Abstract
The current study evaluates the abundance and characteristics (shape, color and chemical composition) of microplastics (MPs) of the Tijuana River Basin, a binational river system shared by Mexico and the USA and located in the northwest corner of Mexico. The investigation was conducted on microplastics in the riverbed and river shoreline of 11 sites along the main tributaries of the Tijuana River. The median abundance of riverbed and river shoreline sediments was 118 and 183 MPs kg−1, respectively. The dominant shape found was fiber (65%) followed by fragments (19%) and films (10%); the predominant identified polymers were PP polypropylene (46%), PE polyethylene (34.3%) and PS polystyrene (11.4), with lower numbers of polyacrylic, polyamide (PA) and polyethylene terephthalate (PET). Eleven colors were recorded, the most abundant being black (43.7%) and transparent (22.6%). Wastewater treatment plant (WWTP) discharges, littering and inappropriate or insufficient solid waste management are the main sources of MPs. The results highlighted the widespread distribution of MPs in the Tijuana River Basin sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2019, approximately 460 million tons (Mt) of plastic was produced, including the resins used for textile fibers, and it is estimated that plastic use will almost triple by 2060 (OECD 2022a). According to a recent study, 22 Mt of plastics were released into the environment in 2019, comprising 88% macroplastics and 12% microplastics (MPs). Moreover, the estimated amounts of plastic accumulated in oceans and rivers globally were 30 and 109 Mt, respectively (OECD 2022b). On the other hand, the plastic recycling rate worldwide is approximately 9% (Wen et al. 2021), varying from country to country. For example, developed economies that encourage recycling have a recycling rate of approximately 30%, and developing countries with a minimum level of industrialization have a recycling rate of approximately 0% (d’Ambrières 2019). In Mexico, the recycling rate is approximately 6% (Calderon 2022). However, the most notable circular economy in Mexico involves the recycling of PET bottles, for which the recycling rate is approximately 60% (Ángeles-Hurtado et al. 2021).
Microplastics are classified as primary or secondary according to their mode of generation. The first consists of small particles designed for commercial use, for example, the microbeads or fragments used in personal care products, the spheres used as virgin material in molding processes and spheres for shot blasting (Eerkes-Medrano et al. 2015; Gregory 1996). Although these products have been banned in the USA (Kettenmann 2016), they are still used in body and face scrubs, shampoos and toothpaste in Mexico. Secondary microplastics result from the fragmentation and environmental degradation of larger plastic items (Cooper and Corcoran 2010), for example, films produced by the fragmentation of bags and wraps (Nor and Obbard 2014).
Rivers and urban runoff have been identified as essential transport routes by which microplastics are conveyed from land to the marine environment (Depledge et al. 2013; Ballent et al. 2016; Piñon-Colin et al. 2020). It is estimated that 80% of the microplastics found in the marine environment originate from land-based sources, which include stormwater runoff, rivers, discharges from wastewater treatment plants, road marking paints, sewage, tire wear and tourism activities (Yonkos et al. 2014; Browne 2015; Avio et al. 2016; Horton et al. 2017). One of these land-based sources is effluent from wastewater treatment plants (WWTPs), which contains fibers from laundry wastewater and microspheres from personal care products (Browne et al. 2011; Hoellein et al. 2017; McCormick and Hoellein 2016; Habib et al. 1996; Zubris and Richards 2005). The amount of microplastics in washing machine effluent has been estimated to be between 8000 and 35,500 fibers/L (Dris et al. 2018; De Falco et al. 2018). The microplastic abundance in WWTPs was found to be between 0.3 and 14,000 particles L−1 (Lin et al. 2018; Mintenig et al. 2017). Recently, Ramírez-Álvarez et al. (2020) found microplastic abundance in WWTP effluent from Ensenada, Mexico, between 81 and 1556 pp/m3, the most abundant being microplastic fibers, which accounted for 65% of the total microplastic content. Treated industrial wastewater is another source of microplastics in receiving water. Although these are highly dependent on industry type, Chan et al. (2020) reported a mean concentration of 362.6 ± 24.5 fibers L−1 in a textile factory. Wang et al. (2020) sampled effluents from 5 industrial wastewater treatment plants and wastewater from 10 industrial plants including several types of industries; the microplastic abundances ranged between 6 and 12 and 8–23 MPs L−1, respectively. Another microplastic source in urban wastewater is hospital effluent. Kamani et al. (2023) reported a microplastic abundance of 15 MPs L−1 in hospital effluent. More alarmingly, MPs from hospital wastewater have been identified as vectors that help antibiotic-resistant microorganism diffuse across long distances (Tuvo et al. 2023). However, no direct hospital wastewater discharge occurs to streams in the Tijuana River Basin. Stormwater runoff has also been identified as a critical microplastic source for rivers. Piñon-Colin et al. (2020) reported median microplastic abundances in stormwater samples from industrial and residential land use sites of 66 and 191 particles L−1, respectively, with estimated annual microplastic loads of 8 × 105 and 3 × 106 particles ha−1, respectively.
Microplastic abundance in rivers or freshwater systems has been linked to urbanization (De Carvalho et al. 2021; Huang et al. 2021; Su et al. 2020), population density (Sekudewicz et al. 2021; Kataoka et al. 2019; Fan et al. 2019) and other specific land cover types, such as industrial land use (Fan et al. 2019; Klein et al. 2015; Liu et al. 2020; Deng et al. 2020). Microplastic abundance in freshwater systems has also been linked to inappropriate and insufficient solid waste management and littering (Akindele et al. 2019; Battulga et al. 2019; Barcelo and Pico 2020).
Considering river dynamics is important for determining the microplastic abundance in river sediments. In areas where the river flow is high, microplastics are transported as fine particles, such as silt and clay. However, in slow-moving rivers, microplastics likely sink along with other sediment particles (Horton and Dixon 2017). This phenomenon is common in rivers located in semiarid areas where wet and dry seasons produce marked differences in the flow of currents and in the MP concentration in sediments (Nel et al. 2018; Xia et al. 2021). Higher concentrations of microplastics occur in the sediments due to low turbulence during low river flow. This promotes MP sedimentation, while high flows lead to resuspension. River sediments are becoming temporary microplastic sinks (He et al. 2020a). Several authors have indicated that microplastic abundance is related to fine river particles (Corcoran et al. 2019; He et al. 2020a).
Microplastics have been linked to several environmental impacts. Due to their small size, these particles can be ingested by many marine and terrestrial organisms (Wright et al. 2013; Cheung et al. 2018; Alvarez-Andrade et al. 2023; Thrift et al. 2022). Other researchers have shown other impacts on marine organisms, such as reduced fecundity, survival rate and hatching success (Cole et al. 2014, 2015; Devriese et al. 2015). Moreover, the presence of microplastics in the food chain and their ubiquity in the environment are risks to human health (Yee et al. 2021). Several studies have shown that micro- and nanoplastics can cause serious adverse effects on the human body. These include inflammation (Brown et al. 2001; Deng et al. 2017), oxidative stress and apoptosis (Inkielewicz-Stepniak et al. 2018; Paget et al. 2015), and metabolic homeostasis (Xia et al. 2016; Jin et al. 2019). Recently, MPs have been detected in lung tissue (Jenner et al. 2022), blood (Leslie et al. 2022), human placenta (Ragusa et al. 2021), feces (Zhang et al. 2021), breastmilk (Ragusa et al. 2022), urine (Pironti et al. 2023) and sputum (Huang et al. 2022). MPs originating from stool and urine are treated in WWTPs. The impact of microplastics on bacteria has been studied recently. For example, Yi et al. (2021) reported that microplastics have an impact on the growth of certain bacteria, such as Escherichia coli. However, recent studies have shown that microplastics in sediments are suitable substrates for biofilm formation. Wu et al. (2020b) reported that the microbial communities in microplastics differ from those in sediments or water and are enriched in specific types of bacteria. Moreover, the authors described the abundance of potentially pathogenic bacteria such as Pseudomonas and Bacillus. Therefore, MPs can be regarded as a new microbial niche in the environment (Yang et al. 2020).
Therefore, it is essential to know the degree to which microplastic contamination exists in different environmental compartments. There are few studies on microplastic abundance in rivers in Mexico and Latin America and none in the border region between the USA and Mexico, where the consumption habits of the population and environmental challenges are different from those in the rest of Mexico. Moreover, there are no published studies on the concentration of microplastics in streams where the flow is made up almost entirely of treated wastewater or urban runoff from informal settlements. In this study, we aimed to quantify and characterize microplastic pollution in the Mexican region of the Tijuana River Basin.
Materials and methods
Study area and sampling sites
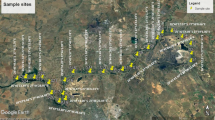
The USA and Mexico share the Tijuana River Basin, which encompasses a watershed area of 4430 km2, approximately 72% of which is in Mexico. The US part of the basin is rural, while in Mexico, the river passes through two cities, Tijuana (1,922,523 inhabitants) and Tecate (108,440 inhabitants) (Inegi 2020). The climate of this region is classified as Mediterranean, with wet winters and hot and dry summers, with annual precipitation ranging from 150 to 500 mm and average annual temperatures ranging between 10 and 16 °C (SDSU-COLEF 2005). The Tijuana River Basin has two main tributaries. The first is Alamar Creek, whose source is in the US Laguna Mountains, where water is received from Cottonwood Creek, Pine Creek and the Tecate River. The other main tributary is Las Palmas Creek, which originates in the Mexican region through the Arroyo Las Calabazas River and begins on the slopes of the Sierra de Juárez at an altitude of 1860 m above sea level (Fig. 1). The tributaries merge in the urban area of Tijuana, flowing approximately 6 km before crossing back into the USA and emptying into the Pacific Ocean 2.2 km north of the border. The Tijuana River and all of its tributaries are intermittent, so under natural conditions, water flows only during storm events. However, discharges from wastewater treatment plants in Tecate and Tijuana and settlements with no sewerage have produced perennial streams of water with poor quality (Wakida et al. 2008). The flow in the Alamar-Tecate tributary is approximately 181 L s−1 and begins in the urban area of Tecate. Its flow is controlled by the discharges of the Tecate WWTP and a brewery factory. On the other hand, Las Palmas Creek has an approximate 386 L−1 discharge product from the two WWTPs.
The primary industry in the area is manufacturing, encompassing a wide range of fields, such as plastics, medical devices, aerospace, toys, automotive, furniture, electronics, metalworking, tools, cables and others (Tijuana 2019).
Eleven sites were sampled in the basin. Eight sites are in the Alamar-Tecate drainage basin, and three are in the Las Palmas drainage basin. Figure 1 shows the sampling site locations. The riverbed at sites S1 and S2 was dry during sampling. These sections of the channel only have water flow during rain events. Site S3 is located downstream of the discharge zone of a brewery plant. Site S4 is located approximately 50 m downstream of Tecate's municipal wastewater treatment plant. All the sites were in the urban area of Tecate. Sites S5 and S6 are in the suburban area of Tecate, and S7 is in a rural area, also known as Tecate. Site S8 is in a suburban area of Tijuana with no sewerage or garbage dumps nearby. Sites S9 and S10 are in the Las Palmas drainage basin in the urban area of Tijuana; these sites receive water from WWTPs. Finally, Site S11 is at Abelardo L. Rodriguez Dam, where there has been little control of domestic wastewater discharge from new housing development in recent years. The general characteristics are shown in Table S1 of Online Resource 1.
Four composite samples were taken at every location—two from sediments along the river shoreline and the others from the riverbed. The sediment samples from the river shoreline were taken from two ten-m-long sections of the shoreline, a meter away from the river, in which multiple subsamples 3 cm deep by 5 cm × 5 cm were taken until approximately 2 kg wet weight of sample was obtained. The samples were collected with a metal trowel. The riverbed sediment composite samples were collected by taking subsamples from the same 10 m sections and the upper 3–5 cm section with a metal bucket. Therefore, at each sampling site, two composite samples of river shoreline sediment (n = 22) and two composite samples of riverbed sediment were collected (n = 22).
MP extraction
The process of microplastic extraction was carried out as follows: A kilogram of sediment or soil sample was dried for 24 h at 40 °C, sieved with a < 1 mm particle size sieve and transferred to a 2-L glass container. Subsequently, 2 L of filtered saturated saline solution (120 g of NaCl per liter) was added, and the mixture was stirred for 15 min. The suspension was allowed to rest overnight if it was cloudy. The supernatant was then transferred to a clean glass container using a vacuum pump, and the liquid was passed through a 25 µm metal sieve. This extraction process was repeated three times. The metal sieve was washed with distilled water prior to filtration (Whatman 969). The filtrate was filtered through a fiberglass filter (Whatman 969). Finally, the fiberglass filter with the MPs was placed in an oven to dry at 40 °C for 24 h to eliminate humidity in the samples before placing it in glass plates.
No plastic equipment was used to avoid plastic contamination throughout the extraction process. Additionally, all glassware utilized in the procedures was rinsed three times with filtered deionized water and covered with aluminum foil to avoid airborne contamination. A 0.4-μm-pore-diameter fiberglass filter (Whatman 969) was used to filter the water used to rinse the glassware and NaCl solutions utilized for the extractions to avoid contamination. Before every extraction process, all surfaces were cleaned with a 70% ethanol solution. The operators washed their hands frequently and used cotton laboratory coats. The filtration processes were conducted in laminar flow chambers, and cotton laboratory coats were used throughout. Three blanks were generated, resulting in a background MP abundance less than three, most of which were blue fibers.
Visual counting and FTIR spectroscopy
Particle shapes were analyzed with a binocular optical stereoscope (Motic Digital Microscope DM143, NTSC System with W10 × 20 lens) to record the number of microplastics and classify them by shape (fibers, spheres, fragments and films) and color. Fibers or microfibers (as other authors call them) are fragile synthetic fibers made of polyester or polyamides (nylon). These particles are detached from clothes made of these materials, either due to washing or use. Spheres were defined as spherical particles used in exfoliating cosmetic products. Microplastics defined as fragments have irregular, sometimes flat, and broken shapes that result from the fragmentation of plastic items. Finally, films are defined as thin plastic films produced by the degradation of larger pieces of plastic, such as plastic bags, packaging film or plastic mulching film (Ruimin et al. 2020). Microplastic identification was conducted using FTIR-IR (Thermo Scientific Nicolet iS5). This analysis was carried out in reflection mode with 30 scans at a resolution of 10 cm−1 and in the range of 400–4000 cm−1. The polymers were identified by comparing bands corresponding to specific chemical functional groups with spectra of reference polymers in the literature.
Statistical analysis
All statistical analyses were conducted using Minitab®15. Using the Kolmogorov‒Smirnov test, we determined that the microplastic abundance data did not follow a normal distribution. Thus, the Mann‒Whitney U test was used to compare the microplastic concentrations in the river shoreline and riverbed sediments. The average and other standard statistical parameters were also calculated for comparison reasons.
Results and discussion
Microplastic abundance in sediments and soils
Table 1 shows the microplastic abundances and the basic statistics of the sampling sites. The MP abundance was between 59 and 586 particles kg−1 in the riverbed sediment and 57 and 469 particles kg−1 in the river shoreline sediments. The relative percentage difference (RPD) is highly variable, ranging between 14 and 116% and 11 and 106% for sediments and river shoreline soils, respectively. This indicates the high variability in MP abundance in sediment and soil. The highest average MP concentrations in the sediment and soil were found at Site S8. This site is in the suburban area of Tijuana, which has many potential sources of microplastics, such as untreated wastewater from informal settlements, illegal trash dumps, urban runoff and MP deposition from the metropolitan area. The second highest abundances in the soil and sediments were observed at Site S5, with 391 and 273 particles kg−1, respectively. This site is located approximately 1.2 km downstream of the Tecate Municipal WWTP. The high abundance of MPs observed could be due to solid sedimentation from self-purification of the stream since these solids can trap microplastics. Hidayaturrahman and Lee (2018) stated that preliminary treatment, including sedimentation, can reduce 56.8–64.4% of microplastics. Moreover, an additional 18.2–27.8% can be eliminated from wastewater by activated sludge treatment. Therefore, sedimentation and biological processes can eliminate suspended solids such as microplastics from water.
Table S1 of Online Resources 1 provides the average size of the particles at the sampling sites. The highest microplastic abundance was observed at the site with the lowest particle size (Site S8). This behavior was also reported by He et al. (2020b) in the Brisbane River in Australia. Moreover, Liu et al. 2021 reported a positive correlation between MP abundance and the silt fraction. This difference may be related to different velocities of river flow that enable finer particles to settle and microplastics to form. However, no correlation was found between sediment particle size (D50) and microplastic abundance, although there was a moderate correlation between microplastic abundance and silt + clay content (r = 0.61).
The mean and median abundances of MPs in the riverbed sediment were 197 and 118 MP kg−1 and 187 and 122 MP kg−1, respectively, in the sediments from the river shoreline. The Kolmogorov‒Smirnov test revealed that the abundances in the soils and sediment did not follow a normal distribution. Therefore, the Mann‒Whitney U test was carried out to compare the medians of the concentrations of microplastics in the sediments and soils of the Tijuana River Basin. No significant difference was found between the concentrations (118 MPs kg−1 in riverbed sediment and 122 MPs kg−1 in shoreline sediment).
Table 2 shows a comparison of the microplastic abundance found in this study with that from other studies. The table also provides general details about the basin and sampling studies and the significant shapes and polymers found in these studies. The microplastic abundance per kilogram found in the Tijuana River Basin sediment is lower than that reported in rivers in densely populated urban areas in China (Huang et al. 2021), the Rhine River (Mani et al. 2019) and the Tisza River in Hungary (Kiss et al. 2021). The microplastic abundances of these rivers are 6–19 times greater than the abundances found in this study. Conversely, the concentrations found in the Lower Ganga River in India (Singh et al. 2021) and Quin River in China (Zhang et al. 2020) were lower than the microplastic abundance found in this study, while the abundances found in the Atoyac River in Mexico (Shruti et al. 2019), Manzhou River in China (Wu et al. 2020a) and Brisbane River in Australia (He et al. 2020a) are within the range found in this study. The variability in microplastic abundance in river sediment may result from sampling methods and seasons, land use and anthropogenic activities in the basin area, and river dynamics (Lagarde et al. 2016; He et al. 2020a; Xia et al. 2021).
Characterization of microplastics
Type and shape
Thirty-five particles were analyzed to determine their chemical composition. The types of polymers identified were propylene (PP), polyethylene (PE), polystyrene (PS), polyacrylic, polyamide (PA) and polyethylene terephthalate (PET). The polymer showing the highest percentage abundance was PP (46%), followed by PE (34.3%) and PS (11.4%). The first two are the polymers most commonly reported in studies of MPs in freshwater systems, along with PET and PS. Rodrigues et al. (2018) reported that PE and PP were identified in more than 50% of analyzed MPs (Table 2). PS, PE and PP were also the dominant polymer types found in the shoreline sediments of the River Rhine in Germany and the Po River Delta in Italy (Klein et al. 2015; Piehl et al. 2019). PP and PE are the most produced polymers worldwide, with projections for 2020 of 75.4 and 117.9 million metric tons, respectively (Statista 2021). These results coincide with those of other reported studies (Klein et al. 2015; Horton et al. 2017; Lin et al. 2018). The spectra of the identified polymers are shown in Fig. 2, and the primary signals are described in the Supplementary information.
The plastic particles were classified into four main morphologies: fibers, fragments, films and spheres. Figure 3 shows some examples of the MPs found in the samples. Figure 4 shows the percentages of various MP morphologies found at different sampling sites. The predominant shape was fibers, with a percentage range between 47 and 81%, the highest percentage being at Site S9 and the lowest at Site S5. The high percentage of fibers reflects the prevalence of wastewater discharge as an MP source at these sites, as shown in Sites S4 (located downstream of the WWTP of Tecate), S7 (rural) and S11 (suburban). No other MP sources are evident at these sites. Site S2 had the lowest percentage of fibers, showing that WWTP discharge did not influence the abundance of MPs since this part of the river remains dry through most of the year. The percentages of the other MP morphologies (fragments and films) found in the sediment and soil samples revealed the variety of MP sources in the study area and the importance of secondary MPs. The sum of the percentages of these two morphologies varied between 5.3 and 50% of the total MPs found. The primary source of spherical MPs could be WWTPs since they are used in cosmetic products such as exfoliators. The highest percentage of spheres was found at Site S7 (approximately 23%). This percentage, in addition to the percentage of fibers, accounts for 94% of the total MPs found at this site, implying that discharge from WWTPs is the main source of MPs since the site is located in a rural area with no other visible sources.
Photographs showing different MP shapes and colors found in the sediments of the Tijuana River Basin: a and b Blue and green fragments resulting from the breakdown of larger plastic pieces. c Transparent film resulting from the fragmentation of the packaging material. d and e White and red fragments from the fragmentation of larger plastic pieces. f and g Pink and blue fibers. h and i Transparent microplastic filaments and white ropes. The horizontal yellow line is 1 mm long
Fibers were the most abundant shape found in the samples (65%), followed by fragments (19%). These findings coincide with those of other studies in which these shapes were predominant (Barrows et al. 2018; Horton et al. 2017; Tien et al. 2020). This is due to the origin of the river flow from WWTP discharges. Sewage is an important source of microfibers generated from the washing of garments made of synthetic fibers (Browne et al. 2011; De Falco et al. 2018). However, the presence of other MP morphologies, such as fragments and films, implies that garbage and solid residues are important sources of MPs, particularly in urban areas. These MPs are the result of the degradation of larger plastic items. Microplastic deposition should also not be ruled out since other studies have shown that the highest percentage of microplastics deposited in the atmosphere are fibers (Wright et al. 2020).
Color
Microplastic color was analyzed in this study because it is considered an important issue since color influences ingestion by marine organisms (Abayomi et al. 2017; Ory et al. 2017). Other authors have related the color of microplastics to anthropogenic sources and the environmental processes to which they are exposed once released into the environment. For example, transparent and blue microplastics have been associated with fishing nets and lines (Peng et al. 2018; Chinfak et al. 2021). On the other hand, white and transparent microplastics are related to plastics degraded due by sunlight and weathering (Hidalgo-Ruz et al. 2012; Neubauer et al. 2017; Singh et al. 2021). Microplastics may retain the original color of the plastic fragmented from packaging material or plastic items. However, approximately 50% of the spheres (beads) particles in this study were yellow or brown. These colors are common characteristics of plastics that have been exposed to environmental factors for some time, especially sunlight, which leads to the photooxidation of some of the components of the plastics (Andrady et al. 1992; Pastorelli et al. 2014; Marti et al. 2020).
Eleven primary colors were found in the microplastics from the sediment and shoreline soil of the Tijuana River Basin: black, transparent, blue, green, red, white, pink, yellow, brown, purple and orange. The most abundant colors were black (43.7%) and transparent (22.6%), followed by blue (9.8%), green (5.8%), red (5.2%), white (4.1%), pink (3.9%), yellow (2.5%), brown (1.8%), purple (0.5%) and orange (0.1%). Although many studies have shown similar color percentages, others have reported white and transparent as the predominant colors (He et al. 2020a; Gerolin et al. 2020; Wu et al. 2020a; Singh et al. 2021). Conversely, the most dominant colors in the sediment from the Tapi-Phumduang River in Thailand were blue (38%), black (32%) and white (18%) (Chinfak et al. 2021). Moreover, Deng et al. (2020) reported that in river sediments in the textile area of Zhejiang Province in China, 60% of the microplastics were black, blue or white.
Figure 5 shows the proportions of microplastics of different colors found among the MPs of different shapes. More colors were observed in fragments and fibers (10 and 9 colors, respectively) than in films and spheres (5 and 5, respectively). This result can be expected due to the large variety of colors of plastic products that can be fragmented in the environment and because clothing is made from artificial fibers. Conversely, the colors found in spheres and films may be limited due to the narrower range of colors used in the original products. The number of microplastic colors detected was high at all sites (9–11 colors), except at Site 7 (6 colors), which was the only site in this study that was in a rural area. This increase in color may imply the influence of urban development and WWTP discharge on the abundance of MPs in sediments.
Future perspectives
The Tijuana River Basin is a complex system. The abundance of microplastics in water and sediments results from several point and diffuse sources. Future actions that are recommended involve improving wastewater treatment involving measures aimed at reducing the discharge of microplastics. Moreover, urban waste management services in the area should be improved, and environmental education should be increased to enhance people´s awareness of microplastic sources; people can then take measures such as reducing their use of single-use plastic items or recycling plastics (Aljerf and Choukaife 2016). These measure can be increased in ecotourism and recreation sites around rivers (Aljerf 2015).
Conclusion
This study confirmed the existence of microplastic pollution in the Tijuana River Basin. The basin has distinctive features because the USA and Mexico have different degrees of economic development. The consumption habits of the population in this border region are consequently different from those in the rest of Mexico and are reflected in the wide range of microplastic colors and morphologies found. The microplastic abundance in the riverbed sediments ranged between 197.4 and 118 MP kg−1 and between 181.5 and 183 MP kg−1 in the shoreline sediments, lower than the microplastic abundance in the sediments of highly urbanized and populated rivers. As expected, the high proportion of fibers confirms that wastewater discharges from WWTPs and slums with no sewerage are the main sources of microplastics in the river sediment. However, secondary microplastics (produced by the breakdown of plastic items such as plastic bottles or packaging materials) are also an important source of microplastics, as evidenced by the high percentage of fragments and films found at some sampling sites. This study highlights the importance of rivers as microplastic sources for marine environments and that riverbeds and shoreline sediments are temporary microplastic sinks.
Availability of data and materials
Not applicable.
References
Abayomi OA, Range P, Al-Ghouti MA, Obbard JP, Almeer SH, Ben-Hamadou R (2017) Microplastics in coastal environments of the Arabian Gulf. Mar Pollut Bull 124:181–188. https://doi.org/10.1016/j.marpolbul.2017.07.011
Akindele EO, Ehlers SM, Koop JHE (2019) First empirical study of freshwater microplastics in West Africa using gastropods from Nigeria as bioindicators. Limnologica 78:125708. https://doi.org/10.1016/j.limno.2019.125708
Alam FC, Sembiring E, Muntalif BS, Suendo V (2019) Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 224:637–645. https://doi.org/10.1016/j.chemosphere.2019.02.188
Aljerf L (2015) Change theories drift conventional tourism into ecotourism. Acta Tech Corviniensis Bull Eng 8(4):101–104
Aljerf L, Choukaife A (2016) Sustainable development in Damascus University: a survey of internal stakeholder views. J Environ Stud 2(2):1–12. https://doi.org/10.13188/2471-4879.1000012
Alvarez-Andrade A, Wakida FT, Piñon-Colin TDJ, Wakida-Kusunoki AT, Castillo-Quiñones JE, García-Flores E (2023) Microplastic abundance in feces of lagomorphs in relation to urbanization. Sci Total Environ 864:161025. https://doi.org/10.1016/j.scitotenv.2022.161025
Andrady AL, Searle ND, Crewdson LFE (1992) Wavelength sensitivity of unstabilized and UV stabilized polycarbonate to solar simulated radiation. Polym Degrad Stabil 35(3):235–247. https://doi.org/10.1016/0141-3910(92)90031-Y
Ángeles-Hurtado LA, Rodríguez-Reséndiz J, Salazar-Colores S, Torres-Salinas H, Sevilla-Camacho P (2021) Viable disposal of post-consumer polymers in Mexico: a review. Front Environ Sci 9:427. https://doi.org/10.3389/fenvs.2021.749775
Avio CG, Gorbi S, Regoli F (2016) Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar Environ Res 128:2–11. https://doi.org/10.1016/j.marenvres.2016.05.012
Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe FJ (2016) Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull 110(1):383–395. https://doi.org/10.1016/j.marpolbul.2016.06.037
Barcelo D, Pico Y (2020) Case studies of macro- and microplastics pollution in coastal waters and rivers: is there a solution with new removal technologies and policy actions? Case Stud Chem Environ Eng 2:100019. https://doi.org/10.1016/j.cscee.2020.100019
Barrows APW, Christiansen KS, Bode ET, Hoellein TJ (2018) A watershed-scale, citizen science approach to quantifying microplastic concentration in a mixed land-use river. Water Res 147:382–392. https://doi.org/10.1016/j.watres.2018.10.013
Battulga B, Kawahigashi M, Oyuntsetseg B (2019) Distribution and composition of plastic debris along the river shore in the Selenga River basin in Mongolia. Environ Sci Pollut Control Ser 26(14):4059–14072. https://doi.org/10.1007/s11356-019-04632-1
Brown DM, Wilson M, MacNee W, Stone V, Donaldson K (2001) Size-dependent proinflammatory effects of ultrafine poly-styrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol 175:191–199. https://doi.org/10.1006/taap.2001.9240
Browne MA (2015) Sources and pathways of microplastics to habitats. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer, Berlin, pp 245–312c
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ Sci Technol 45(21):9175–9179. https://doi.org/10.1021/es201811s
Calderon C (2022) Reciclaje en México: Solo 6% del plástico que se produce en el país se reutiliza (Recycling in Mexico: Only 6% of the plastic that the country produce is reused). El Financiero. August 03, 2022. https://www.elfinanciero.com.mx/empresas/2022/08/02/reciclaje-en-mexico-solo-6-del-plastico-que-se-produce-en-el-pais-se-reutiliza/
Chan CK, Park C, Chan K, Mak DCW, Fang JK, Mitrano D (2020) Microplastic fibre releases from industrial wastewater effluent: a textile wet-processing mill in China. Environ Chem 18(3):93. https://doi.org/10.1071/EN20143
Cheung LT, Lui CY, Fok L (2018) Microplastic contamination of wild and captive flathead grey mullet (Mugil cephalus). Int J Environ Res Public Health 15:597. https://doi.org/10.3390/ijerph15040597
Chinfak N, Sompongchaiyakul P, Charoenpong C, Shi H, Yeemin T, Zhang J (2021) Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci Total Environ 781:146700. https://doi.org/10.1016/j.scitotenv.2021.146700
Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 4:4528. https://doi.org/10.1038/srep04528
Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS (2015) The impact of polystyrene microplastics on feeding, function, and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol 49(2):1130–7. https://doi.org/10.1021/es504525u
Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the Island of Kauai, Hawaii. Mar Pollut Bull 60(5):650–654. https://doi.org/10.1016/j.marpolbul.2009.12.026
Corcoran PL, Belontz SL, Ryan K (2019) Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environ Sci Technol 54(2):818–825. https://doi.org/10.1021/acs.est.9b04896
d’Ambrières W (2019) Plastics recycling worldwide: current overview and desirable changes. Field Actions Sci Rep 19:12–21
De Carvalho AR, Garcia F, Riem-Galliano L, Tudesque L, Albignac M, Ter Halle A, Cucherousset J (2021) Urbanization and hydrological conditions drive the spatial and temporal variability of microplastic pollution in the Garonne River. Sci Total Environ 769:144479. https://doi.org/10.1016/j.scitotenv.2020.144479
De Falco F, Gullo MP, Gentile G, Pace ED, Cocca M, Gelabert L, Brouta-Agnesa M, Rovira A, Escudero R, Villalva R, Mossotti R, Montarsolo A, Gavignano S, Tonin C, Avella M (2018) Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ Pollut 236:916–925. https://doi.org/10.1016/j.envpol.2017.10.057
Deng Y, Zhang Y, Lemos B, Ren H (2017) Tissue accumulation of microplastics in mice and biomarker responses suggest wide-spread health risks of exposure. Sci Rep 7:46687. https://doi.org/10.1038/srep46687
Deng H, Wei R, Luo W, Hu L, Li B, Di Y, Shi H (2020) Microplastic pollution in water and sediment in a textile industrial area. Environ Pollut 58:113658. https://doi.org/10.1016/j.envpol.2019.113658
Depledge MH, Galvani F, Panti C, Caliani I, Casini S, Fossi MC (2013) Plastic litter in the sea. Mar Environ Res 92:279–281. https://doi.org/10.1016/J.Marenvres.2013.10.002
Devriese LI, Van der Meulen MD, Maes T, Bekaert K, Paul-Pont I, Frère L, Vethaak AD (2015) Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar Pollut Bull 98(1–2):179–187. https://doi.org/10.1016/j.marpolbul.2015.06.051
Dris R, Gasperi J, Tassin B (2018) Sources and fate of microplastics in urban areas: a focus on Paris megacity. In: Wagner M, Lamber S (eds) Freshwater microplastics. The handbook of environmental chemistry, vol 58. Springer, Cham, pp 69–83. https://doi.org/10.1007/978-3-319-61615-5_4
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritization of research needs. Water Res 75:63–82. https://doi.org/10.1016/j.watres.2015.02.012
Fan Y, Zheng K, Zhu Z, Chen G, Peng X (2019) Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China. Environ Pollut 251:862–870. https://doi.org/10.1016/j.envpol.2019.05.056
Gerolin CR, Pupim FN, Sawakuchi AO, Grohmann CH, Labuto G, Semensatto D (2020) Microplastics in sediments from Amazon rivers, Brazil. Sci Total Environ 749:141604. https://doi.org/10.1016/j.scitotenv.2020.141604
Gregory MR (1996) Plastic ‘scrubbers’ in hand cleansers: a further (and minor) source for marine pollution identified. Mar Pollut Bull 32:867–871. https://doi.org/10.1016/S0025-326X(96)00047-1
Habib D, Locke DC, Cannone LJ (1996) Synthetic fibers are indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents. Water Air Soil Pollut 103:1–8. https://doi.org/10.1023/A:1004908110793
He B, Goonetilleke A, Ayoko G, Rintoul L (2020a) Abundance, distribution pattern, and identification of microplastics in Brisbane River sediments, Australia. Sci Total Environ 700:134467. https://doi.org/10.1016/j.scitotenv.2019.134467
He B, Wijesiri B, Ayoko GA, Egodawatta P, Rintoul L, Goonetilleke A (2020b) Influential factors on microplastics occurrence in river sediments. Sci Total Environ 738:39901. https://doi.org/10.1016/j.scitotenv.2020.139901
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46(6):3060–3075. https://doi.org/10.1021/es2031505
Hidayaturrahman H, Lee TG (2018) A study on characteristics of microplastic in wastewater of South Korea: identification, quantification, and fate of microplastics during the treatment process. Mar Pollut Bull 146:696–702. https://doi.org/10.1016/j.marpolbul.2019.06.071
Hoellein TJ, McCormick AR, Hattie J, London MG, Scott JW, Kelly JJ (2017) Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw Sci 36(3):491–507. https://doi.org/10.1086/693012
Horton AA, Dixon SJ (2017) Microplastics: an introduction to environmental transport processes. WIREs Water 5(2):1268. https://doi.org/10.1002/wat2.1268
Horton AA, Svendsen C, Williams RJ, Spurgeon DJ, Lahive E (2017) Large microplastic particles in sediments of tributaries of the River Thames, UK: abundance, sources and methods for effective quantification. Mar Pollut Bull 114(1):218–226. https://doi.org/10.1016/j.marpolbul.2016.09.004
Huang D, Li X, Ouyang Z, Zhao X, Wu R, Zhang C, Lin C, Li Y, Guo X (2021) The occurrence and abundance of microplastics in surface water and sediment of the West River downstream, in the south of China. Sci Total Environ 756:43857. https://doi.org/10.1016/j.scitotenv.2020.143857
Huang S, Huang X, Bi R, Guo Q, Yu X, Zeng Q, Huang Z, Liu T, Wu H, Chen Y, Xu J, Wu Y (2022) Detection and analysis of microplastics in human sputum. Environ Sci Technol 56:2476–2486. https://doi.org/10.1021/acs.est.1c03859
Inegi (2020) Comunicado de prensa No 30/21. 26 de enero 2021 (Press release No 30/21 Jan 26, 2021) https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2021/EstSociodemo/ResultCenso2020_BC.pdf (in Spanish)
Inkielewicz-Stepniak I, Tajber BG, Zhang H, Radomski MW, Medina C, Santos-Martinez M (2018) The role of mucin in the toxicological impact of polystyrene nanoparticles. Materials 11:724. https://doi.org/10.3390/ma11050724
Interreg (2018) Tisza River Basin Characterization Report on Surface Water. Interreg, Danube Transnational Programme. http://www.interreg-danube.eu/uploads/media/approved_project_output/0001/37/ed1b7198ffc57c6d2d528717650cd6d94280004e.pdf
Jenner LC, Rotchell JM, Bennett RT, Cowen M, Tentzeris V, Sadofsky LR (2022) Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ 831:154907. https://doi.org/10.1016/j.scitotenv.2022.154907
Jin Y, Lu L, Tu W, Luo T, Fu Z (2019) Impacts of polystyrene microplastic on the gut barrier, microbiota, and metabolism of mice. Sci Total Environ 649:308–317. https://doi.org/10.1016/j.scitotenv.2018.08.353
Kamani H, Ganji F, Ghayebzadeh M (2023) Risk factors and characteristics of microplastics in the hospital wastewater treatment plant. Res Square. https://doi.org/10.21203/rs.3.rs-3365251/v1
Kataoka T, Nihei Y, Kudou K, Hinata H (2019) Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ Poll 244:958–965. https://doi.org/10.1016/j.envpol.2018.10.111
Kettenmann S (2016) Nationwide ban on plastic microbeads in cosmetics. Nat Resour Environ 31(1):58
Kiss T, Forian S, Szatmari G, Sipos G (2021) Spatial distribution of microplastics in the fluvial sediments of a transboundary river: a case study of the Tisza River in Central Europe. Sci Total Environ 785:147306. https://doi.org/10.1016/j.scitotenv.2021.147306
Klein S, Worch E, Knepper TP (2015) Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main Area in Germany. Environ Sci Technol 49(10):6070–6076. https://doi.org/10.1021/acs.est.5b00492
Lagarde F, Olivier O, Zanella M, Daniel P, Hiard S, Caruso A (2016) Microplastic Interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ Pollut 215:331–339. https://doi.org/10.1016/j.envpol.2016.05.006
Leslie HA, Van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH (2022) Discovery and quantification of plastic particle pollution in human blood. Environ Int 163:107199. https://doi.org/10.1016/j.envint.2022.107199
Lin L, Lin-Zi Z, Jin-Pin P, Li-Qi C, Fok L, Yan Y, Heng-Xiang L, Xiang-Rong X (2018) Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci Total Environ 644:375–381. https://doi.org/10.1016/j.scitotenv.2018.06.327
Liu Y, Zhang J, Cai C, He Y, Chen L, Xiong X, Huang H, Tao S, Liu W (2020) Occurrence and characteristics of microplastics in the Haihe River: an investigation of a seagoing river flowing through a megacity in northern China. Environ Pollut 262:114261. https://doi.org/10.1016/j.envpol.2020.114261
Liu Y, Zhang JD, Tang Y, He Y, Li YJ, You JA, Breider F, Tao S, Liu WX (2021) Effects of anthropogenic discharge and hydraulic deposition on the distribution and accumulation of microplastics in surface sediments of a typical seagoing river: the Haihe River. J Hazard Mater 404(Part B):24180. https://doi.org/10.1016/j.jhazmat.2020.124180
Mani T, Primpke S, Lorenz C, Gerdts G, Burkhardt-Holm P (2019) Microplastic pollution in benthic midstream sediments of the Rhine River. Environ Sci Technol 53:6053–6062. https://doi.org/10.1021/acs.est.9b01363
Marti E, Martin C, Galli M, Echevarría F, Duarte CM, Cozar A (2020) The colours of the ocean plastics. Environ Sci Technol 54(11):6594–6601. https://doi.org/10.1021/acs.est.9b06400
McCormick AR, Hoellein TJ (2016) Anthropogenic litter is abundant, diverse, and mobile in urban rivers: insights from cross-ecosystem analyses using ecosystem and community ecology tools. Limnol Oceanogr 61(5):1718–1734. https://doi.org/10.1002/lno.10328
Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G (2017) Identification of microplastic in effluents of wastewater treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res 108:365–372. https://doi.org/10.1016/j.watres.2016.11.015
Nel HA, Dalu T, Wasserman RJ (2018) Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci Total Environ 612:950–956. https://doi.org/10.1016/j.scitotenv.2017.08.298
Neubauer N, Scifo L, Navratilova J, Gondikas A, Mackevica A, Borschneck D, Chaurand P, Vidal V, Rose J, Von der Kammer F, Wohlleben W (2017) Nanoscale coloristic pigments: upper limits on releases from pigmented plastic during environmental aging, in food contact, and by leaching. Environ Sci Technol 51:11669–11680. https://doi.org/10.1021/acs.est.7b02578
Nor NHM, Obbard JP (2014) Microplastics in Singapore’s coastal mangrove ecosystems. Mar Pollut Bull 79(1–2):278–283. https://doi.org/10.1016/j.marpolbul.2013.11.025
OECD (2022a) Global plastics outlook: policy scenarios to 2060. OECD Publishing, Paris. https://doi.org/10.1787/aa1edf33-en
OECD (2022b) Global plastics outlook: economic drivers, environmental impacts and policy options. OECD Publishing, Paris. https://doi.org/10.1787/de747aef-en
Ory NC, Sobral P, Ferreira JL, Thiel M (2017) Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci Total Environ 586:430–437. https://doi.org/10.1016/j.scitotenv.2017.01.175
Paget V, Dekali S, Kortulewski T, Grall R, Gamez C, Blazy K, Aguerre-Chariol O, Chevillard S, Braun A, Rat P et al (2015) Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PLoS ONE 10:0123297. https://doi.org/10.1371/journal.pone.0123297
Pastorelli G, Cucci C, Garcia O, Piantanida G, Elnaggar A, Cassar M, Strlič M (2014) Environmentally induced colour change during natural degradation of selected polymers. Polym Degrad Stabil 107:198–209. https://doi.org/10.1016/j.polymdegradstab.2013.11.007
Peng G, Xu P, Zhu B, Bai M, Li D (2018) Microplastics in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ Pollut 234:448–456. https://doi.org/10.1016/j.envpol.2017.11.034
Piehl S, Mitterwallner V, Atwood EC, Bochow M, Laforsch C (2019) Abundance and distribution of large microplastics (1–5 mm) within beach sediments at the Po River Delta, northeast Italy. Mar Pollut Bull 149:110515. https://doi.org/10.1016/j.marpolbul.2019.110515
Piñon-Colin TDJ, Rodriguez-Jimenez R, Rogel-Hernandez E, Alvarez-Andrade A, Wakida FT (2020) Microplastics in stormwater runoff in a Semiarid Region, Tijuana, Mexico. Sci Total Environ 704:135411. https://doi.org/10.1016/j.scitotenv.2019.13541
Pironti C, Notarstefano V, Ricciardi M, Motta O, Giorgini E, Montano L (2023) First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics 11(1):40. https://doi.org/10.3390/toxics11010040
Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi S, D’Amore E, Rinaldo D, Matta M, Giorgini E (2021) Plasticenta: first evidence of microplastics in human placenta. Environ Int 146:106274. https://doi.org/10.1016/j.envint.2020.106274
Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C, Zucchelli E, De Luca C, D’Avino S, Gulotta A, Carnevali O, Giorgini E (2022) Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers 14:2700. https://doi.org/10.3390/polym14132700
Ramírez-Álvarez N, Rios Mendoza LM, Macías-Zamora JV, Oregel-Vázquez L, Alvarez-Aguilar A, Hernández-Guzmán FA, Sánchez-Osorio JL, Moore CJ, Silva-Jiménez H, Navarro-Olache LF (2020) Microplastics: sources and distribution in surface waters and sediments of Todos Santos Bay, Mexico. Sci Total Environ 703:134838. https://doi.org/10.1016/j.scitotenv.2019.134838
Rodrigues MO, Abrantes N, Gonçalves FJM, Nogueira H, Marques JC, Gonçalves AMM (2018) Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci Total Environ 633:1549–1559. https://doi.org/10.1016/j.scitotenv.2018.03.233
Ruimin Q, Jones DL, Zhen L, Qin L, Changrong Y (2020) Behavior of microplastics and plastic film residues in the soil environment: a critical review. Sci Total Environ 703:134722. https://doi.org/10.1016/j.scitotenv.2019.134722
SDSU (San Diego State University)-Colef (El Colegio de la Frontera Norte) (2005) Atlas de la cuenca del río Tijuana (Tijuana River Basin Atlas). San Diego University Press-Institute for Regional Studies of the Californias, San Diego
Sekudewicz I, Dąbrowska AM, Syczewski MD (2021) Microplastic pollution in surface water and sediments in the urban section of the Vistula River (Poland). Sci Total Environ 762(25):143111. https://doi.org/10.1016/j.scitotenv.2020.143111
Shruti VC, Jonathan MP, Rodriguez-Espinosa PF, Rodríguez-González F (2019) Microplastics in freshwater sediments of Atoyac River basin, Puebla City, Mexico. Sci Total Environ 654:154–163. https://doi.org/10.1016/j.scitotenv.2018.11.054
Singh S, Mondal A, Bagri A, Tiwari E, Khandelwal N, Monikh FA, Darbha GK (2021) Characteristics and spatial distribution of microplastics in the lower Ganga River water and sediment. Mar Pollut Bull 163:111960. https://doi.org/10.1016/j.marpolbul.2020.111960
Statista (2021) Production forecast of thermoplastics worldwide from 2020 to 2050 by type. https://www.statista.com/statistics/1192886/thermoplastics-production-volume-by-type-globally/
Su L, Sharp SM, Pettigrove VJ, Craig NJ, Nan B, Du F, Shi H (2020) Superimposed microplastic pollution in a coastal metropolis. Water Res 168:115140. https://doi.org/10.1016/j.watres.2019.115140
Thrift E, Porter A, Galloway TS, Coomber FG, Mathews F (2022) Ingestion of plastics by terrestrial small mammals Sci. Total Environ 84:156679. https://doi.org/10.1016/J.SCITOTENV.2022.156679
Tibbetts J, Krause S, Lynch I, Sambrook Smith GH (2018) Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 10:1597. https://doi.org/10.3390/w10111597
Tien CJ, Wang ZX, Chen CS (2020) Microplastics in water, sediment, and fish from the Fengshan River system: relationship to aquatic factors and accumulation of polycyclic aromatic hydrocarbons by fish. Environ Pollut 265:114962. https://doi.org/10.1016/j.envpol.2020.114962
Tijuana EDC (2019) Estas son las principales industrias y fábricas en Tijuana (These are the main indstries and factories in Tijuana). https://tijuanaedc.org/es/estas-son-las-principales-industrias-y-fabricas-en-tijuana/ (in Spanish)
Tuvo B, Scarpaci M, Bracaloni S, Esposito E, Costa AL, Ioppolo M, Casini B (2023) Microplastics and antibiotic resistance: the magnitude of the problem and the emerging role of hospital wastewater. Int J Environ Res Public Health 20(10):5868. https://doi.org/10.3390/ijerph20105868
Wakida FT, Lara-Ruiz D, Temores-Peña J, Rodriguez-Ventura JG, Diaz C, Garcia-Flores E (2008) Heavy metals in sediments of the Tecate River, Mexico. Environ Geol 54:637–642. https://doi.org/10.1007/s00254-007-0831-6
Wang F, Wang B, Duan L, Zhang YZ, Zhou YT, Sui Q, Xu DJ, Qu H, Yu G (2020) Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: a case study in Changzhou, China. Water Res 182:115956. https://doi.org/10.1016/j.watres.2020.115956
Wen Z, Xie Y, Chen M, Dinga CD (2021) China’s plastic import ban increases prospects of environmental impact mitigation of plastic waste trade flow worldwide. Nat Commun. https://doi.org/10.1038/s41467-020-20741-9
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ (2020) Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int 136:105411. https://doi.org/10.1016/j.envint.2019.105411
Wu P, Tang Y, Dang M, Wang S, Jin H, Liu Y, Jing H, Zhen C, Yi S, Cai Z (2020a) Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Sci Total Environ 717:135187. https://doi.org/10.1016/j.scitotenv.2019.135187
Wu N, Zhang Y, Zhao Z, He J, Li W, Li J, Xu W, Ma Y, Niu Z (2020b) Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary. Sci Total Environ 708:134876. https://doi.org/10.1016/j.scitotenv.2019.134876
Xia L, Gu W, Zhang M, Chang YN, Chen K, Bai X, Yu L, Li J, Li S, Xing G (2016) Endocytosed nanoparticles hold endo-somes and stimulate binucleated cell formation. Part Fibre Toxicol 13:63. https://doi.org/10.1186/s12989-016-0173-1
Xia F, Yao Q, Zhang J, Wang D (2021) Effects of seasonal variation and resuspension on microplastics in river sediments. Environ Pollut 286:117403. https://doi.org/10.1016/j.envpol.2021.117403
Yang Y, Liu W, Zhang Z, Grossart HP, Gadd GM (2020) Microplastics provide new microbial niches in aquatic environments. Appl Microbiol Biot 104:6501–6511. https://doi.org/10.1007/s00253-020-10704-x
Yee MSL, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY, Tan BK, Wong CY, Leong CO (2021) Impact of microplastics and nanoplastics on human health. Nanomaterials-Basel 11:496. https://doi.org/10.3390/nano11020496
Yi X, Li W, Liu Y, Yang K, Wu M, Zhou H (2021) Effect of polystyrene microplastics of different sizes to Escherichia coli and Bacillus cereus. Bull Environ Contam Toxicol 107:626–632. https://doi.org/10.1007/s00128-021-03215-6
Yonkos LT, Friedel EA, Perez-Reyes AC, Ghosal S, Arthur CD (2014) Microplastics in four estuarine rivers in Chesapeake Bay, USA. Environ Sci Technol 48:14195–14202. https://doi.org/10.1021/es5036317
Yu K, Duan Y, Liao P, Xie L, Li Q, Ning Z, Liu C (2020) Watershed-scale distributions of heavy metals in the hyporheic zones of a heavily polluted Maozhou River watershed, southern China. Chemosphere 239:124773. https://doi.org/10.1016/j.chemosphere.2019.124773
Zhang L, Liu J, Xie Y, Zhong S, Yang B, Lu D, Zhong Q (2020) Distribution of microplastics in surface water and sediments of Qin River in Beibu Gulf, China. Sci Total Environ 708:135176. https://doi.org/10.1016/j.scitotenv.2019.135176
Zhang J, Wang L, Trasande L, Kannan K (2021) Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ Sci Technol Lett 8:989–994. https://doi.org/10.1021/acs.estlett.1c00559
Zubris KA, Richards BK (2005) Synthetic fibers as an indicator of land application of sludge. Environ Poll 138:201–211. https://doi.org/10.1016/j.envpol.2005.04.013
Acknowledgements
Consejo Nacional de CIencia y Tecnología (CONACYT) is gratefully acknowledged for the Ph.D. scholarship to TDJ Piñon-Colin Colin (scholarship No. 691128). The authors also thank to Universidad Autonoma de Baja for the support in this project. This paper was part of the Ph.D. studies by TDJ Piñon-Colin at the MYDCI doctoral program at Universidad Autónoma de Baja California.
Funding
Consejo Nacional de CIencia y Tecnología (CONACYT) for the PhD scholarship to TDJ Piñon-Colin Colin (scholarship No. 691128).
Author information
Authors and Affiliations
Contributions
TJP-C involved in conceptualization, methodology, writing—original draft. FTW took part in conceptualization, methodology, writing—review and editing, supervision. ER-H participated in validation, writing—review and editing, methodology. ATW-K involved in conceptualization, writing—review and editing. EG-F took part in writing—review and editing; HM involved in writing—review and editing, methodology.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Editorial responsibility: Rangabhashiyam S.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
J Piñon-Colin, T., Wakida, F.T., Rogel-Hernandez, E. et al. Microplastics in the sediments of the Tijuana River Basin, Mexico. Int. J. Environ. Sci. Technol. 21, 8361–8374 (2024). https://doi.org/10.1007/s13762-024-05609-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-024-05609-5