Abstract
Purpose
Aortic occlusion (AO) increases proximal perfusion and may improve rates of return of spontaneous circulation (ROSC). The objective of this study was to investigate the hemodynamic effects of cardiopulmonary resuscitation (CPR) and AO by REBOA on patients in traumatic cardiac arrest.
Methods
Patients admitted between February 2013 and May 2017 at a tertiary center who suffered traumatic arrest, had an arterial line placed during resuscitation, and received CPR and REBOA which were included. In-hospital CPR data were obtained from videography. Arterial waveforms were recorded at 240 Hz.
Results
11 consecutive patients were included, 82% male; mean (± SD) age 37 ± 19 years. 55% suffered blunt trauma and the remaining penetrating injuries. 64% arrested out of hospital. During compressions with AO, the mean systolic blood pressure (SBP) was 70 ± 22 mmHg, mean arterial pressure (MAP) 43 ± 19 mmHg, and diastolic blood pressure (DBP) 26 ± 17 mmHg. Nine (82%) had ROSC, with eight having multiple periods of ROSC and arrest in the initial period. In-hospital mortality was 82%. Cardiac ultrasonography was used during arrest in 73%. In two patients with arterial line data before and after AO, SBP (mmHg) improved from 51 to 73 and 55 to 96 during arrest after AO.
Conclusions
High-quality chest compressions coupled with aortic occlusion may generate adequate perfusion pressures to increase the rate of ROSC. New technology capable of transducing central arterial pressure may help us to understand the effectiveness of CPR with and without aortic occlusion. REBOA may be a useful adjunct to high-quality chest compressions during arrest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rates of survival and good neurologic outcome from patients suffering traumatic cardiac arrest have been poor [1,2,3,4]. Severe trauma and hemorrhage leading to cardiac arrest may result in a decreased circulatory volume and the systemic pressures achieved during chest compressions may be inadequate to allow for return of spontaneous circulation (ROSC). Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is a procedure used to obtain proximal aortic control, augment cardiac and cerebral perfusion, and, along with high-quality CPR, may be a beneficial adjunct in traumatic arrest. REBOA has been previously described in the setting of infra-diaphragmatic non-compressible torso hemorrhage, as well as in the setting of traumatic cardiac arrest [5, 6]; however, the utility of REBOA in traumatic cardiac arrest remains unclear.
While non-traumatic cardiac arrest animal models have shown increases in coronary and cerebral perfusion during aortic occlusion (AO) [7,8,9], there has been little clinical investigation into the precise hemodynamic effects of REBOA and closed chest compressions during traumatic cardiac arrest in humans. In addition, owing to the moribund status of these patients and the necessity to perform REBOA emergently, the incidence and use of invasive blood pressure measuring devices (arterial lines) as well as utility of cardiac ultrasonography during cardiac arrest has not been well described.
The objective of this study was to investigate the hemodynamic effects of CPR and AO by REBOA on patients in traumatic arrest using a sophisticated continuous vital sign collection tool. We also aimed to describe the incidence and utility of both invasive arterial line monitoring and cardiac ultrasonography in these patients.
Materials and methods
This study is a retrospective case series utilizing prospectively collected data. Demographics and hospital course data were collected prospectively on all trauma patients, age ≥ 18 years old, who underwent REBOA at the University of Maryland Shock Trauma Center. This study was approved by the Institutional Review Board of the University of Maryland, Baltimore. Patients admitted between February 2013 and May 2017 at a tertiary center who suffered traumatic arrest, had an arterial line in place during arrest, underwent chest compressions, and received REBOA in zone 1 (descending thoracic aorta) were included. Demographics and hospital course data were extracted from the medical record. REBOA-specific and in-hospital CPR-specific procedural timing metrics were recorded by time-stamped videography in the resuscitation areas and operating rooms. Out-of-hospital CPR information was obtained from emergency medical service documentation and the medical record.
Vital signs including arterial blood pressure were recorded from a continuous vital sign monitoring system using a network of GE-Marquette-Solar-7000/8000® (General Electric, Fairfield, CT) patient vital signs monitors as previously described [10]. In brief, all patient vital sign monitors in the trauma resuscitation unit (TRU), operating room, postanesthesia care unit, and intensive care units are networked to provide continuous collection of real-time patient vital sign data. Data are transmitted through a secure intranet connection to dedicated BedMaster® servers (Excel Medical Electronics, Jupiter, FL) and archived. Vital sign data collected included invasive and non-invasive blood pressure, heart rate, end-tidal carbon dioxide (EtCO2) values, and electrocardiographic waveforms, among others. Invasive arterial line waveforms were captured at 240 Hz.
Arterial pressures during cardiac arrest and closed chest compressions were analyzed. Abrupt increases in arterial blood pressure during chest compressions that likely represented ROSC (with confirmation of ROSC after cessation of chest compressions) were excluded from calculations of blood pressure during cardiac arrest with compressions. Non-physiologic arterial line data, such as artifact and times during which the arterial line was recording but not connected to the patient, were excluded.
Multiple variables were analyzed to determine ROSC and rhythm including manual palpation of pulsatile arterial flow, EKG waveform, cardiac ultrasonography, end-tidal carbon dioxide (ETCO2), and invasive arterial line monitoring. ROSC was defined as any spontaneously patient-generated blood pressure, and determined from the vital sign recordings and compared to the medical record and videography.
The source of arterial line data was recorded. During the study period, REBOA was initially performed using a 12 French sheath and the CODA® catheter (Cook Medical, Bloomington, IN). There was a transition to using a smaller 7 French sheath with the FDA approval of a smaller profile catheter, ER-REBOA™ (Prytime Medical, Boerne, TX), which occurred in February 2016. The ER-REBOA™ catheter has an arterial line monitoring port in contrast to the CODA® balloon catheter which does not.
The primary outcome was the blood pressure attained in patients during chest compressions with aortic occlusion. Secondary outcomes included changes in blood pressure caused by AO, describing hemodynamics immediately upon ROSC, comparing blood pressure values during chest compressions with ROSC, and describing the utility of cardiac ultrasonography and arterial line monitoring in these patients.
Unpaired two-sample t test was used for mean comparison. Statistical significance was defined as a p value of 0.05 or less. Statistical analysis was performed using Matlab (R2014b, Natick, MA).
Results
Description of patient population, characteristics, and hospital course
Over the study period, 90 patients were assessed for eligibility, and 11 patients were included (see Fig. 1). Patient demographics and characteristics are listed in Table 1. These patients suffered severe non-compressible infra-diaphragmatic torso injuries and were in extremis from severe hemorrhage as evidenced by their low hemoglobin lab values. In addition, they suffered from severe metabolic derangements as evidenced by their acidosis and high lactate levels. Cardiac ultrasonography was used in 73% of the cases.
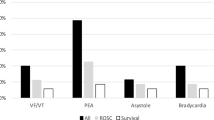
As seen in Table 2, these patients represented a heterogeneous mixture of patients in cardiac arrest at various times and durations before AO. Seven suffered cardiac arrest en-route to the hospital with ROSC being achieved in the out-of-hospital setting in three. Documentation of specific durations of out-of-hospital CPR and ROSC timing were incomplete. Upon arrival to the resuscitation area, three patients not previously in arrest en-route suffered arrest and CPR was initiated, with a total of seven patients arriving to the resuscitation area in arrest. The remaining patients developed cardiac arrest a mean of 2.8 ± 1.8 min after arrival. The first recorded rhythm was pulseless electrical activity (PEA) in 73%, ventricular fibrillation in 9%, and asystole in 18%. The total in-hospital cardiac compression fraction (duration of chest compressions/duration of cardiac arrest) was 84.2 ± 10.7%.
The decision to perform, as well as the performance of REBOA, occurred soon after admission, as shown in Fig. 2. The median [interquartile range] time from admission to start of arterial access was 3.9 [2.7–5.4] min, and median time from admission to AO was 11.7 [9.8–13.2] min. Seven patients had periods of ROSC before AO, with median duration of 4.9 [1.6–10.0] min; however, these patients arrested again before AO. The mean duration of in-hospital CPR before AO was 9.1 ± 4.3 and 10.4 ± 7.9 min after AO. 8 (73%) patients achieved ROSC after AO, with three of these patients having multiple periods of ROSC in between periods of arrest after AO (median duration 5.0 min, range 1.2–28.0 min).
Two patients (both suffered penetrating trauma, one arrested out-of-hospital) survived and were discharged from the hospital. One patient did not suffer any neurologic impairment, while the other suffered an anoxic brain injury with a Glasgow Outcome Score [11] of 3 (conscious but disabled).
Arterial pressure measurements and observations
The mean ± SD (in mmHg) of each patient’s mean systolic (SBP), mean (MAP), and diastolic (DBP) blood pressure during cardiac arrest with AO was 70 ± 22, 43 ± 19, and 26 ± 17, respectively. There was moderate variation in the mean values of all patients’ blood pressures. The range (in mmHg) among all patients’ mean SBP, MAP, and DBP during arrest was 36–96, 16–68, and 6–52 respectively. No association was found with SBP, MAP, or DBP and ROSC (p = 0.64, 0.93, and 0.96, respectively). Blood pressure measurements immediately after ROSC also had moderate variation. The range (in mmHg) of mean SBP, MAP, and DBP after ROSC was 91–159, 84–148, and 59–127.
Two patients had arterial line tracings before and after AO, as shown in Fig. 3. The first patient (Fig. 3a) did not have a femoral arterial line in place, and had arterial access obtained with the purpose of directly upsizing to a 7 Fr sheath to perform REBOA. The catheter was inserted with the arterial line port transduced before the balloon was inflated and AO occurred. A dramatic rise in blood pressure reading occurred upon AO; however, upon manual palpation of the carotid arteries, it was determined that the patient remained in arrest and compressions continued. Review of videography showed that non-physiologic arterial line data were being transduced. After securing the catheter in place and releasing pressure upon the catheter, physiologic arterial data resumed. The mean SBP during compressions immediately before AO was 51 mmHg, compared to approximately 3 min after AO, which increased to 73 mmHg. Approximately 3.7 min after AO, ROSC occurred and was confirmed with cardiac ultrasonography.
Examples of arterial line tracing before and after AO, with ROSC immediately/soon after AO (a, b). Grey shaded areas represent time during chest compressions. Light-grey hatched markings represent non-physiologic arterial line tracings/artifact. Dotted line with AO next to it represents aortic occlusion time
The second patient (Fig. 3b), who had previously been in arrest but had achieved ROSC, remained persistently hypotensive, so the decision was made to exchange an existing femoral arterial line for a 7 Fr sheath and perform REBOA. While this occurred, the patient suffered cardiac arrest and compressions were started. When AO was performed, an immediate increase in systolic blood pressure was seen during CPR, from 55 to 96 mmHg. In addition, the signal quality of the waveform dramatically improved immediately after AO which may, in and of itself, reflect a significant improvement in blood pressure. A brief (2 s) pause determined that ROSC had not been achieved and compressions continued. The blood pressure continued to rise and upon an additional pulse check, ROSC was determined to have been achieved.
Determination of ROSC and rhythm
Three examples are presented to demonstrate the value of invasive arterial line monitoring and cardiac ultrasonography in helping determine ROSC and rhythm. In the first example, the patient was initially in pulseless electrical activity (PEA) arrest, after AO the rhythm changed from PEA to ventricular tachycardia and defibrillation was successful resulting in ROSC with a robust increase in blood pressure, as shown in Fig. 4a. In contrast, in another case, there was a less robust response, with arterial blood pressure being roughly equivalent during CPR as compared to immediately after ROSC, as shown in Fig. 4b. Cardiac ultrasonography was utilized to confirm that the patient had ROSC, despite it being a less robust response as compared to the prior example. In the last example, as shown in Fig. 4c, the blood pressure measured immediately after compressions stopped remained elevated without immediate and rapid decay in blood pressure; however, cardiac ultrasonography demonstrated standstill.
Examples of ROSC with robust increase in blood pressure (a), borderline hypotension after ROSC (b), and moderate arterial pressures despite cardiac standstill (c). Grey shaded areas represent time during chest compressions. Light-grey hatched markings represent non-physiologic arterial line tracings/artifact
Discussion
This study is the first characterization of the blood pressures achieved during traumatic cardiac arrest with closed chest compressions and REBOA. High-quality chest compressions coupled with AO may generate adequate blood pressures to permit ROSC in traumatic cardiac arrest. In addition, aortic occlusion by REBOA may augment central systemic blood pressure during cardiac arrest, as evidenced by two cases in which there was an increase in blood pressure during chest compressions upon AO. Cardiac ultrasonography and invasive arterial pressure monitoring may assist in directing and assessing the adequacy of resuscitation efforts. These findings have important clinical implications for clinicians utilizing REBOA as an adjunct during traumatic cardiac arrest, and provide valuable insight for directing future investigations.
Select cases of ROSC immediately or soon after AO, as well as the demonstration of increased blood pressure during CPR immediately after AO, are highly suggestive of its potential effect and advantage. Despite these findings, the blood pressure generated during CPR after AO was not significantly different in patients who achieved ROSC after AO compared to those who did not. The absence of finding an association between increased blood pressure and ROSC is likely related to a small sample size as well as multiple confounding factors that we were not able to adequately control for such as the duration of arrest and quality of resuscitation up until the point of AO, the nature of the arrest, the severity of concomitant injuries sustained, and the use of vasopressors, among other factors. To our knowledge, a link between systemic arterial pressure attained during chest compressions and ROSC with REBOA has not been described previously; however, animal [12] and human [13] studies have demonstrated an association between increased coronary perfusion pressures and ROSC.
In the setting of patients experiencing multiple episodes of ROSC with subsequent cardiac arrest, it can be difficult to determine whether or not a patient is in cardiac arrest. Palpating a pulse is frequently insufficient, especially in the setting of patients who are borderline hypotensive after ROSC. As shown in Fig. 4, ROSC does not always correlate with a sudden rise in ETCO2. Cardiac ultrasonography and arterial line data are valuable adjuncts which can provide useful information. Placing an arterial line during traumatic arrest and ongoing CPR is extremely challenging. However, the ER-REBOA™ catheter has the ability to provide critical blood pressure measurements and provide AO simultaneously, which eliminates the need to a separate radial or femoral arterial line as was required with initial technology for REBOA. The port, located above the balloon, may transduce central pressure regardless of balloon inflation or deflation. The catheter is FDA approved for isolated use as an arterial monitoring device with or without AO. Obtaining intraaortic pressures can demonstrate dramatic increases in blood pressure during compressions with and without aortic occlusion, and a brief pause with cardiac ultrasonography can confirm ROSC.
As shown in Fig. 4c, we report finding moderate arterial pressures in the setting of aortic occlusion in a patient who had cardiac standstill. The specific mechanism causing this delayed decay in blood pressure after cessation of compressions in the setting of cardiac standstill is unclear, but may be a phenomenon related to the decreased effective capacity of vascular volume secondary to aortic occlusion or may be secondary to an arterial line device flaw. Without cardiac ultrasonography, this episode may have been mistaken for ROSC, and it highlights the utility of cardiac ultrasonography, especially in the setting of AO.
This study has several limitations which are important to discuss. First, the cohort of patients included is small with a significant heterogeneity of injury patterns, mechanisms of injury, and periods of arrest. These limitations ultimately result in an inability to control for multiple confounding variables and hinder the ability to determine the efficacy of REBOA.
Second, the study is limited, secondary to its observational nature, by the diagnostic and therapeutic interventions performed in the course of clinical care. This limitation is, perhaps, highlighted the most by the lack of arterial line monitoring before REBOA in the majority of patients. In addition, the inability to precisely ascertain the patients’ intravascular volume status limits the ability to attribute the specific effects of each intervention such as closed chest compressions, blood product transfusion, inotropic medication administration, and REBOA.
Finally, the lack of control groups limits the ability to determine the benefit of the multiple competing interventions being performed. Use of closed chest compressions is controversial in traumatic arrest [14, 15] given that cardiac output and coronary perfusion may be inadequate to obtain ROSC in the setting of dramatically reduced circulatory volumes (severe hemorrhage). In fact, the use of blood transfusions has been shown to be potentially more beneficial than closed chest compressions [15]. The concurrent use of REBOA and blood transfusions may result in the effective circulatory volume being significantly improved and, therefore, may result in dramatically improved utility in chest compressions. This is supported by the relatively high incidence of ROSC reported in this series; however, without adequate controls, the benefit of REBOA remains unclear. Definitive evidence for the utility of REBOA as an adjunct in traumatic cardiac arrest is under current investigation.
Conclusions
Closed chest compressions coupled with aortic occlusion may generate adequate blood pressures to allow ROSC in traumatic cardiac arrest. New technology capable of transducing central arterial pressure may help us to understand the effectiveness of CPR with and without aortic occlusion. REBOA may be a useful adjunct to high-quality chest compressions during arrest.
References
Gräsner JT, Wnent J, Seewald S, Meybohm P, Fischer M, Paffrath T, Wafaisade A, Bein B, Lefering R, German Resuscitation Registry Working Group. Trauma Registry of the German Society for Trauma Surgery (DGU). Cardiopulmonary resuscitation traumatic cardiac arrest there are survivors. An analysis of two national emergency registries. Crit Care. 2011;15(6):R276.
Harris T, Masud S, Lamond A, Abu-Habsa M. Traumatic cardiac arrest: a unique approach. Eur J Emerg Med. 2015;22(2):72–8.
Zwingmann J, Mehlhorn AT, Hammer T, Bayer J, Südkamp NP, Strohm PC. Survival and neurologic outcome after traumatic out-of-hospital cardiopulmonary arrest in a pediatric and adult population: a systematic review. Crit Care. 2012;16(4):R117.
Seamon MJ, Chovanes J, Fox N, Green R, Manis G, Tsiotsias G, Warta M, Ross SE. The use of emergency department thoracotomy for traumatic cardiopulmonary arrest. Injury. 2012;43(9):1355–61.
Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, Holcomb JB, Scalea TM, Rasmussen TE. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75(3):506–11.
DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, Moore L, Holcomb J, Turay D, Arbabi CN, Kirkpatrick A, Xiao J, Skarupa D, Poulin N, AAST AORTA Study Group. The AAST prospective aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409–19.
Gedeborg R, Rubertsson S, Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation. 1999;40(3):171–80.
Rubertsson S, Bircher N, Alexander H. Effects of intra-aortic balloon occlusion on hemodynamics during, and survival after cardiopulmonary resuscitation in dogs. Crit Care Med. 1997;25:1003–9.
Sesma J, Labandeira J, Sara MJ, Espila JL, Arteche A, Saez MJ. Effect of intra-aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med. 2002;20(5):453–62.
Hu PF, Yang S, Li HC, Stansbury LG, Yang F, Hagegeorge G, Miller C, Rock P, Stein DM, Mackenzie CF. Reliable collection of real-time patient physiologic data from less reliable networks: a “monitor of monitors” system (MoMs). J Med Syst. 2017;41(1):3.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–4.
Reynolds J, Salcido D, Menegazzi J. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14(1):78–84.
Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA J Am Med Assoc. 1990;263(8):1106–13.
Anderson KL, Fiala KC, Castaneda MG, Boudreau SM, Araña AA, Bebarta VS. Left ventricular compressions improve return of spontaneous circulation and hemodynamics in a swine model of traumatic cardiopulmonary arrest. J Trauma Acute Care Surg (Epub 2018 Apr 2).
Watts S, Smith J, Gwyther R, Kirkman E. Closed chest compressions reduce survival in a model of haemorrhage-induced traumatic cardiac arrest. Emerg Med J. 2017;34(12):A866.
Funding
This study was funded in part by a grant from the Department of Defense (Grant number W81XWH-15-1-0025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Megan Brenner is a clinical advisory board member for Prytime Medical Inc. Drs. Philip Wasicek, Shiming Yang, William Teeter, Peter Hu, Deborah Stein, and Thomas Scalea declare that they have no conflicts of interest.
Research involving animal and human participants
This study involved human subjects and was compliant with the 1964 Helsinki Declaration and its later amendments, and was compliant with the Institutional Review Board of the University of Maryland, Baltimore.
Ethical approval
This study was approved by the Institutional Review Board of the University of Maryland, Baltimore.
Informed consent
This was a retrospective observational study of prospective collected data, for which formal consent was not required.
Rights and permissions
About this article
Cite this article
Wasicek, P.J., Yang, S., Teeter, W.A. et al. Traumatic cardiac arrest and resuscitative endovascular balloon occlusion of the aorta (REBOA): a preliminary analysis utilizing high fidelity invasive blood pressure recording and videography. Eur J Trauma Emerg Surg 45, 1097–1105 (2019). https://doi.org/10.1007/s00068-018-0989-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-018-0989-5