Abstract
Background
Continuous manufacturing, a new process that applies the concept of time rather than batch size, is gradually being implemented throughout the pharmaceutical industry. In this process, critical quality attribute (CQA) management strategy for pharmaceutical manufacturing must be established through real-time monitoring technology. Therefore, transitioning from existing offline testing to real-time process analysis techniques (PAT; at-line, on-line, in-line) is essential for ensuring the quality of the intermediate and final products. Additionally, exploring the suitability of PAT must also be considered.
Area covered
In this review, we discuss the application of real-time monitoring technology in the manufacturing process of solid oral dosage forms. We list each manufacturing process for the solid oral dosage form and select each key unit process to be considered when converting to continuous manufacturing while identifying the CQA. We also comprehensively review the real-time monitoring PAT of the continuous manufacturing process studied to the identified CQA. Therefore, this review goal is understanding the status of monitoring enabling quality control and assurance through the listing of real-time PAT that can control CQA in continuous manufacturing.
Expert opinion
In existing studies, there are many individual mentions of real-time monitoring techniques to continuous manufacturing. However, there are relatively few systematic and comprehensive discussions on PAT that can be applied to continuous manufacturing throughout the entire manufacturing process of solid oral dosage forms. Therefore, this review attempts to systematically arrange the real-time monitoring technology applicable to the continuous manufacturing of solid oral dosage forms and lists various examples other than process controls for continuous manufacturing of the drug products listed in ICH guideline Q13. It is hoped that this review will help expand the application of continuous manufacturing in the pharmaceutical industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous manufacturing
The conventional pharmaceutical manufacturing process is a batch process in which individual unit operations (i.e., feeding, powder blending/mixing, granulating, drying, coating) are performed. The batch process is flexible because the manufacturing process can be easily reconfigured. It can be worked empirically according to basic principles, and it is simple to track when a pharmaceutical quality problem occurs (Ierapetritou et al. 2016). However, because quality control through off-line testing is performed among these individual unit operations, in a batch process, the manufacturing line is often stopped and restarted, and the manufacturing line is also cleaned during this time (Helal et al. 2019). In addition, it is essential that equipment and space are sufficient to handle and store intermediates emitted from each unit process. Therefore, the batch process requires a large amount of human resources and manufacturing costs, which have limitations such as batch-to-batch variability due to human factor intervention and technical difficulties in scaling the batch to pilot scale (Ierapetritou et al. 2016).
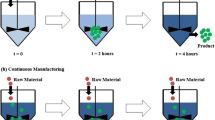
In contrast, continuous manufacturing follows the “one-in, one-out” principle, whereby materials are continuously added, and the final product is discharged at a constant flow rate, with no starting and stopping between each unit operation. Continuous manufacturing can therefore be thought of as a new pharmaceutical manufacturing process with a new concept that can increase batch size over time. Therefore, continuous manufacturing does not require constant updating of equipment required for scale-up research. It is possible to operate the process with only a small number of workers, or it can be unmanned during 24-h processes. In this process, real-time process analytical technology (PAT) is used to ensure the quality of the finished product (Fig. 1). Process analytical technology is a system that designs, analyzes, and controls pharmaceutical manufacturing by timely measuring of raw materials and in-process materials and gauging critical process parameters and performance to ensure final drug product quality (ICH 2009). This manufacturing method can improve manufacturing efficiency, save both time and money, and can easily be scaled up as the understanding of the process increases (Lee et al. 2015; Matsunami et al. 2018).
The size of a batch produced by continuous manufacturing can be defined as the quantity of output material, the quantity of input material, and run time at a defined mass flow rate (ICH 2021). Also, in general terms, the size of a production batch for continuous manufacturing equipment is defined as the unit time (Allison et al. 2015), and because it has fewer scale-up issues, the continuous production equipment can be used at the same scale for drug development, pilot research, clinical trials, and commercial production. Consequently, the overall development time of products developed by continuous manufacturing is significantly reduced, and their time to market is significantly accelerated. In particular, the process of developing a new drug to market occurs over a long period and is hugely expensive. If a pharmaceutical manufacturing process is introduced through a continuous process when developing a new drug, a longer exclusivity period can be enjoyed even if the patent expires. Because the continuous production process is not a technology that anyone dares to imitate, even if the patent expires, you can enjoy a longer patent exclusivity period. So, it means that companies that apply these technologies can reap enormous economic benefits (Fonteyn et al. 2015; Shaver et al. 2011). In addition, there is a possibility of using continuous processes in the extension of patent rights, and gaining a competitive advantage through cost reduction and quality improvement, if continuous manufacturing is used in the development of generic drugs (Ierapetritou et al. 2016). Other advantages of the integrated continuous manufacturing platform are shown in Table 1.
Quality assurance in continuous manufacturing
Previously, there was no specific mention of a continuous process in the ICH guidelines; it was discussed only as an improved manufacturing management method for the actual implementation of quality by design (QbD). However, a draft of specific content for continuous manufacturing was recently mentioned in ICH Q13, released in 2021, which can be used as a reference for detailed guidance on continuous manufacturing (Table 2). This means that in order for us to develop a successful continuous manufacturing technology, we need to understand ICH Q13 and establish management strategies based on the definitions and principles presented in ICH Q8–Q12.
QbD is a systematic approach to drug development, with the overarching goal of providing a consistent supply of drugs with guaranteed critical quality attributes (CQA). The continuous process was understood as a pharmaceutical manufacturing method consistent with the QbD principle, as it can improve drug quality by understanding the production process and ensuring CQA (Nasr et al. 2017). In this regard, examples of the current status of commercialized continuous processes can also be confirmed (Testa et al. 2020).
As the continuous process application develops, PAT is utilized to monitor the CQA of intermediates and processes in real time, making it possible to construct a system that designs, analyzes, and controls the manufacturing process. Therefore, each CQA will need to be included in the appropriate range and limit to ensure the quality of the drug product. The implementation of PAT requires utilizing the following methods: identification of traceability and residence time distribution (RTD) through the PAT framework; continuous monitoring of material properties, critical process parameters (CPP), and real-time release testing (RTRT); and maintaining the process and CQA within the operating range by controlling the drug manufacturing process operation in real-time (Zhong et al. 2020). RTD is defined as the time required for material transport and transformation; ICH Q13 explains that understanding this will be beneficial in material tracking, sampling, and the establishment of conversion strategies.
In addition, it is important to explore regulatory aspects when applying continuous processes in the pharmaceutical industry. ICH Q13, currently under discussion, provides an overview of current regulatory considerations. These considerations are overview of the process, quality control strategy for output, the definition of batch, process model, stability of manufactured drug product, process validation, pharmaceutical quality system (PQS), lifecycle management, and cross-referenced Common Technical Document data. If the manufacturing method is changed from a batch process to a continuous process, these data should be described. Table 3 is currently covered in the ICH Q13 draft. In addition, through major regulatory agencies such as FDA, MHRA, EMA, and PMDA, it is possible to understand the global regulatory environment for continuous processes and to stay up-to-date on support and regulatory status (Wahlich 2021).
The pharmaceutical industry needs to develop a more efficient pharmaceutical manufacturing process, which could mean introducing the continuous process discussed in the ICH guidelines, along with the associated PAT implementation and management strategy development. Therefore, it is necessary to extensively review the PAT to determine what analytical equipment is used for a specific process, and what are the important process quality characteristics. Therefore, we will list and discuss the current state, scope of technology, and mode of application of PAT for commonly produced solid oral dosage forms, with a particular focus on the latest research in this area. We refer to previous reviews (Fonteyne et al. 2015; Vanhoorne et al. 2020; Zhong et al. 2020), and further supplement important information by referring to the continuous manufacturing process for the drug products listed in ICH Q13.
General manufacturing diagram of solid oral dosage forms
A generalized manufacturing process for a solid oral dosage form is shown in Fig. 2. The main unit processes include mixing, wet granulation, dry granulation, spray drying, compression, and coating. However, having a continuous flow of materials is also imperative. Therefore, a control strategy that is suitable for the continuous manufacturing of such a solid oral dosage form is necessary when developing a continuous process and management strategy, meaning that the CQA for each unit process needs to be identified. The various CQA for each unit process are shown in Table 4 and include content uniformity, which is an important CQA that is monitored at almost every step.
Implementing continuous Process Analytical Technology in each unit process
The existing batch process was mainly conducted offline, while quality evaluation was performed on the intermediate and final products. Since this is a post-analysis quality evaluation, the manufacturing process cannot be immediately adjusted, so it is not suitable as an improved process quality control method. However, the PAT applications can measure the CQA related to various unit operations of a continuous process line in real-time (Zhong et al. 2020). Therefore, during the continuous process, CQA is evaluated in real-time via at-line, on-line, and in-line measurements according to the PAT industry guidelines as published by the US Food and Drug Administration (FDA). Each measurement method is described as follows (FDA 2004) (Fig. 3).
-
At-line: measurement where the sample is removed, isolated, and analyzed near the process stream.
-
On-line: measurement where the sample is diverted from the manufacturing process and could be returned to the process stream.
-
In-line: measurement where the sample remains in the process stream and can be either invasive or non-invasive.
The placement of the analyzer’s probe and sensor is an essential consideration when developing and establishing a continuous process control strategy. In this process, in-line measurement can be challenging because of potential contamination of the probe, difficulty in positioning the probe, and difficulty in defining the sampling volume of the powder (Sacher et al. 2022). Both probe and sensor should be positioned to measure the powder flow at a location that does not affect the flow in any way (Fonteyne et al. 2015). If the continuous process manufacturing equipment and PAT tools are appropriately designed or selected in consideration of these points, the process will be simplified, and process monitoring and material conversion can both be facilitated (ICH 2021).
Consequently, this review paper also identifies equipment and process parameters that affect process control based on ICH Q13’s continuous process management strategy. The impact on the material quality characteristics and the unit operating equipment is evaluated as CQA. A description of the PAT according to the manufacturing process flow of solid oral pharmaceuticals is detailed in the next section.
Continuous material streams
An essential element of any continuous process is to ensure a sufficiently constant mass flow (Engisch et al. 2012), since consistently and accurately supplying material from the unit process to the subsequent unit process is important. Therefore, a loss-in-weight (LIW) feeder system that analyzes injector mass flow and variability is typically used for all major routes during a continuous manufacturing process and is also utilized in all major routes of the continuous production lines of solid oral pharmaceuticals, such as direct compression, wet granulation, and dry granulation (Hanson 2018). This is particularly important because LIW feeders can generate fast dynamic disturbances, which are controlled through process monitoring (ICH 2021).
The ICH Q13 guideline states that it is essential to understand the flow of substances (process execution time and RTD) in individual units of equipment design in order to establish the integration process. In particular, because RTD represents the diffusion of material in a continuous process, it is important for the tracking of raw materials during process execution, and it allows downstream control or removal of the affected material in the entire continuous system (Engisch et al. 2016). A detailed overview of the principles and mechanisms of the continuous process feed step can be found in Blackshields et al. (2018).
Consequently, evaluating the homogeneity of the powder stream and the monitoring of the API concentration is conducted during the continuous material stream process. Monitoring techniques usable in continuous processes related to continuous material streams have been developed as follows. In addition, if the monitoring technology is closely intertwined with other major unit processes in the continuous manufacture of solid oral products, it is classified as one of the main unit processes, which are described later in more detail (Table 5).
The monitoring method for evaluating the flowability of powder is generally analyzed, in-line, in a LIW feeder. Additionally, a monitoring method using near-infrared spectroscopy (NIRS), which is the most commonly used technique for process analysis of continuous processes, is also widely used. In particular, as a result of comparing the NIRS monitoring system presented in (Destro et al. 2021) and high performance liquid chromatography (HPLC) and spectroscopic measurements by offline tests, successful in-line measurements can be made, such as active pharmaceutical ingredient (API) concentrations, which tend to have a high level of consistency. Therefore, as a recent research trend, it is in progress that a more detailed study on the influence of supply parameters and the identification and analysis of various CQA during the initial powder supply process. In addition, various combinations of these monitoring approaches are made. Therefore, it is expected that a hybrid form of latent variable modeling and state estimator for fault detection and control will exist from now on (Destro et al. 2020). In addition, an important element of PAT equipment for continuous material stream is the detection of all significant disturbances through high-frequency measurement methods. Therefore, a method of tracing raw materials using RTD data and identifying their characteristics is being developed as a form of corrective action when a disturbance starts, along with utilization of a diagnosis system (Engisch et al. 2016).
Continuous blending
Blend uniformity in the mixing process step is important since it is a prerequisite to ensuring the uniformity of the final drug content. Offline HPLC analysis is often used to evaluate general blend uniformity in the existing batch unit. However, this method has disadvantages such as possible sampling errors, process delays, and increased time and labor (Zhong et al. 2020). Therefore, the following PAT was used in continuous blending monitoring to overcome this limitation. Similarly in the continuous blending step, the RTD identified shows its impact on process dynamics and equipment design in the course of comparing APIs with tracers that have similar flow characteristics to APIs (ICH 2021). ICH Q13 shows that RTD characteristics can provide information on forward and backward blending and the degree of jamming, which can define material traceability and conversion strategies. Additionally, other scientifically validated theories can be used to establish process kinetics knowledge. Based on these characterizations, other CQAs besides blending uniformity are shown in Table 6.
A widely used representative process analysis method, as aforementioned mentioned, is NIR-PLS (partial least squares). In addition, RS (Raman spectroscopy) is a suitable alternative to monitor lower concentrations than NIRS. However, according to (Galata et al. 2021a, b), it is difficult to accurately quantify low concentrations (less than 2w/w%) of API in both of the aforementioned methods. Therefore, there is a need for a PAT tool that can accurately quantify the amount of low-concentration API in the continuous powder blending process.
Recent research is focusing on how PAT is being developed to evaluate the suitability of the current technology for drug development, including low-concentration API. For example, machine vision systems can quantify intensely colored APIs, even in low-density images, when viewed with a digital camera (Galata et al. 2021a, b). There is a study of chemical composition monitoring of metal–organic reaction products using an explosion-proof online NMR sensor with a sampling rate of 15 s. This was introduced as a method that allows access to unstable lithiated intermediates that cannot be calibrated using conventional HPLC analysis methods. In addition, NMR sensors are available as an on-line method for calibrating NIR spectrometers (Kern et al. 2019). Therefore, the transition to a continuous process requires ongoing research to develop PAT tools suitable for all types of API.
Wet granulation
Wet granulation is a process whereby granules are prepared using a liquid binder to improve the fluidity, homogeneity, and compressibility of the mixed powder before tablet compression (Suresh et al. 2017). Twin-screw wet granulators (Huang et al. 2010; Fonteyne et al. 2014a, b), fluid bed granulators (Chablani et al. 2011; El Hagrasy et al. 2013; Fonteyne et al. 2013), and high-shear mixers (Fonteyne et al. 2012; Kumar et al. 2013) are the main methods used during the continuous wet granulation process. The CQA of the wet granulation process includes the solid state, moisture content, and granule size distribution of API and excipients, which is particularly important since particle size, moisture content, and granule density can affect granule fluidity, compressibility, and stability (Fung et al. 2006; Närvänen et al. 2008; Ehlers et al. 2009; Gabbott et al. 2016). It should be noted that for wet granulation, controlling and evaluating the properties of the input material properties during the continuous process may be more important than the actual material specification properties typically considered in batch manufacturing. This is described in ICH Q13 in the section regarding the development of a continuous process control strategy (ICH 2021). Therefore, recognizing the importance to identify and manage the properties of the materials, PAT development should continue apace (Table 7).
During the wet granulation process, the majority of PAT tools are used for the in-line monitoring of granule size distribution. In addition to NIRS and RS, various process analysis tools such as FBRM, SFV, imaging technology, and acoustic emission (AE) have been studied, and their applicability has been proven. However, although the NIRS tool is one of the most commonly used methods, it requires a relatively large amount of data for calibration and can sometimes produce inaccurate data because it can be affected by sensor fouling (Rantanen et al. 2015). In addition, the optical device used in the image processing system can become contaminated, leading to resolution problems when checking the particle size (Suresh et al. 2017). One of the more recently developed tools, AE, is presented as a non-destructive method for particle size adjustment. It is also suitable for the wet granulation process because it is relatively unaffected by pollution and dust and it has a low implementation cost (Abdulhussain et al. 2021). In addition, the particle size analyzer using a customized image analysis-camera also showed excellent similarity when compared with the results obtained offline without major drawbacks, and it was possible to identify the characteristics of the process material according to the CPP. Because the latest algorithm is applied in this process, this method can be applied in a role such as deep learning-based image segmentation in the future (Madarász et al. 2022). Therefore, today's PAT tool, such as AE, is also being touted as an improvement over the existing PAT tools, with the added advantage of being easy to implement during manufacturing while nullifying some of the disadvantages of the existing PAT tools.
Dry granulation
Dry granulation is a granulation process that can improve the flowability of powder by improving particle size or bulk density. However, unlike wet granulation, dry granulation is a relatively simple process with no need for liquid additives (Burcham et al. 2018). In the continuous dry granulation process, a roller compactor compresses the material and then mills it to form a ribbon. The CQA of a roller compactor includes ribbon density, moisture content, particle size distribution, and content uniformity, which is a factor that shows granular properties (Fonteyne et al. 2015).
NIRS, which is a quality control method most commonly used for the roller compaction process applied in the pharmaceutical industry, unlike in the past, tends to lose a large gap between the real-time monitoring value and the standard value at the current technology level (SAMANTA 2012). However, the applicability of new techniques has recently been re-verified due to the shortcomings of NIRS data preprocessing and the need for more sophisticated chemistry (Nasr et al. 2017). In a recent study, the temperature distribution of the ribbon was confirmed by installing a thermal imaging camera on the roller compactor, and the optimal relative temperature uniformity was ensured (Yu et al. 2022). Studies to identify the relationship between process parameters and material properties are being conducted along with the application of PAT. In addition, information on laser diffraction, microwave sensors, infrared thermography, spatial filtering technique (SFT), particle size analyzers, NIRS and microwave resonance sensing is shown in Table 8, whereas more details about the PATs applied in dry granulation, can be found in the paper by Dular Vovko et al. (2020).
Spray drying
Spray drying is a process used to convert a fluid material into a dried particulate form. This process is often used before the direct compression process and has also been utilized in other product manufacturing fields such as for the drying of heat-sensitive materials (Coppi et al. 2002; Picot et al. 2004) and microencapsulation (I Ré 1998). During the spraying process, the CQAs are the particle size distribution, as well as the quantification of the solution composition and residual solvent used in the process. Related PAT tools are shown in Table 9, while a more detailed description of spray drying can be found in Ziaee et al. (2019).
The first unit operating step of spray drying is the preparation of the spray solution (Lee et al. 2019a, b). NIRS can measure the composition of the final spray-drying solution through real-time monitoring at this stage and can correct any problems with the spray volume is incorrect. In this way, the accuracy of the analysis has been proven. In addition, NIRS can be used during intermediate quality control for the quantification of residual solvents and the optimization of drying process cycles (Ikeda et al. 2022). Various studies have been comparatively validated, in-line, at-line, and offline, including the use of an in-line laser diffraction system to monitor particle size distribution, which affects the properties of both the intermediate and final products (Chan et al. 2008).
Compression
When using a tablet press machine during the tablet compression phase, the PAT is mainly applied to the powder feed frame of the tablet press machine. The CQA of this process is the content uniformity of the tablet, the content of the main ingredient, the tableting pressure, and the physical properties (Kim et al. 2021). The process is controlled through monitoring, which determines the quality of the tablets (Table 10).
In the case of NIRS in tablet pressing, the PLS model was designed to quantitatively analyze the concentration of API and its excipients, while a dissolution performance prediction model was also established (Pawar et al. 2016). RS can also be used to determine the CQA of intermediate and final tablets from the tablet press (Nagy et al. 2017), while Terahertz spectroscopy is able to detect tablet weight by measuring the effective refractive index (Bawuah et al. 2014). A report was also written on the combination of two NIRS probes on a tablet press feed frame (Pauli et al. 2019a, b, c): the first probe confirms the API content uniformity of the dried granules, while the second probe allows accurate monitoring of tablet content uniformity at various tableting rates up to 70,000 tablets/h. In addition, according to a recent study, a new NIR-SS hybrid method combining online NIR-PLS and titer soft sensor was successfully performed. This method utilizes the average data of the degree of movement according to the process stay time through a combination of two data streams, and it is possible to diagnose real-time online performance (Cogoni et al. 2021). This means that unsuitable tablets that do not conform to the CQA are distinguished through various combinations rather than through a single utilization of PAT. In addition, the feedback control loop is designed to respond immediately to problems in the process. The overall goal, therefore, should be to establish a system that optimizes current quantitative methods and facilitates improved control strategies for future PAT development in a continuous process.
Coating
The coating process allows for the sustained or controlled release of API, and the stability of tablets. It is an important process that determines the overall appearance of the final product (Carter et al. 2018). Conventional coating thickness measurement methods are gravimetric, dissolution-based, and conducted offline. They are also time-consuming and cannot always provide information relating to tablet uniformity, porosity, and cracking. The CQAs of the coating process include coating thickness, API content, and content uniformity. Examples of real-time PAT development to evaluate these factors are listed below in Table 11.
Various new technologies are applied in the coating process analysis. These include NIRS, RS, SFV, and BARDS, which are spectrum technologies; TPI and OCT, which are imaging technologies; and sound technologies. Among these technologies, in-line OCT has been widely used in recent film coating research; it is a method that can monitor parameters such as tablet coating thickness and content uniformity in real-time using high-resolution imaging. Sacher et al. (2019) explain that OCT can detect defined values and deviations during the coating process faster and more accurately than conventional techniques. A recent study confirmed the high accuracy of tablet moisture content using a PLS model through manual wavelength selection (Shibayama et al. 2021).
The coating process has nevertheless proven to be arguably the most difficult unit operation to convert into a continuous process because of its complexity and its many parameters (Suzzi et al. 2010). In addition, tablets tend to relax after compression, so if the coating process proceeds too quickly, the coating may swell and affect the quality of the finished product. So the holding time before the tablet is coated is an essential process element. However, this holding time conflicts with the characteristics of the continuous process. Therefore, the continuous coating system is known to have developmental limitations meaning that there is a lot of potential for future research and development (Leane et al. 2018; Wahlich 2021). In addition, because the amount of data is not large in the coating stage, it will be necessary to introduce PAT through a selection of optimal hyper-parameters and confirmation of correlation in the future (Shibayama et al. 2021).
Conclusion
This review paper was written based on the management strategy for continuous manufacturing mentioned in the draft ICH Q13 guidelines and discussed the details of process monitoring and management among the elements of the control strategy. Real-time monitoring PAT enables us to establish a scientific understanding of the process and control strategies when implementing a continuous manufacturing process. In particular, NIRS and RS are traditionally widely used as tools for process analysis in the manufacturing process of solid oral dosage forms, and most CQA monitoring is possible using only these two tools. However, as mentioned in the text, both NIRS and RS have their disadvantages. An increasing number of studies are therefore evaluating whether existing tools can be improved upon while determining the applicability of real-time monitoring analysis techniques of new tools. In this process, some of the most recent articles covered in this review paper discusses the detailed analysis of various CQAs through multi-point measurement, and flexibly utilizes various PATs by mixing existing process analytical techniques. Therefore, using PAT in recent studies provides in-depth knowledge about PAT as a real-time monitoring technology.
In addition, the development processes of the majority of continuous manufacturing technologies often only concluded with a review of each unit process. It is therefore necessary to discuss more extensive in-line monitoring to apply and integrate technologies in more diverse unit processes. One of the most recent papers related to this content was written by (Sacher et al. 2021). In addition discussions about the continuous manufacturing process of solid oral pharmaceuticals as well as the formation of an end-to-end system from drug substance synthesis to final formulation are being reported (Wahlich 2021). Therefore, the exploration of real-time monitoring analysis technology utilized in the solid oral dosage form in this text can be referred to as a basic guideline for PAT trends. However, we also need to be aware of and follow the latest discussions about the broader continuous process.
References
Abdulhussain H, Thompson M (2021) Predicting the particle size distribution in twin screw granulation through acoustic emissions. Powder Technol 394:757–766
Alam MA, Liu YA (2021) An agile and robust in-line NIR potency deviation detection method for monitoring and control of a continuous direct compression process. Int J Pharm 601:120521
Alfarsi A, O’mahoney N, Arndt M, Kuntz T, Mcsweeney S, Krüse J, Skalsky B, Fitzpatrick D (2019) A rapid in-process control (IPC) test to monitor the functionality of taste masking polymer coatings using Broadband Acoustic Resonance Dissolution Spectroscopy (BARDS). Int J Pharm 568:118559
Allison G, Cain YT, Cooney C, Garcia T, Bizjak TG, Holte O, Jagota N, Komas B, Korakianiti E, Kourti D (2015) Regulatory and quality considerations for continuous manufacturing. May 20–21, 2014 continuous manufacturing symposium. J Pharm Sci 104:803–812
Alshihabi F, Vandamme T, Betz G (2013) Focused beam reflectance method as an innovative (PAT) tool to monitor in-line granulation process in fluidized bed. Pharm Dev Technol 18:73–84
Alvarado-Hernández BB, Scicolone JV, Ortega-Zuniga C, Román-Ospino AD, Colón-Lugo YM, Aymat E, Sánchez E, Muzzio FJ, Romañach RJ (2020) Method transfer of a near-infrared spectroscopic method for blend uniformity in a poorly flowing and hygroscopic blend. J Pharm Biomed Anal 180:113054
Aoki H, Hattori Y, Sasaki T, Otsuka M (2022) Comparative study on the real-time monitoring of a fluid bed drying process of extruded granules using near-infrared spectroscopy and audible acoustic emission. Int J Pharm 619:121689
Austin J, Gupta A, Mcdonnell R, Reklaitis GV, Harris MT (2013) The use of near-infrared and microwave resonance sensing to monitor a continuous roller compaction process. J Pharm Sci 102:1895–1904
Avalle P, Pollitt M, Bradley K, Cooper B, Pearce G, Djemai A, Fitzpatrick S (2014) Development of Process Analytical Technology (PAT) methods for controlled release pellet coating. Eur J Pharm Biopharm 87:244–251
Bakri B, Weimer M, Hauck G, Reich G (2015) Assessment of powder blend uniformity: comparison of real-time NIR blend monitoring with stratified sampling in combination with HPLC and at-line NIR Chemical Imaging. Eur J Pharm Biopharm 97:78–89
Barimani S, Kleinebudde P (2017) Evaluation of in–line Raman data for end-point determination of a coating process: Comparison of Science-Based Calibration, PLS-regression and univariate data analysis. Eur J Pharm Biopharm 119:28–35
Bawuah P, Silfsten P, Ervasti T, Ketolainen J, Zeitler JA, Peiponen K-E (2014) Non-contact weight measurement of flat-faced pharmaceutical tablets using terahertz transmission pulse delay measurements. Int J Pharm 476:16–22
Besseling R, Damen M, Tran T, Nguyen T, Van Den Dries K, Oostra W, Gerich A (2015) An efficient, maintenance free and approved method for spectroscopic control and monitoring of blend uniformity: the moving F-test. J Pharm Biomed Anal 114:471–481
Blackshields CA, Crean AM (2018) Continuous powder feeding for pharmaceutical solid dosage form manufacture: a short review. Pharm Dev Technol 23:554–560
Boiret M, Chauchard F (2017) Use of near-infrared spectroscopy and multipoint measurements for quality control of pharmaceutical drug products. Anal Bioanal Chem 409:683–691
Burcham CL, Florence AJ, Johnson MD (2018) Continuous manufacturing in pharmaceutical process development and manufacturing. Annu Rev Chem Biomol Eng 9:253–281
Cameron A, Briens L (2019) Monitoring magnesium stearate blending in a V-blender through passive vibration measurements. AAPS PharmSciTech 20:1–11
Carter A, Briens L (2018) Inline acoustic monitoring to determine fluidized bed performance during pharmaceutical coating. Int J Pharm 549:293–298
Chablani L, Taylor MK, Mehrotra A, Rameas P, Stagner WC (2011) Inline real-time near-infrared granule moisture measurements of a continuous granulation–drying–milling process. AAPS PharmSciTech 12:1050–1055
Chan L, Tan L, Heng PW (2008) Process analytical technology: application to particle sizing in spray drying. AAPS PharmSciTech 9:259–266
Cogoni G, Liu YA, Husain A, Alam MA, Kamyar R (2021) A hybrid NIR-soft sensor method for real time in-process control during continuous direct compression manufacturing operations. Int J Pharm 602:120620
Colón YM, Vargas J, Sánchez E, Navarro G, Romañach RJ (2017) Assessment of robustness for a near-infrared concentration model for real-time release testing in a continuous manufacturing process. J Pharm Innov 12:14–25
Coppi G, Iannuccelli V, Bernabei MT, Cameroni R (2002) Alginate microparticles for enzyme peroral administration. Int J Pharm 242:263–266
Dalvi H, Langlet A, Colbert M-J, Cournoyer A, Guay J-M, Abatzoglou N, Gosselin R (2019) In-line monitoring of Ibuprofen during and after tablet compression using near-infrared spectroscopy. Talanta 195:87–96
De Leersnyder F, Peeters E, Djalabi H, Vanhoorne V, Van Snick B, Hong K, Hammond S, Liu AY, Ziemons E, Vervaet C (2018) Development and validation of an in-line NIR spectroscopic method for continuous blend potency determination in the feed frame of a tablet press. J Pharm Biomed Anal 151:274–283
Destro F, Facco P, Muñoz SG, Bezzo F, Barolo M (2020) A hybrid framework for process monitoring: Enhancing data-driven methodologies with state and parameter estimation. J Process Control 92:333–351
Destro F, Muñoz SG, Bezzo F, Barolo M (2021) Powder composition monitoring in continuous pharmaceutical solid-dosage form manufacturing using state estimation–Proof of concept. Int J Pharm 605:120808
Dohi M, Momose W, Yoshino H, Hara Y, Yamashita K, Hakomori T, Sato S, Terada K (2016) Application of terahertz pulse imaging as PAT tool for non-destructive evaluation of film-coated tablets under different manufacturing conditions. J Pharm Biomed Anal 119:104–113
Dular Vovko A, Vrečer F (2020) Process analytical technology tools for process control of roller compaction in solid pharmaceuticals manufacturing. Acta Pharm 70:443–463
Durão P, Fauteux-Lefebvre C, Guay J-M, Abatzoglou N, Gosselin R (2017) Using multiple process analytical technology probes to monitor multivitamin blends in a tableting feed frame. Talanta 164:7–15
Ehlers H, Liu A, Räikkönen H, Hatara J, Antikainen O, Airaksinen S, Heinämäki J, Lou H, Yliruusi J (2009) Granule size control and targeting in pulsed spray fluid bed granulation. Int J Pharm 377:9–15
El Hagrasy A, Cruise P, Jones I, Litster J (2013) In-line size monitoring of a twin screw granulation process using high-speed imaging. J Pharm Innov 8:90–98
Engisch WE, Muzzio FJ (2012) Method for characterization of loss-in-weight feeder equipment. Powder Technol 228:395–403
Engisch W, Muzzio F (2016) Using residence time distributions (RTDs) to address the traceability of raw materials in continuous pharmaceutical manufacturing. J Pharm Innov 11:64–81
Fda (2004) Guidance for Industry: PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. Food and Drug Administration (FDA)
Ficzere M, Mészáros LA, Madarász L, Novák M, Nagy ZK, Galata DL (2021) Indirect monitoring of ultralow dose API content in continuous wet granulation and tableting by machine vision. Int J Pharm 607:121008
Fonteyne M, Soares S, Vercruysse J, Peeters E, Burggraeve A, Vervaet C, Remon JP, Sandler N, De Beer T (2012) Prediction of quality attributes of continuously produced granules using complementary pat tools. Eur J Pharm Biopharm 82:429–436
Fonteyne M, Vercruysse J, Díaz DC, Gildemyn D, Vervaet C, Remon JP, Beer TD (2013) Real-time assessment of critical quality attributes of a continuous granulation process. Pharm Dev Technol 18:85–97
Fonteyne M, Arruabarrena J, De Beer J, Hellings M, Van Den Kerkhof T, Burggraeve A, Vervaet C, Remon JP, De Beer T (2014a) NIR spectroscopic method for the in-line moisture assessment during drying in a six-segmented fluid bed dryer of a continuous tablet production line: validation of quantifying abilities and uncertainty assessment. J Pharm Biomed Anal 100:21–27
Fonteyne M, Gildemyn D, Peeters E, Mortier STF, Vercruysse J, Gernaey KV, Vervaet C, Remon JP, Nopens I, De Beer T (2014b) Moisture and drug solid-state monitoring during a continuous drying process using empirical and mass balance models. Eur J Pharm Biopharm 87:616–628
Fonteyne M, Vercruysse J, De Leersnyder F, Van Snick B, Vervaet C, Remon JP, De Beer T (2015) Process analytical technology for continuous manufacturing of solid-dosage forms. TrAC, Trends Anal Chem 67:159–166
Fonteyne M, Vercruysse J, De Leersnyder F, Besseling R, Gerich A, Oostra W, Remon JP, Vervaet C, De Beer T (2016) Blend uniformity evaluation during continuous mixing in a twin screw granulator by in-line NIR using a moving F-test. Anal Chim Acta 935:213–223
Fung KY, Ng KM, Nakajima S, Wibowo C (2006) A systematic iterative procedure for determining granulator operating parameters. AIChE J 52:3189–3202
Gabbott IP, Al Husban F, Reynolds GK (2016) The combined effect of wet granulation process parameters and dried granule moisture content on tablet quality attributes. Eur J Pharm Biopharm 106:70–78
Galata DL, Könyves Z, Nagy B, Novák M, Mészáros LA, Szabó E, Farkas A, Marosi G, Nagy ZK (2021a) Real-time release testing of dissolution based on surrogate models developed by machine learning algorithms using NIR spectra, compression force and particle size distribution as input data. Int J Pharm 597:120338
Galata DL, Meszaros LA, Ficzere M, Vass P, Nagy B, Szabo E, Domokos A, Farkas A, Csontos I, Marosi G (2021b) Continuous blending monitored and feedback controlled by machine vision-based PAT tool. J Pharm Biomed Anal 196:113902
Ganesh S, Troscinski R, Schmall N, Lim J, Nagy Z, Reklaitis G (2017) Application of X-ray sensors for in-line and noninvasive monitoring of mass flow rate in continuous tablet manufacturing. J Pharm Sci 106:3591–3603
Gavan A, Iurian S, Casian T, Porfire A, Porav S, Voina I, Oprea A, Tomuta I (2020) Fluidised bed granulation of two APIs: QbD approach and development of a NIR in-line monitoring method. Asian J Pharm Sci 15:506–517
Gosselin R, Durão P, Abatzoglou N, Guay J-M (2017) Monitoring the concentration of flowing pharmaceutical powders in a tableting feed frame. Pharm Dev Technol 22:699–705
Gupta A, Austin J, Davis S, Harris M, Reklaitis G (2015) A novel microwave sensor for real-time online monitoring of roll compacts of pharmaceutical powders online—a comparative case study with NIR. J Pharm Sci 104:1787–1794
Hanson J (2018) Control of a system of loss-in-weight feeders for drug product continuous manufacturing. Powder Technol 331:236–243
Harms ZD, Shi Z, Kulkarni RA, Myers DP (2019) Characterization of near-infrared and Raman spectroscopy for in-line monitoring of a low-drug load formulation in a continuous manufacturing process. Anal Chem 91:8045–8053
Harting J, Kleinebudde P (2019) Optimisation of an in-line Raman spectroscopic method for continuous API quantification during twin-screw wet granulation and its application for process characterisation. Eur J Pharm Biopharm 137:77–85
Hattori Y, Sugata M, Kamata H, Nagata M, Nagato T, Hasegawa K, Otsuka M (2018) Real-time monitoring of the tablet-coating process by near-infrared spectroscopy-effects of coating polymer concentrations on pharmaceutical properties of tablets. J Drug Deliv Sci Technol 46:111–121
Helal NA, Elnoweam O, Eassa HA, Amer AM, Eltokhy MA, Helal MA, Fayyaz HA, Nounou MI (2019) Integrated continuous manufacturing in pharmaceutical industry: current evolutionary steps toward revolutionary future. Pharm Patent Anal 8:139–161
Hetrick EM, Shi Z, Barnes LE, Garrett AW, Rupard RG, Kramer TT, Cooper TM, Myers DP, Castle BC (2017) Development of near infrared spectroscopy-based process monitoring methodology for pharmaceutical continuous manufacturing using an offline calibration approach. Anal Chem 89:9175–9183
Hisazumi J, Kleinebudde P (2017) In-line monitoring of multi-layered film-coating on pellets using Raman spectroscopy by MCR and PLS analyses. Eur J Pharma Biopharm 114:194–201
Huang J, Kaul G, Utz J, Hernandez P, Wong V, Bradley D, Nagi A, O’grady D (2010) A PAT approach to improve process understanding of high shear wet granulation through in-line particle measurement using FBRM C35. J Pharm Sci 99:3205–3212
Ich (2008) ICH Guideline Q10 on Pharmaceutical Quality System. International Council for Harmonisation (ICH)
Ich (2009) ICH Guideline Q8 (R2) on Pharmaceutical Development. International Council for Harmonisation (ICH)
Ich (2019) ICH Guideline Q12 on Technical and regulatory considerations for pharmaceutical product lifecycle management. International Council for Harmonisation (ICH)
Ich (2021) ICH Guideline Q13: continuous manufacturing of drug substances and drug products. International Council for Harmonisation (ICH)
Ich (2023) ICH Guideline Q9(R1) on quality risk management. International Council for Harmonisation (ICH)
Ierapetritou M, Muzzio F, Reklaitis G (2016) Wiley Online Library, vol 62, pp 1846–1862
Igne B, Baldasano CN, Airiau C (2021) Feasibility of using light-induced fluorescence spectroscopy for low-dose formulations monitoring and control. J Pharm Innov 16:391–397
Ikeda C, Zhou G, Lee Y-C, Chouzouri G, Howell L, Marshall B, Bras L (2022) Application of online NIR spectroscopy to enhance process understanding and enable in-process control testing of secondary drying process for a spray-dried solid dispersion intermediate. J Pharm Sci 111:2540–2551
Järvinen K, Hoehe W, Järvinen M, Poutiainen S, Juuti M, Borchert S (2013) In-line monitoring of the drug content of powder mixtures and tablets by near-infrared spectroscopy during the continuous direct compression tableting process. Eur J Pharm Sci 48:680–688
Kamyar R, Pla DL, Husain A, Cogoni G, Wang Z (2021) Soft sensor for real-time estimation of tablet potency in continuous direct compression manufacturing operation. Int J Pharm 602:120624
Kern S, Wander L, Meyer K, Guhl S, Mukkula ARG, Holtkamp M, Salge M, Fleischer C, Weber N, King R (2019) Flexible automation with compact NMR spectroscopy for continuous production of pharmaceuticals. Anal Bioanal Chem 411:3037–3046
Kim D-W, Park J-B, Lee S-H, Weon K-Y (2017) Development of a Process Analytical Technology (PAT) method using near-infrared spectroscopy system for evaluating an active coating process for a low-dose drug. J Drug Deli Sci Technol 39:8–15
Kim EJ, Kim JH, Kim M-S, Jeong SH, Choi DH (2021) Process analytical technology tools for monitoring pharmaceutical unit operations: a control strategy for continuous process verification. Pharmaceutics 13:919
Kumar V, Taylor MK, Mehrotra A, Stagner WC (2013) Real-time particle size analysis using focused beam reflectance measurement as a process analytical technology tool for a continuous granulation–drying–milling process. AAPS PharmSciTech 14:523–530
Leane M, Pitt K, Reynolds GK, Dawson N, Ziegler I, Szepes A, Crean AM, Dall Agnol R & Group MCSW (2018) Manufacturing classification system in the real world: factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm Dev Technol 23:964–977
Lee SL, O’connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, Moore C, Yu LX, Woodcock J (2015) Modernizing pharmaceutical manufacturing: from batch to continuous production. J Pharm Innov 10:191–199
Lee WB, Widjaja E, Heng PWS, Chan LW (2019a) Near infrared spectroscopy for rapid and in-line detection of particle size distribution variability in lactose during mixing. Int J Pharm 566:454–462
Lee Y-C, Zhou G, Ikeda C, Chouzouri G, Howell L (2019b) Application of online near infrared for process understanding of spray-drying solution preparation. J Pharm Sci 108:1203–1210
Li Y, Anderson CA, Drennen Iii JK, Airiau C, Igne B (2018) Method development and validation of an inline process analytical technology method for blend monitoring in the tablet feed frame using Raman spectroscopy. Anal Chem 90:8436–8444
Lillotte TD, Joester M, Frindt B, Berghaus A, Lammens RF, Wagner KG (2021) UV–VIS spectra as potential process analytical technology (PAT) for measuring the density of compressed materials: evaluation of the CIELAB color space. Int J Pharm 603:120668
Lin H, May RK, Evans MJ, Zhong S, Gladden LF, Shen Y, Zeitler JA (2015) Impact of processing conditions on inter-tablet coating thickness variations measured by terahertz in-line sensing. J Pharm Sci 104:2513–2522
Lin H, Dong Y, Markl D, Williams BM, Zheng Y, Shen Y, Zeitler JA (2017) Measurement of the intertablet coating uniformity of a pharmaceutical pan coating process with combined terahertz and optical coherence tomography in-line sensing. J Pharm Sci 106:1075–1084
Liu S, Wu Z, Feng X, Ma Q, Qiao Y (2014) In International symposium on optoelectronic technology and application 2014: infrared technology and applications, pp 163–169 (SPIE)
Liu B, Wang J, Zeng J, Zhao L, Wang Y, Feng Y, Du R (2021) A review of high shear wet granulation for better process understanding, control and product development. Powder Technol 381:204–223
Luypaert J, Massart D, Vander Heyden Y (2007) Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta 72:865–883
Madarász L, Köte HB, Csorba K, Kovács V, Lengyel L, Marosi G, Farkas A, Nagy ZK, Domokos A (2022) In-line particle size measurement based on image analysis in a fully continuous granule manufacturing line for rapid process understanding and development. Int J Pharm 612:121280
Manley L, Hilden J, Valero P, Kramer T (2019) Tablet compression force as a process analytical technology (PAT): 100% inspection and control of tablet weight uniformity. J Pharm Sci 108:485–493
Markl D, Zettl M, Hannesschläger G, Sacher S, Leitner M, Buchsbaum A, Khinast JG (2015) Calibration-free in-line monitoring of pellet coating processes via optical coherence tomography. Chem Eng Sci 125:200–208
Martínez L, Peinado A, Liesum L, Betz G (2013) Use of near-infrared spectroscopy to quantify drug content on a continuous blending process: influence of mass flow and rotation speed variations. Eur J Pharm Biopharm 84:606–615
Matsunami K, Miyano T, Arai H, Nakagawa H, Hirao M, Sugiyama H (2018) Decision support method for the choice between batch and continuous technologies in solid drug product manufacturing. Ind Eng Chem Res 57:9798–9809
Mcauliffe M, O’mahony G, Blackshields C, Collins J, Egan D, Kiernan L, O’neill E, Lenihan S, Walker G, Crean A (2015) The use of PAT and off-line methods for monitoring of roller compacted ribbon and granule properties with a view to continuous processing. Org Process Res Dev 19:158–166
Meier R, Harting J, Happel J, Kleinebudde P (2017) Implementation of microwave sensors in continuous powder feeding—a novel tool to bridge refill phases. Pharm Ind 79:576–582
Meng W, Román-Ospino AD, Panikar SS, O’callaghan C, Gilliam SJ, Ramachandran R, Muzzio FJ (2019) Advanced process design and understanding of continuous twin-screw granulation via implementation of in-line process analytical technologies. Adv Powder Technol 30:879–894
Mészáros LA, Galata DL, Madarász L, Köte CK, Dávid Z, Domokos A, Szabó E, Nagy B, Marosi G (2020) Digital UV/VIS imaging: a rapid PAT tool for crushing strength, drug content and particle size distribution determination in tablets. Int J Pharm 578:119174
Nagy B, Farkas A, Gyürkés M, Komaromy-Hiller S, Démuth B, Szabó B, Nusser D, Borbás E, Marosi G, Nagy ZK (2017) In-line Raman spectroscopic monitoring and feedback control of a continuous twin-screw pharmaceutical powder blending and tableting process. Int J Pharm 530:21–29
Nagy B, Petra D, Galata DL, Démuth B, Borbás E, Marosi G, Nagy ZK, Farkas A (2019) Application of artificial neural networks for Process Analytical Technology-based dissolution testing. Int J Pharm 567:118464
Naidu VR, Deshpande RS, Syed MR, Deoghare P, Singh D, Wakte PS (2017) PAT-based control of fluid bed coating process using NIR spectroscopy to monitor the cellulose coating on pharmaceutical pellets. AAPS PharmSciTech 18:2045–2054
Nakano Y, Katakuse Y, Azechi Y (2018) An application of x-ray fluorescence as process analytical technology (PAT) to monitor particle coating processes. Chem Pharm Bull 66:596–601
Närvänen T, Lipsanen T, Antikainen O, Räikkönen H, Yliruusi J (2008) Controlling granule size by granulation liquid feed pulsing. Int J Pharm 357:132–138
Nasr MM, Krumme M, Matsuda Y, Trout BL, Badman C, Mascia S, Cooney CL, Jensen KD, Florence A, Johnston C (2017) Regulatory perspectives on continuous pharmaceutical manufacturing: moving from theory to practice: September 26–27, 2016, international symposium on the continuous manufacturing of pharmaceuticals. J Pharm Sci 106:3199–3206
Nishii T, Matsuzaki K, Morita S (2020) Real-time determination and visualization of two independent quantities during a manufacturing process of pharmaceutical tablets by near-infrared hyperspectral imaging combined with multivariate analysis. Int J Pharm 590:119871
Osorio JG, Stuessy G, Kemeny GJ, Muzzio FJ (2014) Characterization of pharmaceutical powder blends using in situ near-infrared chemical imaging. Chem Eng Sci 108:244–257
Pauli V, Elbaz F, Kleinebudde P, Krumme M (2019a) Orthogonal redundant monitoring of a new continuous fluid-bed dryer for pharmaceutical processing by means of mass and energy balance calculations and spectroscopic techniques. J Pharm Sci 108:2041–2055
Pauli V, Roggo Y, Kleinebudde P, Krumme M (2019b) Real-time monitoring of particle size distribution in a continuous granulation and drying process by near infrared spectroscopy. Eur J Pharm Biopharm 141:90–99
Pauli V, Roggo Y, Pellegatti L, Trung NQN, Elbaz F, Ensslin S, Kleinebudde P, Krumme M (2019c) Process analytical technology for continuous manufacturing tableting processing: A case study. J Pharm Biomed Anal 162:101–111
Pawar P, Wang Y, Keyvan G, Callegari G, Cuitino A, Muzzio F (2016) Enabling real time release testing by NIR prediction of dissolution of tablets made by continuous direct compression (CDC). Int J Pharm 512:96–107
Pawar P, Talwar S, Reddy D, Bandi CK, Wu H, Sowrirajan K, Friedman R, Drazer G, Drennen Iii JK, Muzzio FJ (2019) A “Large-N” content uniformity process analytical technology (PAT) method for phenytoin sodium tablets. J Pharm Sci 108:494–505
Pedersen T, Rantanen J, Naelapää K, Skibsted E (2020) Near infrared analysis of pharmaceutical powders with empirical target distribution optimization (ETDO). J Pharm Biomed Anal 181:113059
Pestieau A, Krier F, Thoorens G, Dupont A, Chavez P-F, Ziemons E, Hubert P, Evrard B (2014) Towards a real time release approach for manufacturing tablets using NIR spectroscopy. J Pharm Biomed Anal 98:60–67
Peters J, Bartscher K, Döscher C, Taute W, Höft M, Knöchel R, Breitkreutz J (2017) In-line moisture monitoring in fluidized bed granulation using a novel multi-resonance microwave sensor. Talanta 170:369–376
Peters J, Taute W, Döscher C, Meier R, Höft M, Knöchel R, Breitkreutz J (2018a) From laboratory-to pilot-scale: moisture monitoring in fluidized bed granulation by a novel microwave sensor using multivariate calibration approaches. Drug Dev Ind Pharm 44:961–968
Peters J, Teske A, Taute W, Döscher C, Höft M, Knöchel R, Breitkreutz J (2018b) Real-time process monitoring in a semi-continuous fluid-bed dryer–microwave resonance technology versus near-infrared spectroscopy. Int J Pharm 537:193–201
Peters J, Taute W, Döscher C, Höft M, Knöchel R, Breitkreutz J (2019) Moisture monitoring in fluid-bed granulation by multi-resonance microwave sensor: applicability on crystal-water containing donepezil granules. AAPS PharmSciTech 20:1–9
Picot A, Lacroix C (2004) Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int Dairy J 14:505–515
Rantanen J, Khinast J (2015) The future of pharmaceutical manufacturing sciences. J Pharm Sci 104:3612–3638
Razavi SM, Callegari G, Drazer G, Cuitino AM (2016) Toward predicting tensile strength of pharmaceutical tablets by ultrasound measurement in continuous manufacturing. Int J Pharm 507:83–89
Ré MI (1998) Microencapsulation by spray drying. Drying Technol 16:1195–1236
Reimers T, Thies J, Dietrich S, Quodbach J, Pein-Hackelbusch M (2019a) Evaluation of in-line particle measurement with an SFT-probe as monitoring tool for process automation using a new time-based buffer approach. Eur J Pharm Sci 128:162–170
Reimers T, Thies J, Stöckel P, Dietrich S, Pein-Hackelbusch M, Quodbach J (2019b) Implementation of real-time and in-line feedback control for a fluid bed granulation process. Int J Pharm 567:118452
Roggo Y, Pauli V, Jelsch M, Pellegatti L, Elbaz F, Ensslin S, Kleinebudde P, Krumme M (2020) Continuous manufacturing process monitoring of pharmaceutical solid dosage form: a case study. J Pharm Biomed Anal 179:112971
Román-Ospino AD, Tamrakar A, Igne B, Dimaso ET, Airiau C, Clancy DJ, Pereira G, Muzzio FJ, Singh R, Ramachandran R (2020) Characterization of NIR interfaces for the feeding and in-line monitoring of a continuous granulation process. Int J Pharm 574:118848
Sacher S, Wahl P, Weißensteiner M, Wolfgang M, Pokhilchuk Y, Looser B, Thies J, Raffa A, Khinast JG (2019) Shedding light on coatings: real-time monitoring of coating quality at industrial scale. Int J Pharm 566:57–66
Sacher S, Poms J, Rehrl J, Khinast JG (2021) PAT implementation for advanced process control in solid dosage manufacturing–a practical guide. Int J Pharm 121408
Sacher S, Poms J, Rehrl J, Khinast JG (2022) PAT implementation for advanced process control in solid dosage manufacturing–a practical guide. Int J Pharm 613:121408
Samanta AK, Karande AD, Ng KY, Heng PWS (2013) Application of near-infrared spectroscopy in real-time monitoring of product attributes of ribbed roller compacted flakes. AAPS PharmSciTech 14:86–100
Samanta AK (2012) A study on the roller compaction of undulated flakes by real-time process monitoring of compaction and cone milling of flakes.
Šašić S, Blackwood D, Liu A, Ward HW, Clarke H (2015) Detailed analysis of the online near-infrared spectra of pharmaceutical blend in a rotary tablet press feed frame. J Pharm Biomed Anal 103:73–79
Shibayama S, Funatsu K (2021) Investigation of preprocessing and validation methodologies for PAT: case study of the granulation and coating steps for the manufacturing of ethenzamide tablets. AAPS PharmSciTech 22:1–15
Silva BS, Colbert M-J, Santangelo M, Bartlett JA, Lapointe-Garant P-P, Simard J-S, Gosselin R (2019) Monitoring microsphere coating processes using PAT tools in a bench scale fluid bed. Eur J Pharm Sci 135:12–21
Suresh P, Sreedhar I, Vaidhiswaran R, Venugopal A (2017) A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chem Eng J 328:785–815
Suzuki Y, Sugiyama H, Kano M, Shimono R, Shimada G, Furukawa R, Mano E, Motoyama K, Koide T, Matsui Y (2021) Control strategy and methods for continuous direct compression processes. Asian J Pharm Sci 16:253–262
Suzzi D, Radl S, Khinast JG (2010) Local analysis of the tablet coating process: Impact of operation conditions on film quality. Chem Eng Sci 65:5699–5715
Testa CJ, Hu C, Shvedova K, Wu W, Sayin R, Casati F, Halkude BS, Hermant P, Shen DE, Ramnath A (2020) Design and commercialization of an end-to-end continuous pharmaceutical production process: a pilot plant case study. Org Process Res Dev 24:2874–2889
Tian G, Wei Y, Zhao J, Li W, Qu H (2018) Application of near-infrared spectroscopy combined with design of experiments for process development of the pulsed spray fluid bed granulation process. Powder Technol 339:521–533
Van Snick B, Holman J, Cunningham C, Kumar A, Vercruysse J, De Beer T, Remon JP, Vervaet C (2017a) Continuous direct compression as manufacturing platform for sustained release tablets. Int J Pharm 519:390–407
Van Snick B, Holman J, Vanhoorne V, Kumar A, De Beer T, Remon JP, Vervaet C (2017b) Development of a continuous direct compression platform for low-dose drug products. Int J Pharm 529:329–346
Vanarase AU, Järvinen M, Paaso J, Muzzio FJ (2013) Development of a methodology to estimate error in the on-line measurements of blend uniformity in a continuous powder mixing process. Powder Technol 241:263–271
Vanhoorne V, Vervaet C (2020) Recent progress in continuous manufacturing of oral solid dosage forms. Int J Pharm 579:119194
Vargas JM, Nielsen S, Cárdenas V, Gonzalez A, Aymat EY, Almodovar E, Classe G, Colón Y, Sanchez E, Romañach RJ (2018) Process analytical technology in continuous manufacturing of a commercial pharmaceutical product. Int J Pharm 538:167–178
Vovko AD, Hodžić B, Hudovornik G, Vrečer F (2021) Implementation of spatial filtering technique in monitoring roller compaction process. Int J Pharm 606:120896
Wahl PR, Fruhmann G, Sacher S, Straka G, Sowinski S, Khinast JG (2014) PAT for tableting: inline monitoring of API and excipients via NIR spectroscopy. Eur J Pharm Biopharm 87:271–278
Wahlich J (2021) (s Note: MDPI stays neutral with regard to jurisdictional claims in published …)
Ward HW, Blackwood DO, Polizzi M, Clarke H (2013) Monitoring blend potency in a tablet press feed frame using near infrared spectroscopy. J Pharm Biomed Anal 80:18–23
Warnecke S, Rinnan AM, Engelsen SB (2015) Fluorescence spectroscopy in process analytical technology (PAT): simultaneous quantification of two active pharmaceutical ingredients in a tablet formulation. Appl Spectrosc 69:323–331
Watano S (2007) Handbook of powder technology, vol 11. Elsevier, pp 477–498
Wiedey R, Kleinebudde P (2018) Infrared thermography—a new approach for in-line density measurement of ribbons produced from roll compaction. Powder Technol 337:17–24
Wiedey R, Kleinebudde P (2019) Potentials and limitations of thermography as an in-line tool for determining ribbon solid fraction. Powder Technol 341:2–10
Wiegel D, Eckardt G, Priese F, Wolf B (2016) In-line particle size measurement and agglomeration detection of pellet fluidized bed coating by Spatial Filter Velocimetry. Powder Technol 301:261–267
Wilms A, Meier R, Kleinebudde P (2021) Development and evaluation of an in-line and on-line monitoring system for granule size distributions in continuous roll compaction/dry granulation based on laser diffraction. J Pharm Innov 16:247–257
Wu H, White M, Khan M (2015) An integrated process analytical technology (PAT) approach for process dynamics-related measurement error evaluation and process design space development of a pharmaceutical powder blending bed. Org Process Res Dev 19:215–226
Yu M, Omar C, Weidemann M, Schmidt A, Litster JD, Salman AD (2020) Roller compaction: infrared thermography as a PAT for monitoring powder flow from feeding to compaction zone. Int J Pharm 578:119114
Yu M, Omar C, Weidemann M, Schmidt A, Litster JD, Salman AD (2022) Relationship between powder properties and uniformity of ribbon property using feeding guider designs with thermography (PAT) in roller compaction. Powder Technol 398:117134
Zaborenko N, Shi Z, Corredor CC, Smith-Goettler BM, Zhang L, Hermans A, Neu CM, Alam MA, Cohen MJ, Lu X (2019) First-principles and empirical approaches to predicting in vitro dissolution for pharmaceutical formulation and process development and for product release testing. AAPS J 21:1–20
Zhao Y, Li W, Shi Z, Drennen Iii JK, Anderson CA (2019) Prediction of dissolution profiles from process parameters, formulation, and spectroscopic measurements. J Pharm Sci 108:2119–2127
Zhong L, Gao L, Li L, Zang H (2020) Trends-process analytical technology in solid oral dosage manufacturing. Eur J Pharm Biopharm 153:187–199
Zhong L, Gao L, Li L, Nei L, Wei Y, Zhang K, Zhang H, Yin W, Xu D, Zang H (2022) Method development and validation of a near-infrared spectroscopic method for in-line API quantification during fluidized bed granulation. Spectrochim Acta Part A 274:121078
Ziaee A, Albadarin AB, Padrela L, Femmer T, O’reilly E, Walker G (2019) Spray drying of pharmaceuticals and biopharmaceuticals: critical parameters and experimental process optimization approaches. Eur J Pharm Sci 127:300–318
Funding
This research was supported by the Industrial Strategic Technology Development Program (20018218, Development of oral drug formulation technology and process technology based on continuous process) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (E.-A. Jung, Y.-J. Park, and J.‑E. Kim) declare no conflicts of interest.
Statement of human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jung, EA., Park, YJ. & Kim, JE. Application of continuous manufacturing for solid oral dosage forms. J. Pharm. Investig. 53, 457–474 (2023). https://doi.org/10.1007/s40005-023-00619-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00619-w