Abstract
Vitamin D is involved in retaining the balance of several minerals in the body, such as calcium and phosphate, which are crucial for the bone formation and development. These physiological effects of vitamin D are mediated by activation of the transcription factor vitamin D receptor (VDR). Moreover, vitamin D is closely related to several pathological conditions including osteoporosis, secondary hyperparathyroidism, cancer, psoriasis and autoimmune diseases, which is not only due to calcemic but also non-calcemic effects of vitamin D such as cell proliferation, differentiation, and immunomodulation. These various abilities of vitamin D have made VDR an attractive therapeutic target. Already, numerous vitamin D analogs have been developed and studied in in vitro disease models or animal models, and some have been in clinical trials or approved for the treatment of certain diseases. In addition, the transcriptional and/or post-transcriptional regulation by VDR activation also affects the genes involved in drug metabolism and disposition, possibly leading to pharmacokinetic changes of several drugs in clinical use. This review provides a detailed summary of therapeutic targets of VDR ligands, and their effects on the transporters and metabolic enzymes, causing in vitro and in vivo pharmacokinetic changes of several drugs. The clinical research is further required for the confirming the clinical relevance of pharmacokinetic drug interactions by VDR ligands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
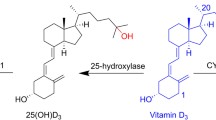
Vitamin D receptor (VDR) is a nuclear receptor that binds 1,25-dihydroxyvitamin D3 (Calcitriol, 1,25(OH)2D3), the active form of vitamin D3 (cholecalcitriol), and mediates its various important biological effects. VDR has been known to be involved in calcemic effects, calcium and phosphorus homeostasis and maintenance of bone content. On the other hands, additional non-calcemic effects such as cell proliferation, differentiation, and immunomodulation have been reported to be mediated by VDR. It has generated the interest in targeting the VDR as various therapeutic targets. The huge amount of vitamin D analogs have been synthesized for the purpose of treating the several diseases such as osteoporosis, secondary hyperparathyroidism, cancer, psoriasis and autoimmune diseases. In the first part of this review, we extensively summarized and discussed the vitamin D related disease and the vitamin D analogs which have been clinically approved or investigated so far (Fig. 1).
It has been reported that VDR regulates metabolic enzymes and transporters via VDR transcriptional and/or post-transcriptional regulation in vitro and in vivo (Thummel et al. 2001; Fan et al. 2009; Eloranta et al. 2012; Chow et al. 2010, 2013; Maeng et al. 2012). Since treatment with VDR ligands modulate the expressional levels of the metabolizing enzymes and transporters, these VDR-mediated regulation could generate the impact on pharmacokinetic changes of the drugs metabolized and/or transported by specific enzymes or transporters affected. Indeed, several studies have reported the pharmacokinetic changes of the drugs by VDR ligands treatment in vitro and in vivo. In the second and third part of this review, we summarized and discussed the modulation of drug transporters and metabolic enzymes in the important absorption and elimination organs by VDR activation in the previous literatures, and further discussed the pharmacokinetic relevance in terms of drug–drug interactions (DDIs) in vitro and in vivo (Fig. 1). It seems that VDR ligand drugs are more used as a second therapy rather than first line therapy. In addition, the diseases treated with VDR ligand drugs are mostly chronic disease, so the patients usually are likely to take other medicines with vitamin D analogs or VDR ligand drugs. Therefore, possible drug interactions should be considered when the VDR ligand drugs are used for the purpose of prevention or treatment of diseases.
VDR as a therapeutic target
A large number of vitamin D analogs (i.e., VDR ligand drugs) have been synthesized by numerous research groups. These analogs have been tested for the purpose of treating or preventing various diseases of the human body, including osteoporosis, secondary hyperparathyroidism, cancer, psoriasis and autoimmune diseases. By looking at the function and the role of vitamin D analogs in these diseases, the potential of VDR as a therapeutic target will be explored.
Bone diseases
A primary role of VDR is to maintain calcium and phosphate homeostasis during bone formation and resorption. Nutritional vitamin D deficiency, altered vitamin D responsiveness due to VDR mutations (hereditary vitamin D-resistant rickets), and impaired production of 1,25(OH)2D3 consequent to CYP27B1 mutations (pseudo-vitamin D deficiency) all have rickets as their major phenotype, showing that vitamin D and 1,25(OH)2D3 are significantly important to bone formation. Vitamin D deficiency may result in lower bone mineral density and increased risk of osteoporosis or bone fracture because a lack of vitamin D alters mineral metabolism in the whole body (Adami et al. 2009; Bell et al. 2010; Holick 2004; LeBoff et al. 1999). Accordingly, VDR ligands have been suggested to be useful for the prevention or the treatment of bone disease such as osteomalacia, osteoporosis, and rickets. A number of reports have demonstrated the prevention or the decrease of vertebral fractures and the increase of mineral density in total body and spine bone in post-menopausal women with 1,25(OH)2D3 treatment (Sunyecz 2008; Papadimitropoulos et al. 2002). 1,25(OH)2D3 has also been shown to treat secondary osteoporosis, especially corticosteroid-induced osteoporosis and post-transplantation bone loss in a clinical trial (Richy et al. 2004; El-Agroudy et al. 2003; Torres et al. 2004). However, the use of 1,25(OH)2D3 in the treatment of osteoporosis is restricted by hypercalcemia, a serious side effect. Hence, attempts have been made to apply less calcemic analogs of vitamin D3 to the treatment of osteoporosis. Alfacalcidol, 1α-hydroxyvitamin D3, a precursor of 1,25(OH)2D3, is an approved drug for the treatment of osteoporosis. Because alfacalcidol does not induce an immediate increase in calcium absorption from gut, it appears to be a safer treatment compared to 1,25(OH)2D3. Alfacalcidol treatment showed therapeutic advantages in vertebral fracture incidence and spinal bone mass in several clinical studies (Fujita et al. 2007; Felsenberg et al. 2011; Francis et al. 1996). 2-(3-hydroxypropoxy)-1,25(OH)2D3 (ED-71) have been shown to be an efficient treatment for osteoporosis, providing higher restoration of mineral density than alfacalcidol in ovariectomized rats (Kubodera et al. 2003). ED71 is also efficient to osteoporosis patients in clinical trials. Lumbar bone mineral density has increased in a dose-dependent manner without producing hypercalcemia and hypercalciuria (Kubodera et al. 2003). 2-methylene-19-nor-(20S)-1,25(OH)2D3 (2MD), a highly potent analog of 1,25(OH)2D3 promoted osteoclast formation and mineralization in human osteoblast cultures in vitro. 2MD also caused an increase in bone mass in ovariectomized rats in vivo (Shevde et al. 2002; Ke et al. 2005). Though the markers for bone formation were increased by 2MD in postmenopausal women, bone mineral density (BMD) was not increased because bone resorption was also stimulated (DeLuca et al. 2011). Ro-26-9228 (1α-fluoro-25-hydroxy-16,23E-diene-26,27-bishomo-20-epi-cholecalciferol), a secosteroidal vitamin D derivative bearing a fluoro group at 1α position decreased bone resorption and increased the number of differentiated osteoblasts in a rat model of osteoporosis (Peleg et al. 2002).
Secondary hyperparathyroidism
Secondary hyperparathyroidism is a parathyroid glands disease characterized by high parathyroid hormone (PTH) levels and low blood calcium concentration. It is an early emerging and a major complication of chronic kidney disease (CKD). Decreased renal function induces the accumulation of serum phosphate level because the kidney is the organ performing the elimination of phosphate from the body. High phosphate level results in the decrease of ionized calcium level, which causes the stimulation of PTH secretion and downregulation of VDR in the parathyroid gland (Goto et al. 2008; DeLuca 2008; Bricker 1972). Defective renal function also induces the decreased production of 1,25(OH)2D3. Vitamin D deficiency results in the enhanced synthesis of PTH and the increased proliferation of the parathyroid cells leading to the excessive secretion of PTH. (DeLuca 2008). Vitamin D deficiency is considered to play a crucial role during the initiation and the maintenance of hyperparathyroidism. Various vitamin D analogs, for example, maxacalcitol, paricalcitol, doxercalciferol, and falecalcitriol have been approved for the treatment of hyperparathyroidism. (Bikle 2014). Therapeutic effects with vitamin D and analogues are closely related to the reduced mortality in CKD patients, particularly in those having secondary hyperparathyroidism. (Duranton et al. 2013). 22-oxacalcitriol (OCT; Maxacalcitol) was shown to be highly potent to suppress PTH in vitro and in vivo (Brown et al. 1989). In the animal models of CKD, 22-oxacalcitriol was slightly less active than 1,25(OH)2D3 in suppressing PTH, but 22-oxacalcitriol’s much smaller calcemic activity has generated a wider therapeutic window (Brown et al. 1990; Hirata et al. 2002; Monier-Faugere et al. 1999; Naveh-Many and Silver 1993). Clinical trial studies for hemodialysis patients have confirmed that 22-oxacalcitriol was effective to suppress PTH levels (Akizawa et al. 2002, 2004; Hayashi et al. 2004; Tamura et al. 2005; Yasuda et al. 2003). Other preclinical studies have shown that 19-norD2 (Paricalcitol) was about 10 times less calcemic and phosphatemic, while only about 3 times less active in suppressing PTH (Slatopolsky et al. 1995). Control of secondary hyperparathyroidism in uremic rats by 19-norD2 could prevent or reverse the high turnover bone disease mediate by PTH (Slatopolsky et al. 2003). Several clinical studies have demonstrated that 19-norD2 effectively reduced PTH with very few hypercalcemic events (Martin et al. 1998a, b). 1α(OH)D2 (doxercalciferol) was also effective in decreasing PTH in predialysis patients (Coburn et al. 2004). Intravenous 1α(OH)D2 showed comparable PTH suppression to that observed with oral 1α(OH)D2. 26,27-F6-1,25(OH)2D3 (falecalcitriol) is more effective than calcitriol in vivo due to its slower metabolism. 23-hydroxylated metabolite of falecalcitriol is resistant to further metabolism and maintains higher activity (Imanishi et al. 1999). In dialysis patient treated with falecalcitriol, PTH was decreased by 25% with minimal change of serum calcium level (Nishizawa et al. 1991).
Cancers
Previous studies have suggest that calcitriol or its analogs have anti-proliferative effect in a wide range of tumor models including those of leukemia (Pepper et al. 2003), bladder (Ma et al. 2010), breast (Colston et al. 1992; Welsh et al. 2003), colon (Cross et al. 1991), kidney (Fujioka et al. 1998), lung (Nakagawa et al. 2005), pancreas (Colston et al. 1997), prostate (Getzenberg et al. 1997), bone (Hara et al. 2001), glioma (Naveilhan et al. 1994), and melanoma (Colston et al. 1981). Several reports have outlined the proposed mechanisms of antitumor activity of vitamin D (Beer and Myrthue 2004; Trump et al. 2010). It includes the inhibition of proliferation and the induction of differentiation (Pálmer et al. 2003; Jiang et al. 2003; Li et al. 2004; Muto et al. 1999; Wang et al. 1996; Zhang et al. 2006; Aguilera et al. 2007; Zhuang and Burnstein 1998; Campbell and Koeffler 1997), apoptosis (Chung et al. 2009; Bernardi et al. 2002; Pendás-Franco et al. 2008; McGuire et al. 2001; Guzey et al. 2002; Pepper et al. 2003), the prevention of invasiveness (Schwartz et al. 1997; Yudoh et al. 1999; Sung and Feldman 2000), and angiogenesis (Majewski et al. 1996; Mantell et al. 2000). Vitamin D and its several analogs such as seocalcitol (22,24-diene-24,26,27-trishomo-1,25(OH)2D3) (Bhatia et al. 2009; Ghous et al. 2008), inecalcitol (19-nor-14-epi-23-yne-1,25(OH)2D3), TX527 (19-nor-14,20-bisepi-23-yne-1,25(OH)2D3) (Ma et al. 2013; Okamoto et al. 2012), paricalcitol (19-nor-1,25(OH)2D3) (Kumagai et al. 2003; Park et al. 2012; Schwartz et al. 2008), doxercalciferol (1α(OH)D2) (van Ginkel et al. 2007; Albert et al. 2004; Grostern et al. 2002), maxacalcitol (22oxa-1,25(OH)2D3) (Seubwai et al. 2010; Kawa et al. 1996), BGP-13 (1R, 3S, 5Z)-5-((8E)-2-((3R)-3-((2R,3S)-3-(5-cyclopropyl-3H-1,2-dioxol-3-yl)-2-ethyl-3-methylcyclohexylidene) ethylidene)-4-methylenecyclohexane-1,3-diol), BXL0124 (20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-1,25(OH)2D3) (So et al. 2011; Lee et al. 2010), MART-10 (19-nor-2α-(3-hydroxypropyl)-1,25(OH)2D3) (Chiang et al. 2013), and 1,25(OH)2D3-3-bromoacetate have been evaluated in rodent models to assess their anticancer activity and safety in vivo, and they have demonstrated the inhibition of tumor growth in various models of tumor types. Based on their promising results of anticancer activity, some analogs have been tested in cancer patients. Unfortunately, however, they did not lead to conclusive results for the beneficial effects (Leyssens et al. 2014; Trump et al. 2010; Hargrove et al. 2014; Narvaez et al. 2014; Marchwicka et al. 2014). Further studies are needed to fully understand the molecular mechanisms of action and the optimal applications of vitamin D analogue in cancer.
Psoriasis
Psoriasis is a chronic autoimmune disease characterized by red, itchy and scaly patches of skin because of the rapid growth of skin cells. It has been reported that vitamin D may play an important role in the skin diseases (Soleymani et al. 2015; Reichrath 2007). Vitamin D controls the proliferation and differentiation of keratinocytes, modulates immune systems and selectively induces apoptosis in the skin (Soleymani et al. 2015; Lehmann et al. 2004; Bollag 2007; Milde et al. 1991). Though the mechanisms mediating the effects of Vitamin D are not fully understood, it is very well-known that those effects are mediated via VDR at least in part (Lehmann et al. 2004; Trémezaygues and Reichrath 2011). VDR is expressed in keratinocytes, fibroblasts, Langerhans cells, sebaceous gland cells, and endothelial cells, which are related to the immune system of skin (Milde et al. 1991). VDR knockout mice show the suppressed level of epidermal differentiation marker and do not respond to the antiproliferative effects of vitamin D analogs in vitro (Bikle et al. 2004; Sakai and Demay 2000). A number of clinical studies have demonstrated that vitamin D and its analogs are effective and safe for treatment of psoriasis (Soleymani et al. 2015; Holick et al. 1996; Pérez et al. 1996; Kragballe et al. 1988, 1991; van de Kerkhof et al. 1989, 2002; Barker et al. 1999; Durakovic et al. 2001; Miyachi et al. 2002; Katayama et al. 2002). Topical application of calcipotriol was shown to be more effective for the treatment of psoriasis than that of bethamethasone 17-valerate (Kragballe et al. 1991). Maxacalcitol has been shown to be a more effective in reducing erythema and scaling than calcipotriol (Barker et al. 1999). Tacalcitol was also effective and safe for the treatment of not only refractory psoriasis having low responsiveness to topical corticosteroids, but also psoriasis vulgaris and plaque psoriasis (Katayama et al. 2002; Miyachi et al. 2002; van de Kerkho et al. 2002).
Cardiovascular diseases
A few studies have demonstrated the relationship between cardiovascular homeostasis and vitamin D (Weishaar et al. 1990; Weishaar and Simpson 1987a, b). Those studies suggested that vitamin D plays a role in retaining cardiovascular homeostasis through not only the direct actions of 1,25(OH)2D3 on VDR in cardiomyocytes but also the indirect actions on circulating calcium and hormones. Vitamin D receptors are expressed in various cells, such as cardiomyocytes, vascular smooth muscle cells and endothelial cells. Through the interaction with the vitamin D receptors on the cardiomyocyte, 1,25(OH)2D3 controls (1) calcium influx into the cell, (2) the amount of free cytosolic calcium available thus modifying contractility of the heart, and (3) cell growth and proliferation (Nibbelink et al. 2007; Walters et al. 1987). The direct effects of vitamin D on the VDR have been examined using VDR knockout mice (Simpson et al. 2007; Xiang et al. 2005; Rahman et al. 2007). In those studies, the data have demonstrated the profound cardiac hypertrophy, the overstimulation of the renin-angiotensin system (RAS), and the increase of both fibrotic lesions and heart weight-to-body weight ratio in VDR knockout mice. A number of studies in human have indicated the association of vitamin D deficiency with cardiovascular disease (Rostand and Drüeke 1999; Wang et al. 2008; Pilz et al. 2008a, b). These data have raised the possibility that supplementation of vitamin D or its analogs could be useful treatments or preventions for cardiovascular diseases. However, only a few studies have suggested that the increment of 25(OH)D levels may provide benefit to patients with heart failure or hypertension (Nemerovski et al. 2009; Schleithoff et al. 2006; Shedeed 2012). Further clinical studies should be needed to confirm that vitamin D supplementation can prevent or treat cardiovascular diseases.
Autoimmune diseases
Both vitamin D metabolizing enzymes and VDRs are also present in a variety of immune cells including antigen-presenting-cells, T cells, B cells and monocytes. These cells can convert 25(OH)D to 1,25(OH)2D3 through CYP27B1 and provide sufficient amount of 1,25(OH)2D3 for its local function (Morán-Auth et al. 2013). It is well known that vitamin D plays an important role in the management of immune functions (Prietl et al. 2013; Wöbke et al. 2014; Zhang et al. 2013). Therefore, impaired or insufficient levels of vitamin D result in the dysregulation of immune responses. It has been reported that several inflammatory autoimmune disease including asthma (Li et al. 2011; Bener et al. 2012), inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease (Ananthakrishnan et al. 2012; Levin et al. 2011), multiple sclerosis (Martinelli et al. 2014; Pierrot-Deseilligny et al. 2012), rheumatic disorder (Shapira et al. 2010), and systemic lupus erythematosus (Abou-Raya et al. 2013) are all related to vitamin D deficiency. Consequently, vitamin D supplementation has been proposed as a hopeful and safe way for the prevention and the treatment of immune diseases (Prietl et al. 2013). Vitamin D seems to present a positive effect on autoimmune disease through the suppression of immune system (Daniel et al. 2008). 1,25(OH)2D3 inhibited the differentiation and maturation of dendritic cells, leading to the suppression of dendritic cell-dependent T cell activation (van Etten and Mathieu 2005). 1,25(OH)2D3 also blocked the secretion of both interleukin (IL)-2 and interferon (IFN)-gamma by T helper (Th) 1 and suppressed the secretion of pro-Th1 cytokine IL-12 by antigen-presenting cells (Lemire 2000). Th17 effector cells also can be affected by immunosuppressive effect of 1,25(OH)2D3 (Daniel et al. 2008). Though a few studies indicated the effectiveness of vitamin D supplementation in autoimmune disease including atopic dermatitis and multiple sclerosis (Åivo et al. 2015; Di Filippo et al. 2015), further and long-term clinical trials are required to determine whether vitamin D supplementation has beneficial effect in immune diseases.
Modulation of drug transporters and metabolic enzymes
VDR is the well-known nuclear receptor, which has significant homology with other xenobiotic nuclear receptors such as the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR). Like PXR and CAR, the VDR could regulate drug transporters and metabolic enzymes. In this section, regulations of drug transporters and metabolic enzymes in the important absorption/elimination organ are summarized in terms of expressional change by VDR ligand drugs in vitro and in vivo.
Drug transporters
Intestine
The regulation of transporters by 1,25(OH)2D3 was first reported for the apical sodium dependent bile acid transporter (Asbt) in the rat intestine (Chen et al. 2006). The treatment of 0.64 nmol/kg/day of 1,25(OH)2D3 for 4 continuous days led to the increased level of Asbt by 2–3 fold in the ileum segment. Consistently, Chow et al. (2009) reported that ileum Asbt was significantly increased in the rat for 2.56 nmol/kg/day of 1,25(OH)2D3. Similarly, an important bile acid transporter, the mRNA of organic solute transporter (Ostα and Ostβ), localized mostly in the ileum, was modestly induced by the low doses (Chow et al. 2009).
The representative observation for the intestinal transporters’ regulation by VDR activation are summarized in Tables 1 and 2. Primary active transporters have been extensively investigated by VDR activation in the intestine. To the best of our knowledge, the first observation for the intestinal efflux transporters by VDR dependent regulation was the colon multidrug resistance-associated protein 3 (Mrp3) in mice, with identified VDR response element (VDRE) for the Mrp3 promoter (McCarthy et al. 2005). In vitro Caco-2 cells, several studies to report expressional change of intestine efflux transporters exist (Fan et al. 2009; Maeng et al. 2012). The ATP-dependent efflux transporter, P-glycoprotein (P-gp), encoded by the multidrug resistance 1 gene (MDR1), and the multidrug resistance-associated proteins (MRPs) such as MRP2 and MRP4 was investigated in the 100 nM VDR ligands (1,25(OH)2D3, 1α-hydroxyvitamin D3, 1α-hydroxyvitamin D2 and 25-hydroxyvitamin D3) (Fan et al. 2009). The treatment of VDR ligands led to higher protein expression of P-gp, MRP2 and MRP4. The increased mRNA levels of P-gp and MRP2 were observed in the VDR ligand treated Caco-2 cells. Interestingly, only the increased change in the protein, not mRNA level of MRP4 was constituently found with 1,25(OH)2D3 in Caoo-2 cells (Fan et al. 2009; Maeng et al. 2012). Moreover, in the rat intestine, protein levels of Mrp2, Mrp3 and Mrp4 were induced with no change of mRNA levels after 1,25(OH)2D3 treatment, whereas P-gp remained unchanged for protein/mRNA levels (Chow et al. 2010). On the other hands, 1α(OH)D2, a synthetic vitamin D2 analog with 1.28 nmol/kg day every other day for 8 days showed a moderate increase in P-gp protein in the ileum of rats (Chow et al. 2011a, b). However, in the ileum segment of 1,25(OH)2D3-treated mice, there was no demonstrable change in the P-gp, Mrp3 and Mrp4 (Chow et al. 2013).
Secondary active transporters including organic anion transporters and the oligopeptide transporter have rarely been investigated so far. PepT1 protein levels in the upper small intestine were significantly increased at the 2.56 nmol/kg/day of 1,25(OH)2D3 (Chow et al. 2010). In addition, organic anion transporter 1 (Oat1) and organic anion transporter (Oat3) in the rat intestine were investigated. Namely, small increase in Oat1 mRNA in the duodenum and significant increase in Oat3 mRNA in the colon were observed with 1,25(OH)2D3 treatment (Chow et al. 2010). However, in the ileum, levels of mRNA for Oat1 and Oat3 were markedly decreased in the 1,25(OH)2D3-treated rats (Chow et al. 2010). Among the organic anion-transporting polypeptides (OATPs), OATP1A2 gene and protein greatly increased in the Caco-2 cells with 500 nM vitamin D3, suggesting that OATP1A2 gene is induced by vitamin D3 at the transcriptional level through VDR (Eloranta et al. 2012).
Kidney
Because the kidney has the highest expressional levels of VDR compared with other organs and play an important role on the excretion of xenobiotics from body, VDR ligands can regulate significant influx and efflux transporters in the kidney. The representative examples for the kidney transporters’ regulation by VDR activation are summarized in Table 3. In terms of efflux transporters, upregulation of mRNA/protein levels of renal P-gp localized in the apical membrane of kidney epithelial cells was reported in the 1,25(OH)2D3-treated rats (Chow et al. 2010). Among the renal Mrps, only Mrp4 mRNA levels were decreased, whereas Mrp2 and Mrp3 remained unchanged in the 1,25(OH)2D3-treated rats (Chow et al. 2010). The treatment of 1α(OH)D2, a synthetic vitamin D2 analog with 1.28 nmol/kg day every other day for 8 days led to increased mRNA levels of P-gp, Mrp2 and Mrp3 in the rats. Only levels of P-gp protein was significantly enhanced 1α(OH)D2-treated kidney (Chow et al. 2011b). In contrast, 1,25(OH)2D3 or 1α(OH)D3 treatment increased P-gp and Mrp4 in the mice kidney (Chow et al. 2013).
In the renal solute carrier (SLC) transporters, levels of Oat1 and Oat3 mRNA and/or protein expression was significantly reduced in the rat kidney pretreated with 1,25(OH)2D3 (Chow et al. 2010; Kim et al. 2014). In addition, renal PepT1 and Pept2 expression levels were markedly decreased in the 1,25(OH)2D3-treated rats (Chow et al. 2010). In comparison, renal PepT1 mRNA expression was also decreased in the 1α(OH)D2-treated rats (Chow et al. 2011b). More recently, the vitamin D-deficiency resulted in the down-regulation of renal Oat3 in the C57BL/6 mouse (Quach et al. 2018).
Liver
It is known that the level of VDR in the rat hepatocytes exist in the very low levels. Therefore, although less effects on the regulation of hepatic transporters by VDR activation is likely to be expected, 1,25(OH)2D3-treatment may exert indirect or secondary effects on the hepatic transporters likely due to the activation or inhibition of other nuclear receptors such as FXR in the liver. It is known that there is little change on the sinusoidal uptake and efflux transporters in the rat liver with 1,25(OH)2D3-treatment (Chow et al. 2009). For examples, for the hepatic uptake transporters, levels of mRNA/protein expression of Oatp1a1, Oatp1a4 and Oatp1b2 remained unaltered in the rat liver with 1,25(OH)2D3-treatment. The sinusoidal efflux transporters, Mrp3 and Mrp4, showed insignificant increase on the protein levels. 1α(OH)D2-treatment also demonstrated hepatic Mrp3 mRNA was induced by twofold in rats (Chow et al. 2011b). However, for the hepatic canalicular transporters, the mRNA levels of Bsep and Mdr1a were found to be increased significantly. The levels of P-gp protein were significantly increased by about 2.5-fold in the rat liver with 1,25(OH)2D3-treatment. Consistently, 1,25(OH)2D3 or 1,25(OH)2D3 showed no significant changes on the expression levels of hepatic transporters in the mice (Chow et al. 2013).
Metabolic enzymes
Phase I enzymes
With respect to cytochrome P450 monooxygenases (CYPs), CYP3A4, the most abundant CYP isoform, was first found to be increased by the levels of mRNA and protein in human intestinal model, Caco-2 cells with 1,25(OH)2D3-treatment (Schmiedlin-Ren et al. 1997). Furthermore, Thummel et al. (2001) demonstrated VDR induces expression of intestinal CYP3A by binding of the activated VDR-RXR heterodimer to the CYP3A PXR response element to induce CYP3A4 transcription. Also, VDRE sequence for human CYP3A4 gene has been identified (Thompson et al. 2002). It is known that the classical VDREs consist of a direct repeat of nuclear receptor half-sites separated by 3 nucleotides (DR3) (Carlberg 1995). In human ileum and liver, 1,25(OH)2D3 induced CYP3A4 expression (Khan et al. 2009). Rat Cyp3a9 (Zierold et al. 2006) and Cyp3a1 (Khan et al. 2009; Chow et al. 2009; Xu et al. 2006) in the intestine consistently have been reported to be induced by VDR ligands (Chow et al. 2009). However, in the rat liver, the mRNA of hepatic Cyp3a1 and Cyp3a9 remained unchanged whereas Cyp3a2 in the cholangiocyte was slightly increased (Chow et al. 2009). The secondary bile acid lithocholic acid (LCA) was reported to induce CYP3A4 expression, which involves detoxification of LCA in human (Makishima et al. 2002) and mice (Cheng et al. 2014). On the other hands, other CYP isoforms by VDR activation has been rarely investigated so far. For examples, in the primary human hepatocytes, CYP2B6 and CYP2C9 could be induced by the 1,25(OH)2D3-treatment (Drocourt et al. 2002). The possibility of regulation for other CYP subfamilies is further required to investigate by VDR activation.
Phase II enzymes
Among the Phase II enzymes, dehydroepiandrosterone sulfotransferase (SULT2A1), abundantly expressed in the liver and intestine, was first reported to be a target of transcription of activation of VDR (Echchgadda et al. 2004). In LS180 colorectal adenocarcinoma cells, the treatment with 1,25(OH)2D3 selectively upregulated human cytosolic SULT1C2 protein levels (Rondini et al. 2014). Moreover, it has been demonstrated that Vit D3-inducible SULT1C2 transcription is mediated through a vitamin D response element (VDRE) in exon 1 (Barrett et al. 2016). Similarly, the regulation of UDP-glucuronosyltransferase (UGT) by VDR activation was reported in a few literatures. UGT1A gene expression is significantly induced by 1,25(OH)2D3 treatment in LS180 cells (Meyer et al. 2012). Two intestinal UGT1A8 and UGT1A10 also have a correlation with transcription levels of VDR and 1,25(OH)2D3 treatment significantly increased transcription of both UGT genes (Wang et al. 2016).
Pharmacokinetic drug interactions with VDR ligands
Pharmacokinetic drug interactions describe how the medication can affect the absorption, distribution, metabolism, or excretion of another medication. VDR ligands are likely to be administered simultaneously with other drugs as adjunctive treatment and nutritional supplements in patients with various diseases because they have various pharmacological and biological activities. Thus, VDR ligands could cause drug interactions by modulation of transporters and metabolic enzymes involved in drug disposition described in the previous section, leading to undesirable effects such as inefficiency and adverse effects. This section summarizes the pharmacokinetic drug interactions by VDR ligands reported in the literature.
Drug interactions via transporter modulations
Digoxin
Recently, drug–drug interaction (DDI) caused by modulation of transporters have been reported to have clinical relevance. In particular, there are several in vitro and in vivo studies demonstrating the clinical significance of P-gp in oral bioavailability of drugs.
Digoxin, the P-gp probe substrate, has been used to evaluate P-gp function from in vitro study or to investigate the in vivo DDI effect. The effect of VDR ligand on the expression and function of P-gp was also studied using digoxin as mentioned above. The apparent basal (B)-to-apical (A) permeability (Papp) of [3H]digoxin was significantly increased in Caco-2 cell treated with 1,25(OH)2D3 for 3 days compared to control group (15.1 × 10− 6 versus 11.8 × 10− 6 cm/s) without changes of the Papp in the A-to-B direction, resulting in increased efflux ratio (from 5.8 to 8.0) (Fan et al. 2009). These results were probably due to increase of P-gp expression by VDR activation. However, the Papp of digoxin in both A-to-B and B-to-A directions was not significantly changed by 1,25(OH)2D3 when the transport was investigated in the rat everted ileac sac, suggesting that functional activity of P-gp had remained unchanged by VDR activation (Maeng et al. 2011). The effect of vitamin D3 on digoxin pharmacokinetics has also been investigated in a clinical setting (Kota et al. 2012). The plasma concentration of digoxin was not significantly changed by coadministration of vitamin D3, which was consistent with the no changes in the A-to-B and B-to-A permeability of digoxin in the above transport study using rat everted ileac sac, suggesting that there was no clinically significant P-gp-mediated drug interaction between digoxin and vitamin D3 (Kota et al. 2012).
However, in in vivo drug–drug interaction study in mice, the area under the concentration–time curve (AUC) of digoxin based on plasma and brain concentration was decreased (24% and 23%, respectively) in 1,25(OH)2D3-treated group, which is caused by increased renal (74%) and total body (34%) clearances of digoxin (Chow et al. 2011a, b). The results suggest the regulation of P-gp by VDR ligand could affect the renal and brain disposition of P-gp substrates, resulting in changes in plasma concentration of digoxin. The reason for interspecies difference in pharmacokinetic changes of digoxin by VDR ligands needs to be clarified for the predictive accuracy of drug–drug interactions.
Adefovir dipivoxil
Effects of 1,25(OH)2D3 on the in vtiro permeability of adefovir dipivoxil (P-gp substrate) and its metabolites, mono(POM)-PMEA and adefovir (MRP4 substrate), were also investigated in Caco-2 cells (Maeng et al. 2012). Basolateral (B) to apical (A) (B-to-A) transport of adefovir dipivoxil, the prodrug of adeforvir, in Caco-2 cells was increased (from 1.97 to 3.19) by 1,25(OH)2D3 treatment, resulting in elevation of efflux ratio (ER) from 1.97 to 3.19. The ER value in 1,25(OH)2D3-treated groups were reduced to 1.57 by verapamil treatment indicating the increased efflux of the drug could be due to upregulation of P-gp by 1,25(OH)2D3. However, the ER ratio of adeforvir dipivoxil was not changed compared with control group because both A to B and B to A permeability of adefovir dipivoxil was increased by 1,25(OH)2D3. The apical efflux of adeforvir, the active metabolite of adefovir dipivoxil, was also increased by 1,25(OH)2D3, which was possibly due to increased MRP4 by 1,25(OH)2D3. The metabolism of adefovir dipivoxil to form an adefovir was unaffected by 1,25(OH)2D3 in this study, but the interpretation of the result should be careful because activity and expression of metabolic enzyme, an esterase, for hydrolysis of adefovir dipivoxil in Caco-2 cells are different from those in vivo (Maeng et al. 2012).
The effect of 1,25(OH)2D3 on the oral absorption and disposition of adefovir dipivoxil was also investigated in rats (Yoon et al. 2015). After intravenous administration of adefovir dipivoxil, there was no significant difference in pharmacokinetic parameters such as AUC, CL, Vss, MRT, and t1/2 of adefovir between control and 1,25(OH)2D3-treated rats suggesting that renal excretion was not affected by 1,25(OH)2D3 because adefovir is eliminated primarily by renal excretion. In addition, in the in situ closed loop study, no significant difference was observed in the remaining fraction of adefovir dipivoxil in the all regions of the intestinal loop between the two groups. However, the AUC and maximum plasma concentration (Cmax) of adefovir were significantly (p < 0.05) increased in 1,25(OH)2D3-treated rats compared with control rats by 64.5 and 109.9%, respectively, resulting in increased bioavailability (21.5% increase). Based on all the results above, the enhanced bioavailability seemed to be due to increased permeability of adefovir across the basolateral membrane via the induction of MRP4 (Yoon et al. 2015).
Cefdinir and cefadroxil
Cefdinir and cefadroxil, the cephalosporin antibiotics, are eliminated via renal OAT1/OAT3. The AUC of cefdinir or cefadroxil after intravenous and oral administration was significantly increased in the 1,25(OH)2D3 treatment group compared with the control group because of decreased CL and CLR caused by significantly decreased kidney uptake and cumulative urinary recovery (Kim et al. 2014). Tissue distribution study also showed the reduced the tissue partition coefficient (Kp) in the kidney. These results suggested that decreased renal elimination of cefdinir and cefadroxil was attributed to downregulation of rOat1/3 by 1,25(OH)2D3 treatment (Kim et al. 2014).
JPB485
JBP485 (cyclo-trans-4-l-hydroxyprolyl-l-serine), a dipeptide compound having liver and gastrointestinal protective effects, has also been reported to be primarily excreted by Oat1 and Oat3 in rat kidney (Guo et al. 2012). The AUC of JBP485 after intravenous administration were significantly increased by 2.6-fold in the1,25(OH)2D3 treatment group. In contrast, the renal clearance of JBP485 and uptake of JBP485 into kidney slices were significantly decreased after 1,25(OH)2D3 treatment. The interaction between JBP485 and 1,25(OH)2D3 could also be due to inhibitory effect of 1,25(OH)2D3 on expression of Oats in rat kidney (Miao et al. 2013).
Drug interactions via metabolic enzymes modulations
Midazolam
Midazolam is a widely used probe substrate for CYP3A4 to investigate drug–drug interaction by modulation of CYP3A4. Treatment of Caco-2 cells with vitamin D analogs led to increased midazolam hydroxylation activity due to enhancement of CYP3A4 mRNA and protein levels in Caco-2 cells (Schmiedlin-Ren et al. 1997). Likewise, dose-dependent increase in midazolam 1′-hydroxylation activity was observed with an increase in CYP3A4 protein in Caco-2 cells treated with 1,25(OH)2D3 for 2 weeks (Thummel et al. 2001). Although midazolam was used to confirm the functional change of CYP3A4 modulation by VDR ligands, the above results suggest a potential of VDR ligand-mediated drug interaction, resulting in concentration change of CYP3A4 substrate.
Atorvastatin
The effect of vitamin D supplementation on pharmacokinetics of atorvastatin, an another CYP3A4 probe substrate, has been investigated (Schwartz 2009). The plasma concentration of atorvastatin and its active metabolites (2-hydroxy-atorvastatin acid and 4-hydroxy-atorvastatin acid) were significantly decreased in patient during vitamin D supplementation. In addition, there was a tendency of negative correlation between total active atorvastatin and 1,25(OH)2D3 concentration. These results could be predicted because increase in CYP3A4 expression by VDR ligands have been previously reported. However, LDL-cholesterol and total-cholesterol was decreased in those patients indicating drug effects was enhanced by unknown mechanism. The mechanism of this controversial results remains to be elucidated.
As reviewed above, the VDR ligands have a potential to elicit drug interaction effects via the regulation of transporters and metabolic enzymes. The in vivo drug interaction effect from various non-clinical and clinical studies are summarized in Table 4.
Conclusion
In summary, this review summarizes the vitamin D related clinically relevant diseases and the vitamin D analogs which have been clinically approved or investigated so far. Importantly, the VDR ligand (i.e, VDR activation) modulates various drug transporters and metabolic enzymes such as CYP3A in vitro and in vivo. Indeed, the VDR ligands have a potential to elicit drug interaction effects via the regulation of transporters and metabolic enzymes. Thus, the development of new VDR ligand drugs may require considering of the potential for drug interactions by modulation of drug transporters and enzymes via VDR activation.
References
Abou-Raya A, Abou-Raya S, Helmii M (2013) The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 40:265–272
Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE, Minisola S, Rossini M (2009) Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int 20:239–244
Aguilera O, Peña C, García JM, Larriba MJ, Ordóñez-Morán P, Navarro D, Barbáchano A, López de Silanes I, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, González-Sancho JM, Muñoz A (2007) The Wnt antagonist DICKKOPF-1 gene is induced by 1α,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 28:1877–1884
Åivo J, Hänninen A, Ilonen J, Soilu-Hänninen M (2015) Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J Neuroimmunol 280:12–15
Akizawa T, Suzuki M, Akiba T, Nishizawa Y, Ohashi Y, Ogata E, Slatopolsky E, Kurokawa K (2002) Long-term effect of 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in haemodialysis patients. One-year administration study. Nephrol Dial Transplant 17(Suppl. 10):28–36
Akizawa T, Ohashi Y, Akiba T, Suzuki M, Nishizawa Y, Ogata E, Slatopolsky E, Kurokawa K (2004) Dose-response study of 22-oxacalcitriol in patients with secondary hyperparathyroidism. Ther Apher Dial 8:480–491
Albert DM, Kumar A, Strugnell SA, Darjatmoko SR, Lokken JM, Lindstrom MJ, Patel S (2004) Effectiveness of vitamin D analogues in treating large tumors and during prolonged use in murine retinoblastoma models. Arch Ophthalmol 122:1357–1362
Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT (2012) Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology 142:482–489
Barker JN, Ashton RE, Marks R, Harris RI, Berth-Jones J (1999) Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo-controlled, double-blind, dose-finding study with active comparator. Br J Dermatol 141:274–278
Barrett KG, Fang H, Kocarek TA, Runge-Morris M (2016) Transcriptional regulation of cytosolic sulfotransferase 1C2 by vitamin D receptor in LS180 human colorectal adenocarcinoma cells. Drug Metab Dispos 44(8):1431–1434
Beer TM, Myrthue A (2004) Calcitriol in cancer treatment: from the lab to the clinic. Mol Cancer Ther 3:373–381
Bell TD, Demay MB, Burnett-Bowie SA (2010) The biology and pathology of vitamin D control in bone. J Cell Biochem 111:7–13
Bener A, Ehlayel MS, Tulic MK, Hamid Q (2012) Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol 157:168–175
Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL (2002) Antiproliferative effects of 1α,25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology 143:2508–2514
Bhatia V, Saini MK, Shen X, Bi LX, Qiu S, Weigel NL, Falzon M (2009) EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther 8:1787–1798
Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21:319–329
Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM (2004) 25-Hydroxyvitamin D 1 α-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122:984–992
Bollag WB (2007) Differentiation of human keratinocytes requires the Vitamin D receptor and its coactivators. J Invest Dermatol 127:748–750
Bricker NS (1972) On the pathogenesis of the uremic state. An exposition of the ‘trade-off hypothesis’. N Engl J Med 286:1093–1099
Brown AJ, Ritter CR, Finch JL, Morrissey J, Martin KJ, Murayama E, Nishii Y, Slatopolsky E (1989) The noncalcemic analogue of vitamin D, 22-oxacalcitriol, suppresses parathyroid hormone synthesis and secretion. J Clin Invest 84:728–732
Brown AJ, Finch JL, Lopez-Hilker S, Dusso A, Ritter C, Pernalete N, Slatopolsky E (1990) New active analogues of vitamin D with low calcemic activity. Kidney Int 29(Suppl. 29):S22–S27
Campbell MJ, Koeffler HP (1997) Toward therapeutic intervention of cancer by vitamin D compounds. J Natl Cancer Inst 89:182–185
Carlberg C (1995) Mechanisms of nuclear signalling by vitamin D3. Interplay with retinoid and thyroid hormone signalling. Eur J Biochem 231(3):517–527
Chen X, Chen F, Liu S, Glaeser H, Dawson PA, Hofmann AF, Kim RB, Shneider BL, Pang KS (2006) Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1α,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol Pharmacol 69:1913–1923
Cheng J, Fang ZZ, Kim JH, Krausz KW, Tanaka N, Chiang JY, Gonzalez FJ (2014) Intestinal CYP3A4 protects against lithocholic acid-induced hepatotoxicity in intestine-specific VDR-deficient mice. J Lipid Res 55(3):455–465
Chiang KC, Yeh CN, Hsu JT, Yeh TS, Jan YY, Wu CT, Chen HY, Jwo SC, Takano M, Kittaka A, Juang HH, Chen TC (2013) Evaluation of the potential therapeutic role of a new generation of vitamin D analog, MART-10, in human pancreatic cancer cells in vitro and in vivo. Cell Cycle 15:1316–1325
Chow EC, Durk MR, Maeng HJ, Pang KS (2009) 1α,25-Dihydroxyvitamin D(3) triggered vitamin D receptor and farnesoid X receptor-like effects in rat intestine and liver in vivo. Biopharm Drug Dispos 30:457–475
Chow EC, Sun H, Khan AA, Groothuis GM, Pang KS (2010) Effects of 1α,25-dihydroxyvitamin D3 on transporters and enzymes of the rat intestine and kidney in vivo.. Biopharm Drug Dispos 31:91–108
Chow EC, Durk MR, Cummins CL, Pang KS (2011a) 1A,25-dihydroxyvitamin D3 up-regulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(-/-) and fxr(+/+) mice and increased renal and brain efflux of digoxin in mice in vivo. J Pharmacol Exp Ther 337:846–859
Chow EC, Sondervan M, Jin C, Groothuis GM, Pang KS (2011b) Comparative effects of doxercalciferol (1α-hydroxyvitamin D2) versus calcitriol (1α,25-dihydroxyvitamin D3) on the expression of transporters and enzymes in the rat in vivo. J Pharm Sci 100:1594–1604
Chow EC, Durk MR, Maeng HJ, Pang KS (2013) Comparative effects of 1α-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on transporters and enzymes in fxr(+/+) and fxr(-/-) mice. Biopharm Drug Dispos 34:402–416
Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS (2009) Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res 69:967–975
Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, Williams ME, Bishop CW (2004) Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis 43(5):877–890
Colston K, Colston MJ, Feldman D (1981) 1,25-Dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology 108:1083–1086
Colston KW, Chander SK, Mackay AG, Coombes RC (1992) Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochem Pharmacol 44:693–702
Colston KW, James SY, Ofori-Kuragu EA, Binderup L, Grant AG (1997) Vitamin D receptors and anti-proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in vivo and in vitro. Br J Cancer 76:1017–1020
Cross HS, Huber C, Peterlik M (1991) Antiproliferative effect of 1,25-dihydroxyvitamin D3 and its analogs on human colon adenocarcinoma cells (CaCo-2): influence of extracellular calcium. Biochem Biophys Res Commun 179:57–62
Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM (2008) Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. Pharmacol Exp Ther 324:23–33
DeLuca HF (2008) Evolution of our understanding of vitamin D. Nutr Rev 66:S73–S87
DeLuca HF, Bedale W, Binkley N, Gallagher JC, Bolognese M, Peacock M, Aloia J, Clagett-Dame M, Plum L (2011) The vitamin D analogue 2MD increases bone turnover but not BMD in postmenopausal women with osteopenia: results of a 1-year phase 2 double-blind, placebo-controlled, randomized clinical trial. J Bone Miner Res 26:538–545
Di Filippo P, Scaparrotta A, Rapino D, Cingolani A, Attanasi M, Petrosino MI, Chuang K, Di Pillo S, Chiarelli F (2015) Vitamin D supplementation modulates the immune system and improves atopic dermatitis in children. Int Arch Allergy Immunol 166:91–96
Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ (2002) Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem 277(28):25125–25132
Durakovic C, Malabanan A, Holick MF (2001) Rationale for use and clinical responsiveness of hexafluoro-1,25-dihydroxyvitamin D3 for the treatment of plaque psoriasis: a pilot study. Br J Dermatol 144:500–506
Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A (2013) Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol 37:239–248
Echchgadda I, Song CS, Roy AK, Chatterjee B (2004) Dehydroepiandrosterone sulfotransferase is a target for transcriptional induction by the vitamin D receptor. Mol Pharmacol 65(3):720–729
El-Agroudy AE, El-Husseini AA, El-Sayed M, Ghoneim MA (2003) Preventing bone loss in renal transplant recipients with vitamin D. J Am Soc Nephrol 14:2975–2979
Eloranta JJ, Hiller C, Jüttner M, Kullak-Ublick GA (2012) The SLCO1A2 gene, encoding human organic anion-transporting polypeptide 1A2, is transactivated by the vitamin D receptor. Mol Pharmacol 82:37–46
Fan J, Liu S, Du Y, Morrison J, Shipman R, Pang KS (2009) Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1α,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J Pharmacol Exp Ther 330:389–402
Felsenberg D, Bock O, Borst H, Armbrecht G, Beller G, Degner C, Stephan-Oelkers M, Schacht E, Mazor Z, Hashimoto J, Roth HJ, Martus P, Runge M (2011) Additive impact of alfacalcidol on bone mineral density and bone strength in alendronate treated postmenopausal women with reduced bone mass. J Musculoskelet Neuronal Interact 11:34–45
Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, Cowan RA, Downes N (1996) A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporos Int 6:284–290
Fujioka T, Hasegawa M, Ishikura K, Matsushita Y, Sato M, Tanji S (1998) Inhibition of tumor growth and angiogenesis by vitamin D3 agents in murine renal cell carcinoma. J Urol 160:247–251
Fujita T, Orimo H, Inoue T, Kaneda K, Sakurai M, Morita R, Yamamoto K, Sugioka Y, Inoue A, Takaoka K, Yamamoto I, Hoshino Y, Kawaguchi H (2007) Clinical effect of bisphosphonate and vitamin D on osteoporosis: reappraisal of a multicenter double-blind clinical trial comparing etidronate and alfacalcidol. J Bone Miner Metab 25:130–137
Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, Dhir R, Shurin Z, Day RS, Trump DL, Johnson CS (1997) Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology 50:999–1006
Ghous Z, Akhter J, Pourgholami MH, Morris DL (2008) Inhibition of hepatocellular cancer by EB1089: in vitro and in vivo study. Anticancer Res 28:3757–3761
Goto S, Komaba H, Fukagawa M (2008) Pathophysiology of parathyroid hyperplasia in chronic kidney disease: preclinical and clinical basis for parathyroid intervention. NDT Plus 1(Suppl 3):iii2–iii8
Grostern RJ, Bryar PJ, Zimbric ML, Darjatmoko SR, Lissauer BJ, Lindstrom MJ, Lokken JM, Strugnell SA, Albert DM (2002) Toxicity and dose-response studies of 1α-hydroxyvitamin D2 in a retinoblastoma xenograft model. Arch Ophthalmol 120:607–612
Guo X, Meng Q, Liu Q, Wang C, Mao Q, Sun H, Peng J, Kaku T, Liu K (2012) Peptide cotransporter 1 in intestine and organic anion transporters in kidney are targets of interaction between JBP485 and lisinopril in rats. Drug Metab Pharmacokinet 27:232–241
Guzey M, Kitada S, Reed JC (2002) Apoptosis induction by 1α,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther 1:667–677
Hara K, Kusuzaki K, Takeshita H, Kuzuhara A, Tsuji Y, Ashihara T, Hirasawa Y (2001) Oral administration of 1α hydroxyvitamin D3 inhibits tumor growth and metastasis of a murine osteosarcoma model. Anticancer Res 21:321–324
Hargrove L, Francis T, Francis H (2014) Vitamin D and GI cancers: shedding some light on dark diseases. Ann Transl Med 2:9
Hayashi M, Tsuchiya Y, Itaya Y, Takenaka T, Kobayashi K, Yoshizawa M, Nakamura R, Monkawa T, Ichihara A (2004) Comparison of the effects of calcitriol and maxacalcitol on secondary hyperparathyroidism in patients on chronic haemodialysis: a randomized prospective multicentre trial. Nephrol Dial Transplant 19:2067–2073
Hirata M, Endo K, Katsumata K, Ichikawa F, Kubodera N, Fukagawa M (2002) A comparison between 1,25-dihydroxy-22-oxavitamin D3 and 1,25-dihydroxyvitamin D3 regarding suppression of parathyroid hormone and calcemic action. Nephrol Dial Transplant 17(suppl 10):41–45
Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362–371
Holick MF, Chen ML, Kong XF, Sanan DK (1996) Clinical uses for calciotropic hormones 1,25-dihydroxyvitamin D3 and parathyroid hormone-related peptide in dermatology: a new perspective. J Investig Dermatol Symp Proc 1:1–9
Imanishi Y, Inaba M, Seki H, Koyama H, Nishizawa Y, Morii H, Otani S (1999) Increased biological potency of hexafluorinated analogs of 1,25-dihydroxyvitamin D3 on bovine parathyroid cells. J Steroid Biochem Mol Biol 70:243–248
Jiang F, Li P, Fornace AJ Jr, Nicosia SV, Bai W (2003) G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J Biol Chem 278:48030–48040
Katayama I, Ohkawara A, Ohkido M, Harada S, Tamaki K, Nakagawa H, Hori Y, Nishiyama S (2002) High-concentration (20 mug/g) tacalcitol ointment therapy on refractory psoriasis vulgaris with low response to topical corticosteroids. Eur J Dermatol 12:553–557
Kawa S, Yoshizawa K, Tokoo M, Imai H, Oguchi H, Kiyosawa K, Homma T, Nikaido T, Furihata K (1996) Inhibitory effect of 220-oxa-1,25-dihydroxyvitamin D3 on the proliferation of pancreatic cancer cell lines. Gastroenterology 110:1605–1613
Ke HZ, Qi H, Crawford DT, Simmons HA, Xu G, Li M, Plum L, Clagett-Dame M, DeLuca HF, Thompson DD, Brown TA (2005) A new vitamin D analog, 2MD, restores trabecular and cortical bone mass and strength in ovariectomized rats with established osteopenia. J Bone Miner Res 20:1742–1755
Khan AA, Chow EC, van Loenen-Weemaes AM, Porte RJ, Pang KS, Groothuis GM (2009) Comparison of effects of VDR versus PXR, FXR and GR ligands on the regulation of CYP3A isozymes in rat and human intestine and liver. Eur J Pharm Sci 37(2):115–125
Kim YC, Kim IB, Noh CK, Quach HP, Yoon IS, Chow ECY, Kim M, Jin HE, Cho KH, Chung SJ, Pang KS, Maeng HJ (2014) Effects of 1α,25-dihydroxyvitamin D3, the natural vitamin D receptor ligand, on the pharmacokinetics of cefdinir and cefadroxil, organic anion transporter substrates, in rat. J Pharm Sci 103:3793–3805
Kota BP, Abdul MI, Allen JD, Kalagara M, Roufogalis BD (2012) Effect of vitamin D3 supplementation on the pharmacokinetics of digoxin—a pilot study. Fundam Clin Pharmacol 26:543–548
Kragballe K, Beck HI, Søgaard H (1988) Improvement of psoriasis by a topical vitamin D3 analogue (MC 903) in a double-blind study. Br J Dermatol 119:223–230
Kragballe K, Gjertsen BT, De Hoop D, Karlsmark T, van de Kerkhof PC, Larkö O, Nieboer C, Roed-Petersen J, Strand A, Tikjøb G (1991) Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet 337:193–196
Kubodera N, Tsuji N, Uchiyama Y, Endo K (2003) A new active vitamin D analog, ED-71, causes increase in bone mass with preferential effects on bone in osteoporotic patients. J Cell Biochem 88:286–289
Kumagai T, O’Kelly J, Said JW, Koeffler HP (2003) Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst 95:896–905
LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J (1999) Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 251:1505–1511
Lee HJ, So JY, DeCastro A, Smolarek A, Paul S, Maehr H, Uskokovic M, Suh N (2010) Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. J Steroid Biochem Mol Biol 121:408–412
Lehmann B, Querings K, Reichrath J (2004) Vitamin D and skin: new aspects for dermatology. Exp Dermatol 13 Suppl 4:11–15
Lemire J (2000) 1,25-Dihydroxyvitamin D3-a hormone with immunomodulatory properties. Z Rheumatol 59(Suppl 1):24–27
Levin AD, Wadhera V, Leach ST, Woodhead HJ, Lemberg DA, Mendoza-Cruz AC, Day AS (2011) Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci 56:830–836
Leyssens C, Verlinden L, Verstuyf A (2014) The future of vitamin D analogs. Front Physiol 5:122
Li P, Li C, Zhao X, Zhang X, Nicosia SV, Bai W (2004) p27(Kip1) stabilization and G(1) arrest by 1,25-dihydroxyvitamin D(3) in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J Biol Chem 279:25260–25267
Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, Litonjua AA, Gao J, Gao X (2011) Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration 81:469–475
Ma Y, Yu WD, Trump DL, Johnson CS (2010) 1,25D3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer 116:3294–3303
Ma Y, Yu WD, Hidalgo AA, Luo W, Delansorne R, Johnson CS, Trump DL (2013) Inecalcitol, an analog of 1,25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle 12:743–752
Maeng HJ, Durk MR, Chow EC, Ghoneim R, Pang KS (2011) 1α,25-dihydroxyvitamin D3 on intestinal transporter function: studies with the rat everted intestinal sac. Biopharm Drug Dispos 32:112–125
Maeng HJ, Chapy H, Zaman S, Pang KS (2012) Effects of 1α,25-dihydroxyvitamin D3 on transport and metabolism of adefovir dipivoxil and its metabolites in Caco-2 cells. Eur J Pharm Sci 46:149–166
Majewski S, Skopinska M, Marczak M, Szmurlo A, Bollag W, Jablonska S (1996) Vitamin D3 is a potent inhibitor of tumor cell-induced angiogenesis. J Investig Dermatol Symp Proc 1:97–101
Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ (2002) Vitamin D receptor as an intestinal bile acid sensor. Science 17:1313–1316
Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE (2000) 1 α,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res 87:214–220
Marchwicka A, Cebrat M, Sampath P, Snieżewski L, Marcinkowska E (2014) Perspectives of differentiation therapies of acute myeloid leukemia: the search for the molecular basis of patients’ variable responses to 1,25-dihydroxyvitamin d and vitamin d analogs. Front Oncol 4:125
Martin KJ, Gonzales EA, Gellens M, Hamm LL, Abboud H, Lindberg J (1998a) 19-Nor-1a,25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 9:1427–1432
Martin KJ, Gonzalez EA, Gellens ME, Hamm LL, Abboud H, Lindberg J (1998b) Therapy of secondary hyperparathyroidism with 19-nor-1a,25-dihydroxyvitamin D2. Am J Kidney Dis 32:S61–S66
Martinelli V, Dalla Costa G, Colombo B, Dalla Libera D, Rubinacci A, Filippi M, Furlan R, Comi G (2014) Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Mult Scler 20:147–155
McCarthy TC, Li X, Sinal CJ (2005) Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J Biol Chem 280(24):23232–23242
McGuire TF, Trump DL, Johnson CS (2001) Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem 276:26365–26373
Meyer MB, Goetsch PD, Pike JW (2012) VDR/RXR and TCF4/beta-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol Endocrinol 26:37–51
Miao Q, Liu Q, Wang C, Meng Q, Guo X, Sun H, Peng J, Ma X, Kaku T, Liu K (2013) Inhibitory effect of 1α,25-dihydroxyvitamin D3 on excretion of JBP485 via organic anion transporters in rats. Eur J Pharm Sci 48:351–359
Milde P, Hauser U, Simon T, Mall G, Ernst V, Haussler MR, Frosch P, Rauterberg EW (1991) Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol 97:230–239
Miyachi Y, Ohkawara A, Ohkido M, Harada S, Tamaki K, Nakagawa H, Hori Y, Nishiyama S (2002) Long-term safety and efficacy of high-concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. Eur J Dermatol 12:463–468
Monier-Faugere MC, Geng Z, Friedler RM, Qi Q, Kubodera N, Slatopolsky E, Malluche HH (1999) 22-oxacalcitriol suppresses secondary hyperparathyroidism without inducing low bone turnover in dogs with renal failure. Kidney Int 55:821–832
Morán-Auth Y, Penna-Martinez M, Shoghi F, Ramos-Lopez E, Badenhoop K (2013) Vitamin D status and gene transcription in immune cells. J Steroid Biochem Mol Biol 136:83–85
Muto A, Kizaki M, Yamato K, Kawai Y, Kamata-Matsushita M, Ueno H, Ohguchi M, Nishihara T, Koeffler HP, Ikeda Y (1999) 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1). Blood 93:2225–2233
Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T (2005) 1 α,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis 26:429–440
Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J (2014) The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Front Physiol 5:213
Naveh-Many T, Silver J (1993) Effects of calcitriol, 22-oxacalcitriol, and calcipotriol on serum calcium and parathyroid hormone gene expression. Endocrinology 133:2724–2728
Naveilhan P, Berger F, Haddad K, Barbot N, Benabid AL, Brachet P, Wion D (1994) Induction of glioma cell death by 1,25(OH)2 vitamin D3: towards an endocrine therapy of brain tumors? J Neurosci Res 37:271–277
Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE (2009) Vitamin D and cardiovascular disease. Pharmacotherapy 29:691–708
Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU (2007) 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 103:533–537
Nishizawa Y, Morii H, Ogura Y, De Luca HF (1991) Clinical trial of 26,26,26,27,27,27-hexafluoro-1,25-dihydroxyvitamin D3 in uremic patients on hemodialysis: preliminary report. Contrib Nephrol 90:196–203
Okamoto R, Delansorne R, Wakimoto N, Doan NB, Akagi T, Shen M, Ho QH, Said JW, Koeffler HP (2012) Inecalcitol, an analog of 1α,25(OH)(2) D(3), induces growth arrest of androgen-dependent prostate cancer cells. Int J Cancer 130:2464–2473
Pálmer HG, Sánchez-Carbayo M, Ordóñez-Morán P, Larriba MJ, Cordón-Cardó C, Muñoz A (2003) Genetic signatures of differentiation induced by 1α,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res 63:7799–7806
Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, Cranney A, Adachi J, Tugwell P, Josse R, Greenwood C, Guyatt G, Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group (2002) Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–569
Park MR, Lee JH, Park MS, Hwang JE, Shim HJ, Cho SH, Chung IJ, Bae WK (2012) Suppressive effect of 19-nor-1α-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J Korean Med Sci 27:1037–1043
Peleg S, Uskokovic M, Ahene A, Vickery B, Avnur Z (2002) Cellular and molecular events associated with the bone-protecting activity of the noncalcemic vitamin D analog Ro-26-9228 in osteopenic rats. Endocrinology 143:1625–1636
Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, González-Sancho JM (2008) DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1α,25-dihydroxyvitamin D3. Oncogene 27:4467–4477
Pepper C, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C (2003) The vitamin D3 analog EB1089 induces apoptosis via a p53-independent mechanism involving p38 MAP kinase activation and suppression of ERK activity in B-cell chronic lymphocytic leukemia cells in vitro. Blood 101:2454–2460
Pèrez A, Chen TC, Turner A, Raab R, Bhawan J, Poche P, Holick MF (1996) Efficacy and safety of topical calcitriol (1,25-dihydroxyvitamin d3) for the treatment of psoriasis. Br J Dermatol 134:238–246
Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, de Paz R, Souberbielle JC (2012) Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord 5:187–198
Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, März W (2008a) Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke 39:2611–2613
Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H (2008b) Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 93:3927–3935
Prietl B, Treiber G, Pieber TR, Amrein K (2013) Vitamin D and Immune Function. Nutrients 5:2502–2521
Quach HP, Noh K, Hoi SY, Bruinsma A, Groothuis GMM, Li AP, Chow ECY, Pang KS (2018) Alterations in gene expression in vitamin D-deficiency: Down-regulation of liver Cyp7a1 and renal Oat3 in mice. Biopharm Drug Dispos 39:99–115
Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU (2007) Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol 103:416–419
Reichrath J (2007) Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol 16:618–625
Richy F, Ethgen O, Bruyere O, Reginster JY (2004) Efficacy of αcalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int 15:301–310
Rondini EA, Fang H, Runge-Morris M, Kocarek TA (2014) Regulation of human cytosolic sulfotransferases 1C2 and 1C3 by nuclear signaling pathways in LS180 colorectal adenocarcinoma cells. Drug Metab Dispos 42(3):361–368
Rostand SG, Drüeke TB (1999) Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 56:383–392
Sakai Y, Demay MB (2000) Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology 141:2043–2049
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R (2006) Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83:754–759
Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Lown KS, Watkins PB (1997) Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1α,25-dihydroxyvitamin D3. Mol Pharmacol 51(5):741–754
Schwartz JB (2009) Effects of vitamin D supplementation in atorvastatin-treated patients: a new drug interaction with an unexpected consequence. Clin Pharmacol Ther 85(2):198–203
Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP (1997) 1α,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev 6:727–732
Schwartz GG, Eads D, Naczki C, Northrup S, Chen T, Koumenis C (2008) 19-nor-1 α,25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther 7:430–436
Seubwai W, Wongkham C, Puapairoj A, Okada S, Wongkham S (2010) 22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma. Cancer 116:5535–5543
Shapira Y, Agmon-Levin N, Shoenfeld Y (2010) Geoepidemiology of autoimmune rheumatic diseases. Nat Rev Rheumatol 6:468–476
Shedeed SA (2012) Vitamin D supplementation in infants with chronic congestive heart failure. Pediatr Cardiol 33:713–719
Shevde NK, Plum LA, Clagett-Dame M, Yamamoto H, Pike JW, DeLuca HF (2002) A potent analog of 1α,25-dihydroxyvitamin D3 selectively induces bone formation. Proc Natl Acad Sci USA 99:13487–13491
Simpson RU, Hershey SH, Nibbelink KA (2007) Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol 103:521–524
Slatopolsky E, Finch J, Ritter C, Denda M, Morrissey J, Brown A, DeLuca H (1995) A new analog of calcitriol, 19-nor-1,25-(OH)2 D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. Am J Kidney Dis 26:852–860
Slatopolsky E, Cozzolino M, Lu Y, Finch J, Dusso A, Staniforth M, Wein Y, Webster J (2003) Efficacy of 19-Nor-1,25-(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int 63:2020–2027
So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N (2011) A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol 79:360–367
Soleymani T, Hung T, Soung J (2015) The role of vitamin D in psoriasis: a review. Int J Dermatol 54:383–392
Sung V, Feldman D (2000) 1,25-Dihydroxyvitamin D3 decreases human prostate cancer cell adhesion and migration. Mol Cell Endocrinol 164:133–143
Sunyecz JA (2008) The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag 4:827–836
Tamura S, Ueki K, Mashimo K, Tsukada Y, Naitoh M, Abe Y, Kawai H, Tsuchida A, Wakamatsu R, Nojima Y (2005) Comparison of the efficacy of an oral calcitriol pulse or intravenous 22-oxacalcitriol therapies in chronic hemodialysis patients. Clin Exp Nephrol 9:238–243
Thompson PD, Jurutka PW, Whitfield GK, Myskowski SM, Eichhorst KR, Dominguez CE, Haussler CA, Haussler MR (2002) Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem Biophys Res Commun 299(5):730–738
Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, Ishizuka H, Kharasch E, Schuetz J, Schuetz E (2001) Transcriptional control of intestinal cytochrome P-4503A by 1α,25-dihydroxy vitamin D3. Mol Pharmacol 60:1399–1406
Torres A, García S, Gómez A, González A, Barrios Y, Concepción MT, Hernández D, García JJ, Checa MD, Lorenzo V, Salido E (2004) Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int 65:705–712
Trémezaygues L, Reichrath J (2011) Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Dermatoendocrinol 3:180–186
Trump DL, Deeb KK, Johnson CS (2010) Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J 16:1–9
van Etten E, Mathieu C (2005) Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97:93–101
van Ginkel PR, Yang W, Marcet MM, Chow CC, Kulkarni AD, Darjatmoko S, Lindstrom MJ, Lokken J, Bhattacharya S, Albert DM (2007) 1 α-Hydroxyvitamin D2 inhibits growth of human neuroblastoma. J Neurooncol 85:255–262
van de Kerkhof PC, van Bokhoven M, Zultak M, Czarnetzki BM (1989) A double-blind study of topical 1 α,25-dihydroxyvitamin D3 in psoriasis. Br J Dermatol 120:661–664
van de Kerkhof PC, Berth-Jones J, Griffiths CE, Harrison PV, Hönigsmann H, Marks R, Roelandts R, Schöpf E, Trompke C (2002) Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br J Dermatol 146:414–422
Walters MR, Ilenchuk TT, Claycomb WC (1987) 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+ uptake by cultured adult rat ventricular cardiac muscle cells. J Biol Chem 262:2536–2541
Wang QM, Jones JB, Studzinski GP (1996) Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res 56:264–267
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS (2008) Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511
Wang X, Wang H, Shen B, Overholser BR, Cooper BR, Lu Y, Tang H, Zhou C, Sun X, Zhong L, Favus MJ, Decker BS, Liu W, Peng Z (2016) 1-A, 25-dihydroxyvitamin D3 alters the pharmacokinetics of mycophenolic acid in renal transplant recipients by regulating two extrahepatic UDP-glucuronosyltransferases 1A8 and 1A10. Transl Res 178:54–62
Weishaar RE, Simpson RU (1987a) Involvement of vitamin D3 with cardiovascular function. II. Direct and indirect effects. Am J Physiol 253:E675–E683
Weishaar RE, Simpson RU (1987b) Vitamin D3 and cardiovascular function in rats. J Clin Invest 79:1706–1712
Weishaar RE, Kim SN, Saunders DE, Simpson RU (1990) Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol 258:E134–E142
Welsh J, Wietzke JA, Zinser GM, Byrne B, Smith K, Narvaez CJ (2003) Vitamin D-3 receptor as a target for breast cancer prevention. J Nutr 133:2425S–2433S
Wöbke TK, Sorg BL, Steinhilber D (2014) Vitamin D in inflammatory diseases. Front Physiol 5:244
Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC (2005) Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288:E125–E132
Xu Y, Iwanaga K, Zhou C, Cheesman MJ, Farin F, Thummel KE (2006) Selective induction of intestinal CYP3A23 by 1α,25-dihydroxyvitamin D3 in rats. Biochem Pharmacol 28:385–392
Yasuda M, Akiba T, Nihei H (2003) Multicenter clinical trial of 22-oxa-1,25-dihydroxyvitamin D3 for chronic dialysis patients. Am J Kidney Dis 41:S108–S111
Yoon IS, Son JH, Kim SB, Choi MK, Maeng HJ (2015) Effects of 1α,25-Dihydroxyvitamin D3 on intestinal absorption and disposition of adefovir dipivoxil and its metabolite, adefovir, in Rats. Biol Pharm Bull 38:1732–1737
Yudoh K, Matsuno H, Kimura T (1999) 1α,25-dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J Lab Clin Med 133:120–128
Zhang Y, Zhang J, Studzinski GP (2006) AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle 5:447–451
Zhang H, Shih DQ, Zhang X (2013) Mechanisms underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol (Bp) 3:237–240
Zhuang SH, Burnstein KL (1998) Antiproliferative effect of 1α,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology 139:1197–1207
Zierold C, Mings JA, Deluca HF (2006) 19nor-1,25-dihydroxyvitamin D2 specifically induces CYP3A9 in rat intestine more strongly than 1,25-dihydroxyvitamin D3 in vivo and in vitro. Mol Pharmacol 69:1740–1747
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016R1D1A1B03931470).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of the interest
These authors declare that they have no conflict of interest.
Human and animal rights participants
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, M.S., Kim, Y.C. & Maeng, HJ. Therapeutic targets of vitamin D receptor ligands and their pharmacokinetic effects by modulation of transporters and metabolic enzymes. J. Pharm. Investig. 50, 1–16 (2020). https://doi.org/10.1007/s40005-019-00429-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-019-00429-z