Abstract
Vascular stent is tubular shaped medical device designed to treat occluded circulatory or digestive systems by opening the blocked vascular sites. Originally stents were made of bare metals, such as stainless steel and cobalt–chromium alloys, due to their inherent corrosion resistance and good mechanical properties. Damage of intimal layer during expansion of stent, however, have caused neointimal hyperplasia resulting in in-stent restenosis which is one of main complications of vascular stent treatment. Drug-eluting stents (DESs) were introduced to alleviate this restenosis by local delivery of anti-proliferative drugs to the implanted or inserted site. The DES systems have been developed over the years by making changes in its main component, namely, drug, polymeric coating layer and expandable scaffold. Nevertheless, late thrombosis caused by drugs themselves and polymeric coating layer still remains as a serious problem of DES systems. Numerous studies, therefore, consistently focus on more advanced DES systems to solve the current issues. In this mini review article, we cast an opinion on how to improve the potential of DES system by means of (i) summarizing the history of DES system developed so far and (ii) updating current advances for the future generation of DES system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stent was named after British dentist Charles T. Stent who invented stent in the late nineteenth century. Originally, stent was a plastic compound material invented to impress patient’s mouth (Hedin 1997). Today, stent is a common name for tubular-shaped medical devices that aim for the patency of blocked or narrowed vascular and non-vascular vessels (Shaikh et al. 2013). The stents are classified into two major categories depending on their application site. One is vascular stent and the other is non-vascular stent. Between those two categories of stents, vascular stents are used for opening of important blood vessels, including coronary artery, peripheral blood vessel and cerebrovasculature (Shaikh et al. 2013).
The early coronary artery stent was called bare metal stent (BMS) because it was made of pure metal, such as stainless steel, cobalt–chromium (Co–Cr), nickel–titanium (Grabow et al. 2010) or platinum–chromium alloys (Jorge and Dubois 2015) without additional chemical treatments. Those BMSs were designed to be expended in the blocked site using ballooning device or self-expandable using shape memory property (Grabow et al. 2010). Unlike balloon angioplasty, after stent implantation, stent remains in the blood vessel and can endure the radial stress from the blood vessel wall. Therefore, stent implantation significantly reduced risk of initial restenosis by recoil of the blood vessel wall (Haude et al. 1993). Compared to coronary artery bypass grafting, there was no differences in the death, stroke and myocardial infraction rates between bypass grafting and stent groups, while stent was about 3000 USD cheaper than bypass grafting (Meraj et al. 2015). The long-term result of stent implantation, however, worse than that of bypass grafting. According to lots of comparison studies, the number of patients who required a secondary revascularization was 4–7 times higher in stent group than bypass grafting group (Meraj et al. 2015). During the expansion of stent, endothelial surface which is located inner most part of blood vessel is damaged and inflammatory process is triggered. Cytokines and growth factors produced by inflammatory cells activate the smooth muscle cells (SMCs) proliferation and migration through the denuded endothelium. These overly proliferated SMCs leads to neointimal hyperplasia resulting in in-stent restenosis (Bhatia et al. 2004; Kornowski et al. 1998; Welt and Rogers 2002), as shown in Fig. 1. To reduce in-stent restenosis, drugs are frequently prescribed frequently with BMS implantation (ten Berg et al. 2001).
Mechanisms of in-stent restenosis after BMS placement; a diseased artery before intervention, b endothelial denudation by stent placement, c, d leucocyte recruitment infiltration and smooth muscle cell proliferation, e neointimal thickening and f long term in-stent restenosis.
Drug-eluting stent (DES) of which surface is coated with drug-loaded layer have been introduced to alleviate the in-stent restenosis caused by BMS implantation. After the first DES was commercialized in the early of 2000s, there have been extensive development in the DES field as summarized in Table 1. The commercialized DESs usually contain paclitaxel (PTX), as an anti-cancer drug, or limus family drugs, as anti-proliferative drugs (Meraj et al. 2015; Stefanini and Holmes 2013). Those drugs prevent neointimal hyperplasia by inhibiting the mechanism of proliferation of SMCs. Although DESs significantly reduced the in-stent restenosis problem, it also caused another problem, i.e. late thrombosis (Joner et al. 2006). The inherent toxicity of those anti-proliferative drugs on endothelial cells delays the healing of endothelial layer and inflammatory reaction by remaining polymer coating layer lead to thrombosis (Joner et al. 2006), the rate of that is higher in DES group than BMS group (Stone et al. 2007).
Therefore, many studies still focus on how to ameliorate these shortcomings of existing DES system or to develop new strategies for advanced DES systems. In this respect, we aim to briefly summarize the development history of DES up to now and update current advances for the future-generation coronary stent or advanced DES systems.
Historical review of DES system
DES systems are composed of three major components; (1) anti-proliferative drug for inhibiting over proliferation of SMCs, (2) polymeric coating layer for controlled release of anti-proliferative drugs and (3) scaffolds for bearing radial stress by blood vessel wall or shear stress from flow of fluids, as shown in Fig. 2. The properties of each component of DES are criteria for classifying the generation of DES systems.
First generation DES
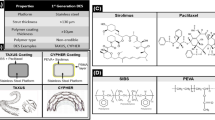
The first generation of DES had stainless steel-based scaffold and durable polymer coating layer which could elute anti-proliferative drugs, namely sirolimus (SRL) and PTX. The first clinical trial of DES was made using Cypher™, manufactured by Cordis Corporation (Rensing et al. 2001), which was approved by US Food and Drug Administration (FDA) in 2003 (Moses et al. 2003). The Cypher™ has dual coating layer on 316L stainless steel scaffold. The dual coating layer of Cypher™ is composed of SRL-incorporated PEVA base layer and PBMA top layer for release rate control. From this coating layer, 80% of loaded SRL was released within 4 weeks and 100% within 6 weeks (Puranik et al. 2013). After 1 year, PTX-eluting Taxus® from Boston Scientific was approved by FDA (Puranik et al. 2013). The Taxus® has a triblock copolymer, poly(styrene-b-isobutylene-b-styrene) (SIBS) coating layer for eluting PTX in a controlled manner. Because there was no top coating layer, Taxus® is supposed to show relatively more initial burst release compared to Cypher™. The initial burst release from Taxus® was controlled by adjusting drug loading amount and the dose of released drug was reasonable for preventing SMCs proliferation (Acharya and Park 2006). In the randomized clinical trial, both first-generation DES groups demonstrated significant reduction in in-stent restenosis rate compared to BMS group (Moses et al. 2003; Stone et al. 2004). When those two first-generation DES was compared to each other, there was no difference in risk of death rate, while Cypher™ showed superiority in the reduction of restenosis and revascularization treatment than Taxus®. Those first-generation DESs, however, showed long-term safety problem, i.e. late thrombosis which occurred after 1 year of stent treatment, and the rate of it was even higher than that in BMS group (Park et al. 2006; Stone et al. 2007). The one reason of late thrombosis is toxicity of the anti-proliferative drugs. Those drugs inhibit proliferation of cells by blocking cell cycle. Because it does not have cell specificity, growth of endothelial cells also is affected and re-endothelialization is delayed by eluted drugs (Finn et al. 2005; Joner et al. 2006). In addition, inflammatory reaction by remaining polymeric coating layer was pointed out as another possible reason of late thrombosis (Finn et al. 2005; Wilson et al. 2009). Those durable polymers are not toxic to cells, however, their hydrophobicity are responsible for adhesion of inflammatory activated monocytes (Heze-Yamit et al. 2008). The second-generation DESs were, thus, introduced to ameliorate of drawback of the first-generation DESs.
Second generation DES
The second-generation DES has been developed by modifying all three major components of first-generation DES systems to reduce its late thrombosis rate which was considered the critical problem of first-generation DES (Puranik et al. 2013). For drug, PTX and SRL were replaced with other limus family drugs, such as zotarolimus and everolimus, which have less toxicity than PTX and SRL. The anti-proliferative mechanisms of zotarolimus and everolimus are basically same as that of SRL, in which inhibit cell cycle by blocking G1/S transition. Also more biocompatible and hydrophilic polymers were introduced as drug-eluting coating materials to alleviate inflammatory reaction from remaining coating layer. Furthermore, mechanical property and radio-opacity were improved by using Co–Cr alloys as scaffold materials. The enhanced mechanical properties of stent also allowed thinner stent strut, which is another advantage of second-generation DESs. Thinner stent strut can reduce blood fluid stagnation or recirculation that results in early thrombosis (Collet et al. 2016) and time required to be covered with endothelial cell layer (Puranik et al. 2013).
Endeavor® stent from Medtronic is zotarolimus-eluting stent which was approved by FDA in 2008 (Puranik et al. 2013). Thin phosphorylcholine (PC)-based coating layer could elute 95% of loaded zotarolimus within 15 days (Bridges and Cutlip 2009). The PC which has zwitterionic nature demonstrated good biocompatibility as well (Bridges and Cutlip 2009). Xience V™ from Abbott is another second-generation DES which could elute everolimus. Everolimus was loaded into biocompatible copolymers, composed of PBMA and PVDF-HFP and 80% of the everolimus was released out within 30 days (Akin et al. 2011). The hydrophilic polymer (PDVF-HFP) was selected for better biocompatibility of the abluminal side of stent (Puranik et al. 2013). Xience V™ stent was also approved by FDA in 2008. In the clinical trial, Xience V™ demonstrated similar or less risk than first-generation DESs, while Endeavor® showed higher risk of revascularization (Stefanini and Holmes 2013). Resolute™ is modified zotarolimus-stent which has similar clinical risk as everolimus-eluting stent (Stefanini and Holmes 2013).
Third generation DES
In the third-generation DES system, extensive modification of each component DES has been proceeded. For reducing inflammatory reaction from remaining coating layer, previously used durable polymers were replaced with biodegradable polymer (Akin et al. 2011). Bio-absorbable materials, such as magnesium alloys or biodegradable polymers were studied as alternative scaffold materials of stainless steel and Co–Cr alloys (Akin et al. 2011; Ernst and Bulum 2014). Also, Biolimus A9, an improved limus family, was used for commercialized third-generation DES (Wiemer et al. 2017).
BioMatrix® by Biosensor International, which has been renamed as Nobori® by Terumo (Japan) after license transfer, has poly(l-lactic acid) (PLLA)-based coating layer was designed for eluting Biolimus A9 and eventually being resorbed within 6–9 months (Akin et al. 2011; Kadota et al. 2012). The clinical trial using the Nobori® stent is currently in progress. For 5-year clinical trial, Nobori® stent showed superior result than PTX-eluting Taxus stent (Chevalier et al. 2015). Furthermore, from the 3-year result, Nobori® stent was not inferior compared with everolimus-eluting stent (Natsuaki et al. 2015). Absorb stent from Abbott is a polymeric scaffold-based DES which is supposed to be degraded completely after implantation. The scaffold of Absorb, composed of PLLA, was coated with everolimus-loaded poly(d-lactic acid) (Ernst and Bulum 2014). Those lactide-based polymers decompose into carbon dioxide and water via Krebs cycle after degradation (Akin et al. 2011) and demonstrated better biocompatibility than conventional durable polymers (Busch et al. 2014). The Absorb was not inferior to second-generation stent Xience in clinical trial (Ellis et al. 2015). Based on this result, the Absorb was approved by FDA recently (Sorrentino et al. 2017).
Although it has not yet been commercialized, there was effort to develop anti-thrombotic drug-eluting stent using curcumin with biodegradable polymeric coating layer. Curcumin is known to its ability to inhibit proliferation of SMCs by arresting G0/G1 phase (Chen and Huang 1998) and thrombosis by preventing aggregation of platelet (Shah et al. 1999). Curcumin was loaded onto stent surface using poly(lactic-co-glycolic acid) (PLGA) as a form of simple film (Pan et al. 2007) or nanoparticle (Nam et al. 2007). The released curcumin from stent effectively inhibited platelet aggregation and fibrinogen adsorption suggesting its potential anti-thrombotic ability (Pan et al. 2007).
Polymer-free DES
A lot of efforts have been made to exclude drug-eluting polymeric coating layers because they have been considered as one of potential risks of late thrombosis caused by inflammatory reaction. One of methods for drug delivering without coating the stent with polymers is to make drug reservoir. Janus stent is first commercialized and CE-marked DES using the reservoir (Puranik et al. 2013). The drug reservoir was fabricated on stent strut using laser and tacrolimus, a limus-family drug was loaded into the reservoir. BioFreedom manufactured by Biosensors International had micro-textured surface on 316L stainless steel-based substrate. The micro-texture was prepared by microabrasion technique and Biolimus A9 was loaded on the surface (Abizaid and Costa 2010). In comparison with polymer-based DES system, reservoir-based DES, however, showed inferior clinical trial results. Only the BioFreedom is known to demonstrate better clinical result than polymer-based DES systems (Chen et al. 2015). Therefore, enormous efforts to improve reservoir-based DES system are still required. More recently, in order to make the drug reservoirs, other nanofabrication techniques such as anodic oxidation (Porta-i-Batalla et al. 2016) and ion-beam erosion have been also tried in the laboratory scale (Park et al. 2016).
Future perspectives of DES
The third-generation DES system, represented by biodegradable polymer-based drug carrier have shown alleviated long-term inflammation compared with durable polymers by being resorbed out. However, the biodegradable polymers used in third-generation DES systems also caused chronic inflammation (Anderson 2001; Böstman and Pihlajamäki 2000). In this chapter, we will review recent advances for alternative or advanced DES systems which have further reduced late restenosis caused by chronic inflammation or can promote healing of blood vessel itself.
Stent containing anti-inflammatory Mg(OH)2
Polylactide (PLA)-based biodegradable polymers, including PLLA, PDLLA and PLGA are currently extensively investigated as a drug-eluting layer (Mani et al. 2007; Zhang et al. 2013) and bioresorbable scaffold in the DES field (Zhang et al. 2013). These polymers are completely degraded in human body by Krebs cycle (Dunne et al. 2000), but also known to cause chronic inflammation (Anderson 2001; Böstman and Pihlajamäki 2000) which might result in late restenosis (Virmani et al. 2002). During the degradation by hydrolysis, PLAs decompose into short chain polymers or monomer lactic acid which decrease pH of environment (Liu et al. 2006; Sung et al. 2004). Weak mechanical properties are another shortcomings of PLA-based polymer when it is used as scaffold (Chen et al. 2009). PLAs are significantly weaker than stainless steel or Co–Cr alloys. Thick strut, therefore, is essential for polymer stent for bearing enough radial force. However, because thick strut is another cause of thrombosis (Collet et al. 2016), the PLA should be reinforced for thinner strut thickness.
One of the easiest ways to reinforce polymeric material is to make combination with mechanically stronger substances, namely ceramics or metals. Magnesium hydroxide, Mg(OH)2, is a basic ceramic substance which can answer the purpose of reinforcing polymer matrices but also neutralizing the acidic byproducts from biodegradable polymers (Fig. 3a) (Kum et al. 2013). Mg(OH)2, a major component of antacid, is easily degradable in acidic environment while it is not in the pure water (Dong et al. 2011). Unlike biodegradable polymers, degradation of Mg(OH)2 rather increase pH of surrounding environment. Mg ion, one of degradation product, is also bio-resorbable (Saris et al. 2000). Effect of size and shape of Mg(OH)2 nanowhisker on physicochemical, mechanical and biological properties of PLLA matrix was recently studied (Kum et al. 2014a). Mg(OH)2 nanorod and nanoplate were respectively prepared by hydrothermal and precipitation method in various sizes (150 and 350 nm for nanorod; 60 and 300 nm for nanoplate) and then composited with PLLA matrix without post treatment. Accelerated degradation test at 80 °C revealed that incorporation of Mg(OH)2 could neutralize media successfully when Mg(OH)2 content was 30 wt%. The neutralizing effect was not dependent on its shape while small particle was slightly effective than larger one. The in vitro biological properties in terms of cytotoxicity and inflammatory responses were evaluated using U937 cells. The cytotoxicity was not dependent on the amount of nanoparticle while increasing the amount of 60 nm Mg(OH)2 nanoplate increased the inflammatory response of U937 cells. Considering pH neutralization, cytotoxicity and inflammatory response, the optimal Mg(OH)2 content in the PLLA matrix was 30 wt% for both shapes. Mg(OH)2 nanorod was much more effective in increasing elastic modulus of PLLA film than Mg(OH)2 nanoplate. The effectiveness of Mg(OH)2 nanorod is seems to be related with its crystal growth toward [001] direction. In the meantime, tensile strength and elongation of PLLA matrix decrease as Mg(OH)2 content irrespective of its shape or size. It was due to weak interfacial compatibility between Mg(OH)2 and PLLA matrix. For mechanical stability, the interfacial compatibility, therefore, was improved by modifying Mg(OH)2 particle surface with organic substance (Kum et al. 2014b). Surface of Mg(OH)2 particle prepared by hydration of MgO particle was decorated with either l or d type of oligolactide and then composited with PLLA matrix. Both l- and d-lactide-grafted Mg(OH)2 were effectively neutralize the pH of media when they were incorporated more than 20 wt% in the matrix and the resulting enhanced cell viability and reduced inflammatory response were also observed. Mechanical properties of Mg(OH)2-incorporated PLLA composite, however, were highly dependent on the graft types. It was observed that tensile strength and elastic modulus of PLLA was improved dramatically when it was incorporated with d-lactide-grafted Mg(OH)2 which can form stereocomplex with l-type PLA substrate while the incorporation of l-lactide-grafted Mg(OH)2 only improved elastic modulus. The formation of stereocomplex between PLLA and d-lactide make higher crystallinity of the matrix which is responsible for its mechanical properties (Tsuji and Ikada 1999). These findings suggested that late inflammatory response and thicker strut thickness of polymeric biodegradable stents due to their acidic degradation products and weaker mechanical properties than metal-based stent could be solved by incorporating surface-modified Mg-based ceramic particles.
Stent with strong coating layer
Improvement of adhesion stability between coating layer and strut surface is another important strategy which can reduce thrombosis by DES implantation. The interface between polymer coating layer and metallic substrate is basically not compatible with each other due to their definitely different physical and chemical properties. Stress during the stent expansion and irregular degradation under physiological condition makes polymer to be cracked or even delaminated from the stent surface (Bedair et al. 2014). And the defect on the polymer coating layer may cause uncontrolled release of drug resulting side effects (Hwang et al. 2005). Thus, Cypher stent also used base poly(p-xylylene) coating layer (Choi et al. 2011) and some attempts to improve the interfacial stability still have been made using polymeric brushes, such as diazonium and methacrylated derivatives (Levy et al. 2009; Shaulov et al. 2009). The improvement of biodegradable coating layer stability was investigated using nanobrushes composed of similar chemistry to coating layer (Bedair et al. 2014; Choi et al. 2011), as shown in Fig. 3b. Because the thickness of base buffer layer is in nanoscale this technique was named ‘nanocoupling’. For this, 4 nm of l-lactide oligomer layer was grafted on O2 plasma-treated stainless steel surface by ring opening polymerization and then PLGA containing SRL was coated on it by electrospraying method. The adhesive strength of nanocoupled coating layer was more than doubled compared to that of PLGA coated on bare or plasma-treated stainless steel (Choi et al. 2011). Formation of stereocomplex can be used to improve the nanocoupling technique. The PLLA brush was grafted on Co–Cr alloy surface and then PDLLA incorporated with SRL was coated on PLLA graft using ultrasonic spray coating. The PDLLA layer with nanocoupling demonstrated higher stability than that coated on other unmodified groups. After 6 weeks of in vitro degradation test, the nanocoupled coating layer showed relatively smoother surface without cracking or pitting. Moreover, when it was applied in real stent, the nanocoupled coating layer was more stable than the coating layer on unmodified one, suggesting the nanocoupling method would be useful method for enhancing the stability of coating layer in DES system (Bedair et al. 2014).
The use of base layer is also applicable to dual coating. A lot of DES studies have tried dual or multiple coatings for delivery multiple drugs (Lim et al. 2016, Su et al. 2013). One example of dual coating techniques is that drug is eluted only one side of stent (either luminal or abluminal side) by covering each side with different materials. As previously mentioned, anti-proliferative drug eluted from DES can not only prevent proliferation of SMCs but also delay the re-endothelialization (Joner et al. 2006). Thus, it would promote the re-endothelialization if the abluminal side of stent that contacts blood vessel wall elutes anti-proliferative drug and the luminal side of stent is coated with biocompatible materials which can provide preferable environment for endothelial cells. Coating of dopamine-mediated hyaluronic acid (HA-DA) and PDLA containing SRL on luminal and abluminal sides of stent was one example of this concept of dual coating (Kim et al. 2016). First, the both sides of Co–Cr stent was coated with HA-DA and then only abluminal side was coated with PDLA. PDLA coating layer was stable because HA-DA layer acted as base buffer layer. There was no cracking or delamination after ballooning the stent. The in vivo test using pig demonstrated that blood vessel expanded by dual coating stent had wider lumen area and reduced inflammatory response than that with BMS after 4 weeks of implantation.
Nitric oxide-eluting stent
Nitric oxide (NO)-eluting stents which can selectively prevent the proliferation of SMCs and promote the growth of endothelial cells have emerged as new platforms of DES. NO, generated by endothelial NO synthase (eNOS) in healthy blood vessel endothelium, is known to maintain patency and homeostasis of blood vessel (Sharif et al. 2012). The NO maintain the homeostasis of blood vessel by suppressing proliferation of SMCs, antherogenesis, and aggregation of platelet and dilating the blood vessel (Sharif et al. 2012). It was also reported that insufficient concentration of NO around implanted stent caused restenosis and thrombosis (Elnaggar et al. 2016). Thus, there have been several studies which focused on stent releasing NO or eNOS. One of the most studied NO donor is N-diazeniumdiolate (NONOate), which generates two equivalents of NO molecule under physiological conditions through hydrolysis (Naghavi et al. 2013). For the effective delivery of NO donor, liposomes are utilized (Elnaggar et al. 2016). Liposomes have been used as drug delivering vehicles due to their sustained releasing ability and similar bilayer structure to cell membrane (Malam et al. 2009; Voinea and Simionescu 2002). The delivery of NO using liposome successfully reduced arterial wall thickening in the rabbits (Huang et al. 2009). Study of prolonged release of NO, however, are still lacking. Recently, layer-by-layer (LBL) methods showed its utility in the prolonged NO releasing system (Elnaggar et al. 2016). In the LBL technique, multiple different coating layers were coated on substrate one by one (Hammond 2010; Thierry et al. 2003). The structure and composition of the coating layer by LBL technique can be controlled easily and accurately. On the HA-DA-coated Co–Cr substrate, (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA NONOate)-incorporated liposome and poly-l-lysine were coated through the LBL technique, as shown in Fig. 3c. While the NO-releasing period by the conventional direct coating method was a few hours, the NO was released for up to 5 days when the DETA NONOate was coated using LBL technique was used. The hydrophilic LBL surface, whose water contact angle was 20°, also effectively prevented adsorption of fibrinogen compared to relatively hydrophobic Co–Cr surface (water contact angle = 65°). On the LBL surface, viability of endothelial cell increased and that of SMC decreased. It was observed that NO-eluting stent group had wider lumen area than BMS group due to reduced thrombosis in the in vivo animal test using pig (Elnaggar et al. 2016). The NO delivery using DETA NONOate was also combined with anti-proliferative drug, PTX (Gallo and Mani 2013). After coating the whole side of stent with phosphonoacetic acid, abluminal side of stent was coated with PTX by spraying and luminal side of stent was coated with DETA NONOate using mandrel. However, the release of PTX and NO was too fast, thus, modification of the coating method for sustained release of those molecules would be required. Recently, Joung and his coworkers reported effectiveness of supported lipid bilayer (SLB) tethered on titanium (Ti) surface as another NO donor delivering method. The tethered SLB (TLB) was formed by rupturing liposome composed of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS) onto surface-treated Ti surface. The lipophilic NO donor 10-nitrooleate (OA-NO2) was easily incorporated into TLB membrane and NO was released out for few hours. The released NO successfully inhibited growth of SMCs while it has endothelial cell compatibility similar to that of bare Ti (Elnaggar et al. 2016).
Biomolecule-eluting or decorated stent
Vascular endothelial growth factor (VEGF) is one of the most extensively investigated growth factors in the stent because it is known to promote angiogenesis by regulating endothelial cell proliferation (Poh et al. 2010). Hepatocyte growth factor (HGF) is another endothelium-specific growth factor which affects migration, proliferation and capillary-like structure formation of endothelial cells (Harada et al. 2002). Meanwhile, a heparin, one of components of extracellular matrix, have been widely used in biomedical applications due to its anti-coagulant property (Clowes and Karnowsky 1977). Heparin strongly interacts with growth factors which have heparin binding domains (Awada et al. 2014). Growth factors, thus, could be loaded easily on to metallic substrate when heparin was coated on it using carriers like HA-DA (Choi et al. 2016). The HA-DA coating itself resulted in enhanced growth of endothelial cells than bare metal surface which was further enhanced by heparin coating. The expression of CD31 and capillary formation of the endothelial cells were upregulated on the VEGF-loaded surface than other surface. The HGF did not strongly promote tubule formation or CD31 expression of endothelial cells than VEGF, however, it induced more upregulated connexin-43 gap junction protein expression (Choi et al. 2016).
Stromal cell derived factor-1α (SDF-1α) is a chemokine which affects homing, mobility and recruitment of stem or progenitor cells (Abbott et al. 2004; Lau and Wang 2011). Endothelial progenitor cells are involved in neovascularization due to its potent differentiation ability into endothelial cells (Asahara et al. 1997). Moreover, endothelial progenitor cells can be incorporated into damaged arteries and help to promote re-endothelialization (Kong et al. 2004a). Like other growth factor systems, the SDF-1α could be loaded onto Co–Cr alloy substrate using heparin-dopamin coating system (Kang et al. 2015). The loaded SDF-1α was released out up to 4 weeks without any initial burst. Migration assay was carried out using fibrin gel where the endothelial progenitor cells were distributed homogenously. When Co–Cr substrate was placed on the cell-laden fibrin gel, the cells were settled down after 7 days. On the other hand, during the same time period, cells migrated toward the SDF-1α releasing substrate even against the gravity (Kang et al. 2015).
Cell capturing using anti-CD34 antibody is another method for healing blood vessel using endothelial progenitor cells. CD34 is a common marker that is abundantly found on endothelial progenitor cell (EPC) surface (Marketou et al. 2014). The development of cell capturing stent was begun at around the same time as first generation DES (Sethi and Lee 2012). Genous™ manufactured by OrbusNeich is most widely studied EPC capturing stent in clinical trials. Its surface was coated with anti-human CD34 antibodies using polysaccharide (Beijk et al. 2010; Garg and Serruys 2010), as shown in Fig. 3d. A lot of animal studies have demonstrated that Genous™ successfully accelerated re-endothelialization, but was not effective in inhibition of intimal hyperplasia (van Beusekom et al. 2012). It might because anti-CD34 antibody response not only with EPCs but also smooth muscle progenitor cells (van Beusekom et al. 2012). In this regard, COMBO dual therapy stent by OrbusNeich is modified version of Genous™, which was designed to capture EPCs and to inhibit SMC proliferation simultaneously by coating luminal and abluminal sides with anti-CD34 antibody and SRL-loaded biodegradable polymer, respectively (Lee et al. 2016). In a 3-year clinical trial on 61 patients, significant late neointima regression was observed on COMBO dual therapy stent, which has never been reported in other mono therapy DES systems (Lee et al. 2016). On the other hand, other specific markers for capturing EPCs have been suggested such as vascular endothelial cadherin (Lim et al. 2011), endoglin (Cui et al. 2014) and CD133 (Sedaghat et al. 2013). However, they did not reveal major improvement of re-endothelialization yet. Our group applied anti-CD146 antibody to capture circulating EPCs and silicon nanofilament to increase surface area and coating efficiency (unpublished). Silicon nanofilament-coated stents with anti-CD146 antibody enhanced cell capturing rate, in vivo re-endothelialization and inhibited neointimal formation in porcine coronary artery.
Gene, a molecular unit of heredity (Tebas et al. 2014), has ability to regulate cell function, which allows it to be widely studied in the regeneration field (Naldini 2015; Rios et al. 2011). Recently, gene-eluting stent has been considered as one of the next generation of coronary stents which can overcome the inherent shortcoming of current DES systems, delayed re-endothelialization. Some small interfering RNAs (siRNA) or messenger RNAs (mRNA) are known to inhibit proliferation of SMC specifically without affecting endothelial cells. These RNAs can be eluted effectively using polymeric materials as DES (Che et al. 2012). Akt1 is an important signaling system that can intermediate PI3K pathway (Vara et al. 2004). The eluted siRNA successfully downregulated in vitro SMC proliferation. Moreover, in vivo animal test using rabbit demonstrated siRNA-eluting stent significantly reduced ISR rate compared with BMS (Che et al. 2012). Gene-eluting stent also accelerated re-endothelialization by delivering plasmid DNA that can encode human VEGF-2 (Walter et al. 2004). This plasmid DNA eluting stent reduced 60% of cross-sectional arterial narrowing compared to PC-coated stent. Gene-eluting stent also alleviated neointimal thickening by inhibiting inflammatory mediators such as monocyte chemoattractant protein-1 and nuclear factor κB (Ohtani et al. 2006). Negatively charged genes are delivered by bulk-immobilizing (Fishbein et al. 2013), surface-immobilizing (Jin et al. 2008) or forming nanocomplex with positively charged polymers (Che et al. 2012). Although gene-eluting stents have showed positive in vivo animal testing results, clinical trials are still lacking.
Endothelium mimicking stent which had high interfacial compatibility with metallic substrate and NO eluting ability for accelerated re-endothelialization was developed using self-assembled peptide-based matrix (Andukuri et al. 2014). Two peptide amphiphiles (PA) which respectively had endothelial cell adhesive ligand (YIGSR) and polylysine NO donor were prepared and then mixed in a 9:1 ratio. This endothelium mimicking nanomatrix showed superior adhesion stability which were examined by applying shear stress using bioreactor system and balloon expansion test. There was no noticeable defect on the coating layer after testing compared to untested one. The uniformly distributed HUVEC on stent surface after 7 days of incubating confirmed the biocompatibility of the endothelium mimicking coating layer. The 4 weeks of in vivo testing using rabbit revealed that neointimal layer which even thinner than stent strut was observed on endothelium mimicking coating layer. Direct delivery of endothelial cells is another method to mimic the endothelium. Several strategies have been proposed to deliver the endothelial cells using magnetic particles (Polyak et al. 2016) and catheter (Kong et al. 2004b; Werner et al. 2003). However, these devices have some limitation which needs additional instrument to successfully deliver the cells. Our group designed cell-derived extracellular matrix coated stents which has synergistic effects of stable cell adhesion and suppression of SMC proliferation (unpublished). Direct delivery of endothelial progenitor cells using extracellular matrix coated stents showed acceleration of in vivo re-endothelialization and successful inhibition of neointimal formation.
Conclusions
The numerous efforts to make better DES systems, have allowed DES to be commercialized and used dominantly in the stent treatment these days. DES system has been evolved by modifying its components to resolve inherent weaknesses including the toxicity of the anti-proliferative drug and the remaining of polymeric coating layer which can cause immune reaction, inflammation and the consequent late-thrombosis. In the next generation of DES, to minimize the side effects after stent implantation, numerous strategies have been investigated. Examples of the strategy are the reduction of inflammation by incorporating of basic ceramic particle and stabilizing of the coating layer, and promotion of blood vessel regeneration by delivering gas and biomolecules or replacing synthetic polymers with biomimetic substance. A lot of subfields in the DES technology, however, still needs further improvement. The above strategies have been developed mainly for the metallic substrate. Therefore, the application to the bioabsorbable polymeric substrate, which was recently commercialized, will meet another challenges. While the vascular DES have made significant progress, progress in the investigation of non-vascular DES for other organs, such as biliary duct, trachea, and urethra are still slow. Because these non-vascular stents have different shape, size and dose of drugs depending on the target organs, the optimization of drug delivering strategies should be adjusted. The efforts are also needed to employ the future techniques in DES, such as 3D printing, nano-fabrication and nano/micro-patterning, that will replace conventional manufacturing methods.
References
Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ (2004) Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110:3300–3305
Abizaid A, Costa JR (2010) New drug-eluting stents. An overview on biodegradable and polymer-free next-generation stent systems. Circ Cardiovasc Interv 3:384–393
Acharya G, Park K (2006) Mechanisms of controlled drug release from drug-eluting stents. Adv Drug Deliv Rev 58:387–401
Akin I, Schneider H, Ince H, Kische S, Rehders TC, Chatterjee T, Nienaber CA (2011) Second- and third-generation drug-eluting coronary stents: progress and safety. Herz 36:190–196
Anderson JM (2001) Biological responses to materials. Annu Rev Mater Res 31:81–110
Andukuri A, Min I, Hwang P, Alexander G, Marshall LE, Berry JL, Wick TM et al (2014) Evaluation of the effect of expansion and shear stress on a self-assembled endothelium mimicking nanomatrix coating for drug eluting stents in vitro and in vivo. Biofabrication 6:035019
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–966
Awada HK, Johnson NR, Wang Y (2014) Dual delivery of vascular endothelial growth factor and hepatocyte growth factor coacervate displays strong angiogenic effects. Macromol Biosci 14:679–686
Bedair TM, Cho Y, Joung YK, Han DK (2014) Biodegradable polymer brush as nanocoupled interface for improving the durability of polymer coating on metal surface. Coll Surf B Biointerfaces 122:808–817
Beijk MAM, Klomp M, Verouden NJW, van Geloven N, Koch KT, Henriques JPS, Baan J et al (2010) Genous™ endothelial progenitor cell capturing stent vs. the Taxus Liberté stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur Heart J 31:1055–1064
Bhatia V, Bhatia R, Dhindsa M (2004) Drug-eluting stents: new era and new concerns. Postgrad Med J 80:13–18
Böstman O, Pihlajamäki H (2000) Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials 21:2615–2621
Bridges J, Cutlip D (2009) Advances in drug eluting stents – focus on the Endeavor® zotarolimus stent. Med Devices (Auckl) 2:1–8
Busch R, Strohbach A, Rethfeldt S, Walz S, Busch M, Petersen S, Felix S et al (2014) New stent surface materials: the impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets. Acta Biomater 10:688–700
Che HL, Bae IH, Lim KS, Song IT, Lee H, Muthiah M, Namgung R et al (2012) Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials 33:8548–8556
Chen HW, Huang HC (1998) Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br J Pharmacol 124:1029–1040
Chen MC, Liu CT, Tsai HW, Lai WY, Chang Y, Sung HW (2009) Mechanical properties, drug eluting characteristics and in vivo performance of a genipin-crosslinked chitosan polymeric stent. Biomaterials 30:5560–5571
Chen W, Habraken TC, Hennink WE, Kok RJ (2015) Polymer-free drug-eluting stents: an overview of coating strategies and comparison with polymer-coated drug-eluting stents. Bioconjug Chem 26:1277–1288
Chevalier B, Wijns W, Silber S, Garcia E, Serra A, Paunovic D, Serruys P (2015) Five-year clinical outcome of the Nobori drug-eluting coronary stent system in the treatment of patients with coronary artery disease: final results of the NOBORI 1 trial. EuroIntervention 11:549–554
Choi J, Cho SB, Lee BS, Joung YK, Park K, Han DK (2011) Improvement of interfacial adhesion of biodegradable polymers coated on metal surface by nanocoupling. Langmuir 27:14232–14239
Choi DH, Kang SN, Kim SM, Gobaa S, Park BJ, Kim IH, Joung YK et al (2016) Growth factors-loaded stents modified with hyaluronic acid and heparin for induction of rapid and tight re-endothelialization. Coll Surf B Biointerfaces 141:602–610
Clowes AW, Karnowsky MJ (1977) Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature 265:625–626
Collet C, Sotomi Y, Cavalcante R, Suwannasom P, Tenekecioglu E, Onuma Y, Serruys PW (2016) Coronary stent thrombosis: what have we learned? J Thorac Dis 8:1398–1405
Cui S, Liu JH, Song XT, Ma GL, Du BJ, Lv SZ, Meng LJ et al (2014) A novel stent coated with antibodies to endoglin inhibits neointimal formation of porcine coronary arteries. BioMed Res Int 2014:428619
Dong C, Song D, Cairney J, Maddan OL, He G, Deng Y (2011) Antibacterial study of Mg(OH)2 nanoplatelets. Mater Res Bull 46:576–582
Dunne M, Corrigan OI, Ramtoola Z (2000) Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials 21:1659–1668
Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR et al (2015) Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med 373:1905–1915
Elnaggar MA, Seo SH, Gobaa S, Lim KS, Bae I-H, Jeong MH, Han DK et al (2016) Nitric oxide releasing coronary stent: a new approach using layer-by-layer coating and liposomal encapsulation. Small 12:6012–6023
Ernst A, Bulum J (2014) New generations of drug-eluting stents-A brief review. EMJ Int Cardiol 1:100–106
Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K et al (2005) Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 112:270–278
Fishbein I, Chorny M, Adamo RF, Forbes SP, Corrales RA, Alferiev IS, Levy RJ (2013) Endovascular gene delivery from a stent platform: gene- eluting stents. Angiol Open Access. https://doi.org/10.4172/2329-9495.1000109
Gallo A, Mani G (2013) A stent for co-delivering paclitaxel and nitric oxide from abluminal and luminal surfaces: preparation, surface characterization, and in vitro drug release studies. Appl Surf Sci 279:216–232
Garg S, Serruys PW (2010) Coronary stents: looking forward. J Am Coll Cardiol 56:S43-S78
Grabow N, Martin DP, Schmitz K-P, Sternberg K (2010) Absorbable polymer stent technologies for vascular regeneration. J Chem Tech Biotechnol 85:744–751
Hammond PT (2010) Thin films: particles release. Nat Mater 9:292–293
Harada M, Takenaka H, Ikenaga S, Zhang H, Zempo N, Esato K, Nagano T et al (2002) Hepatocyte growth factor prevents intimal hyperplasia in rabbit carotid expanded polytetrafluoroethylene grafting. J Vasc Surg 35:786–791
Haude M, Erbel R, Issa H, Meyer J (1993) Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz-Schatz stents. J Am Coll Cardiol 21:26–34
Hedin M (1997) The origin of the word stent. Acta Radiol 38:937–939
Hezi-Yamit A, Sullivan C, Wong J, David L, Chen M, Cheng P, Shumaker D et al (2008) Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J Biomed Mater Res A 90:133–141
Huang SL, Kee PH, Kim H, Moody MR, Chrzanowski SM, MacDonald RC, McPherson DD (2009) Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J Am Coll Cardiol 54:652–659
Hwang CW, Levin AD, Jonas M, Li PH, Edelman ER (2005) Thrombosis modulates arterial drug distribution for drug-eluting stents. Circulation 111:1619–1626
Jin X, Mei L, Song C, Liu L, Leng X, Sun H, Kong D et al (2008) Immobilization of plasmid DNA on an anti-DNA antibody modified coronary stent for intravascular site-specific gene therapy. J Gene Med 10:421–429
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R et al (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48:193–202
Jorge C, Dubois C (2015) Clinical utility of platinum chromium bare-metal stents in coronary heart disease. Med Devices (Auckl) 8:359–367
Kadota K, Muramatsu T, Iwabuchi M, Saito S, Hayashi Y, Ikari Y, Nanto S et al (2012) Randomized comparison of the Nobori Biolimus A9-eluting stent with the sirolimus-eluting stent in patients with stenosis in native coronary arteries. Catheter Cardiovasc Interv 80:789–796
Kang SN, Park C, Kim SM, Park KW, Park BJ, Han DK, Joung YK (2015) Effect of stromal cell derived factor-1α release from heparin-coated Co-Cr stent substrate on the recruitment of endothelial progenitor cells. Macromol Res 23:1159–1167
Kim SM, Park KS, Lih E, Hong YJ, Kang JH, Kim IH, Jeong MH et al (2016) Fabrication and characteristics of dual functionalized vascular stent by spatio-temporal coating. Acta Biomater 38:143–152
Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, Pratt RE et al (2004a) Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation 110:2039–2046
Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC et al (2004b) Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation 109:1769–1775
Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB (1998) In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 31:224–230
Kum CH, Cho Y, Joung YK, Choi J, Park K, Seo SH, Park YS et al (2013) Biodegradable poly(l-lactide) composites by oligolactide-grafted magnesium hydroxide for mechanical reinforcement and reduced inflammation. J Mater Chem B 1:2764–2772
Kum CH, Seo SH, Kang SN, Park BJ, Ahn DJ, Joung YK, Han DK (2014a) Effect of magnesium hydroxide nanoparticles with rod and plate shape on mechanical and biological properties of poly(L-lactide) composites. Macromol Res 22:1032–1041
Kum CH, Cho Y, Seo SH, Joung YK, Ahn DJ, Han DK (2014b) A poly(lactide) stereocomplex structure with modified magnesium oxide and its effects in enhancing the mechanical properties and suppressing inflammation. Small 10:3783–3794
Lau TT, Wang DA (2011) Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther 11:189–197
Lee SW, Lam SC, Tam FC, Chan KK, Shea CP, Kong SL, Wong AY et al (2016) Evaluation of early healing profile and neointimal transformation over 24 months using longitudinal sequential optical coherence tomography assessments and 3-year clinical results of the new dual-therapy endothelial progenitor cell capturing sirolimus-eluting combo stent: the EGO-Combo study. Circ Cardiovasc Interv. https://doi.org/10.1161/CIRCINTERVENTIONS.115.003469
Levy Y, Tal N, Tzemach G, Weinberger J, Domb AJ, Mandler D (2009) Drug-eluting stent with improved durability and controllability properties, obtained via electrocoated adhesive promotion layer. J Biomed Mater Res B Appl Biomater 91:819–830
Lim WH, Seo WW, Choe W, Kang CK, Park J, Cho HJ, Kyeong S et al (2011) Stent coated with antibody against vascular endothelial-cadherin captures endothelial progenitor cells, accelerates re-endothelialization, and reduces neointimal formation. Arterioscler Thrombo Vasc Biol 31:2798–2805
Lim KS, Park JK, Jeong MH, Bae IH, Nah JW, Park DS, Sim JW et al (2016) Optimal coating method for a dual-layer stent with sirolimus and alpha-lipoic acid in a porcine coronary restenosis model. Macromol Res 24:725–733
Liu H, Slamovich EB, Webster TJ (2006) Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int J Nanomed 1:541–545
Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30:592–599
Mani G, Feldman MD, Patel D, Agrawal CM (2007) Coronary stents: a materials perspective. Biomaterials 28:1689–1710
Marketou ME, Kalyva A, Parthenakis FI, Pontikoglou C, Maragkoudakis S, Kontaraki JE, Chlouverakis G et al (2014) Circulating endothelial progenitor cells in hypertensive patients with increased arterial stiffness. J Clin Hypertens 16:295–300
Meraj PM, Jauhar R, Singh A (2015) Bare metal stents versus drug eluting stents: where do we stand in 2015? Curr Treat Options Cardiovasc Med 17:393
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP et al (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349:1315–1323
Naghavi N, de Mel A, Alavijeh OS, Cousins BG, Seifalian AM (2013) Nitric oxide donors for cardiovascular implant applications. Small 9:22–35
Naldini L (2015) Gene therapy returns to centre stage. Nature 526:351–360
Nam SH, Nam HY, Joo JR, Back IS, Park JS (2007) Curcumin-loaded PLGA nanoparticles coating onto metal stent by electrophoretic deposition techniques. Bull Korean Chem Soc 28:397–402
Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, Nakagawa Y, Akasaka T et al (2015) Final 3-year outcome of a randomized trial comparing second-generation drug-eluting stents using either biodegradable polymer or durable polymer. NOBORI biolimus-eluting versus XIENCE/PROMUS everolimus-eluting stent trial. Circ Cardiovasc Interv. https://doi.org/10.1161/CIRCINTERVENTIONS.115.002817
Ohtani K, Egashira K, Nakano K, Zhao G, Funakoshi K, Ihara Y, Kimura S et al (2006) Stent-based local delivery of nuclear factor-κB decoy attenuates in-stent restenosis in hypercholesterolemic rabbits. Circulation 114:2773–2779
Pan CJ, Tang JJ, Shao ZY, Wang J, Huang N (2007) Improved blood compatibility of rapamycin-eluting stent by incorporating curcumin. Coll Surf B Biointerfaces 59:105–111
Park DW, Park SW, Park KH, Lee BK, Kim YH, Lee CW, Hong MK et al (2006) Frequency of and Risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol 98:352–356
Park C, Kim S, Kim HE, Jang TS (2016) Mechanically stable tantalum coating on a nano-roughened NiTi stent for enhanced radiopacity and biocompatibility. Surf Coat Technol 305:139–145
Poh CK, Shi Z, Lim TY, Neoh KG, Wang W (2010) The effect of VEGF functionalization of titanium on endothelial cells in vitro. Biomaterials 31:1578–1585
Polyak B, Medved M, Lazareva N, Steele L, Patel T, Rai A, Rotenberg MY et al (2016) Magnetic nanoparticle-mediated targeting of cell therapy reduces in-stent stenosis in injured arteries. ACS Nano 10:9559–9569
Porta-i-Batalla M, Eckstein C, Xifré-Pérez E, Formentín P, Ferré-Borrull J, Marsal LF (2016) Sustained, controlled and stimuli-responsive drug release systems based on nanoporous anodic alumina with layer-by layer polyelectrolyte recent developments in drug eluting devices with tailored interfacial properties. Nanoscale Res Lett. https://doi.org/10.1186/s11671-016-1585-4
Puranik AS, Dawson ER, Peppas NA (2013) Recent advances in drug eluting stents. Int J Pharm 441:665–679
Rensing BJ, Vos J, Smits PC, Foley DP, van den Brand MJ, van der Giessen WJ, de Feijter PJ et al (2001) Coronary restenosis elimination with a sirolimus eluting stent: first European human experience with 6-month angiographic and intravascular ultrasonic follow-up. Eur Heart J 22:2125–2130
Rios HF, Lin Z, Oh B, Park CH, Giannobile WV (2011) Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J Periodontol 82:1223–1237
Saris NEL, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A (2000) Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta 294:1–26
Sedaghat A, Sinning JM, Paul K, Kirfel G, Nickenig G, Werner N (2013) First in vitro and in vivo results of an anti-human CD133-antibody coated coronary stent in the porcine model. Clin Res Cardiol 102:413–425
Sethi R, Lee CH (2012) Endothelial progenitor cell capture stent: safety and effectiveness. J Interv Cardiol 25:493–500
Shah BH, Nawaz Z, Pertani SA, Roomi A, Mahmood H, Saeed SA, Gilani AH (1999) Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol 58:1167–1172
Shaikh M, Kichenadasse G, Choudhury NR, Butler R, Garg S (2013) Non-vascular drug eluting stents as localized controlled drug delivery platform: preclinical and clinical experience. J Control Release 172:105–117
Sharif F, Hynes SO, McCullagh KJA, Ganley S, Greiser U, McHugh P, Crowley J et al (2012) Gene-eluting stents: non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther 19:321–328
Shaulov Y, Okner R, Levi Y, Tal N, Gutkin V, Mandler D, Domb AJ (2009) Poly(methyl methacrylate) grafting onto stainless steel surfaces: application to drug-eluting stents. ACS Appl Mater Interfaces 1:2519–2528
Sorrentino S, Giustino G, Mehran R, Kini AS, Sharma SK, Faggioni M, Farhan S et al (2017) Everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents. J Am Coll Cardiol 69:3055–3066
Stefanini GG, Holmes DRJ (2013) Drug-eluting coronary-artery stents. N Engl J Med 368:254–265
Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M et al (2004) A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 350:221–231
Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A et al (2007) Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 356:998–1008
Su LC, Chen YH, Chen MC (2013) Dual drug-eluting stents coated with multilayers of hydrophobic heparin and sirolimus. ACS Appl Mater Interfaces 5:12944–12953
Sung HJ, Meredith C, Johnson C, Galis ZS (2004) The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 25:5735–5742
Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK et al (2014) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370:901–910
ten Berg JM, Plokker HWT, Verheugt FWA (2001) Antiplatelet and anticoagulant therapy in elective percutaneous coronary intervention. Curr Control Trials Cardiovasc Med 2:129–140
Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M (2003) Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromol 4:1564–1571
Tsuji H, Ikada Y (1999) Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 40:6699–6708
van Beusekom HMM, Ertaş G, Sorop O, Serruys PW, van der Giessen WJ (2012) The genous™ endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries. Catheter Cardiovasc Interv 79:231–242
Vara JÁF, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30:193–204
Virmani R, Liistro F, Stankovic G, Di Mario C, Montorfano M, Farb A, Kolodgie FD et al (2002) Mechanism of late in-stent restenosis after implantation of a paclitaxel derivate–eluting polymer stent system in humans. Circulation 106:2649–2651
Voinea M, Simionescu M (2002) Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J Cell Molecular Med 6:465–474
Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB et al (2004) Local gene transfer of phVEGF-2 plasmid by gene-eluting stents. Alt Strategy Inh Restenosis Circ 110:36–45
Welt FGP, Rogers C (2002) Inflammation and restenosis in the stent era. Arterioscler Thromb VAsc Biol 22:1769–1776
Werner N, Junk S, Laufs U, Link A, Walenta K, Böhm M, Nickenig G (2003) Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circulation Res 93:e17–e24
Wiemer M, Stoikovic S, Samol A, Dimitriadis Z, Ruiz-Nodar JM, Birkemeyer R, Monsegu J et al (2017) Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow-up. Cardiovasc Diabetol 16:23. https://doi.org/10.1186/s12933-017-0500-3
Wilson GJ, Nakazawa G, Schwartz RS, Huibregtse B, Poff B, Herbst TJ, Baim DS et al (2009) Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in porcine coronary arteries. Circulation 120:141–149
Zhang Y, Bourantas CV, Farooq V, Muramatsu T, Diletti R, Onuma Y, Garcia-Garcia HM et al (2013) Bioresorbable scaffolds in the treatment of coronary artery disease. Med Devices (Auckl) 6:37–48
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by Ministry of Science and ICT (NRF-2014M3A9D3033887 and NRF-2015M3A9E2028580), and Strategic Core Materials Technology Development Program (10062079) funded by Ministry of Trade Industry and Energy (MOTIE), Republic of Korea.
Disclosure
Cheol-Min Han, Kwang-Sook Park and Yoon Ki Joung declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, CM., Park, KS. & Joung, Y.K. Recent alternative approaches of vascular drug-eluting stents. Journal of Pharmaceutical Investigation 48, 153–165 (2018). https://doi.org/10.1007/s40005-017-0378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-017-0378-9