Abstract
Human skin is the largest organ of the body and it provides the first line of the defense system against environmental factors coming in contact by evading our ecosystem. Skin possesses notable regeneration capacity due to the presence of different types of stem cells including epithelial stem cells, melanocyte stem cells, mesenchymal stem-like cells, and progenitor cells. Moreover, the integrity of the skin is mainly maintained by epidermal stem cells. Skin and skin stem cells are more vulnerable toward aging process due to their direct contact with external stimuli including environmental pollutants, infection, and UV irradiation. Aging is a complex and multifactorial process mainly caused by imbalanced redox status, DNA mutation, and telomere shortening. The reactive oxygen species (ROS) overproduction is the major contributor of skin aging as ROS exert oxidative damage to macromolecules and cell organelles, which continuously accumulate and further accelerate aging process. Additionally, UV irradiation induces oxidative stress, overproduction of ROS, and DNA damage which collectively cause photoaging of the skin. This chapter summarizes the overall effects of oxidative stress on skin aging, and several antiaging strategies such as supplementation of nutritional antioxidants and autophagy modulation are also described to slow down the aging process of skin as well as skin diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Skin is the complex and largest organ of the human body that offers protection against environmental stresses and serves as a sensory interface between the body and its surroundings. Human skin is made up of three main layers, viz., the epidermis, dermis, and subcutis. The cells of epidermal layer originate at the basal layer that persistently change their form as they rise to the surface of the skin and are ultimately shed; this is the natural cycle of epidermal layer. Anatomically, epidermal layer can be further divided into four layers, the basal layer, spinous layer, granular layer, and stratum corneum. All these layers made about 0.2-mm-thick, functioning barrier to protect the body from injurious external stimuli (Fisher et al. 1997, 2002; Kang et al. 2001). Skin formation is a continuous process in which new cells are formed at the basal layer and rise up to the stratum corneum. This pattern of cycle is very unique to each individual. Aging of individuals weakens skin metabolism; consequently injuries take a longer time to heal. The slower cyclic pattern of skin leads to the accumulation of dead skin cells on the surface resulting in thin-looking skin with a dull or rough appearance.
The dermal layer consists of hyaluronic acid, collagen, and elastin that are produced from fibroblast cells and help in maintaining skin hydration and firmness. However, this function can gradually decline as a result of skin aging, inflammation, and ultraviolet (UV) radiation exposure. The dermal layer provides firmness and elasticity to the skin. The epidermis is only 0.2 mm thick and comprises about 90% of skin’s thickness. The dermis provides a greater influence on skin firmness and elasticity than the epidermis. The thickness of skin reduces with the progression of age due to loss of dermal collagen. Moreover, UV radiation exaggerates these aging-associated changes which results in coarse wrinkling, pigmentation (solar lentigines), and telangiectasia in UV-exposed areas.
1 Skin Stem Cells
The epidermis constantly self-renewed itself in order to resist the damage caused by their physical, chemical, and biological surroundings. This self-renewal ability of the epidermis is granted by adult stem cells and progenitor cells. Moreover, the dermis is divided into different lineages during embryonic development from morula to blastocyst stage of preimplantation. The first one is the trophectoderm and second is the inner cell mass (Adjaye et al. 2005; Gardner and Beddington 1988). The later one is a supplier of embryonic stem cells which possess self-renewal capability and pluripotency (Thomson et al. 1998). Local stem cells play a significant role in the homeostatic maintenance of many organs and tissues. The best example is the epidermis, in which cellular turnover is high and also has high regenerative potential (Rando 2006). In the adult human body, there are different kinds of stem cells which involve in the renewal of the various cell types in the skin during normal homeostasis or wound repair. Normally, epidermal stem cells and melanoblasts are located in the skin.
Stem Cell Localization in Skin
Different kinds of skin stem cells have been reported in heterogeneous environment of skin (Shi et al. 2006). The epidermal linings of skin are replaced several times in the course of a human lifetime. The undifferentiated stem cells in the basal layer divide continuously and produce differentiated descendants that eventually discarded with the time. Epidermal stem cells have two common features like other stem cells; first, they have self-renewal capacity for extended time periods, and, second, they can form multiple lineages after differentiation (Weissman et al. 2001). The stem cells possess specific cell markers such as p63, β1high/melanoma chondroitin sulfate proteoglycan, and a6high/CD71dim (Suzuki and Senoo 2012; Senoo et al. 2007; Pellegrini et al. 2001).
The multipotent stem cells that produced a series of differentiated cell types in the skin are to be found in a specific area that is adjoining with the epidermis, in the hair follicle, which is called as the bulge (Oshima et al. 2001; Christiano 2004; Morris et al. 2004). The follicle bulge region can develop into hair follicle epithelium, including hair shaft, inner root sheath, and outer root sheath. These cells have specified cellular markers named as Sox9, K15, Lgr5, CD34, Lhx2, NFIB, NFATC1, K15, PHLDA1, K19, CD200, etc. (Liu et al. 2003; Trempus et al. 2003; Jaks et al. 2008; Nowak et al. 2008; Sellheyer and Krahl 2011).
Epidermal Stem Cells
Among a range of skin stem cells, epidermal stem cells are mainly linked to skin regeneration and tissue repair. Several studies suggested that epidermal stem cells are rare and occasionally dividing cells that produce short-lived and fast-dividing cells to accomplish the regeneration of the epidermis. They can be assumed as a key epidermal cell population conscientious for repairing skin injury. Although the majority of epidermal stem cells are resident of the basal layer in the epidermis, a few may also be present in the bulge region of the hair follicle as well as in the base of the sebaceous glands (Watt et al. 2006; Fuchs 2008). Epidermal stem cells, during their entire life cycle, are dispersed in two different cell types; one is the slow-growing cell type, in which epidermal stem cells are dormant. While in another fast-growing cell type, they are fast dividing and produce a large number of cells for the renewal of skin tissue. In the end, they go through several cell divisions prior to terminal differentiation to complete skin renewal. During skin damage, both epidermal stem cells and follicular stem cells play an important role in wound healing (Ito and Cotsarelis 2008; Ito et al. 2005; Taylor et al. 2000). At the time of injury, in the wounded region, hair follicle-derived epidermal stem cells along with progenitor cells start to drift toward the wound spot. Thereafter, epidermal stem cells reactivate response to skin damage and assist to skin rejuvenation at the cellular level (Langton et al. 2008).
Melanocyte Stem Cells
Melanocyte stem cells present in the skin are a supplier of transient fast-growing cells and differentiated melanocytes. There is little known about melanocyte stem cells and their function. The first time, a functional role (niche) for melanocyte stem cells has been reported in the bulge region of the hair follicle, also explained as the lower permanent position (Nishimura et al. 2002; Nishikawa and Osawa 2007). The stem cells are regulated by their microenvironment which includes other adjacent cells, secretary signaling proteins, and scaffold proteins of the extracellular matrix. The niche is very helpful for the population of the stem cell to evade the loss of the stem cell pools; in addition they are promoting dormancy so as to inhibit the overgrowth of cells. Stem cells as well as supportive cells within the niche may show overexpression of adhesion and extracellular matrix proteins including integrins or cadherins for the homeostasis of the cell (Raymond et al. 2009).
Mesenchymal Stem Cell-Like Cells and Neural Progenitor Cells
Mesenchymal stem cell-like cells are present at the dermis, and after division, they form mesodermal parts and some cells of the neural system. They are CD34 negative and CD105, CD90, as well as CD70-specific positive surface markers (Garzón et al. 2013). Moreover, the follicle dermal papillae are the source of neural progenitor cells that can split into the glial and neural lineage, and like other organs or tissues, they shared similar cell markers such as nestin.
Hematopoietic Stem Cells
These cells are situated at the follicle dermal papillae, and after differentiation, they form myeloid and erythroid cell lineages and express similar surface markers like in other organs or tissues.
Shortening of the Telomere, Loss of the Stem Cells, and Skin Aging
Shortening of the telomere is a characteristic of aging which is manifested by the congregation of gene and DNA damage during cell divisions. On the basis of previous findings, it is clear that the nuclease, transferase, and polymerase enzyme activities of telomerase are accountable for the protection of telomeres against programmed cell death in response to DNA damage (Blasco 2005). However, the mechanisms behind the shortening of telomere in skin stem cell aging remain unknown (Counter et al. 2003; Friedrich et al. 2000; Nakamura et al. 2002). Skin contains adequate levels of telomerase enzyme and it is active only in few skin stem cells, basal epidermal cells (Bickenbach et al. 1998; Engelhardt et al. 1997; Taylor et al. 1996), the bulge component of the hair follicle, and keratinocytes (Boukamp 2005; Ramirez et al. 1997). Human epithelial cells or fibroblasts have low or sometimes no telomerase protein (Funk et al. 2000; Nakano et al. 1998; Zouboulis 2003). In dyskeratosis congenital patients, skin shows symptoms like early hair loss, hair graying, poor nail growth, and skin atrophy which are related to shorting of telomeres that lead to defects in proliferation and function of skin stem cells and poor healing of the wound (Mason et al. 2005; Westin et al. 2007).
Consequently, germline and stem cells evolve mechanisms to prevent telomere abrasion and protect them from senescence. In this mechanism, telomerase gets activated, in which TERT, TERC, and dyskerin (DKC) are involved, and can extend the end of the chromosome with specific telomeric DNA sequences (Tomás-Loba et al. 2008; Collins and Mitchell 2002).
Estrogen, Stem Cells, and Skin Aging
Estrogens have a multifunction role in skin aging; it can prevent the loss of collagen, augment the skin thickness, reinstate skin moisture, stop hair loss, as well as improve the wound healing (Brincat 2000; Azzi et al. 2005). Estrogen acts through the estrogen receptors (ERs) and can directly regulate fibroblast function. For collagen production, estrogen increases TGF production by fibroblasts (Ashcroft et al. 1997). Furthermore, estrogen can overturn epidermal atrophy by means of stimulation of the cell proliferation and synthesis of DNA in keratinocytes (Urano et al. 1995). Between menopause and the age of 60 years, the estrogen level 17-E2, DHEA, and progesterone rapidly fall and then exhibit a lower level plateau afterward (Morley 2001; Phillips et al. 2001).
It has been established that estrogen suppresses keratinocyte apoptosis. It also activates cell cycle checkpoint protein cyclin D2, predominantly by means of contact with a cell surface receptor GPR30, that further activates cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway (Kanda and Watanabe 2004).
Estrogen has the ability to affect skin directly but its mechanism of action in regulation of skin progenitor cells is not very clear. On the other hand, at present, the role of estrogen in the regulation of different types of stem cells has already been made known. The alterations in hormone production with age play critical roles in the instigation of skin aging but the exact mechanisms involving skin stem cells as well as hormone deficiency remain unknown.
Reactive Oxygen Species (ROS), Stem Cells, and Skin Aging
Excess of xanthine oxidase and nitric oxide synthase in tissues, under pathological stress conditions, produces heavy endogenous ROS. ROS production is drastically amplified after cigarette smoke, exposure to UV irradiation, and other abuse (Kohen 1999). It is very exciting that ROS mainly produced in mitochondria, as well as other cell organelles, play a crucial role in aging (Chance et al. 1979). In other studies, the exposure of UV irradiation to human skin fibroblasts increases oxidative stress and also increase the expression of signaling molecules p16INK4A and p53/p21WAF1/CIP1 that further cause the early senescence of fibroblasts and the conversion of the fibroblast into myofibroblast (Chen et al. 2008; Ruiter et al. 2002). It is well established that repeated exposure to solar UV irradiation is the principal environmental factor for acceleration of skin aging process. In a study, skin samples were collected from sunlight exposure and sunlight-protected aged individuals and showed that the number of keratinocyte stem cells (KSCs) was considerably lower in photoaged than in the sun-protected skin (Kwon et al. 2008; Rass and Reichrath 2008).
UV Radiation and Photoaging of Skin
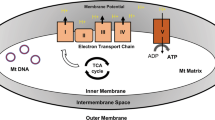
Among the radiation UV radiation is one of natural mutagens liable for the leading proportion of environmentally induced skin diseases, including inflammation and erythema, skin cancer, and age-related changes (Elwood and Jopson 1997). The UV exposure leads to excessive ROS production such as superoxide, hydrogen peroxide, hydroxyl radical, and singlet oxygen which are main contributors to the skin aging (Fig. 1). The effect of UV-induced oxidative stress on the skin aging is usually termed as photoaging. The UVA light mainly alters the physiology of dermis which is accountable for the progression of photoaging (Krutmann and Schroeder 2009). The UV-induced photoaging process also includes oxidative damage of DNA, especially the mtDNA (Berneburg et al. 1997). However, UVA and UVB affect only epidermal cells due to their limited penetration ability and thus contribute to the photoaging process (Krutmann and Schroeder 2009).
Reactive oxygen species (ROS) produced from different sources initiate signaling pathways that contribute to change in skin structures and photoaging. The overproduction of ROS results in imbalanced redox status and oxidative stress which cause damage to various cellular macromolecules and eventually result in the development of photoaging of the skin. Abbreviations: IL interleukin, TNF-a tumor necrosis factor-a, VEGF-A vascular endothelial growth factor-A, MAPK multiple mitogen-activated protein kinase, JNK c-Jun N-terminal kinase, and MMP matrix metalloproteinases
Thus, UV radiation affects the cell’s macromolecules adversely through the production of ROS and other reactive free radicals (Meyskens et al. 2001). Increase in UV radiation in the environment might be an important factor for increasing incident of skin cancer and melanoma from last several years (Armstrong and Kricker 2001; Garbe and Leiter 2009). In childhood, UV protection is very important because it induces deposition of DNA damage in adulthood and leads to skin cancer. There is the significant skin’s anatomical difference between children and adults that makes easier for UV penetration and increases the risk of melanoma with excessive sun exposure before age of 10 years (Volkmer and Greinert 2011). Photoaging of skin is a combined effect of environmental and genetic factors and is mainly predisposed by exposure of UV radiation.
Skin stem cells are also susceptible to various genetic changes caused by harmful agents such as UV radiation; as a result tumor formation may occur (Perez-Losada and Balmain 2003). Interestingly, it has also been demonstrated that cyclobutane pyrimidine dimer (CPD) accumulates in epidermal basal cells upon chronic low-level UVB exposure. CPD-retaining basal cells (CRBCs) were also observed in human skin receiving sporadic sunlight exposure (Mitchell et al. 2001). Thus, CRBCs become targets for UV-induced skin cancer due to accumulation of DNA damage. On the other hand, the specific role of CRBCs in skin cancer induced by UV exposure has not yet been well documented.
2 Current Antiaging Strategies to Slow Down Skin Aging
During last couple of decades, a number of antiaging methods have been developed to slow down skin aging. The antiaging strategies include particularly preventive measurements, cosmetics, topical and systemic therapeutic agents, as well as invasive procedures. Since it is well documented that aging process is the consequent of overproduction of free radicals, nutritional supplements containing antioxidants are the best remedies for aging as they scavenge free radicals and defend the cells from oxidative injure. These nutritional antioxidants comprise of lipoic acid, coenzyme Q, carotenoids, vitamin C, and vitamin E and trace elements including selenium and copper (Fusco et al. 2007; Marini 2011; Berger 2005). There are two safeguards for the skin, endogenous antioxidant (enzymatic antioxidants and synthesis of melanin) and exogenous antioxidant (which we eat in the food). UV-induced photoaging is an outcome of the failure of endogenous anti-oxidative repair processes. After this incident, the skin becomes clinically explicit with age, and functional harm to the skin prevails. At the moment, it becomes necessary to consume supplementary antioxidants or to apply them on the skin in topical preparations (Poljsak et al. 2013).
Vitamin C is a water-soluble vitamin and presents in the skin predominantly that helps in protection of aqueous phase of the cells. Additionally, vitamin E protects the lipid portion of cell membranes and stratum corneum (Thiele 2001). The main concerns about the use of antioxidants include its compatibility, product stability, absorption inside the skin, and activation at the target spot. In the future, exact dosing and administration route will be more important for the use of an antioxidant to give more drug-like effect. UV-induced early photoaging, wrinkling, and pigmentary changes are measured as the most significant cutaneous manifestations. The antiaging strategies against UV-induced photoaging include the use of antioxidants, sunscreens to reduce UV exposure, and retinoids to promote collagen production. Interestingly, combinatorial use of several strategies is the most powerful approach against UV-induced photoaging (Baumann 2007; Trautinger 2001).
Recently, the modulation of autophagy process has been shown to be an effective antiaging strategy against skin aging (Scherfer et al. 2013). Autophagy is a well-conserved cellular process accountable for the continuous removal of oxidatively damaged macromolecules and cell organelles (Singh et al. 2017). The keratinocytes with defective autophagy demonstrate augmented DNA damage, senescence, and anomalous change in lipid profile after oxidative stress (Song et al. 2017). Since autophagy has been demonstrated to regulate skin stem cells, slow down aging process, and deal with oxidative stress and microbial infection, the use of autophagy modulators provides a promising therapeutic strategy for alleviating skin aging and skin diseases (Li et al. 2016). Skin tissue engineering is another novel strategy which provides alternative regenerative medicinal approach for possible management of skin-related problems (Behera et al. 2017).
3 Conclusion
This chapter aims to give an overview of various types of stem cells found in the skin and their role in architecture and function of the skin. Moreover, the role of several factors including telomere shortening, UV irradiation, and oxidative stress in the general pathophysiology of the aging process of skin has also been critically reviewed. The process of aging in the skin is mainly driven by overproduction of reactive oxygen species which eventually lead to oxidative damage of macromolecules and cell organelles. Several antiaging strategies including nutritional antioxidant supplementation, autophagy modulation, and skin tissue engineering have also been discussed to slow down the process of aging in skin and skin diseases.
References
Adjaye, J., Huntriss, J., Herwig, R., et al. (2005). Primary differentiation in the human blastocyst: Comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells, 23, 1514–1525. https://doi.org/10.1634/stemcells.2005-0113.
Armstrong, B. K., & Kricker, A. (2001). The epidemiology of UV induced skin cancer. Journal of Photochemistry and Photobiology B, 63, 8–18.
Ashcroft, G. S., Dodsworth, J., van Boxtel, E., et al. (1997). Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nature Medicine, 3, 1209–1215.
Azzi, L., El-Alfy, M., Martel, C., & Labrie, F. (2005). Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. The Journal of Investigative Dermatology, 124, 22–27. https://doi.org/10.1111/j.0022-202X.2004.23545.x.
Baumann, L. (2007). Skin ageing and its treatment. The Journal of Pathology, 211, 241–251. https://doi.org/10.1002/path.2098.
Behera, S. S., Das, U., Kumar, A., et al. (2017). Chitosan/TiO2composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. International Journal of Biological Macromolecules, 98, 329–340. https://doi.org/10.1016/j.ijbiomac.2017.02.017.
Berger, M. M. (2005). Can oxidative damage be treated nutritionally? Clinical Nutrition, 24, 172–183. https://doi.org/10.1016/j.clnu.2004.10.003.
Berneburg, M., Gattermann, N., Stege, H., et al. (1997). Chronically ultraviolet-exposed human skin shows a higher mutation frequency of mitochondrial DNA as compared to unexposed skin and the hematopoietic system. Photochemistry and Photobiology, 66, 271–275.
Bickenbach, J. R., Vormwald-Dogan, V., Bachor, C., et al. (1998). Telomerase is not an epidermal stem cell marker and is downregulated by calcium. The Journal of Investigative Dermatology, 111, 1045–1052. https://doi.org/10.1046/j.1523-1747.1998.00420.x.
Blasco, M. A. (2005). Telomeres and human disease: Ageing, cancer and beyond. Nature Reviews Genetics, 6, 611–622. https://doi.org/10.1038/nrg1656.
Boukamp, P. (2005). Skin aging: A role for telomerase and telomere dynamics? Current Molecular Medicine, 5, 171–177.
Brincat, M. P. (2000). Hormone replacement therapy and the skin: Beneficial effects: The case in favor of it. Acta Obstetricia et Gynecologica Scandinavica, 79, 244–249.
Chance, B., Sies, H., & Boveris, A. (1979). Hydroperoxide metabolism in mammalian organs. Physiological Reviews, 59, 527–605. https://doi.org/10.1152/physrev.1979.59.3.527.
Chen, W., Kang, J., Xia, J., et al. (2008). p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. International Journal of Molecular Medicine, 21, 645–653.
Christiano, A. M. (2004). Epithelial stem cells: Stepping out of their niche. Cell, 118, 530–532. https://doi.org/10.1016/j.cell.2004.08.024.
Collins, K., & Mitchell, J. R. (2002). Telomerase in the human organism. Oncogene, 21, 564–579. https://doi.org/10.1038/sj.onc.1205083.
Counter, C. M., Press, W., & Compton, C. C. (2003). Telomere shortening in cultured autografts of patients with burns. Lancet, 361, 1345–1346. https://doi.org/10.1016/S0140-6736(03)13042-5.
Elwood, J. M., & Jopson, J. (1997). Melanoma and sun exposure: An overview of published studies. International Journal of Cancer, 73, 198–203.
Engelhardt, M., Kumar, R., Albanell, J., et al. (1997). Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood, 90, 182–193.
Fisher, G. J., Wang, Z. Q., Datta, S. C., et al. (1997). Pathophysiology of premature skin aging induced by ultraviolet light. The New England Journal of Medicine, 337, 1419–1428. https://doi.org/10.1056/NEJM199711133372003.
Fisher, G. J., Kang, S., Varani, J., et al. (2002). Mechanisms of photoaging and chronological skin aging. Archives of Dermatology, 138, 1462–1470.
Friedrich, U., Griese, E., Schwab, M., et al. (2000). Telomere length in different tissues of elderly patients. Mechanisms of Ageing and Development, 119, 89–99.
Fuchs, E. (2008). Skin stem cells: Rising to the surface. The Journal of Cell Biology, 180, 273–284. https://doi.org/10.1083/jcb.200708185.
Funk, W. D., Wang, C. K., Shelton, D. N., et al. (2000). Telomerase expression restores dermal integrity to in vitro-aged fibroblasts in a reconstituted skin model. Experimental Cell Research, 258, 270–278. https://doi.org/10.1006/excr.2000.4945.
Fusco, D., Colloca, G., Lo Monaco, M. R., & Cesari, M. (2007). Effects of antioxidant supplementation on the aging process. Clinical Interventions in Aging, 2, 377–387.
Garbe, C., & Leiter, U. (2009). Melanoma epidemiology and trends. Clinics in Dermatology, 27, 3–9. https://doi.org/10.1016/j.clindermatol.2008.09.001.
Gardner, R. L., & Beddington, R. S. (1988). Multi-lineage “stem” cells in the mammalian embryo. Journal of Cell Science, 10, 11–27.
Garzón, I., Miyake, J., González-Andrades, M., et al. (2013). Wharton’s jelly stem cells: A novel cell source for oral mucosa and skin epithelia regeneration. Stem Cells Translational Medicine, 2, 625–632. https://doi.org/10.5966/sctm.2012-0157.
Ito, M., & Cotsarelis, G. (2008). Is the hair follicle necessary for normal wound healing? The Journal of Investigative Dermatology, 128, 1059–1061. https://doi.org/10.1038/jid.2008.86.
Ito, M., Liu, Y., Yang, Z., et al. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Medicine, 11, 1351–1354. https://doi.org/10.1038/nm1328.
Jaks, V., Barker, N., Kasper, M., et al. (2008). Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genetics, 40, 1291–1299. https://doi.org/10.1038/ng.239.
Kanda, N., & Watanabe, S. (2004). 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. The Journal of Investigative Dermatology, 123, 319–328. https://doi.org/10.1111/j.0022-202X.2004.12645.x.
Kang, S., Fisher, G. J., Voorhees, J. J. (2001). Photoaging: Pathogenesis, prevention, and treatment. Clinics in Geriatric Medicine, 17, 643–659, v–vi.
Kohen, R. (1999). Skin antioxidants: Their role in aging and in oxidative stress–new approaches for their evaluation. Biomedecine Pharmacotheraphy, 53, 181–192. https://doi.org/10.1016/S0753-3322(99)80087-0.
Krutmann, J., & Schroeder, P. (2009). Role of mitochondria in photoaging of human skin: The defective powerhouse model. The Journal of Investigative Dermatology. Symposium Proceedings, 14, 44–49. https://doi.org/10.1038/jidsymp.2009.1.
Kwon, O. S., Yoo, H. G., Han, J. H., et al. (2008). Photoaging-associated changes in epidermal proliferative cell fractions in vivo. Archives of Dermatological Research, 300, 47–52. https://doi.org/10.1007/s00403-007-0812-3.
Langton, A. K., Herrick, S. E., & Headon, D. J. (2008). An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. The Journal of Investigative Dermatology, 128, 1311–1318. https://doi.org/10.1038/sj.jid.5701178.
Li, L., Chen, X., & Gu, H. (2016). The signaling involved in autophagy machinery in keratinocytes and therapeutic approaches for skin diseases. Oncotarget, 7, 50682–50697. https://doi.org/10.18632/oncotarget.9330.
Liu, Y., Lyle, S., Yang, Z., & Cotsarelis, G. (2003). Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. The Journal of Investigative Dermatology, 121, 963–968. https://doi.org/10.1046/j.1523-1747.2003.12600.x.
Marini, A. (2011). Beauty from the inside. Does it really work? Hautarzt Z Dermatol Venerol Verwandte Geb, 62, 614–617. https://doi.org/10.1007/s00105-011-2138-5.
Mason, P. J., Wilson, D. B., & Bessler, M. (2005). Dyskeratosis congenita – a disease of dysfunctional telomere maintenance. Current Molecular Medicine, 5, 159–170.
Meyskens, F. L., Farmer, P., & Fruehauf, J. P. (2001). Redox regulation in human melanocytes and melanoma. Pigment Cell Research, 14, 148–154.
Mitchell, D. L., Volkmer, B., Breitbart, E. W., et al. (2001). Identification of a non-dividing subpopulation of mouse and human epidermal cells exhibiting high levels of persistent ultraviolet photodamage. The Journal of Investigative Dermatology, 117, 590–595. https://doi.org/10.1046/j.0022-202x.2001.01442.x.
Morley, J. E. (2001). Androgens and aging. Maturitas, 38, 61–71. discussion 71–73.
Morris, R. J., Liu, Y., Marles, L., et al. (2004). Capturing and profiling adult hair follicle stem cells. Nature Biotechnology, 22, 411–417. https://doi.org/10.1038/nbt950.
Nakamura, K.-I., Izumiyama-Shimomura, N., Sawabe, M., et al. (2002). Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. The Journal of Investigative Dermatology, 119, 1014–1019. https://doi.org/10.1046/j.1523-1747.2002.19523.x.
Nakano, K., Watney, E., & McDougall, J. K. (1998). Telomerase activity and expression of telomerase RNA component and telomerase catalytic subunit gene in cervical cancer. The American Journal of Pathology, 153, 857–864. https://doi.org/10.1016/S0002-9440(10)65627-1.
Nishikawa, S.-I., & Osawa, M. (2007). Generating quiescent stem cells. Pigment Cell Research, 20, 263–270. https://doi.org/10.1111/j.1600-0749.2007.00388.x.
Nishimura, E. K., Jordan, S. A., Oshima, H., et al. (2002). Dominant role of the niche in melanocyte stem-cell fate determination. Nature, 416, 854–860. https://doi.org/10.1038/416854a.
Nowak, J. A., Polak, L., Pasolli, H. A., & Fuchs, E. (2008). Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell, 3, 33–43. https://doi.org/10.1016/j.stem.2008.05.009.
Oshima, H., Rochat, A., Kedzia, C., et al. (2001). Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell, 104, 233–245.
Pellegrini, G., Dellambra, E., Golisano, O., et al. (2001). p63 identifies keratinocyte stem cells. Proceedings of the National Academy of Sciences of the United States of America, 98, 3156–3161. https://doi.org/10.1073/pnas.061032098.
Perez-Losada, J., & Balmain, A. (2003). Stem-cell hierarchy in skin cancer. Nature Reviews Cancer, 3, 434–443. https://doi.org/10.1038/nrc1095.
Phillips, T. J., Demircay, Z., & Sahu, M. (2001). Hormonal effects on skin aging. Clinics in Geriatric Medicine, 17, 661–672. vi.
Poljsak, B., Dahmane, R., & Godic, A. (2013). Skin and antioxidants. Journal of Cosmetic and Laser Therapy, 15, 107–113. https://doi.org/10.3109/14764172.2012.758380.
Ramirez, R. D., Wright, W. E., Shay, J. W., & Taylor, R. S. (1997). Telomerase activity concentrates in the mitotically active segments of human hair follicles. The Journal of Investigative Dermatology, 108, 113–117.
Rando, T. A. (2006). Stem cells, ageing and the quest for immortality. Nature, 441, 1080–1086. https://doi.org/10.1038/nature04958.
Rass, K., & Reichrath, J. (2008). UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Advances in Experimental Medicine and Biology, 624, 162–178. https://doi.org/10.1007/978-0-387-77574-6_13.
Raymond, K., Deugnier, M.-A., Faraldo, M. M., & Glukhova, M. A. (2009). Adhesion within the stem cell niches. Current Opinion in Cell Biology, 21, 623–629. https://doi.org/10.1016/j.ceb.2009.05.004.
Ruiter, D., Bogenrieder, T., Elder, D., & Herlyn, M. (2002). Melanoma-stroma interactions: Structural and functional aspects. The Lancet Oncology, 3, 35–43.
Scherfer, C., Han, V. C., Wang, Y., et al. (2013). Autophagy drives epidermal deterioration in a Drosophila model of tissue aging. Aging, 5, 276–287. https://doi.org/10.18632/aging.100549.
Sellheyer, K., & Krahl, D. (2011). PHLDA1 (TDAG51) is a follicular stem cell marker and differentiates between morphoeic basal cell carcinoma and desmoplastic trichoepithelioma. The British Journal of Dermatology, 164, 141–147. https://doi.org/10.1111/j.1365-2133.2010.10045.x.
Senoo, M., Pinto, F., Crum, C. P., & McKeon, F. (2007). p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell, 129, 523–536. https://doi.org/10.1016/j.cell.2007.02.045.
Shi, C., Zhu, Y., Su, Y., & Cheng, T. (2006). Stem cells and their applications in skin-cell therapy. Trends in Biotechnology, 24, 48–52. https://doi.org/10.1016/j.tibtech.2005.11.003.
Singh, A. K., Kashyap, M. P., Tripathi, V. K., et al. (2017). Neuroprotection through Rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB Signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and Neurodegeneration in adult rats. Molecular Neurobiology, 54, 5815–5828. https://doi.org/10.1007/s12035-016-0129-3.
Song, X., Narzt, M. S., Nagelreiter, I. M., et al. (2017). Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biology, 11, 219–230. https://doi.org/10.1016/j.redox.2016.12.015.
Suzuki, D., & Senoo, M. (2012). Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors. The Journal of Investigative Dermatology, 132, 2461–2464. https://doi.org/10.1038/jid.2012.165.
Taylor, R. S., Ramirez, R. D., Ogoshi, M., et al. (1996). Detection of telomerase activity in malignant and nonmalignant skin conditions. The Journal of Investigative Dermatology, 106, 759–765.
Taylor, G., Lehrer, M. S., Jensen, P. J., et al. (2000). Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell, 102, 451–461.
Thiele, J. J. (2001). Oxidative targets in the stratum corneum. A new basis for antioxidative strategies. Skin Pharmacology and Applied Skin Physiology, 14(Suppl 1), 87–91. https://doi.org/10.1159/000056395.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147.
Tomás-Loba, A., Flores, I., Fernández-Marcos, P. J., et al. (2008). Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell, 135, 609–622. https://doi.org/10.1016/j.cell.2008.09.034.
Trautinger, F. (2001). Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clinical and Experimental Dermatology, 26, 573–577.
Trempus, C. S., Morris, R. J., Bortner, C. D., et al. (2003). Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. The Journal of Investigative Dermatology, 120, 501–511. https://doi.org/10.1046/j.1523-1747.2003.12088.x.
Urano, R., Sakabe, K., Seiki, K., & Ohkido, M. (1995). Female sex hormone stimulates cultured human keratinocyte proliferation and its RNA- and protein-synthetic activities. Journal of Dermatological Science, 9, 176–184.
Volkmer, B., & Greinert, R. (2011). UV and children’s skin. Progress in Biophysics and Molecular Biology, 107, 386–388. https://doi.org/10.1016/j.pbiomolbio.2011.08.011.
Watt, F. M., Lo Celso, C., & Silva-Vargas, V. (2006). Epidermal stem cells: An update. Current Opinion in Genetics & Development, 16, 518–524. https://doi.org/10.1016/j.gde.2006.08.006.
Weissman, I. L., Anderson, D. J., & Gage, F. (2001). Stem and progenitor cells: Origins, phenotypes, lineage commitments, and transdifferentiations. Annual Review of Cell and Developmental Biology, 17, 387–403. https://doi.org/10.1146/annurev.cellbio.17.1.387.
Westin, E. R., Chavez, E., Lee, K. M., et al. (2007). Telomere restoration and extension of proliferative lifespan in dyskeratosis congenita fibroblasts. Aging Cell, 6, 383–394. https://doi.org/10.1111/j.1474-9726.2007.00288.x.
Zouboulis, C. C. (2003). Intrinsic skin aging. A critical appraisal of the role of hormones. Hautarzt Z Dermatol Venerol Verwandte Geb, 54, 825–832. https://doi.org/10.1007/s00105-003-0581-7.
Acknowledgments
S. S. Tripathi would like to acknowledge DSKPDF scheme of University Grants Commission, New Delhi, India, for providing financial support (F.4-2/2006(BSR)/BL/17-18/0381).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tripathi, S.S., Singh, S., Singh, A.K. (2019). Human Skin Stem Cells, Aging, and Possible Antiaging Strategies. In: Dwivedi, A., Agarwal, N., Ray, L., Tripathi, A. (eds) Skin Aging & Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-13-2541-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-13-2541-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2540-3
Online ISBN: 978-981-13-2541-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)