Abstract
Bare metal stents (BMSs) have been studied in the treatment of tubular organ occlusion, but restenosis remains a problem with this type of therapy. Drug eluting stents (DESs) were developed afterwards to treat and prevent re-stenosis. Clinical studied have proven the efficacy of DESs for the prevention of smooth muscle and cancer cell proliferations. Coating DESs with a polymer facilitates sustained drug release by diffusion, degradation, and osmosis. To control drug delivery in DESs, the drug and polymeric materials-including their physicochemical properties-must be taken into consideration. It is important to understand polymeric materials for the development drug delivery technologies. This review highlights the studies on drug delivery system based on polymeric material used in DESs and the role of polymeric materials in DESs for drug delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stents are tubular medical devices which provide support for a tubular organ. They can be used in the recanalization of vascular and nonvascular occlusions and prevent future stenosis (Shaikh et al. 2013). Stents are divided as vascular and non-vascular depending on the target. Vascular stents are used to clear occlusions in coronary carotid, renal, iliac, superficial femoral and tibial arteries (Machan et al. Machan 2006). Non-vascular stents are used to clear benign and malignant occlusions of the, esophagus, gastrointestinal tract, biliary tree, urethra, and prostate (Shaikh et al. 2013).
In the past, bare metal stents (BMSs) were used to treat stenosis; however, their effect was insignificant. When BMS was implanted, it tended to injure the blood vessel, causing neointimal proliferation (i.e., re-stenosis). Consequently, BMS re-stenosis rates ranged from 16 to 44 % (Duckers et al. 2007). To avoid this problem, drug eluting stents (DES) were developed. Several classes of drugs combined with stents were used, to produce antiproliferative, anti-inflammatory and antimicrobial effects. There- DESs had a significant reduction in restenosis of up to 16 % (Duckers et al. 2007).
The development of DESs relies on polymeric materials to control drug release. Local delivery of paclitaxel (PTX), sirolimus, zotarolimus, everolimus and gemcitabine (GEM) through polymer based DESs proved efficacious in reducing the occurrence of restenosis (Moses et al. 2003; Stone et al. 2004; Babapulle et al. 2004; Fajadet et al. 2006).
In selecting a controlled drug delivery system, both the drug and polymeric material should be taken into consideration specifically, the physicochemical properties of the drug, the duration of the release and the release profiles (Huang and Brazel 2001). It is important to understand the available drug delivery system and how can be used to improve existing DESs and develop new DESs.
This review, highlights different DESs formulations and discusses the role of polymeric materials in DESs with polymeric film or membrane. Its purpose is to serve as a useful resource for those studying DES technologies.
Drugs for DESs
The drugs released from DESs are expected to inhibit inflammation and neointimal growth (i.e., hyperplasia after stenting). Because the inflammatory and hyperplasia responses result from the complex cellular and tissue signaling (Regar et al. 2001), drugs currently used in DESs are PTX, GEM, sirolimus, everolimus, zotarolimus, dexamethasone, and curcumin (Fig. 1).
PTX was first approved by the FDA in 1992 to treat ovarian cancer (Donehower 1996). The FDA also approved the PTX eluting stent in 2004. PTX is a lipophilic molecule with derivatized diterpenoid that prevent microtubule function (Heldman et al. 2001). Microtubules are a component of the cytoskeleton and essential for cell division (Abal et al. 2003). Therefore, PTX leads to the cell death by inhibiting cell division and migration (Wani et al. 1971).
GEM is a hydrophilic drug and an analog of deoxycytidine. After entering the cell, it becomes phosphorylated by deoxycytidine kinase (Hertel et al. 1990). Then, GEM diphosphate and GEM triphosphate inhibit DNA synthesis by replacing cytidine. (Plunkett et al. 1995). GEM was shown to be effective in treating solid tumors including ovarian, colon, breast, bladder, lung and bile duct cancer (Moon et al. 2011). Because of its high water solubility, its pharmaceutical applications have been restricted (Pili et al. 2009).
Sirolimus, everolimus and zotarolimus (Udipi et al. 2008) are limus family compounds used in DESs. They are immunosuppressive agents with anti-migratory and anti-proliferative effects on vascular smooth muscle cells (Puranik et al. 2013). Sirolimus binds the cytosolic protein, FK-binding protein 12 (KFBP12), forming a complex which subsequently binds to its cellular target, mTOR (Brazelton 1996). mTOR is a member of the P13 K-related protein kinase (PIKK) that plays a critical role in the cell cycle (Costa and Simon 2005). As a result, sirolimus inhibits the cell cycle and leads to cell death.
Dexamethasone is an anti-inflammatory compound approved by the FDA 40 years ago. Dexamethasone is a glucocorticoid that readily permeate cell membranes and binds to specific cytoplasmic receptors (Liu et al. 2003; Villa et al. 1994). Continuous treatment with dexamethasone inhibits the inflammatory response and reduces reactive intimal hyperplasia in rabbit and rat restenosis (Berk et al. 1991).
Curcumin (diferuloylmethane), a major chemical component of turmeric, has a low intrinsic toxicity (Arbiser et al. 1998) and possesses a wide range of pharmacological activity, including anti-thrombus, anti-oxidation, anti-proliferation and anti-carcinogenic properties (Chen and Huang 1998; Kumar et al. 1998). Loading curcumin in DESs has blood compatibility issues, due to its hydrophobic property (Pan et al. 2007). Therefore, the coated polymer must be more hydrophilic and have good anticoagulation characteristics to prevent re-stenosis after stent implantation (Pan et al. 2006).
Polymeric materials for DESs
Polymeric materials have been used in the pharmaceutical industry as matrices for drug delivery systems. Polymeric materials mainly serve as physical support and drug reservoirs (Hutmacher 2001; Ansel et al. 1995). It is also used for coating stents. Polymeric materials stay on the stent after stenting and maintain drug release for sufficient time. Polymeric materials are mainly categorized into natural and synthetic polymers. Natural polymers include collagen, fibrin, hyaluronic acid, chondroitin sulfate, pullulan and gelatin. Synthetic polymers include polyesters and ethylene copolymer. Although a wide range of polymer materials have been used to coat stents, only a few have been studied in laboratory and clinical studied (Table 1). This is because the polymeric materials must have a mechanical and biochemical properties suitable for stent deployment and inflammation prevention.
The natural polymer coated DESs
The ACS Multi-Link (Abbott, USA) stent was coated with chondroitin sulfate. It is first coated with PTX by evaporation of a volatile solvent and then dipped in a chondroitin sulfate solution containing glutaraldehyde (Farb et al. 2001). The formation of a polymer film is based on the coacervation of two oppositely charged polymers, gelatin and chondroitin sulfate. The chondroitin sulfate film is prepared under optimized polymer concentrations and crosslinking density was effective in in vivo controlled release of PTX for more than 2 weeks (Farb et al. 2001).
Chen Mei-chin, et al. describes the development of a collagen coated drug eluting stent. Using a spray coating method, collagen and sirolimus were alternately coated layer-by-layer onto the surface of a metallic stent with a topcoat of collagen to control drug release (Chen, M. C. et al. 2005). With high dose sirolimus and the collagen topcoat, drug release was sustained for 4 weeks (Chen et al. 2005).
Moon et al. described the development of an acetylated pullulan coated drug eluting stent. Pullulan is a neural glucan and an edible, biodegradable polymer without any toxicities (Xi et al. 1996; Kim et al. 2008; Lee et al. 2003; Lu et al. 2009). An acetyl group was chemically introduced to pullulan to increase solvent compatibility and improve degradation kinetics (Moon et al. 2011)). The pullulan acetate (PA) was used to coat a polytetrafluoroethylene (PTFE) covered self-expandable metal stent dipped in GEM. Sustained release of GEM from the PA-PTFE membrane lasted approximately 4 weeks. It seemed that hydrogen bonds formed between the hydroxyl group of GEM and acyl group of PA (Le Tien et al. 2003). This means that, PA-PTFE covered stent are capable of prolonged drug release.
Swanson et al. studied phosphocholine-coated stents doped with PTX. Phosphocholine monomers polymerized with hydrophobic monomers to create a highly hydrophobic polymer. (Swanson et al. 2002). The hydrophobic PTX interacted with the phosphocholine in a hydrophobic–hydrophobic manner. The polymer–drug hydrophobic interaction facilitated sustained drug release.
The synthetic polymer coated DESs
Synthetic polymers are one the most commonly used biomedical polymer materials. The typical synthetic polymers are poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone), polyanhydride, poly(orthoester), and poly(vinyl alcohol) (PVA) (Gunatillake et al. 2006).
The Taxus (Boston Scientific, USA) stent used a poly(styrene-b-isobutylene-b-styrene) (SIBS) tripolymer with PTX (Fig. 2a). A styrene layer on the top controls releases of PTX over a prolonged period of time (Acharya and Park 2006). PTX is released from the SIBS polymer matrix directly into the environment by a diffusion-controlled release.
Schematic illustration of the cross-sectional views of the Taxus (a) and Cypher (b) stentS (Acharya and Park 2006)
The Cypher (Johnson & Johnson, USA) stent utilizes poly(ethylene-co-vinyl acetate) (PEVA) and poly(n-butyl methacrylate) (PBMA) as the non-degradable polymer coating that carries the sirolimus (Fig. 2b). A coating of PEVA and PBMA copolymer with the sirolimus in a 2:1 ratio sustained drug release for 30 days (Acharya and Park 2006). The Cypher stent surface was coated with the reservoir layer and then coat with a thin layer of PBMA. The presence of the topcoat results in diffusion-controlled release.
The Xience (Abbott, USA) stent uses a poly(vinylidenefluoride-co-hexafluoropropylene) (PVDF-HFP), PBMA polymer with everolimus (Ding et al. 2009). The Xience stent uses thin struts stent for reduce contact area, resulting in endothelial cells more rapidly covering the stent. The Xience stent surface was coated with PVDF for better biocompatibility and PBMA for sustained drug release (Carter et al. 2006).
Lee et al. described the development of a polyurethane coated drug eluting stent. The polyurethane was coating on PTFE-covered stent with GEM using dip coating method (Lee et al. 2012). GEM is highly soluble in water (15.3 mg per ml) (Pili et al. 2009). To prolong drug release, the GEM was nano-granulated (Fig. 3a). Doing so dramatically decreased the initial burst of GEM; however, only 40 % of the nano-granulated GEM was released after 30 days. To release more GEM, Lutrol F127 was added, increasing the 30 days release to 60 % (Fig. 3b).
Release of nano-granulated gemcitabine (a) and accumulated gemcitabine released from gemcitabine-loaded polyurethane and polyurethane-Lutrol F127 membranes (b) (Lee et al. 2012). Reprinted with permission from Elsevier
Photosensitizer (PS) incorporated polymer coated DESs
Photodynamic therapy (PDT) is a clinically approved method for local treatment of cancers (Dougherty et al. 1998). PDT employs a PS which generates reactive oxygen species (ROS) or singlet oxygen (SO) through direct photochemical reactions with specific wavelengths of light (Chen et al. 2009). ROS and SO have been known to cause cancer cell death by damaging the cell membrane (Schafer and Buettner 1999). Recently, PDT was successfully used in combination with DES to treat various cancers.
Bae et al. describes the development of a PDT stent using a PS as the drug. PA conjugated pheophorbide A was used to coat a self-expanding nonvascular metal stent (Bae et al. 2014). The PDT stent exhibited strong photo-fluorescence (Fig. 4) and is a possible treatment for cholangiocarcinoma. The pheophorbide A remained in membrane coating of the stent for a long period, facilitating the use of PDT for more than 2 months.
DIC (a) and Photo-fluorescence (b) images of a PDT stent (Bae et al. 2014). Reprinted with permission from Elsevier
Additionally, Min, Daehong et al. describes the development of a photosensitizer loaded drug eluting stent. To improve the tissue penetration efficiency of hydrophilic drugs, this group studied photochemical tissue penetration (PTP) DES (Min et al. 2015). The PTP technology using SO damages the epithelial layer. The PTP stents were made using polyurethane, Chlorin e6 and GEM. It inhibited tumor growth almost 3 times as much as the control group (Min et al. 2015).
Drug release control of DESs
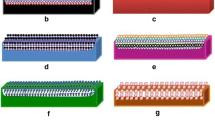
In DESs, polymeric materials and polymer coating are significant to drug release control, because drug release profiles are determined by environmental pH, temperature, and ionic strength (Park 1997; Qiu and Park 2012). Drugs can load directly onto a stent’s metallic surface, but most drugs in DESs are loaded with polymer using topcoating, sublayer coating, reservoir coating and biodegradable polymer coating (Fig. 5).
Schematic representation of different modalities of DES platforms. (a) Non-polymer based drug release, (b) drug diffusion through an additional polymer coating, (c) drug-polymer blend, released by diffusion, (d) combination of topcoat and matrix coating membrane, (e) drug loaded in stent reservoir, (f) drug loaded in reservoir with and additional polymer coating, (g) drug-biodegradable polymer coating released by degradation of the polymer, (h) biodegradable polymer-coated biodegradable stent. (Sousa et al. (2003))
Stent coating methods
Various polymeric materials have been used to cover stent surfaces using a different coating techniques, such as dip coating (Wache et al. 2003), spray coating (Liu et al. 2010), electrospinning (Park et al. 2011), and rolling coating (Jeong et al. 2015). The dip coating method is the most basic coating technique. It is done by dipping a stent into a polymer or polymer–drug solution followed by solvent evaporation. Although the dip coating method is easy to execute, it has a disadvantage in that coating thickness and drug dose is poorly controlled. Spray coating involves spraying microdroplets of a polymer or polymer–drug solution directly onto the stent surface using various spraying devices. It creates a uniform coat and can be used for thin coats; however, there is loss of coating solution and polymer viscosity alters the results. Electrospinning use an electrical field to generate aligned or random nano-fibers from a polymer or polymer-drug solution. It creates uniform coat and fiber thickness can be controlled; however, some coats can be unstable and membranes can be too thick. Rolling coating is similar to spray coating. While the stent is rotating, drug or polymer-drug solution coats it by nozzle or pipet. The coating is uniform and the amount of drug that is loaded can be controlled, but thickness is limited. Because of this, many use spray coating rather than rolled coating.
Drug release system of DESs
The drug release system was classified into physical and chemical mechanisms (Hwang et al. 2001). The physical mechanisms include diffusion of drug through a polymer layer, degradation of a polymer matrix controlling the drug release rate and osmotic pressure for drug release (Craig 2002). The advantage of using physical mechanisms is that the drug release kinetics can be tuned by adjusting the thickness of the polymeric membrane, type of a polymer, and surface area of DESs (Banerjee and Robinson 1991). The chemical mechanisms are based on breaking of covalent bonds, such as the bond between the drug and polymer, by chemical or enzymatic degradation (Langer 1990; Rathbone et al. 2002; Saltzman 2001). To do this, the drug has to be chemically modified for grafting to the polymer. Because of the challenges in synthesis, physical mechanisms have been used more often than chemical mechanisms.
Drug release from the DESs is highly associated with the degradation rate of the polymeric materials used. Biodegradable polymers commonly used in DESs are PLA, PGA, PLGA, poly(ε-caprolactone). They do not dissolve quickly in water, but degrade over weeks and months by hydrolysis. When biodegradable polymers are degraded in DESs, the loaded drugs are slowly released from the polymer matrix, providing sustained and effective therapy. Because stents have to be retained for weeks and months, biodegradable polymers can be used in DESs. The Conor stent from Conor Medsystems, has numerous holes. Each hole is filled with drug and biodegradable polymer, PTX and PLGA (Finkelstein et al. 2003). The PTX release profile can be controlled by adjusting the lactic acid to glycolic acid ratio in the PLGA.
The reservoir system is based on diffusion and utilizes water insoluble polymers (Robinson 1978). It is often combined with a film or membrane. Selection of a biocompatible polymer is important because surface induced thrombosis is one of the major problems with DESs (Amiji and Park 1993). The Cypher stent uses PEVA and PBMA. The top layer of the Cypher stent, a drug free PBMA coating, serves to control drug release and prevent a burst effect. The Taxus stent uses PTX and SIBS polymer; drug release is mediated by diffusion controlled matrix system.
Osmosis-based drug controlled release is defined as the spontaneous movement of a solution from a lower solute concentration to higher solute concentration through a permeable membrane (Gupta et al. 2010). Drugs inside the polymeric membrane of DESs move outside the membrane, where there is a lower drug concentration, by inflow of solvent.
Conclusions
The effectiveness of DES therapy is largely dependent on the drug, polymeric materials, and coating method because these factors significantly influence the drug release system. Many vascular and non-vascular DESs were studied with various polymers and drugs for the treatment of restenosis and gastrointestinal cancer. Although many polymeric materials have been studied in DESs, there are very few commercial DESs available, namely, Taxus, Cypher, and Xience. Additionally, the polymers used in DESs can cause inflammation, and the drugs can cause toxicity. To overcome these problems, natural and synthetic polymers such as collagen, pullulan, PLGA, and PLA were incorporated into DES design. They were attractive substitutes because they have excellent biocompatibility and degradation properties that can be used to control drug release. Drug release depends on its location in the polymeric matrix and the material used to coat the stent. Therefore, the choice of polymer and the coating methods are very important for controlled drug release. The success of controlled drug release will depend on the development of a biocompatible drug releasing polymer and how it is coated on the stent. Although many DESs have been developed, it remains for drug release control to be optimized for efficient treatment of diverse lesions and conditions.
References
Abal M, Andreu JM, Barasoain I (2003) Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets 3:193–203
Acharya G, Park K (2006) Mechanisms of controlled drug release from drug-eluting stents. Adv Drug Deliv Rev 58:387–401
Amiji M, Park K (1993) Surface modification of polymeric biomaterials with poly (ethylene oxide), albumin, and heparin for reduced thrombogenicity. J Biomater Sci Polym Edition 4:217–234
Ansel HC, Popovich NG, Allen LV (1995) Pharmaceutical dosage forms and drug delivery systems. Lippincott Williams & Wilkins, Philadelphia
Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Byers HR et al (1998) Curcumin is an in vivo inhibitor of angiogenesis. Mol Med 4:376
Babapulle MN, Joseph L, Bélisle P, Brophy JM, Eisenberg MJ (2004) A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 364:583–591
Bae BC, Yang SG, Jeong S, Lee DH, Na K, Kim JM, Seo DW et al (2014) Polymeric photosensitizer-embedded self-expanding metal stent for repeatable endoscopic photodynamic therapy of cholangiocarcinoma. Biomaterials 35:8487–8495
Banerjee PS, Robinson JR (1991) Novel drug delivery systems. Clin Pharmacokinet 20:1–14
Berk BC, Gordon JB, Alexander RW (1991) Pharmacologic roles of heparin and glucocorticoids to prevent restenosis after coronary angioplasty. J the Am Coll of Cardiol 17:111–117
Brazelton TR (1996) Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin immunol 8:710–720
Carter AJ, Brodeur A, Collingwood R, Ross S, Gibson L, Wang CA, Virmani R et al (2006) Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc Interv 68:97–103
Chen HW, Huang HC (1998) Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br J Pharmacol 124:1029–1040
Chen K, Preuß A, Hackbarth S, Wacker M, Langer K, Röder B (2009) Novel photosensitizer-protein nanoparticles for photodynamic therapy: photophysical characterization and in vitro investigations. J Photochem Photobiol B Biol 96:66–74
Costa MA, Simon DI (2005) Molecular basis of restenosis and drug-eluting stents. Circulation 111:2257–2273
Craig DQ (2002) The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 231:131–144
Ding NN, Pacetti SD, Tang FW, Gada M, Roorda W (2009) XIENCE V™ stent design and rationale. J Interv Cardiol 22:S18–S27
Donehower RC (1996) The clinical development of paclitaxel: a successful collaboration of academia, industry and the National Cancer Institute. Oncologist 1:240–243
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Peng Q et al (1998) Photodynamic therapy. J Nat Cancer Inst 90:889–905
Duckers HJ, Nabel EG, Serruys PW (2007) Essentials of restenosis. Humana Press, New York
Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Münzel T, Investigators Endeavor II et al (2006) Randomized, double-blind, multicenter study of the endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions clinical and angiographic results of the endeavor II trial. Circulation 114:798–806
Farb A, Heller PF, Shroff S, Cheng L, Kolodgie FD, Carter AJ, Virmani R et al (2001) Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation 104:473–479
Finkelstein A, McClean D, Kar S, Takizawa K, Varghese K, Baek N, Eigler NL et al (2003) Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation 107:777–784
Gunatillake P, Mayadunne R, Adhikari R (2006) Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev 12:301–347
Gupta BP, Thakur N, Jain NP, Banweer J, Jain S (2010) Osmotically controlled drug delivery system with associated drugs. J Pharm Pharm Sci 13:571–588
Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M, Bunge KE et al (2001) Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 103:2289–2295
Hertel LW, Boder GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB (1990) Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res 50:4417–4422
Huang X, Brazel CS (2001) On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release 73:121–136
Hutmacher DW (2001) Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci Polym Ed 12:107–124
Hwang CW, Wu D, Edelman ER (2001) Physiological transport forces govern drug distribution for stent-based delivery. Circulation 104:600–605
Jeong D, Lee DH, Lee DK, Na K (2015) Nonvascular drug-eluting stent coated with sodium caprate-incorporated polyurethane for the efficient penetration of paclitaxel into tumor tissue. J Biomater Appl 29:1133–1144
Kim S, Park KM, Ko JY, Kwon IC, Cho HG, Kang D, Na K et al (2008) Minimalism in fabrication of self-organized nanogels holding both anti-cancer drug and targeting moiety. Colloids Surf B Biointerf 63:55–63
Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB (1998) Curcumin (diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-κB activation. Biochem Pharm 55:775–783
Langer R (1990) New Meth drug deliv Sci 249:1527–1533
Le Tien C, Lacroix M, Ispas-Szabo P, Mateescu MA (2003) N-acylated chitosan: hydrophobic matrices for controlled drug release. J Control Release 93:1–13
Lee ES, Na K, Bae YH (2003) Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release 91:103–113
Lee JW, Yang SG, Na K (2012) Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. Int J Pharm 427:276–283
Liu X, Huang Y, Hanet C, Vandormael M, Legrand V, Dens, J et al (2003) Study of antirestenosis with the BiodivYsio dexamethasone-eluting stent (STRIDE): a first-in-human multicenter pilot trial. Cathet Cardiovasc Interv 60:172–178
Liu SJ, Chiang FJ, Hsiao CY, Kau YC, Liu KS (2010) Fabrication of balloon-expandable self-lock drug-eluting polycaprolactone stents using micro-injection molding and spray coating techniques. Ann Biomed Eng 38:3185–3194
Lu D, Wen X, Liang J, Gu Z, Zhang X, Fan Y (2009) A pH-sensitive nano drug delivery system derived from pullulan/doxorubicin conjugate. J Biomed Mater Res Part B Appl Biomater 89:177–183
Machan L (2006) Clinical experience and applications of drug-eluting stents in the noncoronary vasculature, bile duct and esophagus. Adv Drug Deliv Rev 58:447–462
Min D, Jeong D, Choi MG, Na K (2015) Photochemical tissue penetration via photosensitizer for effective drug penetration in a non-vascular tumor. Biomaterials 52:484–493
Moon S, Yang SG, Na K (2011) An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of gastrointestinal cancer and related stenosis. Biomaterials 32:3603–3610
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Jaeger JL et al (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349:1315–1323
Pan CJ, Tang JJ, Weng YJ, Wang J, Huang N (2006) Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J Control Release 116:42–49
Pan CJ, Shao ZY, Tang JJ, Wang J, Huang N (2007) In vitro studies of platelet adhesion, activation, and protein adsorption on curcumin-eluting biodegradable stent materials. J Biomed Mater Res Part A 82:740–746
Park K (1997) Controlled drug delivery: challenges and strategies. American Chemical society, Washington
Park CG, Kim MH, Park M, Lee JE, Lee SH, Park JH, Choy YB et al (2011) Polymeric nanofiber coated esophageal stent for sustained delivery of an anticancer drug. Macromol Res 19:1210–1216
Pili B, Bourgaux C, Meneau F, Couvreur P, Ollivon M (2009) Interaction of an anticancer drug, gemcitabine, with phospholipid bilayers. J Therm Anal Calorim 98:19–28
Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V (1995) Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 22:3–10
Puranik AS, Dawson ER, Peppas NA (2013) Recent advances in drug eluting stents. Int J Pharm 441:665–679
Qiu Y, Park K (2012) Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 64:49–60
Rathbone MJ, Hadgraft J, Roberts MS (2002) Modified-release drug delivery technology. CRC Press, Boca Raton
Regar E, Sianos G, Serruys PW (2001) Stent development and local drug delivery. Br Med Bull 59:227–248
Robinson JR (1978) Sustained and controlled release drug delivery systems M. Dekker 6
Saltzman WM (2001) Drug delivery: engineering principles for drug therapy. Oxford University Press, Oxford
Schafer FQ, Buettner GR (1999) Singlet oxygen toxicity is cell line-dependent: a study of lipid peroxidation in nine leukemia cell lines. Photochem Photobiol 70:858–867
Shaikh M, Kichenadasse G, Choudhury NR, Butler R, Garg S (2013) Non-vascular drug eluting stents as localized controlled drug delivery platform: preclinical and clinical experience. J Control Release 172:105–117
Sousa JE, Serruys PW, Costa MA (2003) New frontiers in cardiology drug-eluting stents: part I. Circulation 107:2274–2279
Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Popma JJ et al (2004) A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Eng J Med 350:221–231
Swanson N, Javed Q, Hogrefe K, Gershlick A (2002) Human internal mammary artery organ culture model of coronary stenting: a novel investigation of smooth muscle cell response to drug-eluting stents. Clin Sci 103:347–353
Udipi K, Chen M, Cheng P, Jiang K, Judd D, Caceres A et al (2008) Development of a novel biocompatible polymer system for extended drug release in a next-generation drug-eluting stent. J Biomed Mater Res Part A 85:1064–1071
Villa AE, Guzman LA, Chen W, Golomb G, Levy RJ, Topol EJ (1994) Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. J Clin Invest 93:1243
Wache HM, Tartakowska DJ, Hentrich A, Wagner MH (2003) Development of a polymer stent with shape memory effect as a drug delivery system. J Mater Sci Mater Med 14(112):109
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents: VI. The isolation and structure of taxol, a novel antitumor and anitleukemic agent from Taxus brevifolia. J Am Chem Soc 18:242–260
Xi K, Tabata Y, Uno K, Yoshimoto M, Kishida T, Sokawa Y, Ikada Y (1996) Liver targeting of interferon through pullulan conjugation. Pharm Res 13:1846–1850
Acknowledgments
This work was supported by the Technology Innovation Program (10044021) funded by the Ministry of Trade, Industry & Energy (MI, Korea). These authors (Jeongdeok Seo, Jonghwan Lee, and Kun Na) declare that they have no conflict of interest. This article does not contain any studies with human and animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seo, J., Lee, J. & Na, K. Polymeric materials for drug release system in drug eluting stents. Journal of Pharmaceutical Investigation 46, 317–324 (2016). https://doi.org/10.1007/s40005-016-0251-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0251-2