Abstract
Neuroblastoma is the most common extracranial solid tumor in childhood. The poor outcomes of patients with high-risk neuroblastoma have encouraged the search for new therapies. In the current study, the effect of the vitamin D analog 1α-hydroxyvitamin D2 (1α-OH-D2, doxercalciferol) was assessed in a mouse xenograft model of human neuroblastoma. Vitamin D receptor (VDR) expression levels in seven neuroblastoma cell lines were compared using real-time PCR. SK-N-AS cells, which express relatively high levels of VDR, were injected into the flanks of 60 mice. The mice were treated daily via oral gavage for 5 weeks with vehicle (control), 0.15 μg, or 0.3 μg of 1α-OH-D2. The animals were then euthanized, and tumors, sera, and kidneys were collected and analyzed. End tumor volumes were significantly smaller in both the 0.15 μg group (712.07 mm3, P = 0.0121) and 0.3 μg group (772.97 mm3, P = 0.0209) when compared to controls (1,681.75 mm3). In terms of toxicity, serum calcium levels were increased but mortality was minimal in both treatment groups. These results were similar to those previously described in the transgenic (LHβ-Tag) and human xenograft (Y-79) models of retinoblastoma, a related tumor. In vitro cell viability studies of SK-N-AS and NGP cells, which represent two major human neuroblastoma subtypes that differ in their genetic abnormalities as well as their VDR expression levels, show that both are sensitive to calcitriol, the active metabolite of vitamin D3. In conclusion, the present study shows that 1α-OH-D2 can inhibit human neuroblastoma growth in vivo with relatively low toxicity. The safety of 1α-OH-D2 has been extensively studied; the drug is FDA-approved for the treatment of adult kidney patients, and Phase I/II trials have been conducted in adult oncology patients. There should not be major obstacles to starting Phase I and II clinical trials with this drug in pediatric patients with high-risk neuroblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma is a pediatric tumor derived from neuroectodermal cells, and the most common site of occurrence is the adrenal gland [1, 2]. Approximately 9 cases per 1 million children are diagnosed with neuroblastoma each year in the United States, making it the most common extracranial solid tumor in childhood [3]. An overwhelming majority of these cases are sporadic and without any identifiable environmental triggers [4].
Neuroblastomas have a varied clinical course, ranging from spontaneous regression to metastatic disease, often resulting in death [5]. Due to the diverse nature of the disease, patients are stratified into low-, intermediate-, and high-risk groups according to age, clinical stage, and tumor biologic features. Furthermore, the histopathologic features, according to International Neuroblastoma Pathology Classifications, are useful in determining the prognosis [6]. Lastowska et al. have identified different genetic types of neuroblastoma tumors, of which the advanced types fall into three major groups: those with a gain of chromosome 17q, a deletion of chromosome 1p, and amplification of N-myc; those with a gain of 17q, deletion of 1p, but no amplification of N-myc; and those with just a gain of 17q [7].
Although low-risk and intermediate-risk patients generally have a good prognosis, children in the high-risk group have a poor prognosis, with less than a 50% long-term survival rate despite intense multimodal treatment that includes chemotherapy and radiotherapy [8], due to residual disease and multiple drug resistance [9]. In addition, toxic effects of chemotherapy contribute to 15.5% of all deaths in stage 4 neuroblastoma [10] and may increase the risk of secondary leukemia [11]. Radiotherapy has been associated with bowel complications, sterility, and bone growth retardation, as well as increased risk for secondary tumors in adulthood [12, 13]. Although neuroblastomas with N-myc amplifications are considered the most aggressive, recurrent or progressive disease develops in up to 20% of patients with localized or stage 4S neuroblastoma, even without N-myc amplification [14]. The dismal outcomes of high-risk neuroblastoma patients have encouraged the search for new therapies. Vitamin analogs have been among the more promising prospects. Both vitamin A (retinoic acid) and vitamin D compounds reduce the proliferation of cancer cells. Vitamin D compounds have antineoplastic activity against a wide range of human cancer cell lines. In addition, many vitamin D analogs have been tested in animal models of different cancer types [15]. Currently, synthetic retinoids have been undergoing clinical trials that show improved event-free survival rates in high-risk patients [8].
Early studies have shown that both vitamin D2 (ergocalciferol) and calcitriol (1,25 dihydroxy D3), an active physiological metabolite of vitamin D, are toxic in animals due to their strong hypercalcemic effects. The vitamin D analog 1-α-hydroxyvitamin D2 (1α-OH-D2), first synthesized in the 1970s by Lam et al. [16] and subsequently developed for treatment of secondary hyperparathyroidism and metabolic bone disease, has considerably less toxic side effects [17]. In addition, 1α-OH-D2 has a potent antitumor effect in different animal models of retinoblastoma [18–21]. To date, however, this analog has not been tested against neuroblastomas, and only a few studies have examined the efficacy of other vitamin D compounds against neuroblastoma tumors in vivo [22, 23].
To address this issue, we used a mouse xenograft model of human neuroblastoma. After subcutaneous placement of SK-N-AS neuroblastoma cells, 1α-OH-D2 was administered via oral gavage, and the effect of this compound was assessed in terms of its effect on tumor size. In addition, the toxicity of 1α-OH-D2 was determined via its effects on serum calcium level, mortality, body weight, and organ calcification.
Materials and methods
Vitamin D receptor assay
Vitamin D receptor (VDR) levels were quantitated in seven neuroblastoma cell lines: SK-N-AS [24]; NGP [24]; SK-N-LE [25]; SK-N-DZ [26]; KCNR [24]; SH-SY5Y [27, 28]; and NBLS. Total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA, USA). The RNA was treated with DNase I, followed by reverse transcription with oligo dT primers using MMLV reverse transcriptase (Promega, Madison, WI, USA). cDNA was treated with RNAse H and followed by real-time amplification of an 80-bp VDR fragment using specific primers (forward: 5′-GCGCTCCAATGAGTCCTTCA-3′; reverse: 5′-CACGTCACTGACGCGGTACTT-3′); SYBR mix; and an I–cycler (BioRad, Hercules, CA, USA), according to the manufacturer’s instructions. Relative VDR expression was determined using an endogenous reference gene, β-glucuronidase, and specific primers (forward: 5′-GCCCATTATTCAGAGCGAGTATG-3′; reverse: 5′-TGGTGAAACCCTGCAATCGT-3′). All reactions were performed in triplicate. Relative expression levels were calculated using the comparative method as described [29] and normalized to values obtained from the NGP cell line.
Cell culture and inoculation
SK-N-AS and NGP cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, and 1% fungizone, penicillin, and streptomycin. The subcutaneous inoculation of the tumor cells was done using a method similar to the one described by Sabet et al. for studies on retinoblastoma [30]. Briefly, before inoculation of the mice with the cells, the medium containing 1 million SK-N-AS neuroblastoma cells was combined with Matrigel in a 1:1 mixture. This mixture was then injected subcutaneously into the flank of each mouse.
Compound preparation for in vivo study
1α-OH-D2 was provided by Bone Care International, now a part of Genzyme Corp. (Cambridge, MA, USA), in a crystalline form, which was then dissolved with 100% ethanol for a stock solution of 2.98 mg/ml. This solution was diluted in coconut oil to concentrations of 0.15 μg and 0.3 μg per 0.1 ml. Control animals were given 0.1 ml of coconut oil. Spectrophotometric analysis was used to confirm the drug concentrations. Stock solutions of the drug were prepared weekly and stored in amber glass bottles at −40°C to protect the compound from degradation due to temperature or UV light.
Animal studies
Sixty 4- to 5-week-old athymic nude female mice were randomized 2 days after inoculation into one of three treatment groups: vehicle (control); 0.15 μg dose of 1α-OH-D2; or 0.3 μg dose of 1α-OH-D2. This resulted in 3 groups of 20 animals each. To control for variance in initial tumor volumes, the mice were randomized according to estimated initial tumor volume. The mice were kept in conditions consistent with the University of Wisconsin-Madison Research Animals Resources Center (RARC) guidelines and were fed Purina Test Diet deficient in vitamin D and calcium (Purina Mills LLC, St. Louis, MO, USA). Starting 5 days after inoculation, the mice were dosed five times per week for 5 weeks by oral gavage using a 1 in, 1.25 mm-ball diameter steel gavage needle (22GX; Becton Dickinson, Franklin Lakes, NJ, USA).
Mice were weighed twice a week to monitor their health, and dosing was withheld from mice that had a weight loss of more than 15% from baseline. During the treatment period, animals were euthanized under the following conditions: (1) the tumor became ulcerated; (2) the tumor exceeded the allowable size by the RARC standards (greater than 20 mm in any dimension); (3) the animal did not regain lost body weight despite withholding treatment for more than 2 days; or (4) the animal appeared ill, e.g., dehydrated, cold, lethargic, or anorexic. All animals remaining at the end of 5 weeks were then euthanized and underwent necropsy. The tumor, serum, and kidneys were collected from each remaining mouse at this time.
Tumor measurement/evaluation
Tumor sizes were quantified using methods employed in previous studies [30]. First, the tumor was measured in vivo with calipers, providing a means of monitoring both tumor volume and tumor growth during the study. Second, caliper measurement of the tumor was performed after it was removed from the mouse to provide a more accurate end-of-study tumor volume. Third, tumor weight was recorded after excision of the tumor. The tumor was then evaluated histologically for differentiation, necrosis, and calcification.
Toxicity
Toxicity was evaluated as previously described [19]. The parameters examined included mortality, body weight change, serum calcium level, and kidney calcification. At necropsy, serum was drawn and sent for quantitation of total calcium. Kidneys were fixed in formalin, and sections were prepared with von Kossa stain for histologic grading of calcification. The kidneys were graded as follows: grade 0 for no calcification, grade 1 for 1–7 loci of calcification, grade 2 for 8–15 loci, and grade 3 for more than 15 loci.
Statistical analysis
The effect of treatment dose on tumor volume was assessed using analysis of variance. Tumor volume was transformed to the cube root scale to obtain approximately constant variance. All significant tests for effect of treatment dose were followed by pairwise analyses to assess differences between specific treatment groups.
In vitro cell viability assay
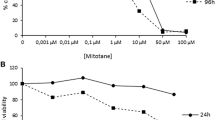
Calcitriol, the active form of vitamin D3, is similar in structure and antiproliferative activity to the principal active metabolite of 1α-OH-D2, and was therefore used to determine the effect of active vitamin D in vitro on growth rates of two neuroblastoma cell lines, NGP and SK-N-AS, using the CellTiter-Blue™ cell viability assay (Promega, Madison, WI, USA). Briefly, 2 × 103 of SK-N-AS and NGP cells were seeded in 96-well plates, incubated at 37C for 48 h, then treated with either vehicle (0.1% DMSO), 25 μM, 50 μM, or 100 μM of calcitriol for 5 days. Medium was replaced with fresh medium containing calcitriol (in 0.1% DMSO) every other day. CellTiter-Blue™ dye was added and incubated at 37C for 90 min. Fluorescence (excitation at 560 nm and emission at 590 nm) was measured with a Spectra Max Gemini spectrofluorometer from Molecular Devices (Sunnyvale, CA, USA). The experiment was repeated three times in triplicate samples. Results are expressed as percent viable cells over control.
Results
VDR assay
The VDR expression levels of seven neuroblastoma cell lines were measured by real time PCR and the values expressed relative to one of the cell lines (NGP). All cell lines expressed VDR, and expression levels ranged from lowest in NGP cells to more than 700-fold higher in SK-N-AS cells (Table 1). Since an earlier study suggested that VDR is required for the antiproliferative activity of vitamin D analogs, the neuroblastoma cell line SK-N-AS was selected for use in vivo to evaluate the antitumor effect of 1α-OH-D2 [31].
Tumor measurements/evaluation
The effect of 1α-OH-D2 on neuroblastoma tumor growth in a SK-N-AS xenograft model was measured by end tumor volume (Fig. 1). A dose–response experiment was performed whereby mice were treated with 0.15 μg or 0.30 μg of 1α-OH-D2. After 5 weeks of oral drug treatment, tumor volume of the 0.15 μg and 0.30 μg drug-treated groups was 59% and 56% less, respectively, than that of the control group. Statistical significance with respect to end tumor volume was noted between the vehicle group and both the 0.15 μg group (P = 0.0121) and 0.3 μg group (P = 0.0209). No statistical significance was found between the two treatment groups (P = 0.829). Histopathologic evaluation of these tumors did not show any differences in differentiation, necrosis, or calcification among the groups.
Of the 60 mice with neuroblastoma xenografts, only one animal (0.15 μg group) died before the end of the study. Six animals in the various groups were euthanized before the end of the study due to tumor sizes exceeding allowable limits set forth by the RARC. One animal from the 0.15 μg group was euthanized due to lethargic appearance (Table 2).
Toxicity
The toxicity of the analog was evaluated by its effects on mortality, serum calcium, body weight change, and kidney calcification. As mentioned previously, only one animal in the entire study died (0.15 μg group). Mean serum calcium levels were as follows: vehicle group—7.02 mg/dl; 0.15 μg group—11.35 mg/dl; 0.3 μg group—12.04 mg/dl (Table 2). Differences in serum calcium were statistically significant between the vehicle-treated group and both the 0.15 μg and 0.3 μg groups (P = 0.0001), but not between the 0.15 μg and 0.3 μg groups. In spite of the increase in serum calcium in the doxercalciferol groups, on average there was weight gain in all 3 groups, although there was significantly less weight gain in the 0.3 μg group when compared to control (P = 0.0037) (Table 2). Two mice in the 0.15 μg group and one mouse in the 0.3 μg group lost weight. The degree of kidney calcification was noted to be dose dependent and ranged from no calcification to low-grade calcification (Table 3).
In vitro cell viability assay
SK-N-AS and NGP cells represent different neuroblastoma subtypes: those that have a gain of 17q, deletion of 1p, but no amplification of N-myc (SK-N-AS); and those with all three traits (NGP), which is considered the most malignant [7]. In addition, VDR expression in SK-N-AS cells is much higher than in NGP cells (by more than 700–fold; Table 1). We therefore measured the effect of calcitriol on cell viability in these two cell lines. The results show a dose-dependent growth inhibition in both SK-N-AS and NGP cell lines, suggesting that vitamin D is effective against different subtypes of neuroblastoma and that the sensitivity to the drug does not correlate with levels of VDR receptor (Fig. 2).
Discussion
There is a need for new therapeutic options for children with neuroblastoma. Current modes of therapy have significant adverse effects, while the event-free survival rate remains poor [8]. Major efforts are being made to develop new methods of treatment, of which vitamin D analogs appear very promising. One of the analogs, 1α-OH-D2, has been approved by the FDA since 2000 for the treatment of secondary hyperparathyroidism in adults with end-stage renal disease [32] and more recently for treatment of secondary hyperparathyroidism in adults with less severe stage 3 or 4 renal disease [33]. In addition, 1α-OH-D2 has already undergone Phase II clinical trials in adults with prostate cancer [34]. We propose 1α-OH-D2 as a possible drug therapy against neuroblastoma.
To determine if 1α-OH-D2 is effective in inhibiting neuroblastoma tumor growth in vivo, a neuroblastoma mouse xenograft model was used. A cell line with relatively high VDR expression was selected, since it has been reported that VDR is required for the antiproliferative activity of vitamin D analogs [31]. In a comparison of seven human neuroblastoma cell lines using real time PCR, SK-N-AS cells had the highest relative levels of VDR expression, almost three orders of magnitude higher than the cell line expressing the lowest amount of VDR (NGP). These results are in agreement with the VDR protein measurements in these cells presented by others [24]. In addition, SK-N-AS cells are a good model for neurobastomas, which have a gain of 17q and deletion of 1p, representing one of the major aggressive genetic subtypes of neuroblastoma [7]. We therefore used the SK-N-AS mouse xenograft model in our in vivo studies.
In our studies, 1α-OH-D2 showed a very potent inhibitory effect on tumor growth in this animal model of human neuroblastoma. After 5 weeks of treatment, average tumor volume in the animal model was almost 60% less than that of the control groups. Statistically significant reductions in SK-N-AS tumor growth were found at 0.15 μg and 0.30 μg doses of 1α-OH-D2 (P = 0.0121 and P = 0.0209, respectively). Drug treatment in these experiments commenced 5 days after inoculation of the tumor cells, when tumor size is minimal. Therefore, these experiments suggest that 1α-OH-D2 can prevent outgrowth of small tumors. Our studies of 1α-OH-D2 in animal models of retinoblastoma show that even after long-term treatment (up to 15 weeks), the antitumor effect of the drug is maintained [19]. The SK-N-AS subcutaneous xenograft model does not metastasize. Future studies are planned, in which tumor cells are implanted at the orthotopic site in the adrenal gland [35], to determine if 1α-OH-D2 can inhibit metastatic growth.
The toxicity of the 1α-OH-D2 doses in the present study was relatively low, with no deaths in the high-dose range (0.3 μg) and only one death in the low-dose range (0.15 μg). On average, all three groups gained weight, although there was significantly less weight gain in the 0.3 μg dose group. There was an elevation in serum calcium in the groups treated with 1α-OH-D2. However, kidney calcification at the end of the study was dose-dependent and relatively uncommon in the low-dose range (0.15 μg). Since better tumor inhibition was observed in the lower (0.15 μg) dosage group, 1α-OH-D2 may be equally or more effective at lower dosages. At the tested dosages, the level of toxicity of 1α-OH-D2 seems minimal, favoring its potential use in the treatment of neuroblastoma. It may be possible to extrapolate toxicity data available from ongoing human trials to adjust dosing for pediatric patients. The current study indicates that 1α-OH-D2 is very potent in its antitumor effects yet much less toxic than the previously tested vitamin D analog calcitriol [36].
Calcitriol, the active metabolite of vitamin D3 with antiproliferative properties similar to the principal active metabolite of 1α-OH-D2, was used to determine its efficacy in inhibiting cell lines representing two major neuroblastoma subtypes, in vitro. Cell viability of NGP cells, which have amplification of the N-myc gene in addition to the gain of 17q and deletion of 1p, was compared to SK-N-AS cells in response to calcitriol treatment. Both SK-N-AS and NGP cells were sensitive to calcitriol, resulting in a decrease in cell viability and suggesting that vitamin D analogs are effective against different neuroblastoma subtypes [7].
Vitamin D analogs’ mechanism for decreased cell viability in vitro and inhibition of tumor growth in vivo, demonstrated in the current study, has not yet been determined. Gumireddy et al. found that vitamin D compounds decrease cell proliferation in neuroblastoma cells [24]. In that study, down-regulation of c-myc and N-myc expression was found in SK-N-AS and NGP cells, respectively. In addition, expression and phosphorylation status of key cell cycle regulators were altered, suggesting that vitamin D mediates antiproliferative effects by regulating key growth control networks involving myc, Id2, and pRB in these cells. Although a number of studies have also found that vitamin D analogs can inhibit proliferation by inducing differentiation in neuroblastoma cell lines [37, 38], this was not apparent in the SK-N-AS tumors in this study, based on histopathology.
We have previously found that 1α-OH-D2 induces apoptosis in retinoblastoma cells in vitro and in vivo through up-regulation of p53 and p21 [39]. In addition, studies in neuroblastoma cells have also shown activation of caspase 3 and induction of apoptosis in response to vitamin D analogs [24]. Therefore, cell viability may decrease both as a result of a decrease in cell proliferation as well as an increase in cell death in neuroblastoma cells.
Recently, we have shown that calcitriol is a potent inhibitor of angiogenesis by inhibiting capillary morphogenesis [40]. Others have shown that calcitriol inhibits proliferation and induces apoptosis of tumor-derived endothelial cells [41–43]. Since tumor angiogenesis is critical for tumors to grow beyond a few millimeters, inhibition of new blood vessel ingrowth by vitamin D analogs may contribute to the inhibition of neuroblastoma growth in the SK-N-AS animal model. Further studies will be necessary to determine the exact mechanisms by which 1α-OH-D2 inhibits the growth of neuroblastoma tumors.
A rational cellular target for mediating the effects of 1α-OH-D2 would be VDR. Zinser et al. [31] reported that VDR is required for the antiproliferative activity of vitamin D analogs. However, others have found that overexpression of VDR in a low expressing cell line did not result in increased sensitivity to vitamin D, even though a vitamin D target gene, CYP24, was activated [44]. The current data also suggests that relative VDR expression levels may not correlate with the sensitivity of the neuroblastoma tumor cells to vitamin D, since both NGP and SK-N-AS cells respond similarly to calcitriol treatment in vitro even though VDR expression levels differ by almost three orders of magnitude. Therefore, levels of VDR alone may not be indicative of the sensitivity of cells to vitamin D analogs. VDR signaling may involve additional epigenetic components that could differ among the cell lines to regulate the extent of the response, including a number of coregulators, the degree of silencing, and the sensitivity of the cell line to proliferative drive [45].
In summary, the present study demonstrates potent antiproliferative activity of 1α-OH-D2 against neuroblastoma tumors with relatively low toxicity in an in vivo model of the disease. The data suggest that 1α-OH-D2 may have significant utility in the treatment of neuroblastoma. Given that its safety has already been established in adult patients, clinical trials to evaluate the safety and effectiveness of 1α-OH-D2 in high-risk pediatric patients with neuroblastoma should be in order.
References
van Noesel MM, Versteeg R (2004) Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene 325:1–15
Bown N (2001) Neuroblastoma tumour genetics: clinical and biological aspects. J Clin Pathol 54(12):897–910
Linet MS, Ries LA, Smith MA, Tarone RE, Devesa SS (1999) Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the united states. J Natl Cancer Inst 91(12):1051–1058
Weinstein JL, Katzenstein HM, Cohn SL (2003) Advances in the diagnosis and treatment of neuroblastoma. Oncologist 8(3):278–292
Gestblom C, Hoehner JC, Hedborg F, Sandstedt B, Pahlman S (1997) In vivo spontaneous neuronal to neuroendocrine lineage conversion in a subset of neuroblastomas. Am J Pathol 150(1):107–117
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B et al (1999) The international neuroblastoma pathology classification (the shimada system). Cancer 86(2):364–372
Lastowska M, Cullinane C, Variend S, Cotterill S, Bown N, O’Neill S et al (2001) Comprehensive genetic and histopathologic study reveals three types of neuroblastoma tumors. J Clin Oncol 19(12):3080–3090
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK et al (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. children’s cancer group. N Engl J Med 341(16):1165–1173
Norris MD, Bordow SB, Marshall GM, Haber PS, Cohn SL, Haber M (1996) Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med 334(4):231–238
Berthold F, Hero B (2000) Neuroblastoma: current drug therapy recommendations as part of the total treatment approach. Drugs 59(6):1261–1277
Kushner BH, Cheung NK, Kramer K, Heller G, Jhanwar SC (1998) Neuroblastoma and treatment-related myelodysplasia/leukemia: the memorial sloan–kettering experience and a literature review. J Clin Oncol 16(12):3880–3889
Ludin A, Macklis RM (2000) Radiotherapy for pediatric genitourinary tumors. its role and long-term consequences. Urol Clin North Am 27(3):553–562
Roarty JD, McLean IW, Zimmerman LE (1988) Incidence of second neoplasms in patients with bilateral retinoblastoma. Ophthalmology 95(11):1583–1587
Kushner BH, Cheung NK (2005) Neuroblastoma – from genetic profiles to clinical challenge. N Engl J Med 353(21):2215–2217
Ordonez-Moran P, Larriba MJ, Pendas-Franco N, Aguilera O, Gonzalez-Sancho JM, Munoz A (2005) Vitamin D and cancer: an update of in vitro and in vivo data. Front Biosci 10:2723–2749
Lam HY, Schnoes HK, DeLuca HF (1974) 1alpha-hydroxyvitamin D2: a potent synthetic analog of vitamin D2. Science 186(4168):1038–1040
Sjoden G, Smith C, Lindgren U, DeLuca HF (1985) 1 alpha-hydroxyvitamin D2 is less toxic than 1 alpha-hydroxyvitamin D3 in the rat. Proc Soc Exp Biol Med 178(3):432–436
Albert DM, Kumar A, Strugnell SA, Darjatmoko SR, Lokken JM, Lindstrom MJ et al (2004) Effectiveness of 1alpha-hydroxyvitamin D2 in inhibiting tumor growth in a murine transgenic pigmented ocular tumor model. Arch Ophthalmol 122(9):1365–1369
Albert DM, Kumar A, Strugnell SA, Darjatmoko SR, Lokken JM, Lindstrom MJ et al (2004) Effectiveness of vitamin D analogues in treating large tumors and during prolonged use in murine retinoblastoma models. Arch Ophthalmol 122(9):1357–1362
Dawson DG, Gleiser J, Zimbric ML, Darjatmoko SR, Lindstrom MJ, Strugnell SA et al (2003) Toxicity and dose-response studies of 1-alpha hydroxyvitamin D2 in LH-beta-tag transgenic mice. Ophthalmology 110(4):835–839
Grostern RJ, Bryar PJ, Zimbric ML, Darjatmoko SR, Lissauer BJ, Lindstrom MJ et al (2002) Toxicity and dose-response studies of 1alpha-hydroxyvitamin D2 in a retinoblastoma xenograft model. Arch Ophthalmol 120(5):607–612
Albert DM, Plum LA, Yang W, Marcet M, Lindstrom MJ, Clagett-Dame M et al (2005) Responsiveness of human retinoblastoma and neuroblastoma models to a non-calcemic 19-nor vitamin D analog. J Steroid Biochem Mol Biol 97(1–2):165–172
Reddy CD, Patti R, Guttapalli A, Maris JM, Yanamandra N, Rachamallu A et al (2006) Anticancer effects of the novel 1alpha, 25-dihydroxyvitamin D3 hybrid analog QW1624F2-2 in human neuroblastoma. J Cell Biochem 97(1):198–206
Gumireddy K, Ikegaki N, Phillips PC, Sutton LN, Reddy CD (2003) Effect of 20-epi-1alpha,25-dihydroxyvitamin D3 on the proliferation of human neuroblastoma: role of cell cycle regulators and the myc-Id2 pathway. Biochem Pharmacol 65(12):1943–1955
Helson L, Helson C (1985) Human neuroblastoma cells and 13-cis-retinoic acid. J Neurooncol 3(1):39–41
Nagai J, Yazawa T, Okudela K, Kigasawa H, Kitamura H, Osaka H (2004) Retinoic acid induces neuroblastoma cell death by inhibiting proteasomal degradation of retinoic acid receptor alpha. Cancer Res 64(21):7910–7917
Celli A, Treves C, Stio M (1999) Vitamin D receptor in SH-SY5Y human neuroblastoma cells and effect of 1,25-dihydroxyvitamin D3 on cellular proliferation. Neurochem Int 34(2):117–124
Jaboin J, Kim CJ, Kaplan DR, Thiele CJ (2002) Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3’-kinase pathway. Cancer Res 62(22):6756–6763
PE applied biosystems (2001) ABI prism 7700 sequence detection system: Relative quatitation of gene expression.user bulletin #2: Rev B, 36 pp
Sabet SJ, Darjatmoko SR, Lindstrom MJ, Albert DM (1999) Antineoplastic effect and toxicity of 1,25-dihydroxy-16-ene-23-yne-vitamin D3 in athymic mice with Y-79 human retinoblastoma tumors. Arch Ophthalmol 117(3):365–370
Zinser GM, McEleney K, Welsh J (2003) Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol 200(1–2):67–80
Brown AJ (2001) Therapeutic uses of vitamin D analogues. Am J Kidney Dis 38(5 Suppl 5):S3–S19
Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM et al (2004) Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis 43(5):877–890
Liu G, Wilding G, Staab MJ, Horvath D, Miller K, Dresen A et al (2003) Phase II study of 1alpha-hydroxyvitamin D(2) in the treatment of advanced androgen-independent prostate cancer. Clin Cancer Res 9(11):4077–4083
Khanna C, Jaboin JJ, Drakos E, Tsokos M, Thiele CJ (2002) Biologically relevant orthotopic neuroblastoma xenograft models: primary adrenal tumor growth and spontaneous distant metastasis. In Vivo 16(2):77–85
Albert DM, Marcus DM, Gallo JP, O’Brien JM (1992) The antineoplastic effect of vitamin D in transgenic mice with retinoblastoma. Invest Ophthalmol Vis Sci 33(8):2354–2364
Veenstra TD, Londowski JM, Windebank AJ, Brimijoin S, Kumar R (1997) Effects of 1,25-dihydroxyvitamin D3 on growth of mouse neuroblastoma cells. Brain Res Dev Brain Res 99(1):53–60
Moore TB, Koeffler HP, Yamashiro JM, Wada RK (1996) Vitamin D3 analogs inhibit growth and induce differentiation in LA-N-5 human neuroblastoma cells. Clin Exp Metastasis 14(3):239–245
Audo I, Darjatmoko SR, Schlamp CL, Lokken JM, Lindstrom MJ, Albert DM et al (2003) Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Invest Ophthalmol Vis Sci 44(10):4192–4199
Albert DM, Scheef EA, Wang S, Mehraein F, Darjatmoko SR, Sorenson CM et al (2007) Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci 48(5):2327–2334
Chung I, Wong MK, Flynn G, Yu WD, Johnson CS, Trump DL (2006) Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res 66(17):8565–8573
Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL (2002) Antiproliferative effects of 1alpha,25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology 143(7):2508–2514
Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE (2000) 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res 87(3):214–220
Peng X, Jhaveri P, Hussain-Hakimjee EA, Mehta RG (2007) Overexpression of ER and VDR is not sufficient to make ER-negative MDA-MB231 breast cancer cells responsive to 1{alpha}-hydroxyvitamin D5. Carcinogenesis 28(5):1000–1007
Abedin SA, Banwell CM, Colston KW, Carlberg C, Campbell MJ (2006) Epigenetic corruption of VDR signalling in malignancy. Anticancer Res 26(4A):2557–2566
Acknowledgements
The research reported in this paper was funded by NIH Grant R01-EY001917, and a Core Grant for Vision Research EY016665 with supplemental funding from Research to Prevent Blindness and Bone Care International, now a part of Genzyme Corp. (Cambridge, Massachusetts, USA).
The authors thank Carol Thiele, PhD, for supplying the various neuroblastoma cell lines; Arthur Polans, PhD, for quantifying VDR levels in the cell lines; and Amit Kumar, MD, Stephen Strugnell, PhD, Christine Damico, Elaina Gates, Mark Hopping, and Kate Fahl for their effort, advice, and support. The authors also wish to thank Peter E. Zage, MD, PhD, at The University of Texas M.D. Anderson Cancer Center, for his consulation and advice in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Ginkel, P., Yang, W., Marcet, M.M. et al. 1 α-Hydroxyvitamin D2 inhibits growth of human neuroblastoma. J Neurooncol 85, 255–262 (2007). https://doi.org/10.1007/s11060-007-9418-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9418-z