG, Morrison JJ, Madureira RM, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): A population based gap analysis of trauma patients in England and Wales. Emerg Med J. 2015;32(12):926–32. https://doi.org/10.1136/emermed-2015-205217.
Bekdache O, Paradis T, Shen YBH, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): Indications: Advantages and challenges of implementation in traumatic non-compressible torso hemorrhage. Trauma Surg Acute Care Open. 2019;4(1):1–7. https://doi.org/10.1136/tsaco-2018-000262.
• Beyer CA, Johnson MA, Galante JM, DuBose JJ. Zones matter: Hemodynamic effects of zone 1 vs zone 3 resuscitative endovascular balloon occlusion of the aorta placement in trauma patients. Injury. 2019;50(4):855–858. https://doi.org/10.1016/j.injury.2019.03.013. Analysis of the AORTA registry revealed a greater change in systolic blood presure for Zone 1 REBOA comapred to Zone 3. Results showed initial Zone 1 REBOA provides maximal hemodynamic support for hypotensive trauma patients and challenges initial Zone 3 REBOA guidelines.
Ribeiro Júnior MAF, Brenner M, Nguyen ATM, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): an updated review. Rev Col Bras Cir. 2018;45(1):1–9. https://doi.org/10.1590/0100-6991e-20181709.
Engdahl AJ, Parrino CR, Wasicek PJ, et al. Anesthetic management of patients after traumatic injury with resuscitative endovascular balloon occlusion of the aorta. Anesth Analg. 2019;129(5):E146–9. https://doi.org/10.1213/ANE.0000000000004130.
Glaser J, Brenner M. The Current Status of REBOA in Traumatic Shock. Curr Surg Rep. 2017;5(10):1–9. https://doi.org/10.1007/s40137-017-0186-1.
Vernamonti JP, Holcomb J, Mick NW, et al. “Step Up” approach to the application of REBOA technology in a rural trauma system. Trauma Surg Acute Care Open. 2019;4(1):1–5. https://doi.org/10.1136/tsaco-2019-000335.
Campagna GA, Cunningham ME, Hernandez JA, Chau A, Vogel AM, Naik-Mathuria BJ. The utility and promise of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in the pediatric population: An evidence-based review. J Pediatr Surg. 2020;(xxxx):1–6. https://doi.org/10.1016/j.jpedsurg.2020.01.052.
• Vella MA, Dumas RP, Dubose J, et al. Intraoperative REBOA: An analysis of the American Association for the Surgery of Trauma AORTA registry. Trauma Surg Acute Care Open. 2019;4(1):1–5. https://doi.org/10.1136/tsaco-2019-000340. Analysis of the AAST AORTA Registry showed that REBOA was employed in the OR for patients with more stable physiology and was not associated with increased in-hospital mortality.
Dumas RP, Holena DN, Smith BP, et al. Resuscitative endovascular balloon occlusion of the aorta: assessing need in an urban trauma center. 2020:413–419. https://doi.org/10.1016/j.jss.2018.08.031.Resuscitative.
Morrison JJ, Rasmussen TE. Noncompressible Torso haemorrhage. A review with contemporary definitions and management strategies. Surg Clin North Am. 2012;92(4):843–58. https://doi.org/10.1016/j.suc.2012.05.002.
Henry R, Matsushima K, Henry RN, et al. Who Would Have Benefited from the Prehospital Use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA)? An Autopsy Study. J Am Coll Surg. 2019;229(4):383-388.e1. https://doi.org/10.1016/j.jamcollsurg.2019.05.025.
Hatchimonji JS, Chipman AM, McGreevy DT, et al. REBOA use in nontrauma emergency general surgery: a multi-institutional experience. J Surg Res. 2020;256:149–55. https://doi.org/10.1016/j.jss.2020.06.034.
•• Romagnoli A, Teeter W, Pasley J, et al. Time to aortic occlusion: It’s all about access. J Trauma Acute Care Surg. 2017;83(6):1161–1164. https://doi.org/10.1097/TA.0000000000001665. This retrospective review demonstrated that once arterial access is achieved, time to aortic occlusion is quicker with REBOA and provides similar stabilization of hemodynamic parameters.
Borger van der Burg BLS, van Dongen TTCF, Morrison JJ, et al. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur J Trauma Emerg Surg. 2018;44(4):535–50. https://doi.org/10.1007/s00068-018-0959-y.
• Cannon J, Morrison J, Lauer C, et al. Resuscitative endovascular balloon occlusion of the Aorta (REBOA) for hemorrhagic shock. Mil Med. 2018;183:55–59. https://doi.org/10.1093/milmed/usy143. This clinical practice guideline provides the indications for REBOA as an accepted management approach to hemorrhagic shock and post-traumatic cardiac arrest.
Morrison JJ. Noncompressible torso hemorrhage. Crit Care Clin. 2017;33(1):37–54. https://doi.org/10.1016/j.ccc.2016.09.001.
Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054–8. https://doi.org/10.1097/TA.0000000000000609.
• Davidson AJ, Russo RM, Reva VA, et al. The pitfalls of resuscitative endovascular balloon occlusion of the aorta: Risk factors and mitigation strategies. J Trauma Acute Care Surg. 2018;84(1):192–202. https://doi.org/10.1097/TA.0000000000001711. This report provides a comprehensive list of anecdotal complications from high-volume REBOA centeters internationally and identifies factors to recognize, mitigate and manage such complications.
Hoehn MR, Hansraj NZ, Pasley AM, et al. Resuscitative endovascular balloon occlusion of the aorta for non-traumatic intra-abdominal hemorrhage. Eur J Trauma Emerg Surg. 2019;45(4):713–8. https://doi.org/10.1007/s00068-018-0973-0.
• Daley J, Morrison JJ, Sather J, Hile L. The role of resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct to ACLS in non-traumatic cardiac arrest. Am J Emerg Med. 2017;35(5):731–736. https://doi.org/10.1016/j.ajem.2017.01.010. The authors review the efficacy and feasibility, indications and outcomes of REBOA use in non-traumatic cardiac arrest.
Júnior MAFR, Maurício AD, Costa CTK, et al. Expanding indications and results for the use of resuscitative endovascular balloon occlusion of the aorta-REBOA. Rev Col Bras Cir. 2019;46(5):1–13. https://doi.org/10.1590/0100-6991e-20192334.
• Knipp BS, Needham KE, Nguyen PT, et al. Leaning forward: Early arterial access promotes resuscitative endovascular balloon occlusion of the aorta utilization in battlefield casualties. J Trauma Acute Care Surg. 2020;89(2S Suppl 2):S88-S92. https://doi.org/10.1097/TA.0000000000002790. This review shows that early arterial access can facilitates REBOA use if indicated and provides invasive continuous blood pressure monitoring.
•• Brenner M, Inaba K, Aiolfi A, et al. Resuscitative Endovascular Balloon Occlusion of the Aorta and Resuscitative Thoracotomy in Select Patients with Hemorrhagic Shock: Early Results from the American Association for the Surgery of Trauma’s Aortic Occlusion in Resuscitation for Trauma and Acu. J Am Coll Surg. 2018;226(5):730–740. https://doi.org/10.1016/j.jamcollsurg.2018.01.044. This landmark study of the AAST AORTA Registry shows that REBOA may have a survival benefit over resuscitative thoracotomy, particularly in patientswho do not require CPR.
Du Bose JJ, Scalea TM, Brenner M, et al. The AAST prospective aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409–19. https://doi.org/10.1097/TA.0000000000001079.
Manzano Nunez R, Naranjo MP, Foianini E, et al. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J Emerg Surg. 2017;12(1):1–9. https://doi.org/10.1186/s13017-017-0142-5.
Bradley MJ, Bonds BW, Chang L, et al. Open chest cardiac massage offers no benefit over closed chest compressions in patients with traumatic cardiac arrest. J Trauma Acute Care Surg. 2016;81(5):849–54. https://doi.org/10.1097/TA.0000000000001227.
•• Brenner M, Teeter W, Hoehn M, et al. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018;153(2):130–135. https://doi.org/10.1001/jamasurg.2017.3549. Prospective review of REBOA use as a minimally invasive alternative to emergent thoracotomy with aortic crossclamping to temporize noncompressible torso hemorrhage for traumatic and nontraumatic hemorrhage control at a large volume center.
•• Brenner M, Bulger EM, Perina DG, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1):1–3. https://doi.org/10.1136/tsaco-2017-000154. The ACS COT and ACEP provide a joint policy statement regarding current practice for REBOA use including indications, potential complications, implementation, pateient management and training of providors.
Brenner ML, Moore LJ, Dubose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013. https://doi.org/10.1097/TA.0b013e31829e5416.
Yamamoto R, Cestero RF, Suzuki M, Funabiki T, Sasaki J. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: A propensity score matching analysis. Am J Surg. 2019;218(6):1162–8. https://doi.org/10.1016/j.amjsurg.2019.09.007.
• Northern DM, Manley JD, Lyon R, et al. Recent advances in austere combat surgery: Use of aortic balloon occlusion as well as blood challenges by special operations medical forces in recent combat operations. J Trauma Acute Care Surg. 2018;85(1S Suppl 2):S98-S103. https://doi.org/10.1097/TA.0000000000001966. This series demonstrates the potential for REBOA to allow austere surgical teams to rapidly stabilize severely injured compat casualities, expant capability and provide enhanced damage control resuscitation while minimizing personnel, resources, and blood product utilization.
Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score / adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721–8. https://doi.org/10.1097/TA.0000000000000578.
Fitzgerald M, Lendrum R, Bernard S, et al. Feasibility study for implementation of resuscitative balloon occlusion of the aorta in peri-arrest, exsanguinating trauma at an adult level 1 Australian trauma centre. EMA - Emerg Med Australas. 2020;32(1):127–34. https://doi.org/10.1111/1742-6723.13443.
• Joseph B, Zeeshan M, Sakran J V., et al. Nationwide Analysis of Resuscitative Endovascular Balloon Occlusion of the Aorta in Civilian Trauma. JAMA Surg. 2019;154(6):500–508. https://doi.org/10.1001/jamasurg.2019.0096. Analysis of the ACS TQIP database with propensity score matching showed that placement of REBOA in severely injured trauma patients was associated with a higher mortality rate when compared to similar cohorts, as well as higher rates of acute kidney injury and lower leg amputation compared to similar cohorts.
Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma - Inj Infect Crit Care. 2011;71(6):1869–72. https://doi.org/10.1097/TA.0b013e31823fe90c.
• Brenner M, Hicks C. Major Abdominal Trauma: Critical Decisions and New Frontiers in Management. Emerg Med Clin North Am. 2018;36(1):149-160. https://doi.org/10.1016/j.emc.2017.08.012. This review describes the REBOA algorithm used at Baltimore Shock Trauma Center, with hyemodynamic status being among the most significant determinants of patient disposition.
Tibbits EM, Hoareau GL, Simon MA, et al. Location is everything: The hemodynamic effects of REBOA in Zone 1 versus Zone 3 of the aorta. J Trauma Acute Care Surg. 2018;85(1):101–7. https://doi.org/10.1097/TA.0000000000001858.
Hoareau GL, Tibbits EM, Simon MA, et al. Renal effects of three endoaortic occlusion strategies in a swine model of hemorrhagic shock. Injury. 2019;50(11):1908–14. https://doi.org/10.1016/j.injury.2019.08.037.
Ribeiro Junior MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13(1):1–6. https://doi.org/10.1186/s13017-018-0181-6.
Bulger EM, Perina DG, Qasim Z, et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: A joint statement from the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians, the N. Trauma Surg Acute Care Open. 2019;4(1):1–6. https://doi.org/10.1136/tsaco-2019-000376.
Adnan SM, Wasicek PJ, Crawford A, et al. Endovascular control of pelvic hemorrhage: concomitant use of resuscitative endovascular balloon occlusion of the aorta and endovascular intervention. J Trauma Acute Care Surg. 2019;86(1):155–9. https://doi.org/10.1097/TA.0000000000002079.
• Pieper A, Thony F, Brun J, et al. Resuscitative endovascular balloon occlusion of the aorta for pelvic blunt trauma and life-threatening hemorrhage: A 20-year experience in a Level i trauma center. J Trauma Acute Care Surg. 2018;84(3):449-453. https://doi.org/10.1097/TA.0000000000001794. A 20-year retrospective review from a Level 1 trauma center demonstrates that REBOA significantly improved SBP from 60-115 mmHg. Vascular complication rate was 19%, but no amputations were required.
Dubose JJ. How i do it: Partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA). J Trauma Acute Care Surg. 2017;83(1):197–9. https://doi.org/10.1097/TA.0000000000001462.
Reva VA, Matsumura Y, Hörer T, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? Eur J Trauma Emerg Surg. 2018;44(4):511–8. https://doi.org/10.1007/s00068-016-0732-z.
Wasicek PJ, Li Y, Yang S, et al. Examination of hemodynamics in patients in hemorrhagic shock undergoing Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Injury. 2019;50(5):1042–8. https://doi.org/10.1016/j.injury.2018.12.030.
Wasicek PJ, Yang S, Teeter WA, et al. Traumatic cardiac arrest and resuscitative endovascular balloon occlusion of the aorta (REBOA): a preliminary analysis utilizing high fidelity invasive blood pressure recording and videography. Eur J Trauma Emerg Surg. 2019;45(6):1097–105. https://doi.org/10.1007/s00068-018-0989-5.
Hilbert-Carius P, McGreevy DT, Abu-Zidan FM, et al. Pre-hospital CPR and early REBOA in trauma patients-results from the ABOTrauma Registry. World J Emerg Surg. 2020;15(1):1–8. https://doi.org/10.1186/s13017-020-00301-8.
• Tiba MH, McCracken BM, Cummings BC, et al. Use of resuscitative balloon occlusion of the aorta in a swine model of prolonged cardiac arrest. Resuscitation. 2019;140(May):106–112. https://doi.org/10.1016/j.resuscitation.2019.05.010. This translational study demonstrated that REBOA significantly increased cerebral perfusion pressure and common carotid artery blood flow in swine models with prolonged cardiac arrest, which ultimately may improve the ability to achieve ROSC.
Nowadly CD, Hoareau GL, Grayson JK, Johnson MA. Zone 3 REBOA does not provide hemodynamic benefits during nontraumatic cardiac arrest. 2020
• Glaser JJ, Neidert LE, Morgan CG, Brenner M, Stigall KS, Cardin S. Resuscitative endovascular balloon occlusion of the aorta for thoracic trauma in the setting of platelet dysfunction: A translational swine study. J Trauma Acute Care Surg. 2020;89(4):708–715. https://doi.org/10.1097/TA.0000000000002882. This translational study showed that Zone 1 REBOA improved survival and decreased blood loss with major thoracic venous injury in a swine model. This suggests that for thoracic bleeding without surgical capability, outcomes may be improved with REBOA, findings that challeng current guidelines on REBOA use in this setting.
Strauss S, Engels P, Harlock J. Distal placement of resuscitative endovascular balloon occlusion of the aorta (REBOA) to Restore Hemodynamic Stability in a Patient With Proximal Aortic Rupture. J Endovasc Ther. 2018;25(2):257–60. https://doi.org/10.1177/1526602818757012.
Sano H, Tsurukiri J, Hoshiai A, Oomura T, Tanaka Y, Ohta S. Resuscitative endovascular balloon occlusion of the aorta for uncontrollable nonvariceal upper gastrointestinal bleeding. World J Emerg Surg. 2016. https://doi.org/10.1186/s13017-016-0076-3.
Lallemand MS, Moe DM, McClellan JM, et al. Resuscitative endovascular balloon occlusion of the aorta for major abdominal venous injury in a porcine hemorrhagic shock model. J Trauma Acute Care Surg. 2017;83(2):230–6. https://doi.org/10.1097/TA.0000000000001548.
Manzano-Nunez R, Escobar-Vidarte MF, Naranjo MP, et al. Expanding the field of acute care surgery: a systematic review of the use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in cases of morbidly adherent placenta. Eur J Trauma Emerg Surg. 2018;44(4):519–26. https://doi.org/10.1007/s00068-017-0840-4.
England EC, Spear CR, Dih D, et al. REBOA as a rescue strategy for catastrophic vascular injury during robotic surgery. J Robot Surg. 2020;14(3):473–7. https://doi.org/10.1007/s11701-019-01011-3.
Goodenough CJ, Cobb TA, Holcomb JB. Use of REBOA to stabilize in-hospital iatrogenic intra-abdominal hemorrhage. Trauma Surg Acute Care Open. 2018;3(1):3–5. https://doi.org/10.1136/tsaco-2018-000165.
Vrancken SM, Borger van der Burg BLS, Vrancken PJEM, Kock GAH, Rasmussen TE, Hoencamp R. A contemporary assessment of devices for Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA): resource-specific options per level of care. Eur J Trauma Emerg Surg. 2020. https://doi.org/10.1007/s00068-020-01382-5.
• Brenner M, Moore L, Teeter W, et al. Exclusive clinical experience with a lower profile device for resuscitative endovascular balloon occlusion of the aorta (REBOA). Am J Surg. 2019;217(6):1126–1129. https://doi.org/10.1016/j.amjsurg.2018.11.029. The authors provide the first description of the newly FDA-approved ER-REBOA catheters and examine associated complications as well as procedural timing and patient outcomes.
Teeter WA, Matsumoto J, Idoguchi K, et al. Smaller introducer sheaths for REBOA may be associated with fewer complications. J Trauma Acute Care Surg. 2016;81(6):1039–44. https://doi.org/10.1097/TA.0000000000001143.
•• Moore LJ, Fox EE, Meyer DE, et al. Prospective Observational Evaluation of the ER-REBOA Catheter at 6 U.S. Trauma Centers. Ann Surg. 2020;Publish Ah(Xx):1–7. https://doi.org/10.1097/sla.0000000000004055. This study is the first prospective, observational, multi-center evaluation of the ER-REBOA catheter, which showed that the device can be used safely and effectively in aortic zones 1 and 3 in diverse patients with varied severity of injury. Placement of ER-REBOA took less than 10 minutes with successful aortic occlusion in more than 97% of cases.
Hoehn MR, Teeter WA, Morrison JJ, et al. Aortic branch vessel flow during resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2019;86(1):79–85. https://doi.org/10.1097/TA.0000000000002075.
Johnson MA, Neff LP, Williams TK, et al. Partial resuscitative balloon occlusion of the aorta (P-REBOA): Clinical technique and rationale. J Trauma Acute Care Surg. 2016;81(5):S133–7. https://doi.org/10.1097/TA.0000000000001146.
Matsumoto S, Funabiki T, Kazamaki T, et al. Placement accuracy of resuscitative endovascular occlusion balloon into the target zone with external measurement. Trauma Surg Acute Care Open. 2020;5(1):1–6. https://doi.org/10.1136/tsaco-2020-000443.
• Eliason JL, Derstine BA, Horbal SR, et al. Computed tomography correlation of skeletal landmarks and vascular anatomy in civilian adult trauma patients: Implications for resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2019;87(1S Suppl 1):S138-S145. https://doi.org/10.1097/TA.0000000000002247. This study used computed tomography morphometry to identify a fixed, optimal REBOA catheter insertion length of 48 cm for zone 1 and 28 cm for zone III for average-height individuals, with torso extent accounting for the majority of variation in vascular distances.
Wasicek PJ, Teeter WA, Brenner ML, Hoehn MR, Scalea TM, Morrison JJ. Resuscitative endovascular balloon occlusion of the aorta: Rupture risk and implications for blind inflation. Trauma Surg Acute Care Open. 2018;3(1):1–6. https://doi.org/10.1136/tsaco-2017-000141.
Simon MA, Tibbits EM, Hoareau GL, et al. Lower extremity cooling reduces ischemia-reperfusion injury following Zone 3 REBOA in a porcine hemorrhage model. J Trauma Acute Care Surg. 2018;85(3):512–8. https://doi.org/10.1097/TA.0000000000001990.
Wasicek PJ, Teeter WA, Yang S, et al. Extended resuscitative endovascular balloon occlusion of the aorta (REBOA)-induced type 2 myocardial ischemia: a time-dependent penalty. Trauma Surg Acute Care Open. 2019;4(1):1–7. https://doi.org/10.1136/tsaco-2018-000194.
Reva VA, Matsumura Y, Samokhvalov IM, et al. Defining degree of aortic occlusion for partial-REBOA: a computed tomography study on large animals. Injury. 2018;49(6):1058–63. https://doi.org/10.1016/j.injury.2018.04.021.
Sadeghi M, Hörer TM, Forsman D, et al. Blood pressure targeting by partial REBOA is possible in severe hemorrhagic shock in pigs and produces less circulatory, metabolic and inflammatory sequelae than total REBOA. Injury. 2018;49(12):2132–41. https://doi.org/10.1016/j.injury.2018.09.052.
Davidson AJ, Russo RM, DuBose JJ, Roberts J, Jurkovich GJ, Galante JM. Potential benefit of early operative utilization of low profile, partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA) in major traumatic hemorrhage. Trauma Surg Acute Care Open. 2016;1(1):1–3. https://doi.org/10.1136/tsaco-2016-000028.
Russo RM, Williams TK, Grayson JK, et al. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2016;80(3):372–80. https://doi.org/10.1097/TA.0000000000000940.
Forte DM, Do WS, Weiss JB, et al. Titrate to equilibrate and not exsanguinate! Characterization and validation of a novel partial resuscitative endovascular balloon occlusion of the aorta catheter in normal and hemorrhagic shock conditions. J Trauma Acute Care Surg. 2019;87(5):1015–25. https://doi.org/10.1097/TA.0000000000002378.
Kauvar DS, Schechtman DW, Thomas SB, et al. Effect of partial and complete aortic balloon occlusion on survival and shock in a swine model of uncontrolled splenic hemorrhage with delayed resuscitation. J Trauma Acute Care Surg. 2019;87(5):1026–34. https://doi.org/10.1097/TA.0000000000002439.
Russo RM, Neff LP, Lamb CM, et al. Partial resuscitative endovascular balloon occlusion of the aorta in swine model of hemorrhagic shock. J Am Coll Surg. 2016;223(2):359–68. https://doi.org/10.1016/j.jamcollsurg.2016.04.037.
• Madurska MJ, McLenithan A, Scalea TM, et al. A Feasibility study of Partial REBOA data in a High-Volume Trauma Center. Eur J Trauma Emerg Surg. 2020;Publish Ah. This retrospective review included 46 patients, 14 of which were treated with P-REBOA. In comparison to complete REBOA, partial REBOA was associated with fewer ventilator days and need for dialysis with significanlty decreased vasopressor requirement.
Williams TK, Tibbits EM, Hoareau GL, et al. Endovascular variable aortic control (EVAC) versus resuscitative endovascular balloon occlusion of the aorta (REBOA) in a swine model of hemorrhage and ischemia reperfusion injury. J Trauma Acute Care Surg. 2018;85(3):519–26. https://doi.org/10.1097/TA.0000000000002008.
Kuckelman JP, Barron M, Moe D, et al. Extending the golden hour for Zone 1 resuscitative endovascular balloon occlusion of the aorta: Improved survival and reperfusion injury with intermittent versus continuous resuscitative endovascular balloon occlusion of the aorta of the aorta in a porcin. J Trauma Acute Care Surg. 2018;85(2):318–26. https://doi.org/10.1097/TA.0000000000001964.
Norii T, Miyata S, Terasaka Y, Guliani S, Lu SW, Crandall C. Resuscitative endovascular balloon occlusion of the aorta in trauma patients in youth. J Trauma Acute Care Surg. 2017;82(5):915–20. https://doi.org/10.1097/TA.0000000000001347.
Theodorou CM, Brenner M, Morrison JJ, et al. Nationwide use of REBOA in adolescent trauma patients: An analysis of the AAST AORTA registry. Injury. 2020;(xxxx):7–11. https://doi.org/10.1016/j.injury.2020.08.009.
Hatchimonji JS, Sikoutris J, Smith BP, et al. The REBOA dissipation curve: training starts to wane at 6 months in the absence of clinical REBOA cases. J Surg Educ. 2020. https://doi.org/10.1016/j.jsurg.2020.05.003.
Theodorou CM, Anderson JE, Brenner M, et al. Practice, practice, practice! effect of resuscitative endovascular balloon occlusion of the aorta volume on outcomes: data from the AAST AORTA registry. J Surg Res. 2020;253:18–25. https://doi.org/10.1016/j.jss.2020.03.027.
Lendrum R, Perkins Z, Chana M, et al. Pre-hospital resuscitative endovascular balloon occlusion of the aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation. 2018;2019(135):6–13. https://doi.org/10.1016/j.resuscitation.2018.12.018.
Zhang J, Watson JD, Drucker C, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) not yet applicable for widespread out-of-hospital use: a case of nonsurvivable complication from prolonged REBOA inflation. Ann Vasc Surg. 2019;56:354.e5-354.e9. https://doi.org/10.1016/j.avsg.2018.08.108.
Barron MR, Kuckelman JP, McClellan JM, et al. Mobile forward-looking infrared technology allows rapid assessment of resuscitative endovascular balloon occlusion of the aorta in hemorrhage and blackout conditions. J Trauma Acute Care Surg. 2018;85(1):25–31. https://doi.org/10.1097/TA.0000000000001932.
Teeter WA, Conti BM, Wasicek PJ, et al. Feasibility of basic transesophageal echocardiography in hemorrhagic shock: Potential applications during resuscitative endovascular balloon occlusion of the aorta (REBOA). Cardiovasc Ultrasound. 2018;16(1):1–7. https://doi.org/10.1186/s12947-018-0129-8.
• Morrison JJ, Madurska MJ, Romagnoli A, et al. A Surgical Endovascular Trauma Service Increases Case Volume and Decreases Time to Hemostasis. Ann Surg. 2019;270(4):612–619. https://doi.org/10.1097/SLA.0000000000003486. This study evaluated the outcomes of a specialty endovascular trauma service at a high volume REBOA center. Results demonstrated that an endovascular trauma service increased case volume and decreased time to hemostasis for trauma patients requiring time sensitive interventions.
Barnard EBG, Manning JE, Smith JE, Rall JM, Cox JM, Ross JD. A comparison of selective aortic arch perfusion and resuscitative endovascular balloon occlusion of the aorta for the management of hemorrhage-induced traumatic cardiac arrest: a translational model in large swine. PLoS Med. 2017;14(7):1–19. https://doi.org/10.1371/journal.pmed.1002349.
Hoops HE, Manning JE, Graham TL, McCully BH, McCurdy SL, Ross JD. Selective aortic arch perfusion with fresh whole blood or HBOC-201 reverses hemorrhage-induced traumatic cardiac arrest in a lethal model of noncompressible torso hemorrhage. J Trauma Acute Care Surg. 2019;87(2):263–73. https://doi.org/10.1097/TA.0000000000002315.
• Madurska MJ, Ross JD, Scalea TM, Morrison JJ. State-of-the-Art Review – Endovascular Resuscitation. Shock. 2020;Publish Ah(x). https://doi.org/10.1097/shk.0000000000001636. This article discusses the emerging catheter-based techniques used in the management of hemorrhagic shock and non-traumatic cardiac arrest. These evolving techniques include REBOA, selective aortic artch perfusion, and extracorporeal membrane oxygenation.
Reynolds CL, Celio AC, Bridges LC, et al. REBOA for the IVC? Resuscitative balloon occlusion of the inferior vena cava (REBOVC) to abate massive hemorrhage in retrohepatic vena cava injuries. J Trauma Acute Care Surg. 2017;83(6):1041–6. https://doi.org/10.1097/TA.0000000000001641.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AM and FA contributed to the literature search and drafting the first manuscript draft. RB, MG, MM, LL, MR, RB, JD and JM drafted and critically revised the final manuscript. RB, MM, and JM worked on the images and captions provided for the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Amanda Marsh MD, Richard Betzold MD, Mira Ghneim MD, Lawrence Lottenberg MD, Mario Rueda MD, Megan Morrow MD, Robert Borrego MD, Joseph DuBose MD, and Faris Azar MD have no financial disclosures or conflicts of interest to report. Jonathan J. Morrison MD PhD is a member of the clinical advisory board of Prytime Medical Inc., a position which includes stock options.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Trauma Surgery.
Rights and permissions
About this article
Cite this article
Marsh, A.M., Betzold, R., Rueda, M. et al. Clinical Use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in the Management of Hemorrhage Control: Where Are We Now?. Curr Surg Rep 9, 5 (2021). https://doi.org/10.1007/s40137-021-00285-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s40137-021-00285-7