Abstract
Purpose of Review
Hemorrhagic shock remains a major cause of potentially preventable death in civilian and military trauma. Balloon occlusion of the aorta has emerged as a viable technique to address non-compressible torso hemorrhage. The purpose of this review is to describe the current state of translational and clinical data on REBOA and help define its role in modern trauma algorithms.
Recent Findings
Recent findings suggest that REBOA is feasible in many clinical areas including prehospital. Robust animal data define a reasonable safety profile and current clinical data suggest that there are subset(s) of patients who may benefit from REBOA over traditional EDT and/or in conjunction with other resuscitation measures.
Summary
Although enthusiasm for the technique may have outpaced high-quality clinical data, ongoing efforts through multicenter trials seek to identify the ideal clinical scenario for REBOA. We also discuss future translational and clinical series for the next generation of REBOA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemorrhagic shock (HS), in particular non-compressible torso hemorrhage (NCTH), remains the major cause of potentially preventable death among the severely injured in both civilian and military settings [1]. In civilian literature, hemorrhage accounts for the largest proportion of mortality occurring within the first hour of trauma center care with NCTH accounting for 60–70% of mortality despite otherwise survivable injuries [2]. Modern wartime data describe that NCTH is the leading cause (50%) of potentially survivable injuries [3]. As NCTH is not amenable to direct pressure control, it is particularly lethal [4,5,6,7].
Initial efforts toward hemorrhage control are paramount, a combined lesson learned between the military and civilian trauma community. In the civilian setting, campaigns similar to the American College of Surgeons Committee on Trauma ‘Stop the Bleeding’ program [8] teach the importance of direct pressure and tourniquet use to prevent exsanguination. The military ‘buddy aid’ acronym has changed from ABC (airway, breathing, circulation) to ‘X-ABC’—attention to controlling exsanguinating hemorrhage is the initial goal [9]. This change is supported by current statistics. Military data collected between 2002 and 2009 reported 1570 vascular injuries, 40% of which were associated with major vascular injuries [10]. Tourniquet use has improved survival in this group with reported overall survival up to 87%, with few complications; however, the use of tourniquets leaves the problem of junctional hemorrhage unsolved [11].
Proximal vascular control in the setting of NCTH must be gained via thoracotomy, laparotomy, or from an extraperitoneal approach. In the setting of pelvic hemorrhage, pre-peritoneal packing of bleeding may be effective and is well described [12, 13]. These options for addressing central and junctional bleeding in a patient in extremis remain a challenge. Resuscitative thoracotomy has a high mortality rate, due largely to the nature of the injuries leading to arrest [14,15,16,17,18,19]. Access to the abdominal aorta through a transabdominal or extraperitoneal approach also carries a high morbidity [20]. The above maneuvers to control bleeding are also best done in the controlled operative setting and attempts to perform these in the emergency room or prehospital will be met with failure without the immediate availability of surgical or interventional resources [21].
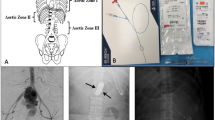
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a technique to stabilize patients in hemorrhagic shock by temporarily occluding the aorta. The described technique refers to the placement of a temporary occlusive balloon in the aorta via a common femoral artery approach, allowing proximal coronary and cerebral perfusion while preventing downstream hemorrhage [22, 23]. It serves as an alternative to aortic clamping or compression via a thoracotomy or laparotomy. REBOA can provide total or partial occlusion of the aorta either just above the diaphragm (Zone I, Fig. 1) or above the iliac bifurcation (Zone III, Fig. 2) depending on the presumed location of hemorrhage. The balloon catheter is inserted through a sheath placed in the common femoral artery (CFA) via ultrasound, blindly, or by surgical cut-down. Contraindications to REBOA include suspected major cardiac, pulmonary, or vascular injury above the diaphragm, all of which may require direct access to the chest for appropriate management, particularly in the setting of arrest.
In cases of traumatic cardiac arrest, or peri-arrest physiology, open thoracotomy provides the access to perform open cardiac massage during resuscitation. Patients who arrest secondary to penetrating thoracic trauma still require an emergency department thoracotomy (EDT) with aortic cross-clamp, as REBOA offers no ability for treatment, and occluding the descending aorta may worsen proximal hemorrhage. In the case of penetrating thoracic trauma, REBOA placement may worsen bleeding at sites proximal to the balloon. In some centers, REBOA has replaced resuscitative thoracotomy for other than significant thoracic trauma. Benefits of this approach include the ability to perform CPR continuously during REBOA placement, especially in light of recent data supporting closed chest CPR as equivalent to open chest CPR at improving markers of resuscitation [24, 25]. Recent data suggest that REBOA may in fact have superior survival benefit to thoracotomy in select patients [26].
Statement of the Problem
The use of a balloon to occlude the aorta for traumatic bleeding was conceptualized very early in trauma, with the first documented use being Lieutenant Colonel Carl Hughes in the Korean War [27]. He reported the use of an intraaortic balloon occluder in 2 critically injured soldiers. He postulated that intra-abdominal aortic tamponade would improve coronary perfusion to improve shock in the moribund patient and noted in his series that earlier use would have likely led to improved outcomes. Although both patients died, blood pressure was temporarily restored in one case. There were no further reports in the trauma literature until the 1980s, when several clinical case series for hemorrhagic shock were described. Low et al. described the use of a Percluder balloon in 15 trauma patients (23 total patients, 5 ruptures AAA, 3 others) in comparison to the military antishock trousers (MAST). Of the 9 patients with vital signs present at the time of occlusion, 100% showed an improvement in blood pressure and allowed time for volume replacement. One trauma survivor later died of ischemic complications after 90 min of aortic occlusion. Overall survival was 26% and re-established feasibility of the technique for hemorrhage [28]. Gupta’s series a year later reported intraaortic balloon occlusion in 21 patients in hemorrhagic shock from penetrating wounds. The technique was successful at occlusion and restoration of blood pressure in 20 patients, and there were 7 long-term survivors [29].
Enthusiasm for the technique was little at the time, other than for vascular surgery applications. In the mid-1990s, the initial case series on endovascular repair for ruptured abdominal aortic aneurysms (rAAA) were published. Aortic occlusion became the first step in the management of rAAA and advances in technology greatly promulgated the technique which has now become standard of care. Despite advancing technology, the primary use of AO occurred in the fluoroscopy suite. It was not until decades later that military and civilian collaboration led to a resurgence of interest and adaptation of the tools and technique to make AO a “bedside” procedure [30, 31].
Translational Data to Support Clinical Use of REBOA
Preclinical translational research supporting the use of REBOA in the 2000s supported growing clinical enthusiasm [32•, 33]. It had been recognized since 1985 that coronary blood flow was augmented during CPR with the addition of aortic occlusion [34]. In a dog model of CPR and ventricular fibrillation arrest, Spence et al. displayed that cerebral blood flow improved by 100% and blood pressure improved by 130% with the addition of aortic balloon occlusion [35]. This data were confirmed by Sesma et al. in swine, with aortic balloon occlusion shown to increase end tidal CO2 (ETCO2), cerebral and coronary perfusion significantly during CPR [36].
Driven by a clinical gap on the battlefield [6, 37], translational work leads to the description of the REBOA technique and the proposed clinical algorithms for its application [38•]. White et al. were the first to describe the use of REBOA in a swine model of NCTH [32]. Using a swine model of class IV shock, groups were randomized to balloon aortic occlusion, thoracotomy and aortic clamping, or no occlusion. Both groups with aortic occlusion showed improved cerebral and coronary perfusion as well as central blood pressure, but the balloon occlusion group also showed less acidosis, improved lactate, and required less fluid and inotropic support (epinephrine) than the open aortic clamping group. The establishment of a reliable swine shock model led to further translation data on REBOA [39]. In particular, the landmark work of Stannard et al. in 2011 [40] that both described the technique as an adjunct for hemorrhagic shock and also provided a clinical algorithm to follow, as well as describing the zones of aortic occlusion that further all clinical and training algorithms have referenced. Markov et al., in the same model, showed a significant lactic acidosis, renal injury, and liver necrosis after 90 min of balloon occlusion in shock as compared to 30 min. Although all parameters recovered to baseline after resuscitation, these data supported the recommendations to keep occlusion time under 90 min [41]. Avaro et al. compared 40 and 60 min occlusion times in a swine model of uncontrolled splenic hemorrhage, showing improved survival from shock and decreased fluid resuscitation requirements (saline) with aortic balloon occlusion. All animals in the 40-min group survived, but 75% of the animals in the 60-min group died upon balloon deflation/reperfusion, suggesting a cumulative metabolic insult over prolonged occlusion times [37]. Scott et al. performed balloon occlusion over 60 min in a pure hemorrhage swine model with acceptable results as well, but this work included a new device. Comparing a fluoroscopy-free balloon device to a traditional aortic occlusion balloon (and a 7Fr vs. 14Fr sheath), all animals displayed improved blood pressure and survival. Of note, lactate peaked 45 min after balloon deflation, consistent with a reperfusion phenomenon, but normalized over the following 48 h of care during the protocol [38•].
Recognizing the potential for ischemia with prolonged inflation times, intermittent occlusion was compared to complete occlusion in another swine model of hemorrhage, a model that included a laparoscopic liver injury. This protocol also incorporated modern damage control practices, including damage control surgery and whole blood resuscitation. Both intermittent and complete occlusion produced higher blood pressure than controls (no occlusion) and had improved survival (75% for complete, 68.5% for intermittent) over controls (100% mortality) [32]. There were no differences noted in metabolic burden (pH, lactate) between complete and intermittent occlusion. This study was also the first to look at the inflammatory burden of REBOA. Although significantly increased from baseline in both groups, no differences were seen between intermittent and complete occlusion for TNF-alpha, IL-6, or IL-8 in this group. Although no significant differences were noted between techniques, it was recognized that the physiologic and metabolic burden of 60 min occlusion time was ameliorated (recovered to baseline) by the addition of a modern damage control resuscitation strategy. In a separate study, IL-6 was noted to significantly rise from baseline in the 60- and 90-min occlusion time points [33].
REBOA has also been evaluated in the setting of life-threatening venous injury (common iliac vein injury). The REBOA group showed an improvement in blood pressure, and survival increased from 4 min in controls to over 40 min with REBOA despite equivalent blood loss. It was concluded from this work that REBOA is effective at extending the survival time and perhaps allowing for definitive surgical bleeding control in the setting of major venous injury [42]. Current animal models of TBI and polytrauma with REBOA as a resuscitation adjunct have shown preserved cerebral blood flow without exacerbating progression of brain injury [43]. Proximal and distal aortic occlusion (Zone 1 vs. Zone 3) has been investigated in swine over a 60 min occlusion time, revealing no statistical difference in mortality or spinal complications—though spinal cord-related mortality was noted to be 12.5% in both groups [44]. REBOA has also been investigated in animal models for safety during CT imaging [45] and in flight/medical evacuation [46].
As described above, balloon occlusion in excess of 90 min has been associated with liver and renal dysfunction. Endpoints evaluated in the lab thus far have included physiologic variables (HR, BP) and basic chemistries (e.g., Lactate, LDH, AST, and Creatinine). While this information is illuminating, it is far from a comprehensive metabolic, proteomic, inflammatory, or immune analysis. Ongoing collaborative protocols between the University of Maryland Shock Trauma Center and military research teams in San Antonio aim to shed light on the ideal duration of aortic occlusion, looking specifically and mesenteric vascular flow (Doppler) over variable durations of occlusion (up to 6 h). Additional outputs include comprehensive inflammation assessment, assessment of coagulopathy, markers of endothelial damage, markers of neurologic and spinal dysfunction, and histological analysis to include brain, lung, kidney, and liver tissue for evidence of ischemia necrosis [47]. Data from this work should shed light on underlying metabolic insult of REBOA and the ideal time points for aortic occlusion as, currently, the acceptable maximum time for aortic occlusion in humans remains unknown [48,49,50].
Clinical Series
There is a growing body of clinical evidence that REBOA, as an adjunct to ‘buy time’ to definitive surgical hemostasis, may improve outcomes including survival. The first clinical series at a large civilian trauma center came from the University of Maryland Shock Trauma Center and University of Texas at Houston in 2013, with a series of 6 patients described [49•]. These patients—4 blunt and 2 penetrating—were treated with REBOA, 3 with zone 1 occlusion and 3 with zone 3 occlusion. In this series, there was a mean increase in systolic blood pressure of 55 mmHg over a mean occlusion time of 18 min. There were no reported hemorrhage-related deaths and no REBOA complications. Although this series was performed at a high-volume center with experts in the technique and was a small series, this demonstrated that the technique was feasible in the civilian setting.
A four-year clinical series, from 2011 to 2015, reported REBOA use in 31 patients at another high-volume trauma center. Balloon occlusion also resulted in a median increased systolic blood pressure of 55 mmHg and spontaneous return of circulation in 60% of patients who had arrested. Early death from hemorrhage was reported at 28%, and only 2 patients expired prior to operative intervention could be attempted [50].
In another combined effort of the 2 early institutional adopters of REBOA, the concept of ‘buying time’ to definitive surgical bleeding control was demonstrated, with increased survival to emergency department to the OR (mortality in ED, OR, ICU for thoracotomy vs. REBOA: 62.5 vs. 16.6%, 8.3 vs. 12.5%, and 19.4 vs. 33.3%). While the thoracotomy statistics are comparable to historical values (9.7%), overall survival was significantly higher at 37.5% for the REBOA group [22].
While REBOA in the United States has become familiar to larger centers, international use has been established for some time. The Japanese National inpatient database was reviewed for REBOA use between 2010 and 2014, comparing REBOA outcomes to thoracotomy within 24 h of admission, excluding penetrating injury. Analyzing 259 patients, no differences were identified in outcomes including mortality, transfusions, ICU length of stay, ventilator free days, or cost [51]. Saito et al. described a clinical series out of Tokyo including 24 patients undergoing REBOA for pelvic fracture or intra-abdominal bleeding with hemodynamic instability. They noted a survival of 30%. Of the survivors, 3 vascular complications (one iliac injury and 2 limb ischemia cases) went on to require amputation. This series describes REBOA as feasible but requiring further safety evaluation [52]. The relative complication of limb ischemia compared to mortality remains a point of discussion.
Further data from the Japanese data bank were analyzed to look at the hazard ratio of REBOA placement. In this series of over 45,000 patients, 452 received REBOA within 24 h of admission. This group had a higher ISS and higher mortality (ISS 35 vs. 13, Mortality 76 vs. 16%). After propensity matching, these authors conclude that REBOA is associated with a higher likelihood of death compared to a matched trauma population who did not receive it. This conclusion however may reflect the lower morbidity of use (as compared to thoracotomy) and its use as a ‘salvage’ maneuver in extreme cases [53].
REBOA has also been successfully demonstrated in the emergency room and ICU setting in Japan. In a series of 25 patients (16 trauma and 9 non-trauma) REBOA was achieved in 22 cases, with an improvement in blood pressure parameters, and reported survival of 20% at 24 h and 12% at 60 days, with no reported complications. This series demonstrated the technical feasibility of REBOA in non-surgical settings [54].
Several significant differences exist in the use of REBOA in Japan compared to our own use in the US. The original data, some of which described limb loss as a complication (Saito et al.), were gathered in patients who received REBOA with larger in-dwelling sheaths which were managed differently. Intra-thoracic hemorrhage is not a contraindication to REBOA in Japan, and some patients received AO as a last ditch effort in resuscitation. The devices used even today are slightly different requiring a guide wire for placement, and the practitioners are all EM doctors performing REBOA in the ED.
Military data from the UK were reviewed to identify casualties that could potentially benefit from REBOA. Over a 10-year dataset, 18.5% of casualties were determined to have a potential indication for REBOA placement, and within this group there were 145 prehospital deaths (n = 174). The median time to death in this cohort was 75 min, making an argument for early, perhaps even prehospital consideration for REBOA placement [55]. The concept of prehospital REBOA is not new, as the London Air Ambulance Services is credited with successful placement of the first prehospital placement of REBOA [56]. However, it is important to recognize differences in local and regional trauma systems when interpreting international data. In the UK, for example, prehospital care is often delivered by emergency physicians or anesthesiologists, versus paramedic equivalents in the US. In Japan, trauma volume is lower as is the availability of in-house trauma surgeons, and there is broad use of endovascular and other non-surgical techniques in the ICU and emergency room settings [57]. These regional differences must be accounted for when interpreting the above datasets.
Many major trauma centers in the US have begun to train and utilize the technique within institution-specific guidelines and protocols. Clinical data are currently being collected and reported both within institutions as well as in multi-institutional collaborations such as AORTA (the Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery) working group [58•]. All adult patients treated with resuscitative aortic occlusion via open or endovascular means in the acute phases after injury, patients with transient or refractory hypotension (SBP < 90) with fluid in the abdomen or pelvic fracture, or those deemed by the attending surgeon to benefit from aortic occlusion are included in the study. In the most recent analysis of this dataset, including 46 REBOA and 68 open procedures, hemodynamic improvement was noted in 62.3% of cases (67.4 REBOA vs. 61.8% open), but a significantly higher number in the REBOA group achieved a stable blood pressure for over 5 min (47.8 vs. 27.9% open). To date, there are no other statistical differences between groups, with comparable mortality (Open vs. REBOA 83.8 vs. 71.7%, p = 0.120) and neurologic outcomes between groups. Complications have been acceptable, with vascular/access complications between 2 and 5% (50% of access was via open cut-down, the vast majority of those in patients in arrest without a palpable femoral pulse), and no limb-threatening ischemia or need for vascular bypass in the REBOA groups.
Increasing enthusiasm for REBOA outside of the trauma arena has led to its widespread use, and case reports are emerging more frequently describing its use in post-partum hemorrhage [59, 60] as a bridge to uterine artery embolization. The largest series includes 36 patients with 100% survival and no major complications [61]. It has been used during caesarian section and gynecological pathology [62,63,64], as an adjunct to control retroperitoneal bleeding [65], oncologic surgery [66], hepatobiliary surgery [67], gastrointestinal bleeding [68], and of course abdominal aneurysmal disease [69, 70].
At large-volume trauma centers, with REBOA experience and expertise, it takes an average of 6.6 min to obtain endovascular aortic occlusion [58•]. CFA access remains the primary challenge, and current recommendations for access are both institution and patient specific and practitioner dependent. Current options include percutaneous and open CFA access, with confirmation of intraaortic placement via plain X-ray, ultrasound [71], manual confirmation (during exploratory laparotomy), fluoroscopy, or without image confirmation [72].
Vascular complications remain a significant concern and one of the hurdles to widespread adoption. A single-institution experience reported by Taylor et al. [73] examined a 5-year dataset, with 48 cases. 38 patients underwent occlusion via a 14Fr sheath and 10 via a 7Fr system. Of the survivors, 19 required arterial repair and none required repair in the 7Fr group. No amputations were required. Another series examined 33 patients with Zone 1 REBOA placement in a Japanese clinical dataset. All access was percutaneous, with placement of a 7Fr sheath and an average sheath dwell time of 28 h in survivors. No complications were related to sheath insertion or removal in this group, nor were any complications identified upon follow-up [74]. Of note, in this series 30/33 REBOA catheters were placed by emergency medicine physicians. It has been demonstrated in a preclinical model that prehospital providers can, without prior training, learn and perform REBOA. Four US Special Operations medical personnel demonstrated safe access and reasonable procedural competency for REBOA placement in a perfused cadaver model after undergoing a basic didactic and hands-on training session [75]. There is general agreement that arterial complications from REBOA (distal embolus, dissection flaps, pseudoaneurysms, distal ischemia, etc.) be addressed expeditiously in consultation with vascular surgery colleagues.
A recent case series of prehospital military REBOA supports the potential use in the far forward environment [76•]. The series describes four cases by a surgically capable forward resuscitation team, with placement by both surgeons and emergency providers. Two patients suffered fragmentation gunshot wounds, one with hemoperitoneum and one in severe (Class 4) shock. REBOA was placed via ultrasound-guided arterial access with 3 balloon inflations in zone 1 and one in zone 3. In all cases, blood pressure was restored allowing for initiation of whole blood resuscitation and performance of emergent surgical bleeding control. Balloon ischemia time ranged from 18 to 60 min. All patients survived transfer to the next level of care and there were no reported REBOA complications. A case report of the first REBOA placed in combat in Afghanistan has been submitted for publication (authors own work). This case reports the use of REBOA for proximal bleeding control, as an adjunct to buy time to obtain definitive surgical control in a case of a transpelvic gunshot wound and sacral plexus injury. This patient survived to hospital discharge. He did suffer gluteal necrosis after pelvic embolization, which required flap coverage. It is unlikely that this was related to the brief duration (5 min) of aortic occlusion.
The Next Evolution of Aortic Occlusion
With the concurrent trends in broad clinical usage and the technologic improvements in REBOA, and the ability of the technique to be used outside of major trauma centers, including the prehospital setting, broadening of the indications and usage can be expected. As discussed above, the creation of a lower profile, wire-free device compatible with a 7Fr sheath has facilitated placement in the prehospital and far forward setting [38•]. FDA approval of the ER-REBOA™ device and expansion of indications in April 2017 to include fluoroscopy-free confirmation will continue to promote the use of the device in other settings [77]. A military-funded, Industry-sponsored multi-institutional grant has begun at several high-volume centers to investigate the use of the newly approved device. It is currently available in 100 level 1 trauma centers in the US (ref: personal communications).
Ongoing preclinical research on partial occlusion and variable occlusion REBOA and industry support of the technical aspects of a ‘smart balloon’ show tremendous promise. It is understood that prolonged balloon inflation, while good for hemorrhage control, can create unacceptable ischemia distally with prolonged use. Additionally, there are cases in the literature illustrating the potential catastrophic effect of proximal aortic and carotid hypertension created with aortic occlusion [78]. Intermittent deflation of the balloon has been successful in the lab [79]. Partial occlusion (pREBOA) seems protective—in animal studies—against the aortic overpressure phenomenon. In one swine study of hemorrhagic shock after induced coagulopathy, blood loss and survival between compete and pREBOA were similar, but complete occlusion led to extreme MAPs and prolonged supra-physiologic carotid blood flow, where partial occlusion preserved these parameters at physiologic levels [80•]. Additionally, rebound hypotension, duodenal ischemia (on histology), and serum lactates were all improved with pREBOA versus complete occlusion.
pREBOA has been described clinically as well [81], with staged deflation of the balloon after definitive surgical control of bleeding, conceptually decreasing the ischemia reperfusion burden of occlusion while avoiding the supra-physiologic pressures created proximally [82]. The duration of AO may be prolonged with intermittent partial AO as described by investigators at Travis Air Force Base [80•, 81], but the question of the ideal techniques to prolong use is still being deciphered.
A hybrid variable occlusion technology has been developed utilizing an extracorporeal circuit interfaced with the endovascular balloon, with automatic feedback used to variably control balloon/aortic wall pressure. Translational work has demonstrated the ability to regulate proximal aortic pressure while simultaneously preserving a physiologic degree of distal blood flow [83]. Theoretic advantages of this technology clearly include prevention of distal ischemia, but can also alleviate concerns for progression of traumatic brain injury [43]. Admittedly, however, the role of balloon aortic occlusion in the setting of TBI is poorly understood. Current research projects, through military and civilian partnerships, are evaluating the utility of variable aortic occlusion in prolonged field care scenarios. Conceptually, a casualty on the battlefield could have an automated aortic balloon placed that would be able to maintain a physiologic balance between hemorrhage and ischemia, while awaiting evacuation, for up to 72 h [84]. The addition of better prehospital imaging (Infrared and automated ultrasound) to assist with arterial access and combining variable occlusion technology with distal and proximal drug delivery designed to preserve preload and afterload, as well as reduce ischemic burden, are all current considerations in REBOA research and funding.
Conclusion
Despite widespread enthusiasm, REBOA is an evolving technique. Preliminary data suggest that there are subset(s) of patients who may benefit from REBOA over traditional EDT and/or in conjunction with resuscitation measures. FDA approval of the ER-REBOA™ has undoubtedly expanded the use of REBOA. Many of the current clinical questions may be answered in the future with maturation of databases such as AORTA. For many trauma and acute care surgeons, REBOA represents a promising option for the treatment of the very real, common, and life-threatening problem of NCTH. It is best considered another tool in the armamentarium of the trauma surgeon and as such will rely on the individual skill, resources, training, and experience of the trauma team to save lives.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kelly JF, Ritenour AE, et al. Injury severity and causes of death from operation Iraqi Freedom and operation enduring freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl):S21–6.
Kauvar DS, Lefering R, et al. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11.
Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am. 2012;92(4):843–58, vii.
Rossaint R, Bouillon B, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14(2):R52. doi:10.1186/cc8943 (Epub 2010 Apr 6).
Eastridge BJ, Mabry RL, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7.
Stannard A, Morrison JJ, et al. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J Trauma Acute Care Surg. 2013;74(3):830–4.
Kisat M, Morrison JJ, et al. Epidemiology and outcomes of non-compressible torso hemorrhage. J Surg Res. 2013;184(1):414–21.
http://stopthebleedingcoalition.org/. Last Accessed 10 May 2017.
US Army Joint Trauma System Clinical Practice Guidelines. http://www.usaisr.amedd.army.mil/pdfs/TCCCGuidelinesforMedicalPersonnel170131Final.pdf. Last accessed 10 May 2017.
White JM, Stannard A, et al. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg. 2011;253(6):1184–9.
Kragh JF Jr, Walters TJ, et al. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249(1):1–7.
Cothren CC, Osborn PM, et al. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma. 2007;62(4):834–9; discussion 839–42.
Tötterman A, Madsen JE, et al. Extraperitoneal pelvic packing: a salvage procedure to control massive traumatic pelvichemorrhage. J Trauma. 2007;62(4):843–52.
Seamon MJ, Pathak AS, et al. Emergency department thoracotomy: still useful after abdominal exsanguination? J Trauma. 2008;64:1–7.
Khorsandi M, Skouras C, Shah R. Is there any role for resuscitative emergency department thoracotomy in blunt trauma? Interact Cardiovasc Thoracic Surg. 2013;16(4):509–16.
Seamon MJ, Fisher CA, et al. Emergency department thoracotomy: survival of the least expected. World J Surg. 2008;32(4):604–12.
Branney SW, Moore EE, et al. Critical analysis of two decades of experience with postinjury emergency department thoracotomy in a regional trauma center. J Trauma. 1998;45(1):87–94; discussion 94–5.
Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma. 1976;16(08):610–5.
Burlew CC, Moore EE, et al. Western Trauma Association critical decisions in trauma: resuscitative thoracotomy. J Trauma Acute Care Surg. 2012;73(6):1359–63.
Hathaway E, Glaser JJ, et al. Exploratory laparotomy for proximal vascular control in combat-related injuries. Mil Med. 2016;181(5 Suppl):247–52.
Rhee PM, Acosta J, et al. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg. 2000;190(3):288–98.
Moore LJ, Brenner M, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523–30.
Brenner M, Teeter W, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a feasible option for proximal aortic control in severe hemorrhage and arrest. JAMA Surg. 2017 (In revisions).
Bradley M, Bonds B, et al. Open chest cardiac massage offers no benefit over closed chest compressions in patients with traumatic cardiac arrest. JTACS. 2016;81(5):849–54.
Brenner M, Teeter WA, et al. “End tidal carbon dioxide (EtCO2) before and after Resuscitative Endovascular Balloon Occlusion of the Aorta with Closed Chest Compression (REBOACCC) is higher compared to Open Chest Cardiac Massage with Aortic Cross-Clamp (OCCMACC)”. Quick shot presentation, Southwestern Surgical Congress, April 2017, manuscript in preparation.
Brenner, M, Aiolfi A, et al. REBOA is superior to resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the AAST AORTA Registry. Accepted for Podium presentation AAST 2017.
Hughes CW. Use of intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery. 1954;36(1):65–8.
Low RB, Longmore W, et al. Preliminary report on the use of the Percluder occluding aortic balloon in human beings. Ann Emerg Med. 1986;15(12):1466–9.
Gupta BK, Khaneja SC, et al. The role of intra-aortic balloon occlusion in penetrating abdominal trauma. J Trauma. 1989;29(6):861–5.
Propper BW, Alley JB, et al. Endovascular treatment of a blunt aortic injury in Iraq: extension of innovative endovascular capabilities to the modern battlefield. Ann Vasc Surg. 2009;23(5):687.e19–22.
Mayer D, Pfammatter T, et al. 10 years of emergency endovascular aneurysm repair for ruptured abdominal aortoiliac aneurysms: lessons learned. Ann Surg. 2009;249(3):510–5.
• White JM, Cannon JW, et al. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400–9. This is the first animal model in the trauma literature to describe the benefits of balloon aortic occlusion and suggest it as an alternative to open occlusion for non compressible torso hemorrhage.
Morrison JJ, Ross JD, et al. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of non-compressible torso hemorrhage. Shock. 2014;41(2):130–7.
Suzuki A, Taki K, et al. Cerebral blood flow during open-chest cardiac massage with occlusion of the descending aorta in dogs. Resuscitation. 1985;13(1):69–75.
Spence PA, Lust RM, et al. Transfemoral balloon aortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res. 1990;49(3):217–21.
Sesma J, Labandeira J, et al. Effect of intra-aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med. 2002;20(5):453–62.
Avaro JP, Mardelle V, et al. Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma. 2011;71(3):720–5; discussion 725–6.
• Scott DJ, Eliason JL, et al. A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg. 2013;75(1):122–8. This translational work established the technology of the first fluoroscopy free REBOA device, as well as one that can be placed though a 7Fr sheath. This animal work describes the device and opened the door for a broader application of REBOA (without requiring a definitive vascular repair from the sheath).
White JM, Cannon JW, et al. A porcine model for evaluating the management of noncompressible torso hemorrhage. J Trauma. 2011;71(1 Suppl):S131–8.
Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71(6):1869–72.
Markov NP, Percival TJ, et al. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848–56.
Lallemand MS, Moe DM, et al. Resuscitative Endovascular balloon Occlusion of the Aorta (REBOA) for major abdominal venous injury in a porcine hemorrhagic shock model. J Trauma Acute Care Surg. 2017 Apr 28.
Johnson MA, Williams TK, et al. The effect of REBOA, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine polytrauma model. J Trauma Acute Care Surg. 2017. doi:10.1097/TA.0000000000001518.
Long KN, Houston R 4th, et al. Functional outcome after resuscitative endovascular balloon occlusion of the aorta of the proximal and distal thoracic aorta in a swine model of controlled hemorrhage. Ann Vasc Surg. 2015;29(1):114–21.
Madurska MJ, Jansen JO, et al. The compatibility of CT scanning and partial-REBOA: a large animal pilot study. J Trauma Acute Care Surg. 2017. doi:10.1097/TA.0000000000001574.
Reva VA, Hörer T, et al. Field and en route REBOA: a feasible military reality? J Trauma Acute Care Surg. 2017. doi:10.1097/TA.0000000000001476.
CDMRP award W81XWH-16-1-0116. Physiologic response to prolonged resuscitative Endovascular balloon occlusion of the thoracic aorta in swine.
Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054–8.
• Brenner ML, Moore LJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75(3):506–11. This is the first clinical series published on REBOA, with 6 patients and 4/6 survivors. This article established REBOA a clinically viable option in US trauma centers.
Moore LJ, Martin CD, et al. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. Am J Surg. 2016;212(6):1222–30.
Aso S, Matsui H, et al. Resuscitative endovascular balloon occlusion of the aorta or resuscitative thoracotomy with aortic clamping for noncompressible torso hemorrhage: a retrospective nationwide study. J Trauma Acute Care Surg. 2017;82(5):910–4.
Saito N, Matsumoto H, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78(5):897–903; discussion 904.
Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721–8.
Tsurukiri J, Akamine I, et al. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24:13.
Morrison JJ, Ross JD, et al. Resuscitative endovascular balloon occlusion of the aorta: a gap analysis of severely injured UK combat casualties. Shock. 2014;41(5):388–93.
Sadek S, Lockey DJ, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: an additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation. 2016;107:135–8.
Qasim Z, Brenner M, et al. Resuscitative endovascular balloon occlusion of the aorta. Resuscitation. 2015;96:275–9.
• DuBose JJ, Scalea TM, et al.; AAST AORTA Study Group. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409–19. This is the most recent data from the US multicenter aortic occlusion study group. It describes data from 114 prospectively collected patients. Comparable outcomes are seen between REBOA and open occlusion in terms of survival and recover of blood pressure. Sustained improvement in blood pressure was higher in the REBOA group. Approximately 50% of REBOAs were placed via a cut down and average time to balloon occlusion was 6.6 minutes. Complications rates were acceptable.
Søvik E, Stokkeland P, et al. The use of aortic occlusion balloon catheter without fluoroscopy for life-threatening post-partum haemorrhage. Acta Anaesthesiol Scand. 2012;56:388–93.
Harma M, Kunt AS, et al. Balloon occlusion of the descending aorta in the treatment of severe post-partum haemorrhage. Aust N Z J Obstet Gynaecol. 2004;44:170–1.
Stensaeth KH, Haakon K, et al. Fluoroscopy-free Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for controlling life threatening postpartum hemorrhage. PLoS ONE. 2017;12(3):e0174520. doi:10.1371/journal.pone.017452.
Paull JD, Smith J, et al. Balloon occlusion of the abdominal aorta during caesarean hysterectomy for placenta percreta. Anaesth Intensive Care. 1995;23:731–4.
Masamoto H, Uehara H, et al. Elective use of aortic balloon occlusion in cesarean hysterectomy for placenta previa percreta. Gynecol Obstet Invest. 2009;67:92–5.
Bell-Thomas SM, Penketh RJ, et al. Emergency use of a transfemoral aortic occlusion catheter to control massive haemorrhage at caesarean hysterectomy. BJOG Int J Obstet Gynaecol. 2003;110(12):1120–2.
Rosenthal MD, Raza A, et al. The novel use of resuscitative endovascular balloon occlusion of the aorta to explore a retroperitoneal hematoma in a hemodynamically unstable patient. Am Surg. 2017;83(4):337–40.
Tang X, Guo W, et al. Use of aortic balloon occlusion to decrease blood loss during sacral tumor resection. J Bone Joint Surg Am. 2010;92(8):1747–53.
Matsuoka S, et al. Temporary percutaneous aortic balloon occlusion to enhance fluid resuscitation prior to definitive embolization of post-traumatic liver hemorrhage. Cardiovasc Intervent Radiol. 2001;24(4):274–6.
Sano H, et al. Resuscitative endovascular balloon occlusion of the aorta for uncontrollable nonvariceal upper gastrointestinal bleeding. World J Emerg Surg. 2016;11:20.
Greenberg RK, et al. An endoluminal method of hemorrhage control and repair of ruptured AAA. J Endovasc Ther. 2000;7(1):1–7.
Malina M, Veith F. Balloon occlusion of the aorta during endovascular repair of ruptured abdominal aortic aneurysm. J Endovasc Ther. 2005;12(5):556–9.
Guliani S, Amendola M, et al. Central aortic wire confirmation for emergent endovascular procedures: as fast as surgeon-performed ultrasound. J Trauma Acute Care Surg. 2015;79(4):549–54.
Pezy P, Flaris AN, et al. Fixed-distance model for balloon placement during fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta in a civilian population. JAMA Surg. 2017;152(4):351–8.
Taylor JR III, Harvin JA, et al. Vascular complications from resuscitative endovascular balloon occlusion of the aorta (REBOA): life over limb? J Trauma Acute Care Surg. 2017;83(1 Suppl 1):S120–3. doi:10.1097/TA.000000000000151.
Teeter WA, Matsumoto J, et al. Smaller introducer sheaths for REBOA may be associated with fewer complications. J Trauma Acute Care Surg. 2016;81(6):1039–45.
Teeter W, Romagnoli A, et al. Resuscitative endovascular balloon occlusion of the aorta: pushing care forward. J Spec Oper Med. Spring. 2017;17(1):17–21.
• Manley JD, Mitchell BJ, et al. A modern case series of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in an out-of-hospital, combat casualty care setting. J Spec Oper Med. Spring 2017;17(1):1–8. This military based case series describes the first use of REBOA in the prehospital and austere setting. Four cases are described, all with normalization of blood pressure, 100% survival, and no REBOA related complications.
http://prytimemedical.com/wp-content/uploads/2017/04/Labeling-Changes-Press-Release.pdf. Last accessed 12 June 2017.
Uchino H, Tamura N, et al. “REBOA”—is it really safe? A case with massive intracranial hemorrhage possibly due to Endovascular Balloon Occlusion of the Aorta (REBOA). Am J Case Rep. 2016;17:810–3.
Davidson AJ, Russo RM, et al. Incremental balloon deflation following complete REBOA results in steep inflection of flow and rapid reperfusion in a large animal model of hemorrhagic shock. J Trauma Acute Care Surg. 2017;83(1):139–43. doi:10.1097/TA.0000000000001502.
• Russo RM, Williams TK, et al. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2016;80(3):372–8; discussion 378–80. This article is a displays a translational swine model of partial balloon occlusion for hemorrhage. While systemic blood pressures were preserved with both partial and complete balloon occlusion, carotid flow was preserved at physiologic levels in the partial occlusion group, versus extreme overpressure in the complete group. Next generation balloon catheters are being designed informed by this data.
Johnson MA, Neff LP, et al. Partial resuscitative balloon occlusion of the aorta (P-REBOA): clinical technique and rationale. J Trauma Acute Care Surg. 2016;81(5 Suppl 2 Proceedings of the 2015 Military Health System Research Symposium):S133–7.
Davidson AJ, Russo RM, et al. Potential benefit of early operative utilization of low profile, partial resuscitative endovascular balloon occlusion of the aorta (P-REBOA) in major traumatic hemorrhage. Trauma Surg Acute Care Open 2016;1(1):e000028.
Williams TK, Neff LP, et al. Extending resuscitative endovascular balloon occlusion of the aorta: endovascular variable aortic control in a lethal model of hemorrhagic shock. J Trauma Acute Care Surg. 2016;81(2):294–301.
http://cdmrp.army.mil/funding/pa/16dmrdpcccrppfcra_pa.pdf. Last accessed 28 June 2017.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.B. is a Clinical Advisory Board member for Prytime Medical Inc. J.G. declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Trauma Surgery.
Rights and permissions
About this article
Cite this article
Glaser, J., Brenner, M. The Current Status of REBOA in Traumatic Shock. Curr Surg Rep 5, 23 (2017). https://doi.org/10.1007/s40137-017-0186-1
Published:
DOI: https://doi.org/10.1007/s40137-017-0186-1