Abstract
Objective
This retrospective observational study examined the implementation of antibiotic stewardship (ABS) on the surgical intensive care unit (SICU) of a specialized academic teaching hospital.
Methods
Application density of antimicrobial agents (ADA), substance class change, development of resistance, and clinical outcomes were investigated with reference to ABS in three intervals over a 10-year period: the pre-intervention phase (2008–2010), the intervention phase (2011–2014), and the post-intervention phase (2015–2017).
Results
Following the introduction of ABS, ADA was reduced from 89.3 recommended daily doses/100 patient days (RDD/100 PD) at the pre-intervention phase to 68.0 RDD/100 PD at the post-intervention phase. The antibiotic ADA (AB-ADA) similarly showed a significant decrease from 83.3 to 62.0 RDD/100 PD (p < 0.0001). The case mix index (CMI), which describes the average case severity across patients and mortality on the SICU was not significantly different comparing intervention and post-intervention phase. It was also possible to achieve a substance class change following the introduction of ABS. There was no obvious change in bacterial resistance rates.
Conclusion
The study demonstrates a sustainable effect of the implementation of ABS, which was sustained through the post-intervention phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2011, a plan of action to counteract antimicrobial resistance was developed by the World Health Organization (WHO), which promoted the prudent use of antimicrobial agents [1]. In Germany, the response to this request by the WHO was to compile the S3 guideline “Strategies for ensuring rational antibiotic use”. This guideline identifies the requirements for implementation of antibiotic stewardship (ABS) and its core strategies [2]. The justification behind these efforts to achieve a more rational use of antimicrobial agents is based on the increasing incidence of antibiotic resistance, which is particularly anticipated in intensive care units (ICUs) [3]. In German ICUs, incidence rates for the following bacteria in particular have increased: vancomycin-resistant enterococci (VRE) and enterobacteriaceae, such as Escherichia coli (E. coli) or Klebsiella pneumoniae (K. pneumoniae), both resistant to third generation cephalosporins and fluoroquinolones. The increase in carbapenem-resistant K. pneumoniae is especially worrying [4]. It has been shown that excessive use of antibiotics, especially of broad-spectrum antibiotics such as carbapenems or third/fourth-generation cephalosporins, is associated with increased development of resistance [5]. In ICUs, large quantities of antibiotics are prescribed. A prevalence study carried out in German hospitals in 2016 demonstrated that 52% of intensive care patients were administered antibiotics, compared to 24.4% of patients on general wards [6]. One reason for the significantly increased use is that the incidence of sepsis in ICUs has increased in recent years [7]. Sepsis is an absolute indication for an anti-infective therapy and often requires high-dose administration, because patients present hyperdynamic circulation and an increased volume of distribution [8, 9]. This retrospective observational study examines the effects of ABS on the use of anti-infective drugs and the clinical outcomes on a surgical intensive care unit (SICU) at a specialized academic teaching hospital across three intervals over a period of 10 years.
Methods
Antibiotic stewardship measures on the SICU
Due to regulated progression, a new head consultant took up the post at the Department for Anesthesiology and Intensive Care Medicine starting 1st January 2011. As part of this process, structures and processes in intensive care medicine were improved. This included implementing the antibiotic stewardship program according to descriptions in many publications [2, 10, 11]. The introduced measures are shown in Fig. 1. In addition, the following measures were implemented.
Agreement regarding cooperation between intensive care doctors, clinical infectious disease specialists, hospital pharmacists, microbiologists, and infection control specialists with frequent rounds on the SICU and ensuring staff are permanently available by telephone to improve prescription quality of anti-infective drugs.
The leading infectious disease specialist is the equal with the senior intensive care physician.
Advanced training of one consultant in intensive care medicine becoming an infectious disease specialist according to the specifications of the German Society of Infectious Diseases (Deutsche Gesellschaft für Infektiologie e.V., DGI) [14, 15]. Furthermore, three consultants of the SICU started training in antibiotic stewardship in accordance with the specifications of the DGI.
Advanced training of two hospital pharmacists as antibiotic stewardship experts/specialist pharmacists in clinical pharmacy and infectious diseases.
Training of two critical care nurses as specialists for infection control who regularly instruct employees of the SICU in hygiene-related topics such as hand hygiene among others.
Quality assurance through participation in the Hospital Infection Surveillance System (ITS-KISS, CDAD-KISS, MRSA-KISS), as well as surveillance of antibiotic use and bacterial resistance in intensive care units (SARI); introduction of usage surveillance for antibiotic application density (ADKA-if, AVS).
Data collection and patient inclusion
ABS was implemented over a 4-year time period from 2011 to 2014 on the SICU of the specialized academic teaching hospital. Three years prior to this (the pre-intervention phase, 2008–2010) and 3 years subsequent to intervention (post-intervention phase, 2015–2017) were also taken into account to assess the baseline situation and the long-term effects of the measures implemented. The effects resulting from the introduction of ABS were examined using different parameters: the application density of antimicrobial agents (ADA) is indicated as the recommended daily dose per 100 patient days (RDD/100 PD) or the defined daily dose per 1000 patient days (DDD/1000 PD). The ADA corresponds to the recommended daily dose (RDD) or the daily dose as defined by the WHO (DDD) of a drug divided by 100 or 1000 patient days and is a suitable benchmark for tracking trends in prescription numbers [36]. The resistance rates for individual bacteria, clinical indicators such as mortality, length of stay, and case mix index (CMI) were analyzed to describe the effects of the modified ADA. When considering the ADA, the focus was on the most frequently administered antibiotics (AB), whereby the development of the AB-ADA of individual substance classes was also described. All patients admitted to the SICU were included. To allow the comparison of AB-ADA and resistance data nationally across Germany, data from the “SARI” (surveillance of antibiotic use and bacterial infections on German intensive care units) [37] and “ARS” (Antibiotic Resistance Surveillance) [38] programs were used. As the SARI data were available as DDD/1000 PD, a further query of AB-ADA was carried out using the aforementioned units. With the exception of this comparison, the ADA was queried in RDD/100 PD, as this is the most commonly used ratio nationally across Germany [16]. The resistance of four indicator strains against the preferred antibiotic for treatment were compared: Escherichia coli (E. coli) and cefuroxime; Pseudomonas aeruginosa (P. aeruginosa) and ceftazidime; Staphylococcus aureus (S. aureus) and oxacillin; Enterococcus faecium (E. faecium) and vancomycin.
An approval by vote of the ethics board of the North-Rhine Medical Association was obtained before the investigation was started (ref. number 273-2016). The study design is a retrospective observational study using pseudonymized data already collected that were completely anonymized prior to publication; therefore no consultation by an ethics committee was required, neither pursuant to §15 of the Professional Code of Conduct for North-Rhine doctors nor by the North-Rhine Medical Association.
Data analysis
The ADA parameters and key values underwent statistical analysis on the basis of overall monthly data for each respective interval. First, descriptive statistical analysis was used to calculate the mean, standard deviation, and 95% confidence interval (CI) of the AB-ADA, the CMI, the length of stay, and mortality for each respective interval. One-way ANOVA with a post hoc test using Bonferroni correction was applied to test for significant differences between the mean values for intervals. The Chi2-four-field test was used to test for significance of the frequency differences relating to mortality in the three time periods. Furthermore, effect size was determined by applying the phi-coefficient. This represents the degree of association between two binary variables. The significance level was set at p < 0.05.
Results
Application density of antimicrobial agents, substance class change, and antibiotic resistance
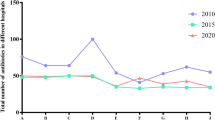
In the 10-year period evaluated, the ADA on the SICU investigated reduced from 89.3 RDD/100 PD at the pre-intervention phase to 68.0 RDD/100 PD at the post-intervention phase. Antibiotics represented the largest proportion of antimicrobial agents over the investigated period. For this reason, AB-ADA was analyzed in greater statistical detail: in the pre-intervention phase, the mean AB-ADA was 83.3 RDD/100 PD (standard deviation (SD) 23.5; 95% CI [75.3–91.2]). This value showed a significant decrease in the intervention phase to 64.8 RDD/100 PD (SD 22.0; 95% CI [58.5–71.2]; p = 0.001). Subsequently, this low number remained constant over time: there was no significant change from the intervention phase to the post-intervention phase (62.0 RDD/100 PD, SD 15.9; 95% CI [56.7–67.4]; p = 0.831). The anti-fungal ADA was comparable in pre- and post-intervention phases at 5.9 and 5.8 RDD/100 PD, respectively. Anti-viral could be disregarded due to a very low ADA (Fig. 2).
From a comparison with 77 ICUs included in the SARI survey [37] , it is clear that use of antibiotics across Germany has fallen slightly from a high level. In 2008, the AB-ADA was 1433 DDD/1000 PD; in 2017 the value was 1377 DDD/1000 PD. This decrease of 56 DDD/1000 PD is equivalent to 3.9% compared to baseline. During the same period, there was a decrease from 1161 to 766 DDD/1000 PD (34.0% compared to baseline) at the SICU investigated. Furthermore, when comparing Fig. 3a, b, a considerably lower AB-ADA starting value for the year 2008 can be seen for the SICU investigated.
Quantitative composition and temporal evolution of ADA of all antibiotics in DDD/1000 PD on: a 77 German ICUs and b the SICU investigated. ADA application density, DDD defined daily dose, PD patient days, ICU intensive care unit, SICU surgical ICU; a modified according to [37]
To verify the success of the substance class change, three classes were selected, of which two were intended to be reduced in the ABS program: cephalosporins, in particular third- and fourth-generation, and fluoroquinolones. From the pre- to the post-intervention phase, ADA of cephalosporins decreased from 13.4 to 5.6 RDD/100 PD or 58.2%. The proportion of third- and fourth-generation cephalosporins decreased overall, with an increased proportion of narrow-spectrum first- and second-generation antibiotics (data not shown).
The ADA of fluoroquinolones fell from 5.4 in the pre-intervention phase to 3.0 RDD/100 PD in the post-intervention phase, a change of 44.4%.
In treating bacterial infections, penicillins are the substance class preferred over the antibiotics listed above. Figure 3b clearly shows that penicillins with beta-lactamase inhibitors (BLI) constitute the greatest proportion of penicillins used on the SICU. Their ADA increased from 17.4 RDD/100 PD at the pre-intervention phase to 22.1 RDD/100 PD at the post-intervention phase, a 27.0% increase.
Despite a decrease in AB-ADA by ABS measures and/or a restrictive antibiotic policy in the 10 year period evaluated, there was no obvious change/reduction in bacterial resistance rates (data not shown).
Clinical outcomes
The total number of patients rose from 2431 at the pre-intervention phase to 2662 patients at the post-intervention phase. The length of stay at the SICU/hospital showed no significant difference from the pre-intervention phase to the post-intervention phase. The average length of stay at the pre-intervention phase was 3.1 days (SD 0.5; 95% CI [2.9–3.2]) on the SICU and 21.8 days (SD 3.6; 95% CI [20.6–23.0]) for the hospital; at the post-intervention phase the average duration was 3.0 days (SD 0.7; 95% CI [2.8–3.3]) on the SICU, and 22.6 days (SD 2.9; 95% CI [21.6–23.5]) for the hospital. Table 1 shows a significant increase in CMI from 3.5 (mean; SD 0.8; 95% CI [3.2–3.8]) at the pre-intervention phase to 4.9 (mean; SD 1.0; 95% CI [4.6–5.2]) at the intervention phase (p < 0.0001). Mortality on the SICU increased significantly from 5.1% (SD 2.0; 95% CI [4.3–6.1]) to 7.3% (SD 2.4; 95% CI [6.4–8.3]) (p = 0.001). From the intervention phase to the post-intervention phase, CMI showed a non-significant decrease to 4.7 (mean; SD 1.0; 95% CI [4.3–5.0]) (p = 0.455), with mortality showing a non-significant change to 8.2% (SD 2.2; 95% CI [7.1–9.3]) (p = 0.23). At the same time that the head consultant in the Department of Anesthesiology and Intensive Care Medicine took over on 1st January 2011, new head consultants also took up posts in the surgical departments. As a result, case numbers increased in the intervention phase (2011–2014) compared to the pre-intervention phase (2008–2010) for diagnoses and procedures with the highest CMI: there was a 164% increase in visceral surgery for malignant neoplasms in the abdomen and inflammatory processes of the colon (average CMI 8.23); a 122% increase in vascular surgery relating to vascular occlusion and patched perforated abdominal aortic aneurysms (average CMI 8.8) and a 25% increase in urology surgeries for malignant neoplasms of the urinary bladder and kidneys (average CMI 4.42). Case numbers remained virtually unchanged in the post-intervention phase (2015–2017). However, increasing complexity of surgical procedures in visceral and vascular surgery, as well as in urology was associated with an increase of 3% in ICU mortality from the pre-intervention phase to the intervention phase, while mortality remained unchanged in the post-intervention phase. In addition, the number of cases presenting with severe sepsis also rose. In the pre-intervention phase, 4.8% of the surgical intensive care patients presented with severe sepsis compared to 11.6% in the intervention phase (p < 0.001); the increase was comparable in the post-intervention phase. However, hospital mortality did not change significantly over the period studied. Similarly, AB-ADA no longer showed a significant change from the intervention phase to the post-intervention phase and remained at a constant and low value (see also Fig. 2). To summarize, it is important to note that mortality showed no significant change from the intervention phase to the post-intervention phase for a comparable patient population (unchanged and high CMI) with consistently low AB-ADA.
Discussion
Impact of antibiotic stewardship
It is clear that ADA of antimicrobial agents was successfully reduced over the 4-year period of ABS implementation, with a 23.9% decrease in RDD/100 PD comparing the pre-intervention phase to the post-intervention phase. ADA also remained at a constantly low rate thereafter, from the intervention phase to the post-intervention phase. This result highlights a sustainable change in prescription. A decrease in ADA brought about by ABS measures and/or a restrictive antibiotic policy has similarly been demonstrated in other recent studies [17, 18]. A systematic review including only studies of effects seen on ICUs also identified an association between ABS and declining AB-ADA. However, the majority of the studies included covered a period from a few months to 2 or 3 years. Only two studies investigated effects over 48 and 54 months, respectively [19]. In another systematic review and meta-analysis, Karanika et al. [20] identified a 39.5% reduction of antibiotic prescription as a result of implementation of ABS on ICUs. One limitation highlighted in that review is that the follow-up period for the majority studies was limited to approximately 1 year. These short observation periods, especially subsequent to implementation of measures, make it difficult to draw conclusions on the sustainability of ABS measures.
In contrast, evidence of the sustainable effects of ABS can be seen at the SICU investigated in this study, both the declining ADA over the 4-year period of the intervention phase, as well as the constantly low ADA during the 3-year follow-up period.
In the ABS S3 guidelines, reduction of ADA is promoted alongside an improvement in prescription quality. This point primarily includes the change of antibiotic class, which also took place at the SICU investigated over the time period studied. As recommended in the guidelines [2], the initially preferred use of cephalosporins and fluoroquinolones declined and other antibiotics (e.g. penicillins) were used as an alternative. The increase in ADA of piperacillin/tazobactam (as the largest representatives of penicillins with BLI), which was prescribed on the basis of the substance class change, can also be seen in ICUs across Germany, with an increase in use of 247% [4]. One review highlighted that treatment according to the guidelines (e.g., using an in-house antibiotics list) is associated with a relative reduction in risk of mortality by 35% [21].
Development of resistance in the four indicator strains was not very apparent at the SICU investigated. No clear trend toward a desired reduction in resistance with reduced use of anti-infective agents was identified. Davey et al. [22] noted that the small number of microbiological results in individual studies may be the reason for the lack of evidence regarding the relationship between ABS measures and a reduction in resistance. External factors (e.g., resistant pathogens acquired on an outpatient basis) may also contribute to the apparent lack of any ABS effect on resistance [23]. Overall, the results in the literature are contradictory. In a systematic review, Schuts et al. [21] used meta-analysis to describe how in many cases favorable results can be found for development of resistance. However, these authors also view the relationship between increasing prescriptions of alternative antibiotics and the increase in other resistances as controversial. They identified inconsistent relationships between antibiotic use and development of resistance, as was found on the SICU in this study. Gastmeier [24] presented international studies that have investigated the influence of ABS measures on the incidence of individual resistant pathogens: the change in the incidence of MRSA subsequent to the introduction of ABS measures varies between studies, but predominantly showed declines, in some cases significant. Some studies identified a reduction in incidence of third-generation cephalosporin-resistant enterobacteriaceae (such as E. coli or K. pneumoniae) following the introduction of different strategies such as restrictions and training courses. Nevertheless, many researchers are skeptical that ABS has beneficial effects, because containment of multi-resistant pathogens has not been successful to date (with the exception of MRSA infections) [25].
Outcome
From the pre-intervention phase to the intervention phase, both the case-mix index (CMI), which describes the average case severity across patients and mortality on the SICU showed significant increases. From the intervention phase to the post-intervention phase, no further significant differences were found. Over this period, mortality is comparable with continued high CMI and stable low ADA of antimicrobial agents. Hospital mortality was comparable in all periods. The length of stay in intensive care was significantly lower in the post-intervention phase compared to the intervention phase. However, the increase in CMI is most likely due to the changing patient population as the complexity of surgical interventions has increased as part of the change in leadership in the surgical departments of the specialized academic teaching hospital. Sjoding et al. [26] observed this trend of changing patient groups and their diseases in a retrospective cohort study of US intensive care units. The main diagnosis sepsis has shot up from eleventh place in 1996 to first place and made up a 10.2% share in 2010. Over the period of the present study, a greater number of co-morbidities and organ dysfunctions were registered as secondary diagnoses, comparable to the data of Sjoding et al. [26] who also showed that invasive measures, such as artificial respiration or hemodialysis, have increased considerably. A significant association has been described in the literature between the severity of diseases and CMI [25, 27]. This also applies to the relationship between CMI and mortality. It is therefore reasonable to assume that the increase in mortality on the SICU from the pre-intervention phase to the intervention phase was due to increasing case severity and was not a result of antibiotic stewardship intervention, particularly as the hospital mortality in all investigated periods remained stable and the length of stay on the intensive care unit in the post-intervention phase compared to the intervention phase decreased significantly. If the restricted use of antibiotics would have led to higher (re-)infection rates, an increased length of stay would have been observed.
As such, ABS seems to be safe: several studies have demonstrated that decreasing use of antibiotics has no negative impact on mortality and some studies also found no negative impact on length of stay (days) [22, 28, 29]. Studies carried out exclusively on ICUs have also shown that deescalation therapies, for example in patients with hospital-acquired pneumonia, are safe for patients; the mortality rate in these studies was even found to be significantly lower [30].
The development of clinical outcomes of critically ill patients is influenced only in part by the implementation and results of ABS strategies. Many different factors have an impact on patients. The risk of infection with multidrug-resistant bacteria (MDR), for example, which is associated with a higher mortality rate, can further be a result of invasive procedures (which increase with longer stays on the ICU), general contact with care staff, the presence of a serious underlying disease, as well as other factors [31]. The number of patients at risk of infection will further increase in the future; one of the reasons for this is that the number of immunosuppressed patients and those undergoing organ transplants will continue to rise due to advances in diagnostics and treatment [16]. In addition, the average age of intensive care patients has increased in recent years as part of demographic change. More elderly and more multi-morbid patients undergo surgical interventions more and more frequently. These patients must be admitted more frequently to ICU following surgery and longer admissions may result [32].
Baur et al. [33] have highlighted the synergistic effect between ABS and hospital hygiene, which contributes to infection control. In their systematic review and meta-analysis, they compared studies in which there was also a program for hand hygiene (additional to ABS) with others, where only ABS measures were implemented. There was a 66% reduction in resistances by combining a program for hand hygiene and ABS. On the other hand, exclusive ABS measures decreased resistances by just 17%. This essential close cooperation between the two areas of ABS and hospital infection control represents a limitation in this study, with respect to conclusions about the effects of ABS alone. When interpreting the results, it must be noted that influencing effects result from a combination of ABS and infection control measures on the SICU. Understanding the individual effects of ABS from a bundle of measures is difficult, since in reality there are always several aspects that are applied that affect the overall result [21]. A further limitation of the study may result from the fact that the change of the leading physicians in intensive care medicine and surgery had a positive effect on consumption of antibiotics in addition to the ABS measures. The 10-year study period at the SICU of the specialized hospital investigated is significantly longer than other studies [17, 18, 34]. However, the retrospective nature of the analysis presents a further limitation. Nevertheless, a long-term and sustained effect could be demonstrated. As has been pointed out by several authors, large multi-center trials with comparable study designs conducted over the course of several years are required to prove the benefits of long-term ABS on patient outcomes and the development of resistance [35].
References
Regionalkomitee für Europa. Strategischer Aktionsplan zur Bekämpfung von Antibiotikaresistenzen in der Europäischen Region 2011;1–14.
de With K, Wilke K, Kern WV, Strauß R, Kramme E, Freidrichs A, et al. S3-Leitlinie Strategien zur Sicherung rationaler Antibiotika-Anwendung im Krankenhaus. AWMF online: Das Portal der wissenschaftlichen Medizin 2018;4–52
Kollef MH, Bassetti M, Francois B, Burnham J, Dimopoulos G, Garnacho-Montero J, et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med. 2017;43:1187–97.
Remschmidt C, Schneider S, Meyer E, Schroeren-Boersch B, Gastmeier P, Schwab F. Surveillance der Antibiotika-Anwendung und Resistenzentwicklung auf Intensivstationen (SARI). Dtsch Arztebl Int. 2017;114:858–65.
Zhang Y-Z, Singh S. Antibiotic stewardship programmes in intensive care units: why, how, and where are they leading us. World J Crit Care Med. 2015;4:13–28.
Behnke M, Aghdassi SJ, Hansen S, Alberto L, Diaz P, Gastmeier P, et al. Prävalenz von nosokomialen Infektionen und Antibiotika-Anwendung in deutschen Krankenhäusern. Dtsch Arztebl Int. 2017;114:851–7.
Richter DC, Heininger A, Brenner T, Hochreiter M, Bernhard M, Briegel J, et al. Bakterielle Sepsis Diagnostik und kalkulierte Antibiotikatherapie. Anaesthesist. 2017;66:737–61.
Bode-Böger SM. Optimierung der Antiinfektivatherapie mittels therapeutischen Drug-Monitorings. Nephrologe. 2014;9:457–64.
Cotta MO, Roberts JA, Lipman J. Antibiotic dose optimization in critically ill patients. Med Intensiva. 2015;39:563–72.
Pickens CI, Wunderink RG. Principles and practice of antibiotic stewardship in the ICU. Chest. 2019;156:163–71.
Luyt C-E, Bréchot N, Trouillet J-L, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18:1–12.
Hohn A, Heising B, Schütte J-K, Schroeder O, Schröder S. Procalcitonin-guided antibiotic treatment in critically ill patients. Langenbecks Arch Surg. 2017;402:1–13.
Hohn A, Schroeder S, Gehrt A, Bernhardt K, Bein B, Wegscheider K, et al. Procalcitonin-guided algorithm to reduce length of antibiotic therapy in patients with severe sepsis and septic shock. BMC Infect Dis. 2013;13:1–9.
Deutsche Gesellschaft für Infektiologie e. V. Fortbildungskonzept «Infektiologe (DGI)». 2017;1–8.
Deutsche Gesellschaft für Infektiologie e. V. Checkliste für die Antragstellung auf Zertifizierung der Zusatzbezeichnung «Infektiologe (DGI)»/«Infektiologin (DGI)» Ärzte, die an einem «Zentrum für Infektiologie (DGI)» tätig sind: 2018;49:3980193.
Schweickert B, Kern WV, De With K, Meyer E, Berner R, Kresken M, et al. Antibiotika-Verbrauchs-Surveillance: Ausführungen und Erläuterungen zur Bekanntmachung ‘Festlegung der Daten zu Art und Umfang des Antibiotika-Verbrauchs in Krankenhäusern nach §23 Abs. 4 Satz 2 IfSG’. Bundesgesundheitsblatt 2013;56:903–12.
Okumura LM, da Silva MMG, Veroneze I. Effects of a bundled antimicrobial stewardship program on mortality: a cohort study. Braz J Infect Dis. 2015;19:246–52.
Wu CT, Chen CL, Lee HY, Chang CJ, Liu PY, Li CY, et al. Decreased antimicrobial resistance and defined daily doses after implementation of a clinical culture-guided antimicrobial stewardship program in a local hospital. J Microbiol Immunol Infect. 2017;50:846–56.
Kaki R, Elligsen M, Walker S, Simor A, Palmay L, Daneman N. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother. 2011;66:1223–30.
Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother. 2016;60:4840–52.
Schuts EC, Hulscher MEJL, Mouton JW, Verduin CM, Stuart JWTC, Overdiek HWPM, et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:847–56.
Davey P, Marwick C, Scott C, Charani E, Mcneil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Datab Syst Rev. 2017
Kern WV. Antibiotic stewardship-Programme in Akutkrankenhäusern. Klinikarzt. 2018;47:272–6.
Gastmeier P. Antibiotic stewardship und hygiene—2 Seiten einer Medaille. Anasthesiol Intensivmed Notfallmedizin Schmerztherapie. 2017;52:248–59.
Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;43:1464–75.
Sjoding Michael W, Prescott Hallie C, Wunsch Hannah, Iwashyna Theodore J, Cooke Colin R. Longitudinal changes in ICU admissions among elderly patients in the United States. Crit Care Med. 2016;44:1.
Kuster SP, Ruef C, Bollinger AK, Ledergerber B, Hintermann A, Deplazes C, et al. Correlation between case mix index and antibiotic use in hospitals. J Antimicrob Chemother. 2008;62:837–42.
Gonzalez L, Cravoisy A, Barraud D, Conrad M, Nace L, Lemarié J, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17:1–8.
Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15:1–11.
Joung MK, Lee JA, Moon SY, Cheong HS, Joo EJ, Ha YE, et al. Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit Care. 2011;15:10–7.
Karam G, Chastre J, Wilcox MH, Vincent JL. Antibiotic strategies in the era of multidrug resistance. Crit Care. 2016;20:1–9.
Schuster M, Ferner M, Bodenstein M, Laufenberg-Feldmann R. Palliative Therapiekonzepte in der Intensivmedizin. Anaesthesist. 2017;66:233–9.
Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Döbele S, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990–1001.
Bonsignore M, Balamitsa E, Nobis C, Tafelski S, Geffers C, Nachtigall I. Antibiotic stewardship an einem Krankenhaus der Grund- und Regelversorgung. Anaesthesist. 2018;67:47–55.
Mertz D, Brooks A, Irfan N, Sung M. Antimicrobial stewardship in the intensive care setting—a review and critical appraisal of the literature. Swiss Med Wkly. 2015;145:1–10.
Internet links
Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen 2018: webKess—Portal für KISS [National Reference Centre for Surveillance of Hospital-Acquired Infections 2018: webKess—KISS portal]. https://webkess.charite.de/webKess2/de-DE//Home/Index (queried on 22/09/2019).
Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen (2018) National Reference Centre for Surveillance of Hospital-Acquired Infections, SARI Antibiotika- und Antimykotikaverbrauch im Zeitverlauf [SARI antibiotic and anti-fungal use over time]. http://sari.eu-burden.info/auswertung/down/AD-ZEIT.pdf (queried on 22/09/2019).
Robert Koch Institut (RKI) (2017) Antibiotika Resistenz Surveillance (ARS). Antibiotic Resistance Surveillance. https://ars.rki.de/Content/Database/ResistanceOverview.aspx (queried on 22/09/2019).
Acknowledgement
The authors would like to thank Thomas Ploch of COMESTA (Consulting Methods and Statistics) of Marburg, Germany, for his excellent advice and support in applying the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors indicate that no conflicts of interest exist.
Rights and permissions
About this article
Cite this article
Schröder, S., Klein, MK., Heising, B. et al. Sustainable implementation of antibiotic stewardship on a surgical intensive care unit evaluated over a 10-year period. Infection 48, 117–124 (2020). https://doi.org/10.1007/s15010-019-01375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-019-01375-6