Abstract
Ten sites along the Tecate River, Mexico were sampled to evaluate the cadmium, lead, nickel and chromium concentrations in sediments. The result shows contamination for cadmium in most of the sites, where two sites were class 4 (polluted to strongly polluted) according to geoaccumulation index proposed by Muller. Two sites were found polluted for all the heavy metals analyzed (Cr, Cd, Pb and Ni), indicating the effect of anthropogenic activities. A correlation between Ni and Cd concentration had been found indicating a common source. These metals are usually used in electroplating industry. The results of this study can be used for decision makers to prioritize measures to control the pollution for these metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of elevated concentrations of trace metals in sediments found at the bottom of the water column can be a good indicator of man induced pollution rather than natural enrichment of the sediment by geological weathering (Davies et al. 1991, Chang et al. 1998). It is well known that an important proportion of metals are associated with suspended or bottom sediments dependant of sorption processes (Irion 1991; Wang et al. 1997). The accumulation of metals in sediments is controlled by a number of environmental factors that include pH, Eh, anthropogenic input, the type and concentration of organic and inorganic ligands, hydraulic processes within the stream and the available surface area for adsorption caused by the variation in grain size distribution (Axtmann and Luoma 1991; Davies et al. 1991; Sondi et al. 1994).
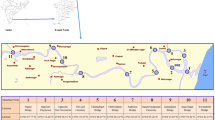
The Tecate river is part of the Tijuana River Basin (4,430 km2), which is a binational basin shared by Mexico and the US (Fig. 1). The major part of this basin is in Mexico (72%). The Tecate River in natural conditions is an ephemeral stream this means that water flows only in storm water events and its associated floods. However, discharges from wastewater treatment plants located in Tecate have produced a perennial stream of poor water quality downstream from these discharges. The average discharge is 170 L s−1, of which 150 L s−1 comes from the municipal wastewater plant and 20 L s−1 corresponds to a brewery company. Discharge from the municipal wastewater plant has a very low quality. The average concentration of chemical demand of oxygen (COD) was approximately 528 mg L−1 and the biochemical demand of oxygen (BOD) was 183 mg L−1 in the period from January 2002 to June 2004. The low quality of the municipal wastewater plant of Tecate is because the wastewater generation has exceeded the capacity of the plant. However, there are plans to update the capacity of the plant to cope with this problem. Conversely, the discharge from the brewery company has a good quality with an average BOD of 23 mg L−1 in the same period (Ponce 2004). The main industry in Tecate is the maquiladora sector (in-bond manufacturing and assembly plants) According to INEGI (2001) they encompass mainly basic metallurgy industry and furniture manufacturing. A great number of these basic metal industries use electroplating processes, so their wastewater or storm water can be a heavy metal source.
Placchi (1998) evaluated the heavy metal concentrations in runoff in the upper Tijuana River Basin, as well as in the Tecate River. As expected, the highest levels of heavy metals were found in the sites with urban and industrial land use. The storm water runoff in the urban site contained Cd (0.4–1.6 μg L−1), Cr (33–104 μg L−1), Pb (3–41 μg L−1) and Ni (91–215 μg L−1) and the maximum concentrations found in the industrial site were for Cd (16 μg L−1), Cr (36 μg L−1), Pb (40 μg L−1) and Ni (22 μg L−1).
Meyer and Gersberg (1997) stated that in the period from 1989 to 1997, the concentration values in the top 3 cm of sediment from 40 sites in the Tijuana River Estuary increased approximately four times for cadmium, copper and nickel. In the case of lead and zinc the concentrations were threefold higher. All are attributed to the increment of the maquiladora industry in the Tijuana River Basin. However, Weis et al. (2001) indicated that the load of heavy metal to the estuary has not shown any consistent long-term increment trend. It may reflect the recent progress in pollution prevention in the basin.
This study reports data on Cr, Ni, and Cd and Pb levels in sediments the Tecate River in order to identify polluted zones.
Methods
Ten sites along a 14 km long stretch of the Tecate River were selected for the present study. The site characteristics are shown in Table 1. The sampling events were conducted in August and November 2003. These dates represent the dry season and the wet season. The samples of the first 5–10 cm of the river deposits were taken from the middle or banks of the active river channel. The samples were analyzed also for pH, texture and organic matter content. In addition three samples were taken from three different undisturbed sites. These samples were analyzed and the average was taken to be the natural concentrations for the metals analyzed for the fraction <63 μm.
The sample preparation was carried out using the method described by Binning and Baird (2001). The samples were dried at 80°C for 48 h. The fraction <63 μm was used for the analysis of the metals. The fine grain sizes (usually <63, <20 or <2 μm) are routinely used for the analysis of trace metals and it is assumed the metals are found in these fractions. Two grams of the sample was placed in a 250 mL beaker with 20 mL of aqua regia (1:3 HNO3:HCl) and allowed to stand overnight; a 5 M HNO3 solution was added and the samples were allowed to stand overnight and then were filtered through Whatman No 41 filter paper. The filtrates were transferred to a 100 mL volumetric flask and made up to the mark with 0.5 M HNO3 solution. The water samples for metal analysis were prepared using nitric acid digestion 3030E (APHA 1995). A hundred milliliters of a well-mixed sample were transferred to an Erlenmeyer flask and 5 mL of HNO3 was added. The mixture was brought to slow boiling and evaporated on a hot plate to about 10–20 mL. The remaining solution was transferred to a 100-mL volumetric flask, diluted to the mark and mixed thoroughly.
The heavy metal determinations of the solutions were conducted using an atomic absorption spectrophotometer with a graphite furnace. Quality control was provided by parallel analysis of prepared spiked matrix samples with a certified standard from Environmental Research Associates (ERA 508). Sediment samples were spiked with a known concentration of the certified standard and treated in the same way as the samples. The percentage of recovery ranged from 87 to 95% for metals and the variation coefficients ranged from 5 to 14%. The sediment particle analysis was carried out by the pipette method (Rowell 1994).
Results
Results of a t-test showed that there was no significant difference (p > 0.05) between the two sampling events for lead, nickel and chromium and a significant difference for cadmium. This means that Cd concentrations in the first sampling are higher than in the second sampling. This may be due to rainfall producing a higher flow in the river, leading to a disturbance of the river base and the transport of sediment downstream. Cadmium in the runoff has been found associated to an appreciable degree with colloidal material (Harrison and Wilson 1985), which can be easily transported by the river flow. Cadmium is utilized by the electroplating industry, which discharges their treated effluents to the municipal sewers and the Cd present in water may adsorbed in organic matter suspended in the wastewater discharged to the river due to the poor treatment. Table 2 shows the heavy metal concentration, organic matter, pH and the silt plus clay content of the ten sites.
The average and ranges for the two sampling events in mg kg−1 were as follows: Pb 4.5 (0–11.5); Cr 5 (1.7–12.7), Ni 3.3 (0–16.7) and Cd 1.23 (0–5.25). In water samples only cadmium was detected with an average concentration of 54 μg L−1. The small amounts or absence of dissolved metals in water samples may be related to the pH values observed in the Tecate River. At pH 8.0, most metals are precipitated in the sediments as metal hydroxides.
The sediment pH in most of the sampling sites was alkaline and it was found that the discharges of the brewery have increased the sediment pH in a stretch of the river (site three and four) in which the values were between 9.2 and 9.8. This may be as a result of the NaOH used to clean the process pipes.
The role of organic matter and sediment grain size in relation to the accumulation of heavy metals to the sediments has been emphasized (Davies et al. 1991, Sakai et al. 1986, Thorne and Nickless 1981). Increases in heavy metal concentrations are associated with finer grain sediments sizes and organic matter and this can be seen at site two where the highest concentrations of heavy metals were found and the highest percentage of silt plus clay (35%) and organic matter (1.56%) were also found in this site. Site two is located in an urban area with a mix of residential, commercial and industrial land uses. It should be pointed out that this site is adjacent to a parking lot for lorries of a brewery company.
The highest concentration of lead was also found in site three (28 mg kg−1) adjacent to a treated wastewater discharge from a brewery. Therefore, it can be seen that this discharge is contributing in the lead accumulation in sediments at this point. Site two also showed high concentration of lead with respect to the other sites (9.5 and 11.5 mg kg−1). This can be the result that these sites being downstream of a high traffic street.
Correlations
The correlation coefficient value denotes if there is a relationship between two groups of values. Table 3 shows the correlation coefficient values between heavy metals and other physicochemical parameters such as percentage of organic matter and sediment particle size.
There was a high correlation between nickel and organic matter, as well as cadmium. However, for the second sampling there was no correlation between these heavy metals concentrations and percentage of organic matter.
In the case of the correlation between heavy metal and fine size particles (clay + silt), there was a high correlation between lead and nickel in the second sampling representing the rainy season (95 and 85%, respectively). This suggests that concentrations of these metals could be linked to particulate matter transported in storm water runoff from urban and industrial areas.
Correlations between metals indicate whether there is any common source of pollution. In the first sampling event (dry season), Tables 4, 5 shows the matrices of correlation values between metals for the sampling events. For the first event a high positive correlation was found between nickel and cadmium, indicating a possible common source that could be the electroplating industry, because this type of industry uses these metals and it is the predominant industry in the study area. Lead is used in welding operations in the electronic manufacture industry, although its use is diminishing due to its substitution in these processes by other less toxic materials.
A high correlation between nickel and lead was found in the second sampling representing the rainy season. It can indicate a common source of metal after the rain events bring the storm water runoff. This common source may be traffic because nickel can be generated by the wear of bearings, bushings and other moving parts in engines, while lead is used as a filler material in tires (Makepeace et al. 1995).
Geoaccumulation index
The geoaccumulation index proposed by Muller (1979) has been utilized to evaluate the pollution by heavy metals in sediments (Audry et al. 2004; Santos Bermejo et al. 2003; Munendra et al. 2002).
The index proposed by Muller (1979) is:
where C.M is the concentration in the fraction analyzed of the sample (<63 μm) and C.B. is the concentration in the same fraction of the sediments in the background site and the factor 1.5 is used to take into account the possible litological variability).
The Muller index has seven classes depending of its value: <0 or 0 no pollution, values from 0 to 1 not polluted to moderately polluted (class 1), 1 to 2, moderately polluted (class 2); 2 to 3, moderately polluted to polluted (class 3); 3 to 4, polluted to strongly polluted (class 4); 4 to 5, strongly polluted (class 5); 5 to 6 strongly polluted to very polluted (class 6); and >6 very polluted (class 7).
Table 6 shows the Muller geoaccumulation index for the different sites and sampling events. Most of the values for Pb were negative, which indicated no pollution in the sites. According to these values site 2 and 8 fell in class 1 (no polluted to moderately polluted) and site 3 fell in class 2 (moderately polluted) for Pb.
Site two and eight were class 4 (polluted to strongly polluted) for cadmium, with exemption to site 5, the rest of the sites fell in the class 2 and 3 in the first sampling, indicating moderately polluted to polluted for cadmium. For chromium the values of geoaccumulation index showed values from 0.4 to 2.10 indicating values for not polluted to moderately polluted. Site 2 and 8 were the only sites for nickel to show a moderate pollution, the rest of the sites were found not polluted for this metal.
The results showed that the sites with moderately polluted to strongly polluted were site 2 and site 8. Although site 3 can be pointed out as a moderately to polluted site for Pb, Cd and Cr. Site 2 shows the impact of urban storm water since this part of the river is dry for most of the year. High metal concentration in site 8 may be the result of the highway and industrial runoff.
The values of geoaccumulation of heavy metals in sediments may be higher than the values found in this study, but it is believed that the transport of the smallest fraction of sediment downstream due to the river velocity contributes to the accumulation of heavy metals in lower parts of this basin such as the Alamar River zone.
Conclusions
The main sources of heavy metals in the Tecate River are the urban and industrial wastewater. The main source of heavy metals in wastewater seem to be the electroplating industry since there was a high correlation between cadmium and nickel concentrations, which are used in the processes of this industry. Another important source of metal may be the urban surface runoff, specifically from industrial and junkyards. The results showed geoaccumulation for cadmium in almost of the sampling points. The highest geoaccumulation values were observed at site two and eight (class 4). Only two of ten sites were considered class 1 for nickel and lead and one considered class 2 for lead based on the Muller geoaccumulation index. These results showed that there is anthropogenic impact in the sediment quality of this stream.
References
APHA (1995) Standard methods for examination of water and wastewater. American Health Public Association
Audry S, Schafer J, Blanc G, Jouanneau JM (2004) Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoirs (France). Environ Pollut 132(3):413–426
Axtmann E, Luoma S (1991) Large scale distribution of metal contamination in the fine-grained sediments of the Clark Fork River, USA. Appl Geochem 6:75–88
Bermejo Santos JC, Beltran R, Gomez Araiza JL (2003) Spatial variations of heavy metals contamination in sediments from Odiel River (Southwest Spain). Environ Int 29(1):69–77
Binning K, Baird D (2001) Survey of heavy metals in the sediments of the Swartkops River Estuary, Port Elizabeth South Africa. Water SA 27(4):461–466
Chang JS, Yu KC, Tsai LJ, Ho ST (1998) Spatial distribution of heavy metals in bottom sediment of Yenshui river, Taiwan. Water Sci Technol 38(11):159–167
Davies CA, Tomlinson K, Stephenson T (1991) Heavy metals in River Tees estuary sediments. Environ Technol. 12:961–972
Harrison RM, Wilson SJ (1985) The chemical composition of highway drainage waters III. Runoff water metal speciation characteristics. Sci Total Environ 43:89–102
Instituto Nacional de Estadística, geografía e Informática (INEGI) (2001) “Sistemas de información empresarial mexicano, SIEM”. (Systems of Mexican business information) http://www.inegi.gob.mx. Cited 21 September 2006
Irion G (1991) Minerals in rivers. In: Degens E, Kempe F, Richey J (eds) Biogeochemistry of major world rivers, SCOPE 42. Wiley, New York, pp 356
Makepeace DK, Smith DW, Stanley SJ (1995) Urban stormwater quality: summary of contamination data. Crit Rev Environ Sci Technol 25(2):93–139
Meyer SF, Gersberg R (1997) Heavy metals and acid-volatile sulfide in sediments of the Tijuana Estuary. Bull Environ Contam Toxicol 59:113–119
Muller G (1979) Schwermetalle in den Sedimenten des Rheins Veranderungen seit 1971. Umschau 79(24):778–783
Munendra S, Müller G, Sinhg B (2002). Heavy metals in freshly deposited stream sediments of rivers associated with urbanization of the Ganga plain, India. Water, Air Soil Pollut 141:35–54
Placchi CA (1998) Land use and water quality in the upper reaches of the Tijuana River. Master thesis. San Diego State University
Ponce VM 2004, Feasibility of pumping scheme to provide water for Tecate River park, http://ponce.sdsu.edu/tecate_rio_parque_report_september2004.html. Accessed 12 May 2005
Rowell DL (1994) Soil science, methods & applications. Logman Essex, England
Sakai H, Kojima Y, Saito K (1986) Distribution of heavy metals in water and sieved sediments in the Toyohira river. Water Res 20:559–567
Sondi Y, Juracic M, Prohic ME, Pradvdic V (1994) Particulates and the environmental capacity for trace metals. A small river as a model for land-sea transfer system The Rasa River Estuary. Sci Total Environ 155:173–185
Thorne LT, Nickless G (1981) The relation between heavy metals and particle size fractions within the Severn estuary (UK) inter-tidal sediments. Sci Total Environ 19:207–213
Wang F, Cheng J, Forsling W (1997) Modeling sorption of trace metals on natural sediments by surface complexation model. Environ Sci Technol 31:448–453
Weis DA, Callaway JC, Gersberg RM (2001) Vertical accreation rates and heavy metal chronologies in wetland sediments of the Tijuana Estuary. Estuaries 24(6):840–850
Acknowledgments
This research has been conducted with the support of Universidad Autonoma de Baja California. The authors would like to thank Mario Del Valle for correcting the draft of this paper and the anonymous reviewers for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wakida, F.T., Lara-Ruiz, D., Temores-Peña, J. et al. Heavy metals in sediments of the Tecate River, Mexico. Environ Geol 54, 637–642 (2008). https://doi.org/10.1007/s00254-007-0831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-0831-6