Abstract
Senescence is an inevitable natural life process that involves structural and functional degeneration of tissues and organs. Recently, the process of skin aging has attracted much attention. Determining a means to delay or even reverse skin aging has become a research hotspot in medical cosmetology and anti-aging. Dysfunction in the epidermis and fibroblasts and changes in the composition and content of the extracellular matrix are common pathophysiological manifestations of skin aging. Reactive oxygen species and matrix metalloproteinases play essential roles in this process. Stem cells are pluripotent cells that possess self-replication abilities and can differentiate into multiple functional cells under certain conditions. These cells also possess a strong ability to facilitate tissue repair and regeneration. Stem cell transplantation has the potential for application in anti-aging therapy. Increasing studies have demonstrated that stem cells perform functions through paracrine processes, particularly those involving exosomes. Exosomes are nano-vesicular substances secreted by stem cells that participate in cell-to-cell communication by transporting their contents into target cells. In this chapter, the biological characteristics of exosomes were reviewed, including their effects on extracellular matrix formation, epidermal cell function, fibroblast function and antioxidation. Exosomes derived from stem cells may provide a new means to reverse skin aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Senescence is an unavoidable natural process of life that causes structural and functional degeneration of tissues and organs. As the largest organ of the human body, skin inevitably faces problems associated with aging [1]. Recently, skin aging has attracted a great deal of attention. Establishing a strategy to delay or even reverse skin aging has become a research hotspot in medical cosmetology and anti-aging. Stem cells are pluripotent cells that possess the ability to self-renew and can differentiate into various types of functional cells under certain conditions; these cells possess a strong ability to facilitate tissue repair and regeneration [2]. There is potential for the application of stem cell transplantation in the field of anti-aging treatment [3]. The implantation of stem cells in vivo significantly delayed senescence, but surprisingly, the implanted stem cells were not detected within the tissues; these findings suggest that their anti-senile effect may be achieved through paracrine activities [4]. Based on this, much attention has been focused on the study of exosomes, as they are the most important structure involved in paracellular secretion. As an alternative to cell-free therapy, exosomes avoid the risks of immune rejection, stemness maintenance, cell senescence and tumorigenesis in stem cell transplantation [3]. These advantages make exosomes ideal for use in novel anti-aging strategies. The role of stem cell-derived exosomes in reversing skin aging was reviewed in this chapter.
2 Biological characteristics of exosomes
In the 1980s, Canadian scientists discovered a nanoscale vesicle-like structure, which they termed exosomes while examining the process of reticulocytes maturing into red blood cells [5]. The diameter of exosomes is approximately 40–100 nm. Under an electron microscope, they are double concave dish-shaped or cup-shaped. Exosomal vesicles are formed by inward budding of the limiting membrane of early endosomes and mature into multivesicular bodies (MVBs) during the process [6,7,8]. Almost all human cells can secrete exosomes, which are widely distributed in various body fluids and carry a variety of proteins, mRNAs, miRNAs, lipids, etc. So exosomes form a brand new cell–cell information transfer system, which can participate in cell communication, cell migration, angiogenesis and tumour cell growth [9]. Exosomes can be preserved for long periods at –20ºC. These structures can also undergo multiple freeze–thaw cycles without any alteration in their volume [10]. There are many separation methods specific for exosomes; among these methods, density gradient centrifugation is the most common. The isolated particles can be identified by transmission electron microscopy, nanoparticle tracing or flow cytometry [10]. Since the first detection of mesenchymal stem cell (MSC) exosomes in 2010, a variety of stem cell-derived exosomes have been isolated [11]. The umbilical cord MSC exosomes, first reported in 2013, contain more growth factors than exosomes derived from other sources [12, 13]. The main contents of exosomes are proteins, RNA and lipid components [14]. Exosomal RNA cannot completely represent the total RNA of origin cells but only selectively contains part of the total cellular RNA [15]. Moreover, the RNA contained within serum exosomes is not the same as that in tissues [16]. Exosomes typically express CD9, CD63, CD81 and CD82, as well as adhesion molecules that are similar to those of their source cells. For example, CD29, CD44 and CD73 were also detected in mesenchymal stem cell-derived exosomes [10]. Exosomes influence a series of pathophysiological processes by participating in cell–cell communication [10]. Mushrooming basic research examining stem cell exosomes found that these structures function in tissue damage repair, chronic wound healing, inhibition of wound scar formation and anti-aging [17,18,19].
3 Physiological and pathological mechanisms underlying skin aging
The skin consists of the dermis and the epidermis, which is separated by a basement membrane (BM), a specialized extracellular matrix (ECM) structure consisting primarily of laminins, collagens and nidogens [20]. The composition of the ECM determines not only its adhesive and mechanical properties (stiffness, elasticity, topology) but also serves as a reservoir for cytokines [21, 22]. Skin aging is divided into intrinsic and extrinsic aging [23]. The former is primarily characterised by dry skin, fine wrinkles, reduced collagen and elastic fibres, and thinner subcutaneous fat. Intrinsic aging is normal skin aging that gradually occurs with increasing age. Extrinsic aging is caused by environmental factors, particularly ultraviolet rays, and may present as dry skin, large and deep furrows, loss of skin elasticity, coarse skin and uneven pigmentation [23]. Skin aging can reflect the degree of aging experienced by a given living organism [24]. The decreased proliferation and division ability of keratinocytes, abnormal function of fibroblasts, flattening of the papillary layer and changes in the ECM composition and content are common pathophysiological changes that occur during endogenous and exogenous skin aging [23]. The researchers revealed progressive accumulation of photoaging-related changes and increased chronic inflammation with age by performing single-cell RNA sequencing of human eyelid skin. At the same time, they found that genetic activation of HES1 or pharmacological treatment with quercetin alleviated cellular senescence of dermal fibroblasts [25]. Declined stem cell (SC) function is a hallmark of aging and is linked to impaired regeneration and damage-induced repair. Aged epidermal SCs show a reduced proliferation rate, lowered fitness and regenerative potential [26,27,28]. Rübe et al. found that human skin aging is associated with increased expression of the histone variant H2A.J in the epidermis [29]. Cellular senescence, chronic inflammation, matrix metalloproteinases (MMPs), reactive oxygen species (ROS) and hormones (sex, growth, insulin-like growth factors and other synthetic and metabolic hormones) are involved in this process [23, 30]. Therefore, possible ways to prevent skin aging include the use of antioxidants and the regulation of epidermal SCs, ECM and fibroblast function.
4 Mechanism of stem cell exosome activity against skin aging
4.1 Effect and molecular mechanism of exosomes on epidermal cells function
The epidermis is the outermost layer of mammalian skin and comprises a multi-layered epithelium, the interfollicular epidermis, with associated hair follicles, sebaceous and eccrine sweat glands. The epidermis is mainly made of keratinocytes (95%) [31]. The differentiated cells of the epidermis are dead, frequently anucleate cells. Epidermal maintenance depends on the proliferation of epidermal SCs, which are cells with an extensive self-renewal capacity and the ability to produce daughter cells that undergo further differentiation [31]. The thickness of the epidermis is reduced by about 6.4% during each decade of aging and by the reduction of the glycoprotein levels [32]. The thinning may also be caused by the decrease in the keratinocyte proliferation rate. Zhou et al. found that aquaporin (AQP)5 significantly decreased in the epidermal SCs with age by transcriptome sequencing and could promote the proliferation and dedifferentiation of HaCaT, but did not influence cell apoptosis [33]. Induced pluripotent SC (iPSC)-derived MSCs (iMSCs) serve as a unique source for cell therapy. Kim et al. found that iMSC-Exo promotes the proliferation of HaCaTs and human dermal fibroblasts (HDFs) by stimulating ERK1/2 and highlighting the application of iMSCs to produce exosomes [34]. Ma et al. found that ADSC-Exos could prompt cell proliferation and migration of HaCaT cells and repress their cell apoptosis, possibly via Wnt/β-catenin signalling [35]. Zhang et al. confirmed that ADSC-Exos promote the proliferation and migration of HaCaT cells by regulating the activation of the Akt/HIF-1α signalling pathway, thus promoting wound healing [36]. Wu Peipei et al. found that hucMSC-Ex protects skin keratinocytes from oxidative stress and delivers 14–3-3ζ protein, which promotes SIRT1 expression in HaCaT cells under oxidative stress conditions [37]. In addition, Duan et al. found that epidermal SC-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing [38]. The activities and mechanisms of SC exosomes against epidermal SCs have been poorly studied, so more research is needed.
4.2 Effect and molecular mechanism of exosomes on fibroblast function
Fibroblasts are the main cellular components of the dermis, and they produce collagen and other ECM components [39, 40]. Typically, fibroblasts proliferate at a low rate in vivo, and their impaired function and resultant changes in the ECM are closely related to exogenous skin aging [24]. The effects of exosomes on fibroblast function have been widely reported. However, the exact mechanisms are unclear. MSC exosomes primarily gather around the nucleus after entering the fibroblasts [17]. Exosomes derived from human umbilical cord blood plasma can transport miR21-3p into cells to downregulate the expression of phosphatase and tensin homolog (PTEN) and sprouty homolog 1 (SPRY1), thereby activating the PI3K/Akt and ERK1/2 signalling pathways and regulating cell function. Interestingly, the intensity of the effects exerted by exosomes gradually decreases with time [18]. Besides mRNA, the cytokines in exosomes also play roles in modulating fibroblasts’ function. For example, platelet-derived growth factor (PDGF) involved in exosomes can be delivered to fibroblasts and binds to the corresponding receptor, then the mitogen-activated protein kinase (MAPK) signalling pathway is activated to promote cell proliferation [39]. The reactivity of senescent fibroblasts to PDGF is decreased significantly, and upregulation of Akt-1 kinase can restore the sensitivity of senescent cells to PDGF [41]. Exosomes from iPSCs can enhance the proliferation of fibroblasts in a concentration-dependent manner and reverse UVB-induced fibroblast senescence through reducing expression of SA-β-Gal [42]. However, the effect of exosomes on endogenous aging factor-induced cell senescence was not obvious [43]. Other studies have found that MSC exosomes exert a stronger effect on the proliferation and migration of HDFs derived from normal tissue sources than they do on fibroblasts derived from diabetic wound sources. However, the latter was found to be more sensitive to exosomes according to cell migration tests [17]. Studies have shown that 3D culture of HDF globular cells is a key step in restoring the functional characteristics of passage fibroblasts, and globular exosomes derived from dermal fibroblasts help mediate this process [44]. However, the effect of exosomes on cell proliferation and migration is related to the origin of exosomes. The exosomes secreted by bone marrow and umbilical cord MSCs have strong induction ability [45]. These results suggest that the effect of exosomes on fibroblasts is selective. The proliferation of fibroblasts, together with the decrease in the ECM regeneration, results in accelerated tissue collapse to produce exogenous aging features, such as wrinkles [46]. In the presence of exosomes, western blotting results revealed a significant increase in the expression of Akt, signal transducers and activators of transcription (STAT) and ERK1/2 in normal fibroblasts. This increase was particularly high regarding STAT3, which activates the expression of a range of genes such as cyclins and growth factors (such as hepatocyte growth factor [HGF], insulin-like growth factor-1 [IGF1], nerve growth factor [NGF] and stromal-derived growth factor-1 [SDF1]) [17]. The STAT3, PI3K/Akt, ERK1/2 and MAPK signalling pathways are all essential pathways that regulate cell growth, proliferation and differentiation in vivo. Among these, ERK is a member of the MAPK family, and cross-linking exists among these signalling pathways to form a signal network where MAPK plays a key role. In vivo and in vitro experiments have shown that exosome mRNAs promote wound healing; however, exosome proteins do not promote this process [47]. Umbilical cord blood MSCs (UCB-MSCs) can improve wound healing, which can be, in part, through miR-21-5p- and miR-125b-5p-mediated TGF-β receptor inhibition [48]. Kim et al. found that USC-CM has various growth factors associated with skin rejuvenation, and in vitro results showed that USC-CM-Exos integrate with HDFs and consequently promote cell migration and collagen synthesis of HDFs [13]. Li et al. found that hMSC cells secreting miR-26a exosomes inhibited the proliferation, migration and transdifferentiation of high glucose-induced BJ cells and promoted cell apoptosis, which may be related to the TLR4/NF-κB signalling pathway [49].
4.3 Effect and molecular mechanism of exosomes on matrix metalloproteinase (MMP)
MMPs are ubiquitous peptide-chain enzymes found within living organisms. They are named due to their ability to bind metal ions at their active sites. The most important MMPs in the skin are MMP1, MMP3 and MMP9, and these enzymes can degrade almost all the molecules in the ECM of the dermis, particularly the collagen and elastin fibres that make up the dermal structure [50]. Based on this, MMP activities may represent the primary mechanism that regulates the photoaging of the skin [50,51,52]. In 1996, Fisher first observed in vivo that exposure of human skin to UVB irradiation induces the expression and activation of MMP by upregulating the transcription factors AP-1 and NF-B in keratinocytes and fibroblasts in a dose-dependent manner [53]. MMP expression and activity have been shown to be increased during both endogenous and exogenous skin aging. In unexposed skin, older patients possess more MMP than younger patients [54], and aging skin contains relatively few tissue inhibitors of metalloproteinases (TIMPs) [55]. TIMPs selectively inhibit MMP activity through non-covalent binding-based interactions. It was found that the conditioned medium from human dermal SCs could reduce the expression of MMP1 in senescent fibroblasts and could upregulate TIMP1 in these cells [56]. Further in vitro experiments revealed that SC exosomes not only downregulated the expression of MMP in natural senescent fibroblasts, but they also significantly inhibited the overexpression of MMP1 and MMP3 in UVB-induced fibroblasts [13, 42]. Exosomes from adipose-derived SCs (ADSC) can significantly reduce the secretion of MMP1, MMP2, MMP3 and MMP9 in corneal stromal cells, and these exosomes can upregulate extracellular matrix-related proteins, including four types of collagen and fibrin [57]. Exosomes carry MMP1, MMP2, MMP3, MMP9 and MMP13 on their surfaces. By regulating the structure of the ECM, they create space for newly formed blood vessels and vascular-related migratory cells and promote new angiogenesis and wound repair [58]. However, another study found that ADSC-derived exosomes increased MMP3 levels by activating the ERK/MAPK signalling pathway, and MMP3 increased proportionally with an increase in exosome concentration, ultimately resulting in the degradation of over-proliferating fibres to facilitate wound healing without scarring [59]. These interesting results indicate that the regulation of MMP by exosomes is bidirectional. Based on the imperfect repair mechanism and chronic imbalance in the ECM synthesis and degradation, the damage of elastic and collagen fibres accelerates the breakdown of the dermal structure. This breakdown is the main molecular feature of dermal aging [13].
4.4 Effect and molecular mechanism of exosomes on reactive oxygen species
ROS are by-products of cellular metabolism, and examples include hydrogen peroxide, superoxide anions, hydroxyl radicals and others. Low concentrations of ROS are involved in cell proliferation, differentiation and signal transduction, processes that are beneficial to the organism. High concentrations of ROS cause oxidative stress damage to DNA, proteins and lipids [60,61,62]. Ultraviolet radiation can produce a large number of ROS that can affect the function of dermal fibroblasts, induce MMP synthesis and damage the ECM components such as collagen and elastin [30]. Abnormal regulation of the redox response pathways involved in ROS metabolism is also associated with senescence [30, 61]. Senescent fibroblasts produce more ROS than normal cells [30, 60, 62]. The expression of AQP-1 and AQP-3 in fibroblasts and the secretion of hyaluronic acid make AQP-1 have a good moisturizing effect on the skin. However, oxidative stress decreased AQP-1 and AQP-3 mRNA expression and hyaluronic acid secretion, which was reversed by exosomes [63]. Conditioned medium (CM) from human dermal stem/progenitor cells (HDSPCs) reduced ROS levels by upregulating superoxide dismutases-2 (SOD2) [56]. Malondialdehyde (MDA) is a marker of lipid peroxidation, and it can reflect the degree of cytotoxic damage. Through its paracrine mechanism, ADSC upregulates SOD and downregulates MDA levels to reverse galactose-induced skin oxidative stress [64]. Glutathione peroxidase 1 (GPX1) is the primary ROS scavenging enzyme system, and nicotinamide adenine dinucleotide phosphate oxidase (NOX) is the key ROS production enzyme system in vivo. Studies have found that exosomes derived from ADSCs can inhibit the expression of NOX1 and NOX2, thus reducing ROS production [65]. Studies examining exosomes that promote the healing of diabetic foot ulcers have found that NOX1 and NOX4 expressions and ROS production are decreased [66]. Exosomes derived from umbilical cord MSCs can reduce ROS in liver cells through GPX1 transport [67]. Exosomes target and silence CaMKII expression through their rich miR-214 content, thereby inhibiting ROS and preventing oxidative stress injury in cardiomyocytes [68]. A recent study found that exosomes promote axonal regeneration by regulating ROS production through NOX2 transport to nerve axons [69]. In conclusion, SC exosomes possess certain functions in ROS production and clearance, and the specific mechanism is worthy of further study. The downregulation of SIRT1 led to increased acetylated p53 expression over time induced by H2O2 in fibroblasts, which induced the expression of p21, a downstream molecule of p53, and arrested the cell cycle leading to cell senescence. MSC-Exo enhanced these signal transduction systems. MSC-Exo was also effective at blocking the increase of SA-β-Gal activity and accumulation of ROS in cells [63].
5 Conclusion and prospect
Stem cell derived exosomes enhance the proliferation and migration of skin fibroblast (FB) and inhibit the production of ROS and MMP. UVB can induce fibroblast senescence, which can be reversed by exosomes; exosomes can enhance proliferation and migration ability of FB through activating MAPK, AKT, STAT3 and ERK1/2 signaling pathways. Senescent FB produce more ROS and MMP, exosomes can inhibit this process and thus alleviate senescence.
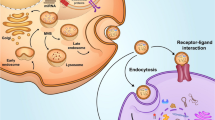
In summary, by activating multiple signalling pathways, SC exosomes are involved in regulating the proliferation of epidermal cells, fibroblasts, restoring the ECM components, lowering ROS levels and ultimately reversing skin aging (Fig. 1). Compared with cell-based therapy in regenerative medicine, MSC exosome therapy is more attractive for clinical development. Firstly, MSC exosome has a low amount of major histocompatibility complex[70], and its immunogenicity is lower than that of its parent cells, which may make MSC exosome not easy to cause immune response in foreign hosts. Secondly, many security issues and ethical constraints associated with stem cell transplantation can be mitigated by replacing live cells with exosomes secreted by them; third, new evidence suggests that exosomes are atomizable and can be cryopreservation at a temperature of 20 °C for 6 months without losing their biochemical activity. More importantly, unlabelled exosomes did not induce significant alterations in cellular and biochemical blood parameters, or any morphological changes in the heart, kidney, lung, spleen or liver tissue. In sum, UCB-MNC-Exo has no significant toxicity in vitro or in vivo, even when administered repeatedly at high concentrations [71].
Stem cell derived exosomes enhance the proliferation and migration of skin fibroblast (FB) and inhibit the production of ROS and MMP. UVB can induce fibroblast senescence, which can be reversed by exosomes; exosomes can enhance proliferation and migration ability of FB through activating MAPK, AKT, STAT3 and ERK1/2 signaling pathways. Senescent FB produce more ROS and MMP, exosomes can inhibit this process and thus alleviate senescence
Based on this, exosomes are providing new hope for anti-aging treatment. However, it is still uncertain if exosomes can completely replace and simulate the function of SCs, and the long-term risk of exosome treatment remains unknown [3]. The separation and purification of exosomes must be further improved [10]. Ultimately, exosomes containing specific contents may be produced in the future for precise treatment. All of these are worthy of further exploration.
References
Farage MA, Miller KW, Elsner P, Maibach HI. Functional and physiological characteristics of the aging skin. Aging Clin Exp Res. 2008;20:195–200.
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68.
Raik S, Kumar A, Bhattacharyya S. Insights into cell-free therapeutic approach: Role of stem cell “soup-ernatant.” Biotechnol Appl Biochem. 2018;65:104–18.
Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83.
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019;8:727–51.
Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–57.
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22.
Patel AN, Vargas V, Revello P, Bull DA. Mesenchymal stem cell population isolated from the subepithelial layer of umbilical cord tissue. Cell Transplant. 2013;22:513–9.
Kim YJ, Yoo SM, Park HH, Lim HJ, Kim YL, Lee S, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun. 2017;493:1102–8.
Fatima F, Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin J Cancer. 2015;34:541–53.
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127.
Lee BR, Kim JH, Choi ES, Cho JH, Kim E. Effect of young exosomes injected in aged mice. Int J Nanomed. 2018;13:5335–45.
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24:1635–47.
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–84.
Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–7.
Watt FM. Mammalian skin cell biology: at the interface between laboratory and clinic. Science. 2014;346:937–40.
Aumailley M. Laminins and interaction partners in the architecture of the basement membrane at the dermal-epidermal junction. Exp Dermatol. 2021;30:17–24.
Walma DAC, Yamada KM. The extracellular matrix in development. Development. 2020;147:dev175596.
Kanaki T, Makrantonaki E, Zouboulis CC. Biomarkers of skin aging. Rev Endocr Metab Disord. 2016;17:433–42.
Tigges J, Krutmann J, Fritsche E, Haendeler J, Schaal H, Fischer JW, et al. The hallmarks of fibroblast ageing. Mech Ageing Dev. 2014;138:26–44.
Zou Z, Long X, Zhao Q, Zheng Y, Song M, Ma S, et al. A single-cell transcriptomic atlas of human skin aging. Dev Cell. 2021;56:383-397.e388.
Doles J, Storer M, Cozzuto L, Roma G, Keyes WM. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 2012;26:2144–53.
Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–9.
Solanas G, Peixoto FO, Perdiguero E, Jardí M, Ruiz-Bonilla V, Datta D, et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell. 2017;170:678-692.e620.
Rübe CE, Bäumert C, Schuler N, Isermann A, Schmal Z, Glanemann M, et al. Human skin aging is associated with increased expression of the histone variant H2AJ in the epidermis. NPJ Aging Mech Dis. 2021;7:7.
Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29.
Kretzschmar K, Watt FM. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold SPRING Harbor Perspect. Med. 2014;4:ao13631.
Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11:221–35.
Zhou J, Dong Y, Liu J, Ren J, Wu J, Zhu N. AQP5 regulates the proliferation and differentiation of epidermal stem cells in skin aging. Braz J Med Biol Res. 2020;53:e10009.
Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Molecular Sci 2018;19:3119–35.
Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120:10847–54.
Zhang Y, Han F, Gu L, Ji P, Yang X, Liu M, et al. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. J Mol Histol. 2020;51:375–83.
Wu P, Zhang B, Han X, Sun Y, Sun Z, Li L, et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging. 2021;13:11542–63.
Duan M, Zhang Y, Zhang H, Meng Y, Qian M, Zhang G. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res Ther. 2020;11:452.
Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–81.
Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdisciplinary Rev Dev Biol. 2018;7:307–26
Diez C, Nestler M, Friedrich U, Vieth M, Stolte M, Hu K, et al. Down-regulation of Akt/PKB in senescent cardiac fibroblasts impairs PDGF-induced cell proliferation. Cardiovasc Res. 2001;49:731–40.
Oh M, Lee J, Kim YJ, Rhee WJ, Park JH. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mole Sci. 2018;19:1715–31.
Wang T, Guo S, Liu X, Xv N, Zhang S. Protective effects of adipose-derived stem cells secretome on human dermal fibroblasts from ageing damages. Int J Clin Exp Pathol. 2015;8:15739–48.
Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU, et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13:11273–82.
Hoang DH, Nguyen TD, Nguyen HP, Nguyen XH, Do PTX, Dang VD, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. 2020;7:119.
Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96–102.
Zhao B, Li X, Shi X, Shi X, Zhang W, Wu G, et al. Exosomal MicroRNAs derived from human amniotic epithelial cells accelerate wound healing by promoting the proliferation and migration of fibroblasts. Stem Cells Int. 2018;2018:5420463.
Zhang Y, Pan Y, Liu Y, Li X, Tang L, Duan M, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res Ther. 2021;12:434.
Li Q, Huang P, Chen W, Bi J. Mechanism of bone marrow mesenchymal stem cells secreting miR-26a exosomes affecting high glucose-induced skin fibroblasts function by regulating TLR4/NF-κB signaling. Inflamm Res. 2021;70:811–21.
Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864:2015–25.
Sárdy M. Role of matrix metalloproteinases in skin ageing. Connect Tissue Res. 2009;50:132–8.
Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mole Sci. 2016;17:868.
Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–9.
Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–6.
Ashcroft GS, Herrick SE, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Human ageing impairs injury-induced in vivo expression of tissue inhibitor of matrix metalloproteinases (TIMP)-1 and -2 proteins and mRNA. J Pathol. 1997;183:169–76.
Jung JY, Shim JH, Choi H, Lee TR, Shin DW. Human dermal stem/progenitor cell-derived conditioned medium improves senescent human dermal fibroblasts. Int J Mol Sci. 2015;16:19027–39.
Shen T, Zheng QQ, Shen J, Li QS, Song XH, Luo HB, et al. Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin Med J. 2018;131:704–12.
Hu P, Chiarini A, Wu J, Freddi G, Nie K, Armato U, et al. Exosomes of adult human fibroblasts cultured on 3D silk fibroin nonwovens intensely stimulate neoangiogenesis. Burns Trauma. 2021;9:tkab003.
Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47.
Labunskyy VM, Gladyshev VN. Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal. 2013;19:1362–72.
Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radical Biol Med. 2013;60:1–4.
Matsuoka T, Takanashi K, Dan K, Yamamoto K, Tomobe K, Shinozuka T. Effects of mesenchymal stem cell-derived exosomes on oxidative stress responses in skin cells. Mol Biol Rep. 2021;48:4527–35.
Zhang S, Dong Z, Peng Z, Lu F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by D-galactose. PLoS One. 2014;9:e97573.
Sun CK, Chen CH, Chang CL, Chiang HJ, Sung PH, Chen KH, et al. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Trans Res. 2017;9:1543–60.
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1–14.
Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, et al. hucMSC exosome-derived GPX1 Is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25:465–79.
Wang Y, Zhao R, Liu D, Deng W, Xu G, Liu W, et al. Exosomes derived from miR-214-enriched bone marrow-derived mesenchymal stem cells regulate oxidative damage in cardiac stem cells by targeting CaMKII. Oxid Med Cell Longev. 2018;2018:4971261.
Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol. 2018;20:307–19.
Park D, Yang G, Bae DK, Lee SH, Yang YH, Kyung J, et al. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J Neurosci Res. 2013;91:660–70.
Rodrigues SC, Cardoso RMS, Gomes CF, Duarte FV, Freire PC, Neves R, et al. Toxicological profile of umbilical cord blood-derived small extracellular vesicles. Membranes 2021;11:647–60
Acknowledgements
The work was supported by Grants from (1) Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16040400); (2) Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (Project No 19441909900); (3) National Natural Science Foundation of China (82172218); 4) Taizhou Technology Support Program (TS202008); (5) National Key Research and Development Program, China (No.2018YFA0108202): (6) National Science Foundation, China (No. 61971410, 61801464 and 61801465).
Author information
Authors and Affiliations
Contributions
Jin-Yan Wu, Li-Ping Zhang and Sai-Nan Wu draft the manuscript, they contributed equally to this work, Ruo-Yue Yuan, Xian-Sheng Zhao, Yue Li, Jian-Lan Liu, Qu-Yang Yang, Hong-Ju Mao and Ning-Wen Zhu participated in the paper modification and generated the figure. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, JY., Wu, SN., Zhang, LP. et al. Stem Cell-Derived Exosomes: A New Method for Reversing Skin Aging. Tissue Eng Regen Med 19, 961–968 (2022). https://doi.org/10.1007/s13770-022-00461-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00461-5