Abstract
Assessing the effects of an antimicrobial stewardship program (ASP) implemented in a 78-bed Internal Medicine ward of an Italian mid-sized acute care hospital of 296 beds (26,820 bed days/year in 2015 and 26,653 in 2016). The ASP, implemented in May 2016, included: (a) formulation and dissemination of local guidelines on empiric antibiotic therapy; (b) educational training; and (c) restrictive control on the use of carbapenems. We included in the study all the patients who had received at least one systemic antibiotic as empiric therapy and who were discharged in two comparable time periods (Oct–Nov 2015: period 1 and Oct–Nov 2016: period 2), before and after the implementation of the ASP. Clinical data were collected to compare the two study periods. The percentage of patients treated with antibiotics was significantly lower in period 2 (272/635 = 42.8% vs 238/648 = 36.7%, − 6.1%, p < 0.01). A similar reduction was observed in terms of defined daily doses per 100 bed days (from 49.5 to 46.9; − 5.3%). In period 2, we observed a significant reduction of patients treated with carbapenems (5.7 vs 2.1%, p < 0.05). The length of hospital stay and in-hospital mortality was similar in the two study periods. The implementation of an ASP in our Internal Medicine ward has been associated with a significant reduction of patients treated with antibiotics. The reduction was particularly relevant for carbapenems, antibiotics which should be used only in selected cases. These results have been obtained without increasing length of hospital stay and in-hospital mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing spread of antimicrobial resistance is an issue of the utmost importance on a global level, so much so that several health and government organizations have proclaimed a “call to action” to counteract the phenomenon [1,2,3,4,5]. The antimicrobial resistance rates in Europe show wide variations depending on the bacterial species, antimicrobial group, and geographical region. In general, lower resistance percentages are reported by countries in the north, while higher percentages are reported in the south and east of Europe [6]. In the European context, Italy is one of the Member States with the highest level of antibiotic resistance. In particular, the hyper-endemic levels of carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii are of considerable concern [7]. Moreover, data published on antimicrobial resistance and antibiotic consumption during the year 2014 show that in Tuscany, one of the most populated Italian regions, resistance rates and antibiotic use are higher than the Italian average [8]. For example, Klebsiella pneumoniae is carbapenem resistant in 46.2% of the cases (Italian average: 32.9%) and Escherichia coli is resistant to third-generation cephalosporins in 43.9% and to fluoroquinolones in 55% of the cases (Italian average: 28.7 and 43.9%). The same document shows that in our hospital (San Giovanni di Dio Hospital, Florence, Italy), more antibiotics are used compared to the average of Tuscan hospitals (116 vs 88.7 defined daily doses (DDD)/bed days) and confirmed the high frequency of multidrug resistant isolates. A 1-day survey performed in our Internal Medicine ward detected a high prevalence (45%) of patients on antibiotics (unpublished data).
The analysis of these data pushed our Hospital Management and the Department of Internal Medicine to create a multidisciplinary team to design and implement an antimicrobial stewardship program (ASP) suitable for the Internal Medicine ward. The present study is aimed to assess the effects of this ASP on the antibiotics use. To this purpose, we retrospectively compared two 2-month sample time periods, before and after the ASP implementation.
Materials and methods
This study was approved by the Department of Internal Medicine and by the Hospital management.
Clinical setting
This single-center retrospective cross-sectional study was conducted at the Internal Medicine ward of San Giovanni di Dio Hospital, Florence, Italy, a medium size, acute care, general hospital (296 beds). The Internal Medicine ward consists of 78 beds for acute patients, mostly admitted from the Emergency Department (92% in 2015 and 90% in 2016). In 2015 and 2016, the total number of patients discharged from the ward was 3887 and 3754 with an occupancy rate of 95.1 and 93.9%, respectively. The ward is equipped with a full electronic clinical records system (ARGOS software, Dedalus, Italy).
Antimicrobial Stewardship Program
In April 2016, an Antimicrobial Stewardship Program (ASP) was introduced in the Internal Medicine ward of our hospital, with the aim of improving the appropriateness in the use of antibiotics and reducing their consumption. The ASP was designed by a multidisciplinary team consisting of internists, infectious disease specialists, microbiologists, and pharmacists. The intervention model was mainly educational, with the addition of a restrictive intervention on carbapenem prescriptions.
The ASP included:
-
1.
Elaboration and dissemination of local guidelines on the proper use of antibiotics Based on international guidelines and local resistance data, the ASP team elaborated a schematic guideline document containing empirical antibiotic treatment recommendations adapted to the specific hospital epidemiological context. This guideline is composed of: (a) generic indications about antibiotic choice, timing, dosing, de-escalation, treatment duration, microbiological testing, and procalcitonin use and (b) targeted advices for the empirical treatment of intraabdominal infections, pneumonia, skin and soft tissue infections, or urinary tract infections. Particular emphasis was given on the need to re-evaluate antibiotic therapy at least every 72 h, and to modify or discontinue therapy when suggested by clinical or microbiological data. The guideline document was sent via e-mail to all the physicians attending the Internal Medicine ward. Local guidelines on the use of antibiotics can be made available on request.
-
2.
Education In the month preceding the ASP implementation, the ASP team organized two educational meetings directed to all the physicians attending the Internal Medicine unit, with the aim of: (a) share data of local antibiotic consumption and local antibiotic-resistance rates; (b) present the program and discuss its rationale and objectives; (c) train physicians in the management of infections; and (d) promote the appropriate use of antibiotics, on the basis of the local guidelines. After implementation of the ASP, quarterly meetings were held in which: (a) the concepts of appropriateness in the use of antibiotics were reinforced; (b) clinical cases and personal performance were discussed; and (c) using benchmarking comparison to similar institutions, feedback was provided on the overall and specific consumption of antibiotics, on the appropriateness of carbapenem prescriptions and on the local antimicrobial resistance rates (the latter data updated every 6 months). The meetings were scheduled as part of the annual training program for doctors.
-
3.
Restriction of carbapenem use All the physicians attending the Internal Medicine unit were authorized to start carbapenem therapy. Within 72 h, the appropriateness of carbapenem prescription had to be assessed by a member of the ASP team experienced in antibiotic therapy (advisor), who decided whether to continue or discontinue it. The two advisors selected from the hospital Internal Medicine team, spent about 1 h a week each on the program. The advisors individually assessed the appropriateness of carbapenem prescriptions. To meet the need of a standardized process, a multi-item questionnaire was used to evaluate the prescriptions [9].
-
4.
Monitoring of local antibiotic-resistance rates The ASP team established a collaboration with the laboratory of microbiology of our institution, which was designated to provide a 6-month hospital antibiogram, informing about antimicrobial resistance rates of the principle pathogens isolated at the hospital level. Local antibiotic-resistance rates can be made available on request.
-
5.
Monitoring of antibiotic consumption The hospital pharmacy quarterly provided a report on consumption of all the antibiotic drugs for systemic use in the Internal Medicine wards. Antibiotic consumption was expressed in DDDs/1000 occupied bed days; DDDs were calculated following the Anatomical Therapeutical Chemical Classification/Defined Daily Doses (ATC/DDD) methodology recommended by the WHO [10].
Patients
Two trained medical students, supervised by two clinicians of the ASP team, retrospectively reviewed the clinical records of all patients discharged from the Internal Medicine ward in two sample periods of 2 months each: from 1 October to 30 November, 2015 (period 1) and from 1 October to 30 November, 2016 (period 2), before and after the activation of the ASP (Table 1). We include in the study all patients who received at least 24 h of systemic antibiotics as empiric therapy during hospitalization. Patients who had received antibiotic therapy for prophylactic treatment were excluded.
The following data were collected: age, gender, class of antibiotic used, changes and duration of antibiotic therapy, length of hospital stay, and in-hospital mortality.
Numerical data were reported as mean ± standard deviation.
Sample size calculation
A 1-day survey performed in our Internal Medicine ward in October 2015 shows that 45% of patients are on antibiotic therapy (unpublished data). Based on this assumption, the sample size was calculated to detect an 8% reduction of the patients on antibiotic therapy after implementation of the ASP, from 45% in period 1–37% in period 2, with a 5% probability of type I error (alpha 0.05) and a 20% probability of type II error (beta 0.2). The number of observations required was at least 1184 (592 per each period).
Statistical analysis
Statistical analysis was performed using the Student’s t test for parametric data and by means of Chi-square test or exact Fisher test for nonparametric data, when appropriate. Differences were considered significant for a value of p < 0.05. All analyses were carried out using MedCalc® version 12.3.0 (MedCalc Software; Mariakerke, Belgium).
Results
Description of study samples
The flow chart generating the two study samples is shown in Fig. 1. In period 1, 272 out 635 patients discharged from our unit received a systemic antibiotic as empirical therapy. In period 2, 238 out of 648 patients discharged from our unit received a systemic antibiotic as empirical therapy.
Main results
The percentage of patients treated with antibiotics was 42.8% (272/635) in period 1 and 36.7% (238/648) in period 2; the 6.1% reduction was statistically significant (p < 0.05) (Table 2).
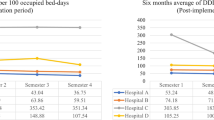
Overall antibiotic consumption decreased by 5.3%, from 49.5 DDD per 100 bed days in period 1–46.9 in period 2.
The main classes of antibiotic prescribed as empiric therapy are reported in Table 3, both as percentage of treated patients and as number of DDD per 100 bed days. The percentage of patients treated with carbapenems has more than halved, from 5.7% in period 1–2.1% in period 2 (p < 0.05). Similarly, the number of DDD per 100 bed days of carbapemens decreased from 3.0 to 0.6 (− 80%). The percentage of patients treated with metronidazole was also significantly reduced, while differences in the use of other classes of antibiotics were not significant.
The majority of patients were treated with antibiotic monotherapy in both periods (68 and 64%) and the most frequent route of administration was the intravenous (Table 4). Antibiotic therapy has been changed more frequently in period 2 compared to period 1 (36 vs 24%, p < 0.05), with a similar proportion of de-escalation, escalation, and maintenance of the same spectrum. No significant differences were observed with respect to duration of therapy and switching from intravenous to oral route of administration (Table 4).
The length of hospital stay and the rate of in-hospital mortality did not significantly change from period 1 to period 2 (Table 5).
Discussion
Main results
The main finding of this study is that the implementation of a multifaceted ASP is able to reduce the consumption of antibiotics in an internal medicine ward of an Italian medium-sized hospital. This result is obtained both in terms of reduced percentage of treated patients (− 6.1%) and in the number of DDD per 100 bed days (− 5.3%), and is particularly relevant for carbapenems (− 80%). Limiting the use and increasing the appropriateness of antibiotic treatment is of straightforward clinical importance in Italy, where the hospital antibiotic consumption and the multi-resistant bacteria rate are among the highest in Europe [6]. The greatest concern is due to the high resistance, with a dramatic increase of the percentage of carbapenem-resistant Klebsiella pneumoniae isolates in recent years, from less than 1% in 2008 to 33% in 2015 [11]. In our region (Tuscany), this resistance rate reached 46.2% in 2014 [8]. The significant reduction in the use of the carbapenems that was obtained may contribute to hinder the further emergence of resistance. These antibiotics are among the few means that we have to fight against many multi-resistant bacteria, such as the Extended Spectrum Beta-Lattamase (ESBL) producing species, and one of the main interventions of the ASP was to make doctors aware that carbapenems should be reserved for selected cases of suspected or confirmed infection by bacteria resistant to the most common antibiotics.
Currently, there is no reference standard on the amount of antibiotics used in the Italian Internal Medicine wards. The literature data show a great variability between different types of hospital and between different hospital settings. A prospective multicenter observational study on the antibiotic use in Internal Medicine wards of a different Italian region (Lazio) reports that 58.2% of the in-hospital patients are on antimicrobial treatment [12], with wide variability between the different centres (range 17–71%). This value is higher than those we have observed in the present study, both before and after ASP implementation (42.8 and 36.7%), and confirms the wide heterogeneity existing between the Italian units of internal medicine. A recent survey [13] shows that antibiotics are used by 33.2% of patients admitted to 53 medical wards of Southern Europe; lower values are observed in Western (23.4%), Northern (29.8%), and Eastern Europe (11.6%).
The length of the in-hospital antibiotic treatment is not substantially modified by ASP implementation; however, it was already short and in line with the recommendations of the literature before the ASP intervention (6 + 4 days), so that it was unlikely and not necessarily appropriate to get a further reduction. Another interesting result of the ASP implementation is the increase in changes of antibiotic therapy during hospitalization (from 24 to 36% of patients). This is probably due to the more frequent revision of the therapeutic schemes, according to our local guidelines, which recommend a critical control of the therapy, based on clinical and microbiological data, at least every 72 h. The multidisciplinary team intends to make the periodical revision of the therapy mandatory, by an alert included in the electronic medical record.
A concern about the ASPs is the possibility that the reduction of antibiotic use could determine an increase in the length of hospital stay or mortality. Although none of these events are observed in the present study, it should be underlined that the size of the patients’ sample was not calculated for this purpose. However, a large review including 221 randomized and non-randomized studies indicates that interventions to improve antibiotic prescribing practices for in- hospital patients can reduce antibiotic use without adversely affecting the length of stay and mortality [14].
Antimicrobial Stewardship Program
When our multidisciplinary team began the design of the ASP (October 2015), there were no National or regional rules and guidelines on the use of antibiotics. Most hospitals had their own regulation, usually focused on controlling the prescription of high-cost antibiotics. At that time, ASPs were designed and implemented at the level of individual hospitals or individual departments, without a national or regional coordination. Therefore, we designed an ASP suitable for our Internal Medicine ward following the international recommendations [15, 16].
In the following years, regional documents [17, 18] and a National plan on the fight against antibiotic resistance were published [11]. These documents include antimicrobial stewardship interventions and antimicrobial resistance monitoring to be implemented in the coming years.
Various modalities of antimicrobial stewardship interventions have been used to obtain a reduction of unnecessary antibiotic use (educational, restrictive, structural, or mixed) [14]. We have chosen a mixed intervention program, consisting of a restrictive control over the use of carbapenems associated with a multifaceted educational method. What is the most effective stewardship method is still the subject of debate [14]. Restriction in the use of an antibiotic is a rapidly effective method [19], but, if not associated with educational intervention, may rapidly lose its effectiveness after discontinuation [14, 19]. On the other hand, although effective in various clinical settings [20,21,22,23,24], the educational method also has some limitations. Namely, the results are obtained slowly, a large use of time and resources is required and some studies have not shown a significant clinical efficacy when used alone [25, 26]. The combination of restrictive and educational interventions is probably the best way to obtain fast and lasting results over time [14]. With a mixed educational and restrictive method similar to that used in our study, a reduction in the consumption of antibiotics, particularly of carbapenems, cephalosporins, and fluoroquinolones, was obtained in a university hospital in northern Italy [27].
According to our experience, all steps of the ASP must be implemented carefully. A crucial point was to establish a stable relationship with the pharmacists and microbiologists of our hospital to obtain periodic of data about antibiotic consumption and local microbial resistance. Equally important, as widely described in the literature [23, 28,29,30], were the periodic meetings with all doctors of the ward, during which feedback on local data compared with those of similar institutions and on personal performance were provided.
Costs
The costs have not been measured in detail. However, we estimate that the pharmacist and two advisors in charge of checking the appropriateness of the carbapenems prescription had spent on the program about 1 h a week each. Considering the savings obtained with the reduction in the use of antibiotics, we believe that the cost of the program is not particularly high and is overall sustainable.
Strengths and limitations of the study
We have shown that a multifaceted educational and restrictive program is able to reduce the consumption of antibiotics in an Internal Medicine ward of a medium-sized Italian hospital in a relatively short time and with sustainable costs. These results are of a considerable importance in Italy, a nation with a dramatic and increasing spread of antimicrobial resistances, and in which a global plan to combat antimicrobial resistance has been formulated only a few months ago and is still far from being implemented [11]. Our ASP may serve as a model for other Italian Internal Medicine units to address the issue of antibiotic therapy appropriateness [31], keeping in mind that each program should be tailored according to the specific characteristics of the single hospital.
The present study has some limitations. First, this is a monocentric study performed on a relatively small number of patients, so we are not sure that the program could be applied with the same results in other hospitals. Second, the outcome of the ASP was analyzed in a single period of time after its implementation and no information is currently available on the long-term duration of these effects. To obtain such data, the analysis of time series will be necessary with more observations at regular time intervals.
Another limitation of this study is the lack of evaluation of some relevant clinical indicators, in particular the impact of the ASP on the diffusion of nosocomial infections (e.g., C. difficile) and on the diffusion of resistant pathogens. We decided not to evaluate these indicators at the current stage, because the ASP was applied only in our unit, which counts about one quarter of the beds of the entire hospital, for a relatively short time. The ASP efficacy in minimizing nosocomial infections and reducing resistant pathogens will be more properly evaluated when the intervention will be implemented throughout the hospital for longer time, as we plan to do. Available data look encouraging, because antibiotic stewardship programs have been shown to significantly reduce the incidence of infections and colonization with antibiotic resistant bacteria and C. difficile infections in hospitalized patients [32].
Conclusions
In conclusion, the results of this study indicate that the ASP we implemented, consisting in educative and restrictive interventions, has been able to significantly modify the use of antibiotic therapy in an Internal Medicine ward. We obtain a significant reduction of antibiotic consumption, particularly of carbapenems, and this result is of great value in the attempt to limit the spread of antimicrobial resistance. The interventions included in our ASP may serve as a model for other Internal Medicine units to address the topic of antibiotic therapy appropriateness. Further assessments are needed to confirm these findings and hopefully ameliorate the program over time.
References
World Health Organization (2011) Antimicrobial resistance: no action today, no cure tomorrow. World Health Organization, Geneva, Switzerland. http://www.who.int/world-health-day/2011/en/. Accessed 22 Apr 2018
O’Neill J, Wellcome Trust and UK Government. Review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. Accessed Dec 2014
National action plan for combating antibiotic-resistant bacteria. The White House. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed Mar 2015
A European One Health Action Plan against Antimicrobial Resistance (AMR) (2017). https://ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf. Accessed 22 Apr 2018
Centers for Disease Control and Prevention (2017) Be antibiotic aware. https://www.cdc.gov/antibiotic-use/index.html. Accessed 22 Apr 2018
European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2016. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2017. https://ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016. Accessed 27 Apr 2018
European Centre for Disease Prevention and Control. ECDC country visit to Italy to discuss antimicrobial resistance issues. Stockholm: ECDC; 2017. https://ecdc.europa.eu/en/publications-data/ecdc-country-visit-italy-discuss-antimicrobial-resistance-issues. Accessed 30 Mar 2018
L’utilizzo di antibiotici e l’antibiotico-resistenza in Toscana Secondo report della Rete di Sorveglianza dell’Antibiotico Resistenza in Toscana (SART). Documenti della Agenzia Regionale di Sanità della Toscana Ottobre 2015, n. 84. https://www.ars.toscana.it/collana-documenti-ars/pubblicazioni-2015/3190-l-utilizzo-di-antibiotici-e-l-antibiotico-resistenza-in-italia-2015.html. Accessed 8 Feb 2018
Cisneros JM, Neth O, Gil-Navarro MV et al (2014) Global impact of an educational antimicrobial stewardship programme on prescribing practice in a tertiary hospital centre. Clin Microbiol Infect 20(1):82–88
WHO Collaborating Centre for Drug Statistics Methodology (2018). International language for drug utilization research. https://www.whocc.no/. Accessed 20 Jan 2018
Piano Nazionale di Contrasto dell’Antimicrobico-Resistenza (PNCAR) 2017–2020. Ministero della Salute (October 2017). http://www.salute.gov.it/imgs/C_17_pubblicazioni_2660_allegato.pdf. Accessed 24 Apr 2018
Martolini D, Galiè M, Santoro AM, Monno D, Santini C, Terracina D, On behalf of FADOI Lazio—Area of Infectious Diseases (2017) Antibiotic use in Departments of Internal Medicine of Lazio. Ital J Med 11:364–370
Versporten A, Zarb P, Caniaux I, On behalf of the Global-PPS network et al (2018) Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. https://doi.org/10.1016/S2214-109X(18)30186-4. www.thelancet.com/lancetgh. Accessed 19 Apr 2018
Davey P, Marwick CA, Scott CL et al (2017) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2:CD003543
Dellit TH, Owens RC, McGowan JE Jr et al (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177
Centers for Disease Control and Prevention (CDC). Core elements of hospital antibiotic stewardship programs, 2014. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed 14 Feb 2016
Prevenzione dell’antibioticoresistenza e delle infezioni in ambito assistenziale. Regione Emilia-Romagna. Agenzia sanitaria e sociale regionale (2016). http://assr.regione.emilia-romagna.it/it/ricerca-innovazione/prevenzione-antibioticoresistenza-infezioni. Accessed 30 Apr 2018
Programma Regionale di Lotta alla sepsi. Regione Toscana (2017). http://www.regione.toscana.it/bancadati/atti/Contenuto.xml?id=5152917&nomeFile=Delibera_n.752_del_10-07-2017-Allegato-A. Accessed 30 Apr 2018
de With K, Allerberger F, Amann S et al (2016) Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Infection 44:395–439
Serisier DJ, Bowler SD (2007) Effect of a simple educational intervention on the hospital management of community-acquired pneumonia. Respirology 12:389–393 (III)
Zabarsky TF, Sethi AK, Donskey CJ (2008) Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a longterm care facility through an educational intervention. Am J Infect Control 36:476–480
Akter SF, Heller RD, Smith AJ, Milly AF (2009) Impact of a training intervention on use of antimicrobials in teaching hospitals. J Infect Dev Ctries. 3:447–451 (II)
Butler CC, Simpson SA, Dunstan F et al (2012) Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ 344:d8173. https://doi.org/10.1136/bmj.d8173
Chang Y, Chen H, Lin C et al (2017) Implementation and outcomes of an antimicrobial stewardship program: effectiveness of education. J Chin Med Assoc 80(6):353–359
Knox MC, Edye M (2016) Educational antimicrobial stewardship intervention ineffective in changing surgical prophylactic antibiotic prescribing. Surg Infect 17(2):224–228. https://doi.org/10.1089/sur.2015.194
Hulscher MEJ, Prins JM (2017) Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect 23(11):799–805. https://doi.org/10.1016/j.cmi.2017.07.017
Giacobbe D, Del Bono V, Mikulska M et al (2017) Impact of a mixed educational and semi-restrictive antimicrobial stewardship project in a large teaching hospital in Northern Italy. Infection 45(6):849–856
Marwick CA, Guthrie B, Pringle JE et al (2014) A multifaceted intervention to improve sepsis management in general hospital wards with evaluation using segmented regression of interrupted time series. BMJ Qual Saf 23(12):e2
Nathwani D, Lawson W, Dryden M et al (2015) Implementing criteria-based early switch/early discharge programmes: a European perspective. Clin Microbiol Infect Suppl 2:S47–S55. https://doi.org/10.1016/j.cmi.2015.03.023 (Epub 2015 Jul 18)
Pulcini C, Binda F, Lamkang AS et al (2018) Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2018.03.033 (Epub ahead of print)
Menichetti F, Tagliaferri E (2012) Antimicrobial resistance in internal medicine wards. Intern Emerg Med Suppl 3:S271–S281
Baur D, Gladstone BP, Burkert F et al (2017) Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet 17(9):990–1001
Funding
This ASP and the present study were performed as part of our routine work. No external funding was obtained for the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Statement of human and animal rights
The authors declare that all procedures performed in this study are in accordance with ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This is a retrospective study and for this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Fortini, A., Faraone, A., Di Pietro, M. et al. Antimicrobial stewardship in an Internal Medicine ward: effects on antibiotic consumption and on the use of carbapenems. Intern Emerg Med 13, 1219–1226 (2018). https://doi.org/10.1007/s11739-018-1916-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1916-9