Abstract
Purpose of Review
This narrative review introduces key elements of cough neural control, function, dysfunction, and measurement for physicians and speech-language pathologists. Its goal is to guide integrated approaches to the assessment of cough and facilitate differential diagnosis of cough dysfunction among people with dysphagia.
Recent Findings
Research has shown that cough and swallow dysfunction have high co-occurrence, especially in neurodegenerative populations. Both sensory and motor components of cough dysfunction can be evaluated using high and low-tech equipment (e.g., handheld peak flow meters). The evaluation of cough function is vital given the known benefit of targeted dystussia treatments for people with dysphagia.
Summary
Intact airway protection requires both functional swallowing and cough. However, clinicians report that objective cough evaluations are not commonly included in assessments of airway protection. This review provides an overview of cough neurobiology, describes its measurement, and presents case vignettes to illustrate the benefits of integrating cough assessments into clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A cough is an airway protective mechanism that serves to expel material from the upper and lower airways to ensure pulmonary clearance and health [1]. Hypotussic, or downregulated cough, refers to the inability to adequately sense or effectively remove aspirate material that enters the airway, resulting in a high risk of aspiration pneumonia [2,3,4,5,6]. Aspiration pneumonia is a serious consequence which can lead to increased healthcare costs, decreased quality of life, increased mortality rates, and other adverse health outcomes [2,3,4, 7,8,9,10,11,12,13]. Aspiration pneumonia risk is also particularly high in the presence of disordered swallowing, which exists at the opposite end of the continuum of airway protection from cough [1, 9, 11, 14,15,16,17,18,19]. Many individuals with swallowing disorders, or dysphagia, are at risk for aspiration and demonstrate decreased sensitivity to detect foreign material that errantly enters the airway, also known as “silent” aspiration [18, 20,21,22]. Additionally, many individuals with dysphagia exhibit a reduced ability to cough effectively to expel foreign material from the airway, even when sensation is present [23,24,25]. When the sensory or motor components of cough are impaired in the presence of impaired swallowing function, aspirate is repeatedly introduced into the respiratory system and is unable to be removed, contributing to uncontrolled colonization of bacteria [26,27,28]. Therefore, it is of critical importance that clinicians who work with individuals at risk for dysphagia understand when it is appropriate to refer, evaluate, and treat these individuals for impairment of sensorimotor cough function.
At present, many clinicians who treat individuals with dysphagia report limited education related to cough [29•]. In fact, over 97% of speech-language pathologists (SLPs) surveyed in a recent study reported an interest in learning more about cough assessment [29•]. Therefore, the aim of this review is to provide a basic introduction to cough function, describe its neurobiology in both healthy and disordered cough, and focus on the implementation of cough assessments in clinical practice. We will then conclude this review with case vignettes to exemplify how cough assessments may facilitate differential diagnoses in airway protection deficits and impact dysphagia management.
The cough literature frequently categorizes cough behaviors as either voluntary or reflex. These labels are important to understand theoretically and are relevant for clinical practice and cough research. Voluntary cough is initiated on command (e.g., after a verbal cue to cough), whereas reflex cough is triggered in response to stimulation of the airway [22, 30•, 31, 32]. There are a variety of external or endogenous stimuli which can trigger a reflex cough, one of which is the introduction of aspirate into the airway. Reflex cough can also be stimulated by aerosolized materials inhaled into the airway, which is how reflex cough is typically studied in research settings. Despite these labels, reflex and voluntary cough behaviors do not always exist as discrete entities. It is known that there is some degree to which individuals can sense and potentially even modulate cough behaviors [33]. This also relates to an important feature of reflex cough, known as the “urge to cough” or UTC. The UTC is the intensity of a person’s perception of a cough-inducing laryngeal or tracheal stimulus and can contribute to the ability to either up or down-regulate the cough response [33,34,35,36,37]. For example, if a subject perceives a faint airway irritant, they might have the choice to either produce a strong cough, stimulate a throat clear, or suppress a response altogether. However, if a subject feels a strong laryngeal irritant, this may result in a reflex cough response that is more difficult to modulate or suppress.
Neurological Control of Cough
Cough is a behavior which typically involves the coordination of afferent laryngeal sensory mechanisms and efferent cortical, subcortical, and brainstem activation systems. Two types of neurons facilitate the transfer of somatosensory cough information: mechanoreceptors and chemoreceptors [38]. Mechanoreceptors, which are primarily located in the distal airways (i.e., lungs and bronchi), respond to mechanical pressure, specifically touch-like stimuli. They are often described as “cough receptors,” “irritant receptors,” or “rapidly adapting mechanoreceptors.” Chemoreceptors, on the other hand, are primarily located in the proximal airways (i.e., trachea and main bronchi). These neurons include slow-conducting unmyelinated C-fibers that selectively respond to chemical stimuli, such as capsaicin (a tussigenic, or cough-provoking, stimulus) [39, 40]. Chemoreceptors respond to different types of stimuli depending on the afferent pathway and are responsible for inducing cough via the jugular afferent pathway [41].
Brainstem Processing of Reflex Cough
Airway protective reflexes are first sensed through the stimulation of laryngeal and tracheobronchial irritant receptors as well as vagal inputs through pulmonary stretch receptors [42, 43]. Polysynaptic pathways responsible for the integration of afferent input and sequencing of the respiratory pump motor patterns exist in the caudal medullary raphe nuclei [42]. This pathway connects to dorsal (DRV) and ventral respiratory groups (VRG), which in turn have mutual interactions with several brainstem regions [42, 44]. Specific regions of the medulla and pons have been identified as key areas of the brainstem responsible for controlling the magnitude of respiratory muscle activation to produce a cough [42, 45, 46].
Central and Subcortical Control of Reflex Cough and Voluntary Cough
Studies have demonstrated that a UTC always precedes the activation of a reflex cough, and a UTC can be detected at lower thresholds than stimuli that would prompt reflex cough responses [34]. The UTC sensation is a process dependent on both discriminative (i.e., discrete components such as location) and affective (i.e., emotional) processing. This multifactorial process includes the integration of respiratory afferent information, respiratory motor drive, affective state, attention, experience, and learning [34, 35, 37, 47].
In conscious humans, respiratory afferent information associated with a UTC typically ascends from the brainstem to the cortex for higher-order processing prior to efferent motor output of reflex cough [35]. Brain regions including the orbitofrontal cortex, supplementary motor area, insula cortex, anterior midcingulate cortex, and cerebellum have been associated with the activation of an UTC [48]. Two unique pathways have been proposed in relation to the cortical and subcortical integration of UTC afferent pathways; however, it is generally understood that there is overlap and integration [41, 45, 49, 50]. Jugular or superior afferent pathways receive inputs from the proximal airways and then project to the paratrigeminal nucleus of the brainstem [51]. These then continue to the primary sensory cortex and are important in the perception of UTC [50]. Nodose or inferior afferent pathways first project to the nucleus tractus solitarius of the brainstem [34, 49]. From there, nuclei project to the orbitofrontal cortex, cingulate cortex, and other limbic regions [41, 48]. This pathway is important for many aspects of the sensorimotor cough response following airway irritation [50]. Subcortically, structures such as the cerebellum and basal ganglia are also involved in controlling the coordination, timing, amplitude, and force of movement, which plays an important role in the production of cough [41, 52,53,54].
Conceptually, voluntary and reflex cough represent similar rapid expulsive pulmonary clearing behaviors. However, research now supports distinct cortical pathways for these two cough archetypes [45, 55]. In contrast with reflex cough, which can occur solely through brainstem control, neuronal activation of voluntary cough activates desire motivational pathways [35]. These are primarily accessed through cortical and subcortical pathways, and include areas of the motor cortex, premotor cortex, supplementary motor area, insula, anterior and mid-cingulate cortex, ventral and dorsomedial thalamus, inferolateral sensorimotor cortex, and the striatum [44, 52].
Peripheral Motor Control of Cough
The production of an effective cough requires precise coordination across three distinct motoric phases: inspiration, compression, and expulsion [56]. In the inspiration phase, there is contraction of the external intercostal muscles, which brings the rib cage upward and outward, resulting in expansion of the thoracic cavity. This action results in a reduction in lung pressure, allowing up to 2.5 L of air to fill the lungs [57]. In the compression phase, the vocal folds are firmly pressed in the closed position through adduction of the laryngeal cricoarytenoid and transverse arytenoid muscles [57]. The internal intercostals also contract, and this motion is followed by the contraction of the abdominal muscles, which push against the diaphragm. The compression phase results in the generation of subglottic pressure of 100 mmHg or more, which is necessary to produce an effective cough response [57, 58]. In the third phase (expulsion), there is a sudden glottal opening followed by continued dynamic compression of the intra-thoracic region. Air velocity during this third expulsive phase can reach speeds of 75 to 100 miles per hour [57].
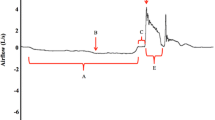
There are important physiologic differences between reflex and voluntary cough. For example, healthy adults typically initiate voluntary cough responses at higher lung volumes than reflex cough. Voluntary coughs are also associated with higher expulsive phase airflow rates (i.e., peak expiratory flow rate or PEFR; “e” in Fig. 1) [59]. Additionally, expiratory muscles tend to be activated sooner than accessory muscles during voluntary cough, whereas reflex cough demonstrates simultaneous activation of expiratory and accessory muscles and greater overall muscle activation for shorter periods of time [59].
A cough waveform is frequently used to represent and measure cough airflow parameters related to the cough phases of inspiration (a and b), compression (c), and expulsion (d–f) via spirometry. In this graph, the y-axis represents the airflow velocity measurement in liters per second, while the x-axis represents time in seconds. As one breathes in for the inspiratory phase, the graph slopes in the negative direction, indicating airflow into the lungs. During the compression phase, the waveform slope should become relatively flat, representing no change in airflow rate. Then, as the expulsive phase begins, the waveform slopes in the positive direction rapidly arriving at a peak. The peak then quickly returns to baseline as a breath is returned to the lungs after the cough is completed
In addition to the differences between reflex and voluntary cough, there are also functional and physiological differences between single and sequential coughs. This is notable because a reflex cough commonly forms a pattern including a sequence of expulsive cough events or a cough epoch [56]. While single coughs have been found to be effective at expelling debris from the upper airway, sequential coughs are more effective than single coughs at removing material from the lower airways [58, 60, 61]. This phenomenon is due to the dynamic compression that is generated during sequential cough production, which results in a reduction in the area of the tracheobronchial lumen and a subsequent increase in the velocity of airflow at the site of compression [60, 61].
Cough Measurement and Assessment
An effective cough response requires intact sensory and motor function as well as precise coordination of neural and peripheral mechanisms in order to effectively eject foreign material from the airway. Therefore, clinicians should consider both motor and sensory aspects when comprehensively evaluating cough. Several objective and patient-reported cough data can be obtained to measure cough sensorimotor function.
A subjective perceptual cough assessment is a widely used strategy that can be conducted as part of a clinical swallow evaluation [29•]. With this type of assessment, a patient would be asked to cough following a cue (e.g., “cough as if something went down the wrong pipe”) [30•]. Cough effectiveness could then be subjectively described using words such as “weak” or “abnormal” [29•, 62]. However, recent research has demonstrated that perceptual ratings of cough effectiveness have only moderate interrater reliability [62, 63]. It is unclear whether this is because perceptual cough information is too inconsistent to be used as a proxy for objective measures, or because clinicians lack a universal rating system and training curriculum. In either case, the pitfalls of unstructured perceptual cough ratings highlight the need for more objective approaches to cough measurement.
Cough Airflow Measurements
Spirometry is the gold standard for lung function measurement. It can be used to provide quantitative data on the strength and coordination of various cough components (Fig. 1). A spirometry setup includes a pneumotachograph, which can be outfitted onto a facemask that covers the subject’s nose and mouth. This then is connected via tubing to hardware for data acquisition and software to visualize the cough airflow. Unfortunately, cost and lack of portability remain barriers to the collection and analysis of spirometry data in most clinical practices which assess and treat dysphagia.
Fortunately, research has supported the use of handheld peak flow meters in place of the gold standard pneumotachograph, with no statistical differences found in mean peak cough airflow [22, 64]. Cough peak flow rates can be obtained with small analog or digital peak flow meters without significantly affecting reliability [22, 64]. These devices are cost-effective, portable, and easy-to-use. They provide a single numeric data point: PEFR (‘e’ in Fig. 1). This objective measure of cough strength has been found to be correlated with the ability to clear aspirated material from the airway [30•]. Obtaining PEFR has the potential to be a robust clinical tool, as objective data can be compared to normative ranges in healthy adults and tracked over time to examine disease progression or improvement with treatment [65, 66].
Reflex Cough Testing
The assessment of reflex cough typically involves administering cough-inducing, or tussive, agents to assess reflex cough sensory and motor integrity. These tussive agents are airway irritants, and they can be aerosolized and delivered to the airway in measured concentrations. In the case of hypotussic cough evaluation, these tussive agents are typically meant to imitate aspiration [67]. To obtain these measurements, a nebulizer, dosimeter, and compressor can be utilized in-line with a spirometry system. The nebulizer and compressor work to create and administer specific concentrations of aerosol particles, while a dosimeter can be used to time its delivery with inspiration [68]. Tussive agents that are most frequently used include capsaicin or citric acid [69]. When assessing reflex cough, key outcomes often include the presence or absence of a cough to a particular concentration of tussive agent (i.e., indicative of an intact sensory response), as well as cough airflow outcomes of interest (e.g., the strength of the motor response). Interestingly, even something as simple as the presence or absence of a cough reflex has been shown to be difficult for experienced clinicians to judge based on audiovisual information alone [62]. Therefore, there is a need for clear guidelines and training if this type of testing is to be adopted in a clinical setting.
Cough reflex testing (CRT) is a specific approach to the assessment of reflex cough which has garnered significant research and clinical interest. This type of measurement can be used to determine the lowest amount of tussive stimulus that elicits a consistent cough reflex [35, 69, 70]. CRT measurements can either be obtained to find the natural cough threshold at the lowest stimulus dose, or to find the threshold at which cough can no longer be suppressed [37, 71]. This is clinically relevant because research has found that lowered cough reflex thresholds are associated with a higher aspiration risk for individuals with dysphagia [2]. Additionally, CRT has been found to improve the sensitivity of instrumental assessments of swallowing to identify individuals at risk for silent aspiration [72, 73]. For this reason, it has become increasingly incorporated in clinical dysphagia care in countries such as New Zealand, Australia, Japan, and the UK [74, 75]. For a full description of a CRT protocol, see Wallace et al. [76•]. However, there is still work that needs to be done in comparing cough reflex thresholds across patient populations, and to understand how this can be used to guide clinical dysphagia practices [69, 74, 77•, 78, 79].
While understanding cough reflex thresholds across a variety of patient populations is important to further our understanding of cough sensitivity and aspiration risk, in the United States this type of testing is largely limited to research settings. This is in part due to the high cost associated with this equipment, as well as the lack of familiarity with cough measurement in the United States in general [29•]. However, a cheaper and more portable form of delivery can potentially be achieved with a portable or handheld nebulizer device. Some research on handheld nebulizers has revealed reliable cough thresholds in healthy controls as well as those at risk for aspiration [67, 80,81,82]. Additionally, using aerosolized solutions of distilled water (e.g., fog) in cough reflex testing can offer an appealing option for facilities with restrictions on other forms of tussigenic stimulation, though some studies have shown measurement variability with fog, potentially due to differences in nebulizer output [82,83,84]. Research is ongoing to develop the ideal methodology for a sensitive, specific, and feasible reflex cough assessment protocol that will be widely accessible in the United States.
Instrumental Assessments of Swallowing
Cough effectiveness can also be assessed during instrumental assessments of swallowing. When airway invasion occurs, patients can be asked to assess their UTC using a modified Borg CR10 scale [85]. This will provide information about whether the patient is sensing the aspirate or penetrant but are not responding effectively or whether they do not perceive the airway invasion at all. The UTC can also be used to assess the perception of cough inducing stimuli during reflex cough testing. Additionally, the effectiveness of a reflex cough or voluntary cough following airway invasion can be assessed by utilizing the penetration-aspiration scale (PAS) or visual analysis of swallowing efficiency and safety (VASES) outcomes to report changes in laryngeal residue from before to after the cough [86, 87].
Clinical Case Vignettes
Here, we briefly summarize the literature on hypotussic cough in three key clinical populations: amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and head and neck cancer (HNC). Clinical case vignettes will highlight the assessment of different cough profiles within a variety of clinical settings and the integration of these findings into a comprehensive airway protection (i.e., dysphagia and dystussia) management plan.
Clinical Case Vignette 1: Amyotrophic Lateral Sclerosis (ALS)
ALS is a neurodegenerative disease of the upper motor neurons of the cortex, and the lower motor neurons of the brainstem and spinal cord which results in global weakness [88, 89]. At disease onset, this corresponds with more focal weaknesses in the limbs or as bulbar symptoms, but the progressive course continues to global weakness. Mortality in ALS is primarily due to pneumonia-related complications resulting in respiratory failure [90]. Relatedly, it is known that individuals with bulbar ALS are at greater risk for airway protection impairments including dysphagia and dystussia [64, 91].
A 55-year-old female with a 1-year history of bulbar-onset ALS presents to an interdisciplinary clinic appointment. She consumes an unrestricted regular diet with thin liquids at home. The patient has recently lost around 30 lb and reports fatigue during meals as well as difficulty swallowing liquids. As a part of the interdisciplinary evaluation, the patient completes a cough evaluation with a handheld peak flow meter. The patient’s maximum PEFR out of 5 trials is 2 L/s, which is associated with an increase in risk for airway invasion in the ALS population (PEFR < 3.97 L/s) [31]. Additionally, this value is well below thresholds which has been determined to promote cough clearance of aspirate material in a heterogenous group of individuals with neurodegenerative diseases. For example, clinically meaningful cutoffs for effective airway clearance in the literature are values greater than 3.23 L/s [30•].
Clinical Takeaways
Low-tech and cost-effective evaluation of cough with a handheld peak flow meter can serve to identify patients at risk for airway invasion, distinguish patients whose cough is too weak to clear aspirate material, and serve as a baseline measure to track changes in cough due to disease or treatment.
Clinical Case Vignette 2: Parkinson’s Disease (PD)
PD is a neurodegenerative disorder involving the loss of dopaminergic neurons in the substantia nigra pars compacta [54]. The substantia nigra pars compacta is one of several circuits of the basal ganglia, and the progressive reduction of dopaminergic neurons in this brain region contributes to bradykinesia (e.g., slowness of movement) and increased muscle tone (e.g., rigidity), which are common symptoms of PD that affect movement timing and amplitude [41, 54, 92, 93]. These impairments also contribute to the high prevalence of dysphagia and dystussia in PD, likely due to sensory and neuromotor changes [20, 32, 70, 94,95,96]. It is also well-documented that individuals with PD are known to underreport dysphagia due to these sensory impairments [97].
A 65-year-old male with a 5-year history of PD and dysphagia presents to an SLP clinic for a comprehensive evaluation of airway protective function. An instrumental assessment of swallowing identifies consistent aspiration of thin liquids without a cough response and with blunted UTC. When cued to perform a voluntary cough to clear the aspirate material, the patient’s cough is ineffective. The patient’s maximum PEFR out of 5 trials is 3.5 L/s, which is associated with increased risk of airway invasion in PD (PEFR values of < 7.49 L/s are associated with penetration, while values < 5.24 L/s are associated with aspiration) [30•, 32, 70, 94, 96]. However, the patient’s maximum expiratory pressure (MEP) is adequate at 120 cm H2O [98].
Clinical Takeaways
In this individual with PD, the comprehensive evaluation of airway protection revealed the presence of airway invasion in the context of disordered cough. Specifically, cough function was ineffective in clearing airway invasion, UTC was blunted, PEFR was low, but MEP was adequate. Given the results of the evaluation, the patient’s diagnosis, and likely pathophysiology of dysfunction, future treatment should target both swallowing and cough. Specifically, improved perception of cough stimuli and enhanced coordination of cough with cough skill training should be considered for this patient [99•, 100•, 101•].
Clinical Case Vignette 3: Head and Neck Cancer (HNC)
Physiological changes in HNC are mainly peripheral in nature [102]. The extent of these changes may depend on adjuvant therapies including radiation, chemotherapy, or surgery. Acute and long-term toxicities associated with airway protection deficits in HNC include pain, edema, salivary changes, fibrosis, and mucositis [17, 103,104,105,106,107,108,109,110]. Emerging research in cough testing has demonstrated that sensory changes in the upper airway can contribute to reduced sensation of penetrated and aspirated material [25].
A 70-year-old male patient with a diagnosis of HNC presents to a laryngology clinic. This patient has recently completed primary chemoradiation treatment for a posterior lingual mass. The patient reports no difficulty swallowing solids or liquids during meals but has had pneumonia recently. The ENT completes an examination which reveals increased post-radiation fibrosis of the oral and pharyngeal structures. The ENT refers this patient to SLP services. During this patient’s SLP visit, PEFR trials with a handheld peak flow meter reveal cough inefficiency with a maximum peak flow value of 4.0 L/s across 5 trials. MEP is also found to be inadequate at 45 cmH2O. Research has demonstrated that peak flow values less than 6.3 L/s and MEP values less than 100.8 cm H2O are associated with aspiration risk in the head and neck cancer population [25]. Further swallowing assessment is planned to follow this initial cough assessment, given these findings as well as the clinical history of repeated pneumonia.
Clinical Takeaways
The pathophysiology of HNC and its associated treatments including chemoradiation can contribute to reduced force and range of oropharyngeal structures, which can impact both swallow safety and cough effectiveness. Treatment approaches such as expiratory muscle strength training may be an appropriate option to consider for these patients [111].
Conclusions
This narrative review aims to provide clinicians with a framework for the neurobiology of cough as well as how the measurement of sensorimotor cough function can be assessed with a variety of equipment and measurement approaches. Clinically, increasing the utilization of structured assessment of cough sensation and motor function can help to identify and treat disordered airway protection across a variety of populations at risk for dysphagia. Additionally, it is beneficial for allied providers, including referring physicians, to understand ways in which SLPs can offer cough assessment and treatment with the goal of reducing the incidence of aspiration pneumonia and improving the health and quality of life in patients with dysphagia.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Troche MS, Brandimore AE, Godoy J, Hegland KW. A framework for understanding shared substrates of airway protection. J Appl Oral Sci. 2014;22:251–60. https://doi.org/10.1590/1678-775720140132.
Addington WR, Stephens RE, Gilliland K, Rodriguez M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80:150–4. https://doi.org/10.1016/S0003-9993(99)90112-0.
Nakamori M, Imamura E, Kuwabara M, Ayukawa T, Tachiyama K, Kamimura T, et al. Simplified cough test can predict the risk for pneumonia in patients with acute stroke. PLoS ONE. 2020;15:e0239590. https://doi.org/10.1371/journal.pone.0239590.
Niimi A, Matsumoto H, Ueda T, Takemura M, Suzuki K, Tanaka E, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58:152–3. https://doi.org/10.1136/thorax.58.2.152.
Sancho J, Servera E, Díaz J, Marín J. Predictors of ineffective cough during a chest infection in patients with stable amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2007;175:1266–71. https://doi.org/10.1164/rccm.200612-1841OC.
Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135:769–77. https://doi.org/10.1378/chest.08-1122.
Semenov YR, Starmer HM, Gourin CG. The effect of pneumonia on short-term outcomes and cost of care after head and neck cancer surgery. Laryngoscope. 2012;122:1994–2004. https://doi.org/10.1002/lary.23446.
Ottosson S, Lindblom U, Wahlberg P, Nilsson P, Kjellén E, Zackrisson B, et al. Weight loss and body mass index in relation to aspiration in patients treated for head and neck cancer: a long-term follow-up. Support Care Cancer. 2014;22:2361–9. https://doi.org/10.1007/s00520-014-2211-6.
Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. https://doi.org/10.1007/PL00009559.
Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159:2058–64. https://doi.org/10.1001/archinte.159.17.2058.
Allen J, Greene M, Sabido I, Stretton M, Miles A. Economic costs of dysphagia among hospitalized patients. Laryngoscope. 2020;130:974–9. https://doi.org/10.1002/lary.28194.
D’Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, Salemi G, et al. Long–term survival of Parkinson’s disease. J Neurol. 2006;253:33–7. https://doi.org/10.1007/s00415-005-0916-7.
Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8:CR241-6.
Akbar U, Dham B, He Y, Hack N, Wu S, Troche M, et al. Incidence and mortality trends of aspiration pneumonia in Parkinson’s disease in the United States, 1979–2010. Parkinsonism Relat Disord. 2015;21:1082–6. https://doi.org/10.1016/j.parkreldis.2015.06.020.
Manabe T, Teramoto S, Tamiya N, Okochi J, Hizawa N. Risk factors for aspiration pneumonia in older adults. PLoS ONE. 2015;10:e0140060. https://doi.org/10.1371/journal.pone.0140060.
Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–36. https://doi.org/10.1378/chest.124.1.328.
Nguyen NP, Frank C, Moltz CC, Vos P, Smith HJ, Bhamidipati PV, et al. Aspiration rate following chemoradiation for head and neck cancer: an underreported occurrence. Radiother Oncol. 2006;80:302–6. https://doi.org/10.1016/j.radonc.2006.07.031.
Pikus L, Levine MS, Yang Y-X, Rubesin SE, Katzka DA, Laufer I, et al. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. Am J Roentgenol. 2003;180:1613–6. https://doi.org/10.2214/ajr.180.6.1801613.
van der Maarel-Wierink CD, Vanobbergen JNO, Bronkhorst EM, Schols JMGA, de Baat C. Meta-analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res. 2011;90:1398–404. https://doi.org/10.1177/0022034511422909.
Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in Parkinson disease. Chest. 2014;146:1294–9. https://doi.org/10.1378/chest.14-0066.
Hammond CAS, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–6. https://doi.org/10.1212/WNL.56.4.502.
Silverman EP, Carnaby-Mann G, Pitts T, Davenport P, Okun MS, Sapienza C. Concordance and discriminatory power of cough measurement devices for individuals with Parkinson disease. Chest. 2014;145:1089–96. https://doi.org/10.1378/chest.13-0596.
Sohn D, Park G-Y, Koo H, Jang Y, Han Y, Im S. Determining peak cough flow cutoff values to predict aspiration pneumonia among patients with dysphagia using the citric acid reflexive cough test. Arch Phys Med Rehabil. 2018;99:2532-2539.e1. https://doi.org/10.1016/j.apmr.2018.06.015.
Bianchi C, Baiardi P, Khirani S, Cantarella G. Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am J Phys Med Rehabil. 2012;91:783–8. https://doi.org/10.1097/PHM.0b013e3182556701.
Hutcheson KA, Barrow MP, Warneke CL, Wang Y, Eapen G, Lai SY, et al. Cough strength and expiratory force in aspirating and nonaspirating postradiation head and neck cancer survivors. Laryngoscope. 2018;128:1615–21. https://doi.org/10.1002/lary.26986.
Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, et al. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age Ageing. 2012;41:376–81. https://doi.org/10.1093/ageing/afs006.
Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116:858–65. https://doi.org/10.1177/000348940711601112.
Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life – a population-based study. Aliment Pharmacol Ther. 2008;27:971–9. https://doi.org/10.1111/j.1365-2036.2008.03664.x.
• Mir MJ, Wheeler HK. A survey of speech-language pathologists’ experience with clinical cough assessment. Perspect ASHA Spec Interest Groups. 2021;6:1627–40. https://doi.org/10.1044/2021_PERSP-21-00144. A survey of SLPs and SLP students on how cough assessments are implemented in clinical settings, and to assess interest in additional cough assessment training.
• Borders JC, Troche MS. Voluntary cough effectiveness and airway clearance in neurodegenerative disease. J Speech Lang Hear Res. 2022;65:431–49. https://doi.org/10.1044/2021_JSLHR-21-00308. A retrospective analysis of measures of cough airflow and degree of airway clearance in individuals with neurodegenerative conditions.
Plowman EK, Watts SA, Robison R, Tabor L, Dion C, Gaziano J, et al. Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia. 2016;31:383–90. https://doi.org/10.1007/s00455-015-9687-1.
Pitts T, Troche M, Mann G, Rosenbek J, Okun MS, Sapienza C. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138:1426–31. https://doi.org/10.1378/chest.10-0342.
Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J Appl Physiol. 2012;113:39–46. https://doi.org/10.1152/japplphysiol.01299.2011.
Davenport PW. Clinical cough i: the urge-to-cough: a respiratory sensation. In: Chung KF, Widdicombe J, editors. Pharmacol. Ther. Cough Berlin, Heidelberg: Springer; 2009. p. 263–76.
Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186:107–11. https://doi.org/10.1007/s00408-007-9045-7.
Hutchings HA, Morris S, Eccles R, Jawad MSM. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993;87:379–82. https://doi.org/10.1016/0954-6111(93)90052-2.
Perry SE, Troche MS. Dual tasking influences cough sensorimotor outcomes in healthy young adults. J Speech Lang Hear Res JSLHR. 2019;62:3596–606. https://doi.org/10.1044/2019_JSLHR-H-19-0122.
Canning BJ. Afferent nerves regulating the cough reflex: mechanisms and mediators of cough in disease. Otolaryngol Clin North Am. 2010;43(15–25):vii. https://doi.org/10.1016/j.otc.2009.11.012.
Holzer P. Acid-sensitive ion channels and receptors. In: Canning BJ, Spina D, editors. Sens. Nerves Berlin, Heidelberg: Springer; 2009. p. 283–332.
Ho C-Y, Gu Q, Lin YS, Lee L-Y. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–24. https://doi.org/10.1016/S0034-5687(01)00241-9.
Mazzone SB, Farrell MJ. Heterogeneity of cough neurobiology: clinical implications. Pulm Pharmacol Ther. 2019;55:62–6. https://doi.org/10.1016/j.pupt.2019.02.002.
Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulm Pharmacol Ther. 2004;17:369–76. https://doi.org/10.1016/j.pupt.2004.09.022.
Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–27. https://doi.org/10.1152/japplphysiol.00252.2006.
Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152:266–81. https://doi.org/10.1016/j.resp.2006.02.016.
Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–58. https://doi.org/10.1113/jphysiol.2003.057885.
Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–35. https://doi.org/10.1152/jappl.1998.84.6.2020.
Janssens T, Silva M, Davenport PW, Van Diest I, Dupont LJ, Van den Bergh O. Attentional modulation of reflex cough. Chest. 2014;146:135–41. https://doi.org/10.1378/chest.13-2536.
Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176:327–32. https://doi.org/10.1164/rccm.200612-1856OC.
Mazzone SB, McGovern AE, Yang S-K, Woo A, Phipps S, Ando A, et al. Sensorimotor circuitry involved in the higher brain control of coughing. Cough. 2013;9:7. https://doi.org/10.1186/1745-9974-9-7.
Driessen AK, Farrell MJ, Mazzone SB, McGovern AE. Multiple neural circuits mediating airway sensations: recent advances in the neurobiology of the urge-to-cough. Respir Physiol Neurobiol. 2016;226:115–20. https://doi.org/10.1016/j.resp.2015.09.017.
Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev. 2016;96:975–1024. https://doi.org/10.1152/physrev.00039.2015.
Simonyan K, Saad ZS, Loucks TMJ, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage. 2007;37:401–9. https://doi.org/10.1016/j.neuroimage.2007.05.021.
Xu F, Frazier DT, Zhang Z, Baekey DM, Shannon R. Cerebellar modulation of cough motor pattern in cats. J Appl Physiol Bethesda Md. 1985;1997(83):391–7. https://doi.org/10.1152/jappl.1997.83.2.391.
Hallett M. Physiology of basal ganglia disorders: an overview. Can J Neurol Sci J Can Sci Neurol. 1993;20:177–83. https://doi.org/10.1017/S0317167100047909.
Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31:2948–58. https://doi.org/10.1523/JNEUROSCI.4597-10.2011.
Fontana GA. Before we get started: what is a cough? Lung. 2008;186:S3-6. https://doi.org/10.1007/s00408-007-9036-8.
Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. 12th ed. Philadelphia, Pa: Saunders/Elsevier; 2011.
Smith JA, Aliverti A, Quaranta M, McGuinness K, Kelsall A, Earis J, et al. Chest wall dynamics during voluntary and induced cough in healthy volunteers. J Physiol. 2012;590:563–74. https://doi.org/10.1113/jphysiol.2011.213157.
Brandimore AE, Troche MS, Huber JE, Hegland KW. Respiratory kinematic and airflow differences between reflex and voluntary cough in healthy young adults. Front Physiol. 2015. https://doi.org/10.3389/fphys.2015.00284.
Hegland K, Troche M, Davenport P. Cough expired volume and airflow rates during sequential induced cough. Front Physiol. 2013;4:167. https://doi.org/10.3389/fphys.2013.00167.
Ross BB, Gramiak R, Rahn H. Physical dynamics of the cough mechanism. J Appl Physiol. 1955;8:264–8. https://doi.org/10.1152/jappl.1955.8.3.264.
Miles A, McFarlane M, Huckabee M-L. Inter-rater reliability for judgment of cough following citric acid inhalation after training. Speech Lang Hear. 2014;17:204–9. https://doi.org/10.1179/2050572814Y.0000000040.
Laciuga H, Brandimore AE, Troche MS, Hegland KW. Analysis of clinicians’ perceptual cough evaluation. Dysphagia. 2016;31:521–30. https://doi.org/10.1007/s00455-016-9708-8.
Sancho J, Servera E, Díaz J, Marín J. Comparison of peak cough flows measured by pneumotachograph and a portable peak flow meter. Am J Phys Med Rehabil. 2004;83:608–12. https://doi.org/10.1097/01.PHM.0000133431.70907.A2.
Suárez AA, Pessolano FA, Monteiro SG, Ferreyra G, Capria ME, Mesa L, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil. 2002;81:506–11. https://doi.org/10.1097/00002060-200207000-00007.
Cook NR, Evans DA, Scherr PA, Speizer FE, Vedal S, Branch LG, et al. Peak expiratory flow rate in an elderly population. Am J Epidemiol. 1989;130:66–78. https://doi.org/10.1093/oxfordjournals.aje.a115324.
Barber CM, Curran AD, Bradshaw LM, Morice AH, Rawbone R, Fishwick D. Reproducibility and validity of a yan-style portable citric acid cough challenge. Pulm Pharmacol Ther. 2005;18:177–80. https://doi.org/10.1016/j.pupt.2004.11.009.
Ruppel GL. Aerosol use in the pulmonary function lab. Respir Care. 2015;60:931–40. https://doi.org/10.4187/respcare.03493.
Wallace E, Guiu Hernandez E, Ang A, Hiew S, Macrae P. A systematic review of methods of citric acid cough reflex testing. Pulm Pharmacol Ther. 2019;58:101827. https://doi.org/10.1016/j.pupt.2019.101827.
Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, et al. Impaired efficacy of cough in patients with Parkinson disease. Chest. 2003;124:1009–15. https://doi.org/10.1378/chest.124.3.1009.
Wallace E, Guiu Hernandez E, Ang A, Macrae P. Quantifying test-retest variability of natural and suppressed citric acid cough thresholds and urge to cough ratings. Pulm Pharmacol Ther. 2019;58:101838. https://doi.org/10.1016/j.pupt.2019.101838.
Miles A, Moore S, McFarlane M, Lee F, Allen J, Huckabee M-L. Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav. 2013;118:25–31. https://doi.org/10.1016/j.physbeh.2013.05.004.
Kallesen M, Psirides A, Huckabee M-L. Comparison of cough reflex testing with videoendoscopy in recently extubated intensive care unit patients. J Crit Care. 2016;33:90–4. https://doi.org/10.1016/j.jcrc.2016.02.004.
Miles A, Zeng ISL, McLauchlan H, Huckabee M-L. Cough reflex testing in dysphagia following stroke: a randomized controlled trial. J Clin Med Res. 2013;5:222. https://doi.org/10.4021/jocmr1340w.
Miles A. Silent aspiration and cough reflex testing [Internet]. Dysphagia Cafe. 2015.
• Wallace ES, Huckabee M, Macrae P. Cough reflex testing in clinical dysphagia practice. Adv Commun. Swallowing IOS Press; 2022:Preprint:1–9. https://doi.org/10.3233/ACS-220008. A review of cough reflex testing and step-by-step instructions on how it can be administered in a clinical setting.
• Perry SE, Miles A, Fink JN, Huckabee M-L. The dysphagia in stroke protocol reduces aspiration pneumonia in patients with dysphagia following acute stroke: a clinical audit. Transl Stroke Res. 2019;10:36–43. https://doi.org/10.1007/s12975-018-0625-z. A comparison of clinical outcomes post-implementation of a structured cough reflex testing protocol in 4 hospital settings in New Zealand.
Field M, Wenke R, Sabet A, Lawrie M, Cardell E. Implementing cough reflex testing in a clinical pathway for acute stroke: a pragmatic randomised controlled trial. Dysphagia. 2018;33:827–39. https://doi.org/10.1007/s00455-018-9908-5.
Holmes S. A service evaluation of cough reflex testing to guide dysphagia management in the postsurgical adult head and neck patient population. Curr Opin Otolaryngol Head Neck Surg. 2016;24:191–6. https://doi.org/10.1097/MOO.0000000000000256.
Curtis JA, Troche MS. Handheld cough testing: a novel tool for cough assessment and dysphagia screening. Dysphagia. 2020;35:993–1000. https://doi.org/10.1007/s00455-020-10097-z.
Wakasugi Y, Tohara H, Nakane A, Murata S, Mikushi S, Susa C, et al. Usefulness of a handheld nebulizer in cough test to screen for silent aspiration. Odontology. 2014;102:76–80. https://doi.org/10.1007/s10266-012-0085-y.
Hegland KW, Troche MS, Brandimore A, Okun MS, Davenport PW. Comparison of two methods for inducing reflex cough in patients with Parkinson’s disease, with and without dysphagia. Dysphagia. 2016;31:66–73. https://doi.org/10.1007/s00455-015-9659-5.
Morice AH, Fontana GA, Belvisi MG, Birring SS, Chung KF, Dicpinigaitis PV, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–76. https://doi.org/10.1183/09031936.00101006.
Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson’s disease. Am J Respir Crit Care Med. 1998;158:458–64. https://doi.org/10.1164/ajrccm.158.2.9705094.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. https://doi.org/10.1249/00005768-198205000-00012.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. https://doi.org/10.1007/bf00417897.
Curtis JA, Borders JC, Perry SE, Dakin AE, Seikaly ZN, Troche MS. Visual analysis of swallowing efficiency and safety (VASES): a standardized approach to rating ppharyngeal residue, penetration, and aspiration during FEES. Dysphagia. 2021. https://doi.org/10.1007/s00455-021-10293-5.
van den Bos MAJ, Geevasinga N, Higashihara M, Menon P, Vucic S. Pathophysiology and diagnosis of ALS: insights from advances in neurophysiological techniques. Int J Mol Sci. 2019;20:2818. https://doi.org/10.3390/ijms20112818.
Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci. 2010;31:2247–65. https://doi.org/10.1111/j.1460-9568.2010.07260.x.
Corcia P, Pradat P, Salachas F, Bruneteau G, le Forestier N, Seilhean D, et al. Causes of death in a post-mortem series of ALS patients. Amyotroph Lateral Scler. 2008;9:59–62. https://doi.org/10.1080/17482960701656940.
Ruoppolo G, Schettino I, Frasca V, Giacomelli E, Prosperini L, Cambieri C, et al. Dysphagia in amyotrophic lateral sclerosis: prevalence and clinical findings. Acta Neurol Scand. 2013;128:397–401. https://doi.org/10.1111/ane.12136.
Lo Bianco C, Ridet J-L, Schneider BL, Déglon N, Aebischer P. α-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci. 2002;99:10813–8. https://doi.org/10.1073/pnas.152339799.
Massano J, Bhatia KP. Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harb Perspect Med. 2012;2:a008870. https://doi.org/10.1101/cshperspect.a008870.
Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23:297–301. https://doi.org/10.1007/s00455-007-9144-x.
Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301–8. https://doi.org/10.1378/chest.08-1389.
Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192:601–8. https://doi.org/10.1007/s00408-014-9584-7.
Michou E, Baijens L, Rofes L, Sanz P, Clavé P. Oropharyngeal swallowing disorders in Parkinson’s disease: revisited. Int J Speech Lang Pathol Audiol. 2013;1:76–88. https://doi.org/10.12970/2311-1917.2013.01.02.5.
Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax. 1984;39:535–8. https://doi.org/10.1136/thx.39.7.535.
• Troche MS, Curtis JA, Sevitz JS, Dakin AE, Perry SE, Borders JC, et al. Rehabilitating cough dysfunction in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2022. https://doi.org/10.1002/mds.29268. A randomized controlled trial comparing strength and skill-based approaches to improving airway protection in PD.
• Sevitz JS, Borders JC, Dakin AE, Kiefer BR, Alcalay RN, Kuo S-H, et al. Rehabilitation of airway protection in individuals with movement disorders: a telehealth feasibility study. Am J Speech Lang Pathol. 2022;31:2741–58. https://doi.org/10.1044/2022_AJSLP-22-00063. A study evaluating the implementation of EMST and cough skill training via telehealth with individuals with neurodegenerative movement disorders.
• Curtis JA, Dakin AE, Troche MS. Respiratory–swallow coordination training and voluntary cough skill training: a single-subject treatment study in a person with Parkinson’s disease. J Speech Lang Hear Res. 2020;63:472–86. https://doi.org/10.1044/2019_JSLHR-19-00207. A single-subject study with large effect sizes following a treatment plan using respiratory-swallow coordination and cough skill training.
Sroussi HY, Epstein JB, Bensadoun R-J, Saunders DP, Lalla RV, Migliorati CA, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6:2918–31. https://doi.org/10.1002/cam4.1221.
Awan MJ, Mohamed ASR, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, et al. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol. 2014;50:746–52. https://doi.org/10.1016/j.oraloncology.2014.05.003.
Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–43. https://doi.org/10.1200/JCO.2006.06.0079.
Wall LR, Ward EC, Cartmill B, Hill AJ. Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: a systematic review. Dysphagia. 2013;28:481–93. https://doi.org/10.1007/s00455-013-9491-8.
Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MWS, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–9. https://doi.org/10.1002/cncr.27631.
Kraaijenga SAC, van der Molen L, Jacobi I, Hamming-Vrieze O, Hilgers FJM, van den Brekel MWM. Prospective clinical study on long-term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. Eur Arch Otorhinolaryngol. 2015;272:3521–31. https://doi.org/10.1007/s00405-014-3379-6.
Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA. Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia. 2014;29:223–33. https://doi.org/10.1007/s00455-013-9500-y.
Xiong J, Krishnaswamy G, Raynor S, Loh KS, Kwa ALH, Lim CM. Risk of swallowing-related chest infections in patients with nasopharyngeal carcinoma treated with definitive intensity-modulated radiotherapy. Head Neck. 2016;38:E1660–5. https://doi.org/10.1002/hed.24296.
Xu B, Boero IJ, Hwang L, Le Q-T, Moiseenko V, Sanghvi PR, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer. 2015;121:1303–11. https://doi.org/10.1002/cncr.29207.
Hutcheson KA, Barrow MP, Plowman EK, Lai SY, Fuller CD, Barringer DA, et al. Expiratory muscle strength training for radiation-associated aspiration after head and neck cancer: A case series. Laryngoscope. 2018;128:1044–51. https://doi.org/10.1002/lary.26845.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Troche reports the following: Financial: Salary — employment — Teachers College, NIH/NINDS — grant, and MedBridge Inc. — Royalty — Consulting. Non-financial: Dysphagia Research Society — professional — board membership, and Journal of Speech Language and Hearing Research — editorial board member. The other authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on LARYNGOLOGY: Update on Dysphagia
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lowell, E.R., Borders, J.C., Sevitz, J.S. et al. A Primer on Hypotussic Cough: Mechanisms and Assessment. Curr Otorhinolaryngol Rep 11, 182–191 (2023). https://doi.org/10.1007/s40136-023-00446-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-023-00446-5