Abstract
The escalating global concern over water pollution, predominantly propelled by industrial activities, underscores the urgency for effective environmental remediation strategies. Of particular, concern is the discharge of synthetic colorants into water bodies, a prevailing issue notably pervasive in textile industries. Azo dyes, ubiquitous in various industrial sectors, including food and textile manufacturing, constitute a significant portion of dye-contaminated wastewater, thereby contributing to soil and water pollution, especially in emerging and economically challenged nations. The deleterious impact of azo dyes on human health and aquatic ecosystems heightens the urgency of addressing this environmental menace. In response to the imperative need for mitigating the adverse effects of azo dyes on the environment, human health, and natural water resources, a spectrum of physio-chemical technologies has been proposed for azo dye degradation. However, a noteworthy paradigm shift toward environmentally friendly approaches is increasingly imperative. This comprehensive review critically examines various strategies employed for the degradation of Acid Orange 7, a representative azo dye, encompassing physical, chemical, and biological interventions. In the realm of biological treatment, the focus is predominantly on microorganisms such as fungi, yeast, bacteria, and algae, which have emerged as promising agents due to their inherent eco-friendly nature. This article provides an in-depth exploration of microbial remediation strategies, highlighting recent advancements in degradation techniques and elucidating the intricate mechanisms underlying azo dye degradation by bacteria. This review aims not only to present a consolidated overview of existing technologies but also to underscore the importance of harnessing the potential of microorganisms in the treatment of azo-dye-containing wastewater. The comprehensive insights provided herein contribute to the evolving discourse on sustainable and environmentally benign approaches for mitigating the impact of azo dyes on global water resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ever-increasing threat of environmental pollution poses a monumental challenge to the delicate balance of ecosystems and the well-being of humanity (Shen et al. 2023; Saharan et al. 2023; Ke Wang 2023, Kim et al. 2013). Defined as any human activity causing the natural environment's deterioration or loss of quality, pollution has evolved from a historical concern into the paramount factor in environmental morbidity and mortality (Ukaogo et al. 2020; Radhika 2019; Kaur et al. 2023; Su et al. 2023). This review embarks on an exhaustive exploration of the intricate web of environmental pollution, with a special focus on the profound implications of azo dyes emanating from the textile industry. The urgency of this review is underscored by the critical need for sustainable and innovative strategies to address the global ramifications of textile dye-associated pollution.
Environmental pollution is a complex phenomenon, manifesting across various dimensions including air, water, and soil/land pollution (Zhang et al. 2023; Kumar et al. 2023). The release of hazardous substances into the environment, such as toxic metals, gaseous pollutants, particulate matter, and a myriad of anthropogenic activities like sewage, industrial effluents, agricultural runoff, and electronic waste disposal, collectively contributes to the overarching menace of pollution (Jiku et al. 2021; Imran et al. 2023; Kamalam et al. 2023). A concerning aspect is the ineffective textile dyeing techniques, resulting in the discharge of 15–50% of azo dyes into generated wastewater, thereby exacerbating water pollution. These pollutants, classified as primary and secondary, infiltrate the atmosphere through domestic, industrial, and transportation sources, marking the distinction between directly released primary pollutants (e.g., CO, CO2, NO2, SO2) and secondary pollutants, which form in the atmosphere from primary pollutants (Lin et al. 2022a, b; Kaur et al. 2021; Abdullah et al. 2023).
Underground water sources face pollution risks from naturally occurring ores rich in toxic metals like copper, lead, iron, and silver, further amplifying the complexity of the pollution landscape (Yan et al. 2022). Soil pollution, arising from the migration of organic pollutants such as polycyclic aromatic hydrocarbons (PAHs), pesticides, herbicides, and volatile organic compounds (VOCs), adds another layer to the intricate tapestry of environmental degradation (Ma et al. 2021; Singh et al. 2023; Gupta et al. 2023). Anthropogenic sources, including open-air trash disposal, waste burning, and inadequate landfills, significantly contribute to soil pollution, marking three primary causes of this environmental concern (Li et al. 2019).
The worldwide economy and environmental pollution share an inextricable link, with the textile industry playing a pivotal role in this interplay (Olisah et al. 2021; Ramanpreet Kaur et al. 2021). China's ascendancy as the largest manufacturer of dye stuffs since 1995, producing over 200 kt annually, underscores the global significance of the textile industry in the pollution paradigm (Rafi et al. 1990). The historical attraction of humanity to color, expressed through the use of natural substances for dyeing, has evolved into a complex scenario with the advent of agriculture, permanent communities, and the utilization of textiles.
The modern textile industry, driven by a multitude of water-soluble dyes, introduces an array of environmental challenges. Dyes and pigments, integral to various sectors, are manufactured using organic solvents that chelate with metals, resulting in effluents containing acids, alkalis, salts, and nitro compounds (Gao et al. 2018; Sharma et al. 2020; Li et al. 2022a, b). The indispensable role of dyes in human life, evident in kitchenware, furniture, painted walls, and colored meals, necessitates a thorough examination of the environmental consequences of their production and usage.
The textile industry, a dynamic hub of innovation and creativity, relies on a diverse range of natural and synthetic fibers, including acrylic, wool, cotton, silk, polyester, and polyamide (Silva et al. 2021; Lin et al. 2022a, b). However, at various stages of the textile production process, the industry employs a vast array of harmful chemicals for finishing, smoothing, whitening, and sizing, further contributing to the environmental footprint (Gao et al. 2022; Kishor et al. 2021; Kaur et al. 2019; Sherwani et al. 2022). The discharge of textile dyes as untreated waste into marine ecosystems, including rivers, oceans, lakes, streams, and ponds, poses significant ecotoxicological risks with profound consequences for living organisms (Parmar et al. 2022; Meng et al. 2022).
The staggering removal of tons of azo dyes annually, driven by concerns related to cancer and mutation risks, forms a critical socioeconomic and environmental issue (Solovchenko et al. 2020; Bawazeer et al. 2022). It is against this backdrop of urgent and interconnected challenges that the need for a comprehensive review on the degradation of azo dyes arises.
The novelty of this review lies in its comprehensive exploration of the multifaceted dimensions of environmental pollution, focusing specifically on the impact of azo dyes from the textile industry. As pollution continues to escalate and pose a formidable threat to global ecosystems, the need for innovative and sustainable solutions is more pressing than ever. Traditional methods of wastewater treatment and pollution control are proving insufficient, necessitating a paradigm shift toward environmentally favorable technologies.
A critical examination of the techniques employed for the degradation of azo dyes becomes imperative in this context. This review serves as a repository of knowledge, dissecting treatment processes, physical and chemical interventions, and biological methods that hold promise in mitigating the adverse effects of azo dyes on the environment. The detailed exploration of factors influencing dye degradation, including pH, dye concentration, temperature, and structural attributes, contributes to a nuanced understanding essential for the formulation of effective remediation strategies.
The elucidation of the mechanisms orchestrating azo dye degradation by bacteria and white rot fungi adds a layer of sophistication to our understanding, paving the way for tailored and targeted interventions. Furthermore, the review ventures into other strategies utilized for azo dye degradation, providing a comprehensive overview of the evolving landscape in this critical field of study.
As the world grapples with the consequences of industrialization and globalization, the role of the textile industry in environmental pollution cannot be understated. This review, in its timeliness, seeks to bridge the existing knowledge gaps, offering insights into the current state of affairs and paving the way for future advancements in sustainable textile production and pollution control.
This review stands at the intersection of environmental science, chemistry, and engineering, presenting a holistic perspective on the challenges posed by azo dyes in the textile industry. Its significance lies not only in the critical examination of existing methodologies but also in the identification of emerging strategies that align with the principles of environmental sustainability. As we embark on this scientific journey, the urgency of addressing the environmental consequences of textile dyeing practices becomes palpable, highlighting the crucial role of this review in shaping the discourse on sustainable and eco-friendly solutions for the textile industry and beyond.
In the vast realm of synthetic dyes, the azo group stands as a distinctive and chemically significant entity, marked by the presence of the nitrogen-to-nitrogen double bond (-N = N-). This review embarks on an intricate exploration of azo dyes, delving into their chemical intricacies, industrial applications, and profound implications for environmental sustainability. A critical understanding of azo dyes is paramount, given their prevalence, vibrant coloring, and extensive use across diverse industries.
Azo dyes
Chemically characterized by the (-N = N-) azo chromophore group, azo dyes encapsulate a broad spectrum of compounds where one to three azo bonds unite phenyl and/or naphthyl rings. The versatility of these compounds is highlighted by the substitution possibilities with groups such as amino, chloro, nitro, and hydroxyl (Shindhal et al. 2021). Synthetic procedures, including hydrogenation and oxidation, yield varied azo compounds, with their vivid colors distinguishing them as a predominant class constituting nearly two-thirds of contemporary synthetic dyes (Bell et al. 2000; Chung 2016; Liu et al. 2022a, b; Ritika et al. 2018).
The chemical structure of azo dyes is not merely an aesthetic consideration; it extends to functional aspects, particularly in metal complexation. The ortho positions adjacent to the azo group provide a coordination site for metal ions, with copper(II), cobalt(III), chromium(III), etc. emerging as prominent choices for commercial metal complexes (Ghosh et al. 2016; Suo et al. 2022). This intersection of chemical structure and metal coordination underscores the versatility and applicability of azo dyes in a myriad of industrial processes.
Azo dyes, celebrated for their vibrant hues, find extensive application across diverse industries, with the textile sector serving as a prominent canvas for their expression. They play a pivotal role in coloring textiles composed of protein fibers, cellulose, and synthetic counterparts like polyester and nylon. Beyond textiles, their influence extends to culinary, printing, leather, pharmaceutical, and cosmetic domains (Oon et al. 2017; Chaudhary et al. 2018; Liu et al. 2022a, b). Figure 1 encapsulates the pervasive role of azo dyes in textile production, illustrating the various wet processes involved, including dyeing, finishing, printing, and scouring. However, this multifaceted utility comes at a cost, as the contaminants released during these processes pose environmental and health risks if not adequately treated.
The intricate wet processes depicted in Fig. 1 unveil the complexity of textile production, emphasizing the potential contaminants released during dyeing, finishing, printing, and scouring. It serves as a visual testament to the profound impact of azo dyes on the environment and underscores the necessity for rigorous treatment before their release.
A deeper comprehension of azo dyes necessitates an exploration of their chemical structure, depicted in Fig. 2. The intricate arrangement of atoms in the azo chromophore embodies the essence of these compounds. Unraveling this structural complexity is pivotal for understanding their reactivity, interactions, and, importantly, their environmental fate.
Figure 2 elucidates the chemical architecture of an azo dye, providing a visual representation of the nitrogen-to-nitrogen double bond and the phenyl/naphthyl rings. This structural insight lays the foundation for a more nuanced exploration of their behaviors and transformations in various environments.
As we stand at the intersection of technological advancement and environmental consciousness, the significance of this review becomes evident. The widespread use of azo dyes in diverse industries demands a comprehensive evaluation of their chemical intricacies, industrial applications, and, crucially, their environmental impact. The urgency of this review is underscored by the evolving landscape of environmental regulations, consumer awareness, and the imperative for sustainable industrial practices. In an era where the ecological footprint of industrial processes is under unprecedented scrutiny, a review focusing on the chemical nuances of azo dyes and their environmental implications is both timely and imperative. This work seeks to unravel the complexities surrounding azo dyes, providing a valuable resource for researchers, industry professionals, and policymakers navigating the intricate intersection of chemistry, industry, and environmental stewardship.
Acid Orange 7
In the realm of synthetic colorants, Acid Orange 7 (AO7) stands out as a ubiquitous azo dye, boasting a distinctive orange hue employed in the vibrant coloring of leather, paper, and a spectrum of textile materials, including wool, silk, and nylon. Functioning as a direct printing agent, AO7's chromatic allure is deeply intertwined with its molecular architecture and the intricate interplay of auxochromes and chromophores (Anliker et al. 1980). Auxochromes, pivotal in the color expression of dyes, act as electron-donating or electron-withdrawing substituents. They not only produce but also amplify the color of chromophores, the atomic groups responsible for imparting color to dyes. In the case of AO7, notable auxochromes include hydroxyl (OH), amine (NH3), and carboxyl (COOH), while key chromophores encompass azo (NN), sulfonate (SO3H), nitro (NO3), carbonyl (CO), and methine (CH). The environmental implications of AO7 are profound, especially when discharged untreated into water bodies. This unbridled release leads to a surge in water color and a consequential decline in water quality. Of particular concern is the anaerobic breakdown of AO7 by microorganisms within water bodies, culminating in the production of aromatic amines. These amines, recognized for their carcinogenic and mutagenic properties, pose a severe threat to both human health and the delicate balance of aquatic ecosystems.
The impact of high salinity levels, reaching 2.0% salinity, adds another layer of complexity to the AO7 conundrum. This elevated salinity adversely affects the viability of microbial cells, thereby diminishing the efficacy of AO7 removal and nutrient elimination. Addressing this challenge necessitates a strategic approach, balancing rapid adsorption and deliberate biodegradation processes. In the realm of AO7 removal strategies, a nuanced combination of rapid adsorption and deliberate, albeit slow, biodegradation processes emerges as a viable solution. The biodegradation of AO7 unfolds gradually within the anaerobic granular sludge (AGS) system, involving intricate processes such as desulfurization, deamination, decarboxylation, and hydroxylation. This stepwise mineralization of AO7 underscores the complexity of its breakdown pathways within a controlled biological environment. The imperative for dedicated research on the degradation mechanisms of AO7 becomes evident, driven by the urgent need to mitigate the environmental impact of this widespread azo dye. Understanding the intricacies of AO7 degradation pathways equips researchers and environmental stewards with valuable insights, paving the way for sustainable solutions to counteract its deleterious effects on aquatic ecosystems. In this pursuit, the scientific community plays a pivotal role in unraveling the mysteries of AO7 degradation, contributing to the broader goal of ensuring environmental health and sustainability.
A comprehensive classification of azo dyes
In the kaleidoscopic realm of synthetic dyes, azo dyes reign supreme, exhibiting a versatility that stems from their diverse origin, intricate structures, and targeted applications across various industries. This comprehensive classification, shaped by insights from Holkar et al. (2016) and Akpomie et al. (2020), delves into the nuanced categories of azo dyes, shedding light on their molecular intricacies and industrial significance. Azo dyes exhibit a rich diversity that extends beyond mere color. Structurally, these dyes can be categorized based on the number of azo bonds present. Mono-azo molecules, with a single N=N azo bond, dominate the palette, imparting color to a myriad of materials. Beyond this, the complexity unfolds with di-azo dyes, featuring two N=N bonds, and poly-azo counterparts, boasting three or more azo bonds. This structural diversity becomes a canvas for the vibrant hues that azo dyes contribute to the world of synthetic coloring (Benkhaya et al. 2020; Bharathi et al. 2022; Liu et al. 2022a, b).
The expansive utility of azo dyes becomes apparent as we explore their application across various sectors. Industries such as cosmetics, textiles, leather, paints, and printing harness the vivid properties of azo dyes extensively, weaving them into the fabric of everyday life. This broad classification underscores the ubiquitous nature of azo dyes, emphasizing their pivotal role in the coloration of diverse materials (Varjani et al. 2020). Within the azo dye spectrum, acid colors emerge as one of the most diverse groups, predominantly characterized by their anionic nature. Applied extensively to color wool and cloth, acid dyes form a bond with the cationic functional group (NH4+) of fibers. Found both in nature and synthetically produced, acid dyes encompass the azoic (red), anthraquinone (blue), and triarylmethane variants, illustrating the extensive chromatic range within this category. Reactive dyes carve a unique niche by forming covalent bonds with the "–OH," "–NH," or "–SH" groups of materials like cotton, wool, silk, or nylon. Aptly named, these reactive colors showcase a chromophore responsiveness, aligning with the substrate fiber. However, a caveat arises, as 40% to 50% of reactive dyes persist in hydrolyzing in the aqueous phase, contributing to colored water effluent. A subset of acid and reactive dyes takes on a distinctive character by incorporating metal complexes. These metal-complex dyes, although not explicitly distinguished in the Color Index, wield significant influence. Their application extends across diverse industries, bringing forth a nuanced interplay of color and metallic elements. Direct dyes showcase a high affinity for cellulose fibers, drawn together by Van der Waals forces. Meanwhile, basic dyes, characterized by their cationic nature, lend their vibrant hues to artificial fabrics such as polyester, silk, and wool. The adherence of basic dyes necessitates the use of dye mordants, contributing to their extensive use in histological research for tissue and cell staining.
Disperse dyes, predominantly azo or anthraquinone-based, exhibit low water solubility, making them ideal for specific applications. Vat dyes, initially insoluble, undergo reduction and become water-soluble with sodium dithionite treatment. Sulfur dyes, sharing similarities with vat dyes, undergo reduction and oxidation processes during textile dyeing, contributing to their unique coloration properties. Within the vast tapestry of azo dyes, solvent dyes take center stage in coloring substrates like plastic, wax, varnish, ink, and fat. Solubilized in these mediums, solvent dyes impart color to materials that remain otherwise insoluble.
Figure 3 encapsulates the essence of azo dye classification, offering a visual representation of mono-azo, di-azo, and poly-azo bonds. This molecular tapestry captures the structural intricacies that define the chromatic richness of azo dyes. The classification of azo dyes transcends color, delving into the very essence of molecular structures and industrial applications. This expansive categorization, spanning mono to poly-azo bonds and diverse application strategies, unravels the intricate tapestry of azo dyes, portraying them as indispensable contributors to the vivid palette of synthetic coloring agents.
Impact and toxicity of textile dyes
The vibrant and diverse world of textile dyes conceals a darker reality as their unbridled release into the environment wreaks havoc on ecosystems and poses a looming threat to both aquatic life and human well-being. This section delves into the multifaceted impacts and inherent toxicity of textile dyes, shedding light on the intricate web of consequences that ensue from their unregulated disposal. Textile wastewater, laden with azo dyes, emerges as a potent environmental pollutant, staining water bodies with vivid hues and threatening aquatic life. Zebrafish, a sensitive indicator of water quality, bears the brunt of this pollution. Research findings by Ahlström et al. (2005) reveal a spectrum of maladies inflicted upon zebrafish embryos exposed to varying concentrations of Azo dye. From hatching issues to genetic abnormalities, including heart enlargement, decreased heart rate, placental enlargement, and spinal distortions, the impact is profound. At higher concentrations of 100 mM, the lethal repercussions underscore the urgency of addressing azo dye contamination in aquatic ecosystems.
The insidious infiltration of Azo dyes into the soil disrupts the delicate balance of nitrogen and carbon cycles (Rehman et al. 2018). Regulatory measures, exemplified by the European Commission's restrictions on specific dyes, underscore the gravity of the issue. The prohibition of certain dyes in textiles above 30 ppm concentration and the restriction of leather and textile items containing hazardous amines, especially those in direct skin contact, aim to mitigate the environmental and health risks associated with Azo dyes (Wesenberg et al. 2003; Sood et al. 2015a, b; Tang et al. 2022).
The nexus between textile dyes and human health is fraught with peril, primarily driven by the presence of carcinogens like benzidine and other aromatic chemicals produced during microbial metabolism (Nikulina et al. 1995). Water-soluble colors, including Azo dyes, become conduits for human exposure through ingestion or direct contact. The repercussions are severe, encompassing physiological afflictions such as intermittent fever, hypertension, kidney damage, and cramping. The insidious integration of these toxins into water bodies and aquatic species through the food chain amplifies the urgency of addressing this health and environmental crisis (Asad et al. 2007).
Azo dyes, characterized by their chemical stability and synthetic nature, pose a formidable challenge to conventional wastewater treatment processes. Their slow breakdown and resistance to microbiological attacks perpetuate their persistence in the environment, emphasizing the need for innovative strategies to tackle their recalcitrance (Vinitnantharat et al. 2008). In underdeveloped nations, the use of untreated industrial effluents for agricultural irrigation presents a stark agricultural conundrum. The repercussions are felt as crop germination rates plummet, and soil quality deteriorates. This alarming trend underscores the urgency of implementing treatment plans to safeguard both agriculture and the overarching ecosystem, ensuring sustainability for future generations (Sojobi et al., 2022; Manickam et al., 2021; Lamba et al. 2015; Sood et al. 2015a, b).
The imperative for sustainable practices echoes loudly in the face of escalating environmental degradation caused by textile dye contaminants. Addressing the toxicity and carcinogenicity of these substances requires concerted efforts, innovative treatment methodologies, and stringent regulations. As textile dyes continue to infiltrate the intricate tapestry of ecosystems, the call for sustainable practices and holistic solutions becomes more urgent than ever (Mudhoo et al. 2020).
The impact and toxicity of textile dyes extend far beyond the realms of aesthetics, unraveling a narrative of environmental peril, human health risks, and agricultural vulnerabilities. Mitigating these threats demands a collective commitment to sustainable practices, innovative technologies, and unwavering regulatory measures to safeguard the delicate balance of our ecosystems.
The captivating array of colors that enrich our fabrics belies a concealed threat, as textile dyes, when not meticulously managed, can unleash a myriad of health hazards. Khan et al. (2014) underscore the potential impact on the immune system, unveiling a phenomenon known as respiratory sharpening. The consequences range from mild irritations, such as tingling, watering eyes, and sniffling, to more severe asthmatic symptoms, including wheezing and hacking. Beyond the immediate discomfort, the long-term ramifications are ominous, with the potential to escalate to heightened immune reactivity upon subsequent exposure.
The sinister alliance between azo dyes and various cancers further intensifies the health risks. Azo dye consumption has been linked to elevated risks of bladder cancer, splenic sarcomas, and hepatocarcinoma, painting a dire picture of the potential consequences for human health (Mishra et al. 2014). Skin ailments, allergies, and DNA damage add another layer to this complex narrative, with the latter paving the way for the insidious growth of cancerous tumors. Methyl Yellow dye, once used illicitly to color butter, serves as a chilling example of the latent dangers, inducing liver cancer in rats after mere months of exposure. Similarly, Amaranth, implicated in DNA damage in mouse colon epithelial cells, serves as a poignant reminder of the genotoxic potential of certain dyes (Ali et al. 2020).
Figure 4 demonstrates the comprehensive spectrum of effects that azo dyes can impose on human health, underscoring the urgency of addressing these health hazards. The list includes tartrazine, associated with angioedema, nasal congestion, itchy skin, and hives, as well as the potential to bind to albumin, induce neurotoxicity, and impair mental abilities (Zanoni et al. 2013). The hepatocarcinogenicity of printer dye 4-aminobenzene, also known as aniline yellow, further accentuates the range of adverse health outcomes linked to dye exposure (Chung 2016). Sudan III, commonly used in coloring non-polar materials, adds to the ominous tally, with documented links to cancer (Puvaneswari et al. 2006).
The deleterious impact of textile dye pollution necessitates urgent remediation strategies. Physico-chemical treatments, including electrocoagulation, coagulation–flocculation, and adsorption, have been employed to address the issue. However, these methods bear significant drawbacks, ranging from high costs and substantial sludge production to the generation of harmful substances, such as aromatic amines, which pose disposal challenges (Bayramonglu et al., 2007; Wang 1990; Ghaly et al. 2014). Figure 4 encapsulates the complex effects of azo dyes on human health, urging the exploration of alternative and more sustainable solutions.
The quest for affordable and effective solutions to tackle textile wastewater pollution has spurred a paradigm shift toward bioremediation. This biological method emerges as a promising avenue, boasting advantages of lower costs, increased sustainability, and enhanced safety when compared to chemical and physical reclamation methods. Bioremediation, a process that leverages the natural capabilities of microorganisms to break down pollutants, offers a more eco-friendly approach to addressing the menace of dye pollution (Bayramonglu et al., 2007).
The significance of biodegrading azo dyes extends beyond immediate remediation. It provides a sustainable and eco-friendly avenue for addressing the chronic issue of dye pollution, offering insights into the mechanisms that drive the breakdown of these persistent compounds. This understanding becomes a catalyst for developing more efficient and effective bioremediation methods, ensuring a holistic and sustainable approach to environmental stewardship. In a world increasingly focused on sustainable production, bacterial bioremediation emerges as a crucial player in the endeavor to balance industrial progress with ecological responsibility. As we navigate the complexities of textile dye pollution, the importance of embracing innovative and eco-conscious solutions becomes more apparent, steering us toward a future where environmental health and industrial activity coexist harmoniously.
Techniques for azo dye degradation
As we grapple with the intricate challenge of azo dye degradation, the selection of an appropriate treatment technology becomes a pivotal decision. It is a multidimensional choice, intricately tied to the presence of additional inorganic and organic elements, their toxicity, and the mandated environmental discharge levels (Li et al. 2022a, b; Zhang et al. 2019). The daunting task is further exacerbated by the remarkable water solubility of azo dyes, making their removal through conventional techniques a formidable challenge (Varjani et al. 2021).
Figure 5 serves as a visual testament to the myriad factors that intricately interplay when determining the most suitable technology for textile dye degradation. The decision-making process involves a delicate balance, considering not only the nature of the dye but also the broader environmental implications. This intricate dance between elements underscores the need for a nuanced approach to azo dye treatment.
The vast landscape of dye wastewater treatment unfolds through three primary categories: chemical, physical, and biological treatment. This triad forms the backbone of the industrial arsenal against dye pollution, with a spectrum of treatments employed at various stages of the effluent management process. The journey begins with preliminary treatment, encompassing equalization and neutralization, laying the foundation for subsequent interventions (Atul et al. 2012). Sedimentation, screening, chemical coagulation, flocculation, and floating constitute the initial steps, orchestrating the removal of impurities and preparing the effluent for further refinement. The secondary treatment phase comes into play, introducing methods such as physical–chemical separation and biological oxidation to target organic molecules and elevate the treatment efficiency (Mandal et al. 2020). It is a delicate choreography, a symphony of processes working in harmony to transform the polluted effluent into a more benign form. The crescendo of this treatment symphony is the tertiary treatment, a pivotal phase that significantly enhances the overall efficacy of effluent treatment. As illustrated in Fig. 6, this stage acts as a refining touch, addressing residual contaminants and ensuring that the effluent released into the environment meets stringent standards (Mandal et al. 2020). The array of treatment processes for azo dye degradation showcased in Fig. 6 underscores the diverse strategies employed, each tailored to the unique demands of the effluent at hand.
The journey through the intricacies of azo dye degradation treatment is a testament to the ongoing efforts to harmonize industrial progress with environmental responsibility. The selection of treatment technologies is not merely a technical decision; it is a conscientious choice that echoes through the delicate ecosystems impacted by dye pollution. As we navigate the complexities of dye wastewater treatment, the imperative remains to seek sustainable and effective solutions that safeguard our environment for future generations.
Efficient wastewater treatment is a multistage process, crucial for mitigating the environmental impact of dye pollutants. Each stage plays a distinctive role in ensuring the transformation of contaminated effluent into a form compatible with environmental standards.
Preliminary treatment
The journey commences with the equalization and mixing of wastewater streams from diverse manufacturing operations. This preliminary stage ensures a harmonious blend, establishing consistent temperature, pollutant load, and pH characteristics. This equalization process sets the stage for subsequent treatments, laying the foundation for a more efficient and streamlined purification process.
Primary treatment
Primary treatments unfold as a symphony of screening, sedimentation, and flotation. While these methods effectively tackle many pollutants, fine, suspended, and colloidal impurities pose challenges. Chemical coagulation emerges as a key player, employing substances like alum, polyelectrolyte, ferrous sulfate, ferric chloride, and lime. This not only aids in flash mixing but also facilitates the reduction of COD and BOD. However, the chemical coagulation process proves more effective in decolorizing insoluble dyes than their soluble counterparts (Xue et al. 2019; Khouni et al. 2011).
Secondary treatment/biological treatment
Secondary wastewater treatment takes center stage in stabilizing color and dissolved organic components. Microbial wonders, including bacteria, fungi, and yeast, engage in a vital dance to complete this intricate process. Both anaerobic and aerobic processes come into play, providing a versatile approach. Anaerobic processes facilitate waste digestion, contingent on factors such as temperature, pH, oxygen levels, and the presence of toxic materials. In contrast, aerobic processes showcase the transformative prowess of microorganisms, progressively altering colloidal substances, oxidizing organic molecules, and converting organic nitrogenous substances. Though aerobic treatment has proven less effective for azo dyes, its utilization remains situational (Carmen et al. 2012; Popli and Patel 2015).
Tertiary treatment/chemical treatment
Tertiary treatment takes the effluent refinement to its zenith, addressing organic colorants, dissolved solids, and adsorption through membrane filtration methods. The wastewater from the dye industry, laden with hazardous colors, demands an enhanced treatment approach. The use of oxidizing agents like ozone in tertiary treatment proves instrumental in eliminating various contaminants, providing a final touch to the purification process.
Physical and chemical treatment of azo dye
The arsenal of methods for azo dye treatment includes both physical and chemical techniques, a testament to the multifaceted challenge posed by these synthetic colors. Physical approaches encompass filtration and adsorption, with activated carbon emerging as a preferred yet cost-prohibitive choice. Membrane filtration methods such as reverse osmosis, nano-filtration, and ultrafiltration find application for water reuse and chemical recovery, albeit with challenges like high upfront costs and the potential for membrane fouling.
Chemical treatment, on the other hand, deploys various substances and methods, including electrochemical processes, ozone, coagulation–flocculation, and Fenton's reagent. Flocculation and coagulation are succeeded by sedimentation in typical wastewater treatment facilities, although challenges persist, with some dyes proving resistant to conventional remedies. This underscores the urgency of exploring environmentally acceptable methods to manage synthetic colors, particularly the stubborn azo dyes.
As depicted in Fig. 7, diverse physicochemical methods, such as coagulation, adsorption, membrane separation, ion exchange, and more, contribute to the intricate tapestry of azo dye degradation. This visual representation serves as a comprehensive guide, outlining the array of physical and chemical techniques employed in the pursuit of sustainable and effective solutions.
As the treatment processes for azo dye degradation unfold, the imperative remains to navigate the complexities with a commitment to sustainability. Balancing industrial progress with environmental responsibility, the ongoing quest for safer, more efficient methods underscores the dynamic nature of wastewater treatment in the face of evolving challenges. Each stage in this journey is a testament to our collective responsibility, steering toward a future where the impact of dye pollutants is mitigated for the well-being of both ecosystems and future generations.
Coagulation
In the intricate symphony of wastewater treatment, coagulation stands out as a powerful pre-treatment process, renowned for its efficiency, simplicity, and minimal initial investment (Liang et al. 2014). This technique proves invaluable in the removal of turbidity particles and organic debris from the aquatic environment. A key application of coagulation emerges in the realm of dye removal, where it orchestrates the formation of flocs, destabilizing dye solution systems (Fig. 8) (Zhao et al. 2018).
Conventional metal coagulants, such as ferrous sulfate and ferric chloride, are gradually losing favor due to their limited applicability, propensity for excessive sludge formation, and sensitivity to pH variations (Zheng et al. 2020). The evolving landscape of coagulation has witnessed the emergence of various polymeric substances, both natural and synthetic, with exceptional flocculation capabilities. Notable examples include modified chitosan- and dextran-based substances, some even graft-modified, showcasing their prowess in the formation of effective flocs (Anjaneyulu et al. 2005).
In a study by Shi et al. (2007), coagulation with aluminum chloride (AlCl3), polyaluminum chloride (PAC), and pure Al13 demonstrated efficacy in removing Direct black 19, Direct red 28, and Direct blue 86. The removal rate exhibited an upward trajectory with increasing coagulant doses, with the efficiency ranking as Al13 > PAC > AlCl3. Man et al. (2012) delved into the realm of thermolytic coagulation–flocculation for curing G. Malachite color from contaminated water. The investigation revealed that coagulation–flocculation treatment of fresh green dye aqueous solution surpassed thermolysis, resulting in enhanced malachite green dye treatment. The removal of approximately 90% of the color was achieved at basic pH levels.
As depicted in Fig. 8, the coagulation technique is visually encapsulated, showcasing its prowess in precipitating contaminants from wastewater. This visual representation serves as a concise guide, emphasizing the significance of coagulation in the larger framework of wastewater treatment. The coagulation process, with its nuanced ability to destabilize and remove pollutants, stands as a foundational pillar in the pursuit of cleaner and more sustainable water treatment processes. Its versatility and efficacy make it a crucial tool in the ongoing endeavors to address the environmental challenges posed by dye pollutants.
Ion exchange
In the intricate tapestry of wastewater treatment, the ion exchange process emerges as a strategic player, aiming to transform the molecular composition of wastewater. The fundamental objective is to guide wastewater through ion exchanger resin until each binding site saturates. While this method has demonstrated efficacy, a notable drawback surfaces—the ion exchanger's limited capacity to handle a diverse range of dyes. Consequently, its application is somewhat restricted in treating effluents laden with dyes, especially considering the large sludge-producing capacities associated with this technique (Burakov et al. 2018).
Figure 9 provides a visual depiction of the ion exchange process for wastewater treatment. This schematic representation encapsulates the essence of the ion exchange technique, showcasing its operational principles in addressing wastewater challenges. The ion exchange process operates on the principle of selective exchange of ions between a solid phase (ion exchange resin) and a liquid phase (wastewater). This dynamic interaction facilitates the removal of specific ions or contaminants from the wastewater, paving the way for a more refined and purified effluent. While the ion exchange process may not be the panacea for all wastewater challenges, its targeted approach to ion removal renders it a valuable asset in the broader spectrum of wastewater treatment methodologies. As innovations continue to shape the landscape of environmental remediation, the ion exchange process stands resilient in its contribution to the pursuit of sustainable and effective wastewater treatment.
Adsorption
The multifaceted phenomenon of adsorption stands as a pivotal paradigm in the realm of wastewater treatment, intricately weaving molecular dynamics and surface interactions. Defined by two distinct processes—chemisorption and physisorption—adsorption unveils a captivating molecular ballet, dictating the enthralling interplay between contaminants and solid adsorbent surfaces (Saravanan et al. 2022a, b). A symphony of forces governs the adsorption process, each force playing a unique role in the molecular tango. Electrostatic interactions, arising from the charge distribution of molecules, contribute to the initial attraction. Van der Waals forces, characterized by dipole–dipole interactions and London dispersion forces, accentuate the cohesive binding. Hydrophobic interactions further influence the adsorption landscape, orchestrating the affinity of non-polar molecules. Hydrogen bonding, a testament to molecular diplomacy, enhances the adhesive bonds between adsorbent and adsorbate (Saravanan et al. 2022a, b).
The ensemble of adsorbents participating in this molecular ballet is diverse, each bringing its unique characteristics to the performance. Zeolites, with their crystalline structures, display selective adsorption capabilities. Alumina, recognized for its surface reactivity, partakes in the adsorption repertoire. Silica gel, with its porous matrix, facilitates molecular entrapment. Activated carbon, the virtuoso of adsorbents, steals the limelight owing to its reusability and high adsorption efficiency (Samsami et al. 2020).
Among the virtuosos, activated carbon emerges as a luminary, offering a captivating performance in the adsorption spectacle. The meticulously crafted dance of methylene blue adsorption onto activated carbon derived from oil palm fiber epitomizes the nuanced equilibrium of pH, contact time, concentration, and temperature. With a BET surface area of 1354 m2/g and a porous architecture, activated carbon unveils a mesmerizing act in rapidly attaining equilibrium during the adsorption process (Tan et al. 2009; Gomez et al. 2007).
In a parallel act, the bio-composite ensemble, comprising sugarcane bagasse chitosan, starch, and polyaniline, emerges as a dynamic participant. The performance unfolds with pH, retention durations, initial dye concentration, and temperature influencing the adsorption choreography. This bio-composite symphony, marked by exothermic nature, positions itself as an eco-friendly contender in the realm of dye removal (Noreen et al. 2020).
The walnut shell, an unconventional yet effective adsorbent, takes center stage in its own overture. Its prowess in removing Acid Red dye from aqueous solutions underscores the potential diversity in materials contributing to the grand tapestry of wastewater treatment methodologies (Mokri et al. 2015).
Figure 10 provides a visual crescendo, capturing the essence of molecular choreography in contaminant removal. This captivating dance not only showcases the scientific intricacies but also holds promise for sustainable and effective wastewater treatment modalities. The molecular ballet of adsorption continues to unfold, presenting a spectrum of possibilities in the pursuit of environmental stewardship.
Advanced oxidation
In the pursuit of decolorizing water tainted with the stubborn vestiges of pigments, advanced oxidation stands as a beacon of transformative environmental alchemy (Banerjee et al. 2018). This sophisticated arsenal of techniques encompasses an array of processes such as photolysis, photocatalysis, sonolysis, sonocatalysis, ozonolysis, moist air oxidation, and the Fenton process. Within this orchestration of environmental alchemy, photocatalysis emerges as the virtuoso, displaying unparalleled efficacy in the annihilation of dye residues from wastewater (Gayathri et al. 2019). The crown jewel within the realm of advanced oxidation, photocatalysis orchestrates a symphony of light-induced degradation. Leveraging semiconductors, often titanium dioxide (TiO2) or zinc oxide (ZnO), as catalysts, this process harnesses the transformative power of photons to initiate radical generation. The ensuing radicals, such as hydroxyl radicals (•OH), embark on a molecular crusade, dismantling dye molecules with precision and efficacy (Cotillas et al. 2018). Delving into the avant-garde, Cotillas et al. (2018) unfurled the prowess of conductive-diamond electrolytic oxidation in the removal of Red MX-5B dye from wastewater. The catalytic dance of diamond electrodes, under the influence of electrolysis, engendered the electrogeneration of oxidizing chemicals. This catalytic choreography rendered Red MX-5B dye utterly vanquished, marking a triumph in the realm of electrolytic oxidation.
Membrane separation
In the dynamic landscape of wastewater treatment, the avant-garde prowess of membrane separation emerges as a beacon of precision and efficiency, particularly in the removal of recalcitrant azo dyes. This sophisticated physical technology harnesses the principle of selective permeability to orchestrate the removal of dye molecules, heralding a new era in water purification (Behera et al. 2021). At the forefront of membrane separation, nanofiltration (NF) stands as a technological marvel with membranes characterized by minuscule pores, typically ranging between 0.5 and 0.2 nm in diameter (Dasgupta et al. 2015). This meticulous engineering allows for the separation of color molecules in polluted water solutions based on both size and electrostatic repulsion principles. The result is a dye-free solution, epitomizing the finesse of nanofiltration technology (Wang et al. 2020a).
In tandem with membrane filtration, reverse osmosis (RO) unfurls its capabilities as a stalwart in industrial water purification. The membranes utilized in RO, acting as selective barriers, facilitate the concentration and separation of contaminants without necessitating a change in state or the deployment of thermal energy (Wu et al. 2022). This elegant process ensures the production of high-quality water, substantiating its widespread application in the treatment of water laden with dyes (Wang et al. 2020b; Liang et al. 2021).
Delving into the specifics, Wu et al. (2008) delved into the efficacy of cation exchange membranes in the removal of methyl violet dye (2B) from aqueous solutions. Employing a batch approach, the investigation unfolded with a focus on estimating desorption efficiency. The findings highlighted that cation exchange membranes wield formidable prowess in efficiently removing cationic dye methyl violet from water, ensuring maximal dye recovery through desorption. Figure 11 unfolds the tapestry of membrane separation techniques, each thread weaving a narrative of precision and selectivity. As environmental custodians navigate the intricate realm of membrane separation, the promise of water purification and azo dye degradation stands resilient, offering a glimpse into a purified and sustainable future.
Biological methods for the discoloration of azo dyes
Amidst the evolving landscape of textile wastewater treatment, the spotlight turns toward the realm of biological methods, where the orchestration of microbial symphonies becomes a cost-effective and environmentally responsible choice. Within this paradigm, the paramount goal is the breakdown, mineralization, and detoxification of pernicious azo dyes, transmuting them into forms less menacing to the ecosystem. The biological ballet unfolds through biodegradation, where intricate hues are decolonized, and complex color structures are dismantled into more manageable constituents.
Biodegradation, the virtuoso performance orchestrated by populations of bacteria, fungi, algae, and yeasts, manifests its efficacy in both oxygenic and non-oxygenic conditions. This environmentally conscious approach champions the breakdown of azo dyes, charting a course toward remediation of the world's wastewater challenges (Shah 2014). The ascendancy of biological methods in wastewater treatment is underpinned by their economic viability and eco-friendly disposition.
Dye degradation unfolds harmoniously in diverse environmental conditions, encompassing both anaerobic and aerobic milieus. This versatility empowers microbial communities to thrive and perform their biotransformational feats, demonstrating the adaptability and resilience of biological approaches in diverse wastewater scenarios. At the heart of this microbial alchemy lies a repertoire of microbial enzymes, each playing a pivotal role in the decolorization of azo dyes. Azo reductases, NADH-DCIP reductase, and ligninolytic enzymes such as laccase (Lac), manganese peroxidase (MnP), and lignin peroxidase (LiP) emerge as the enzymatic virtuosos orchestrating the transformative dance of dye molecules (Kalyani et al. 2009). The substrate specificity and catalytic finesse of these enzymes contribute to the efficiency of dye decolorization, unveiling the biochemical ballet underlying the microbial remediation of azo dyes.
Biological methods stand as stalwart custodians of the environment, offering a symphonic solution to the challenges posed by azo dyes in textile wastewater. As microbial maestros continue their transformative play, the stage is set for a sustainable and eco-friendly future in wastewater treatment.
Decolorization and deterioration of azo dye by bacteria
In the realm of wastewater treatment, bacteria emerge as versatile virtuosos, capable of adapting to diverse environmental conditions encompassing variations in oxygen levels, pH, and temperature. Their prowess in industrial wastewater treatment is underscored by their agility and efficiency, often surpassing fungi in the decolorization process (Carvalho et al. 2008; Lin et al., 2008). Bacterial decolorization, notably faster than fungal counterparts, becomes especially pronounced in anoxic environments, eliciting the production of aromatic amines (Carliell et al. 1994).
Despite the inherent resilience of azo dyes to bacterial attack in oxygenated conditions, certain aerobic bacteria deploy azo reductases to initiate the breakdown of azo bonds (Olukanni, 2009). The reductive cleavage of the -N = N bond serves as the catalyst for bacterial degradation of synthetic dyes. Bacterial cohorts, spanning various taxonomic groups, exhibit the capacity to decolorize azo dyes under diverse conditions including non-oxygenic (methanogenic), anoxic, and oxygenic environments. This diverse spectrum of bacteria sourced from unmixed cultures, mixed cultures, anaerobic sediments, granular anaerobic sludge, and activated sludge under non-oxygenic conditions, illustrates the broad applicability of bacterial involvement in the degradation of azo dyes (Wuhrmann et al. 1980; Carliell et al. 1994; Ali et al. 2019). The interplay between bacterial prowess and environmental conditions is exemplified in instances such as Reactive dye decolorization by Micrococcus species, showcasing a discrepancy in decolorization periods between anaerobic (24 h) and aerobic (6 h) environments (Walker 1970).
Various bacterial species, including but not limited to Klebsiella, Bacillus, Rhodococcus, and Shigella, have been identified as capable agents in the breakdown of azo dyes (Erden et al. 2011; Guo et al. 2020; Chen et al. 2021a, b; Wang et al. 2022). The utilization of bacteria in azo dye degradation is accentuated by their ease of cultivation, faster specific growth rates compared to other microbes, and a diverse catalytic repertoire for the mineralization of azo dyes in contaminated water (Patil et al. 2022).
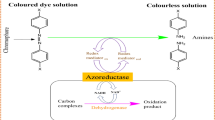
Figure 12 demonstrates a typical schematic for the bacterial-assisted biodegradation of azo dyes. Initially, the azo dye molecules permeate the aquatic environment, originating from industrial wastewater effluents. These synthetic hues, though visually striking, pose environmental hazards. The bacterial warriors, portrayed as resilient microorganisms, are the key players in this ecological drama. These bacteria, carefully selected for their adeptness in azo dye degradation, wield enzymatic tools to commence the intricate process. Enzymes such as azo reductases, NADH-DCIP reductase, and ligninolytic enzymes stand as the weaponry in the bacterial arsenal. These enzymes, secreted by the bacterial forces, target the azo bonds within the dye molecules. A pivotal moment in the narrative unfolds as the bacterial enzymes engage in precise azo bond cleavage. This biochemical maneuver disintegrates the azo bonds, breaking down the complex dye structures into simpler, less harmful components. The byproduct of azo bond cleavage includes aromatic amines. Under anaerobic conditions, reducing equivalents generated during the microbial degradation process contribute to the creation of aromatic amines. The process culminates in the complete biodegradation of azo dyes, rendering the once vivid pollutants into innocuous substances. This marks a triumph for bacterial-assisted biodegradation in mitigating the environmental impact of azo dyes.
This schematic encapsulates the essence of the intricate ballet orchestrated by bacteria in dismantling the chromatic threat of azo dyes, emphasizing the pivotal role of microbial communities in sustainable wastewater treatment practices. Table 1 exhibits a list of bacteria used for the degradation of azo dye with their % decolorization. Table 1 stands as a testament to the prowess of various bacterial species enlisted in the noble quest of azo dye degradation. Each entry in this tableau represents a bacterial champion, armed with enzymatic weaponry, striving to decolorize and detoxify industrial wastewater tainted by vibrant synthetic dyes. The percentages of decolorization associated with each bacterial hero serve as metrics of their efficiency in dismantling the chromatic threat. As we delve into the microbial hall of fame, we witness the diverse bacterial forces contributing to sustainable and eco-friendly solutions in the battle against azo dye pollution.
Decolorization and deterioration of azo dye by fungi
Fungi, with their intricate and adaptive metabolisms, emerge as adept artisans in the biodegradation ballet of azo dyes. Flourishing in diverse environments such as soil, active plants, and organic waste, filamentous fungi is ubiquitous, offering potential as both biosorbents and biodegraders of dyes. The ability of mushrooms to swiftly modulate their metabolism in response to varying carbon and nitrogen sources is crucial for their survival.
Fungi, characterized by their filamentous structure, represent an advantageous bioadsorbent owing to their abundant biomass, cost-effectiveness, and remarkable chemical and mechanical stability in both acidic and alkaline environments. The rigid structure of macrofungi enhances biosorption, with temperature and dye reaction kinetics influencing their adsorptive capacity. However, challenges such as adsorbent surface deactivation or a reduction in the number of active sites can be encountered during the process (Asgher et al. 2008).
The employment of fungi as a sensible approach for dye decolorization is underscored by its cost-effectiveness, environmental safety, and the potential for complete dye degradation. Fungi exhibit a rich repertoire of intracellular and extracellular enzymes that contribute to the degradation of complex organic pollutants, including polyaromatic hydrocarbons, organic waste, azo dye effluents, and steroid chemicals (Bhatia et al. 2022; Jiku et al. 2021).
Among the fungi with noteworthy decolorizing capabilities, those inducing white rot, such as Phanerochaete chrysosporium, stand out as particularly effective (Lim et al. 2010; Robinson et al. 2001). Despite their prowess, the use of fungi for color removal from textile effluents presents challenges, including prolonged developmental and sludge retention phases required for complete decoloration and the need for nitrogen-less conditions. Table 2 provides a list of fungi used for the degradation of azo dyes with their % decolorization.
Decolorization and deterioration of azo dyes by algal species:
The realm of azo dye remediation expands to embrace the chlorophyll-rich allies—algae. Table 3 unfolds the botanical warriors enlisted in the cause, showcasing their formidable decolorization capabilities against the vibrant onslaught of synthetic dyes. Algae, including species like Chlorella vvulgaris, Oscillatoria rubescens, Elakatothrix viridis, Lyngbya lagerlerimi, Nostoc linckia, and Volvox aureus, emerge as key players in this eco-friendly battle against dye pollution.
These photosynthetic powerhouses utilize their diverse cell wall functional groups, featuring carboxy, carbonyl, hydroxy, phosphoryl, and amide groups, as strategic arsenals for dye decolorization (Mishra et al. 2020). The enzymatic prowess of azoreductase, found within algae, becomes a catalyst for the breakdown of azo dyes into less harmful aromatic amines (Khataee et al. 2011). In aquatic ecosystems, these algal superheroes not only combat the detrimental impact of dye-contaminated water on marine life but also stand as cost-effective champions due to their ability to harness solar energy for photosynthesis. The percentages of decolorization associated with each algal species in Table 3 shed light on their individual contributions to the collective mission of azo dye degradation.
Decolorization and deterioration of azo dyes by yeast
In the vast kingdom of microorganisms, yeasts stand out as swift responders and survivors in challenging environmental conditions. With a repertoire that includes both adsorption and enzymatic disintegration, yeasts, akin to fungi, join the ranks of nature's champions in the crusade against azo dye pollution (Dilarri et al. 2018). The yeast arsenal boasts key players such as Galactomyces geotrichum and Saccharomyces cerevisiae, exhibiting their prowess in absorbing significant concentrations of dye. Glucose emerges as a vital carbon source for the yeast-driven degradation of dyes, setting the stage for the development of oxidases, reductases, and yeast NADH-DCIP reductase in response to azo dye exposure (Singh and Singh 2015).
Thriving in harsh environments and matching the rapid multiplication rates of bacteria, yeasts from the Candida genus take center stage in the process of dye decolorization. Employing diverse strategies such as biosorption, biodegradation, or bioaugmentation, these yeasts, showcased in Table 4, contribute their unique percentages of decolorization to the collective symphony of biological azo dye degradation. Figure 13 encapsulates the biological degradation for the removal of azo dyes from our ecosystems.
Factors affecting the dye degradation
In the intricate world of microbial treatment, various parameters wield a significant influence on the efficacy of dye degradation. For a microbial orchestra to achieve its highest rate of dye reduction, a delicate balance in oxygen content, temperature, pH, initial dye concentration, types of dye, and redox potential must be maintained within optimal ranges.
Oxygen Content: The availability of oxygen is a critical factor, determining whether the microbial players engage in aerobic or anaerobic degradation pathways. The oxygen dance profoundly impacts the efficiency of dye reduction.
Temperature: The temperature of the microbial habitat acts as a maestro guiding the tempo of microbial activities. Fluctuations in temperature can either invigorate or impede the microbial symphony, influencing the rate of dye decomposition.
pH Levels: The pH of the milieu serves as the conductor's baton, directing the microbial ensemble. Optimal pH conditions ensure that the microbial performers are in harmony, accelerating the reduction of dyes.
Initial Dye Concentration: The starting concentration of dyes in the wastewater sets the stage for microbial action. Microbes exhibit varying degrees of proficiency based on the intensity of the color challenge they encounter.
Types of Dye: The diverse array of dye genres, from acidic and basic to direct, dispersion, metal-complex, reactive, sulfur, and vat, poses a palette of challenges. Understanding the microbial capacity to tackle this spectrum is crucial for effective treatment.
Redox Potential: The redox potential orchestrates the electron exchange ballet, influencing microbial reactions. A balanced redox environment is pivotal for optimal dye degradation by microbial agents.
Textile Wastewater Composition: The composition of textile wastewater is akin to the musical composition of a symphony, with organics, nutrients, salts, sulfur compounds, poisons, and colors playing diverse roles. The microbes' ability to navigate this diverse composition impacts their effectiveness in global cycles of carbon, nitrogen, and sulfur.
As depicted in Fig. 14, these factors converge in a complex interplay, affecting the biological degradation of azo dyes. The understanding of these parameters serves as a compass, guiding the optimization of microbial treatments and paving the way for sustainable and effective solutions in the realm of dye pollution.
Impact of pH
The pH of microbial media is akin to the conductor of a symphony, orchestrating the intricate interplay of decolorization and dye degradation. This crucial parameter profoundly influences not only the degree of decolorization but also the solubility of the dye and the overall hue of the solution.
In the microbial world, fungi and bacteria showcase distinct pH preferences in their decolorization endeavors. Fungi typically exhibit heightened decolorization and degradation activities in acidic or neutral pH environments, while bacteria showcase optimal prowess in basic or neutral pH conditions. The movement of dye molecules across cell membranes, a pivotal phase in the decolorization process, is intricately linked to pH dynamics.
For fungi, the acidic range emerges as the sweet spot for optimal dye decoloration. Effluent pH plays a pivotal role in dye biodegradation, with the highest dye-degrading capacity observed in neutral to slightly alkaline pH conditions (Ghaly et al. 2014; Pinheiro et al. 2022). A nuanced understanding of the effluent pH becomes imperative for successful bacterial growth, as dormant bacteria render dye degradation impossible. Strategic adjustments to effluent pH or the selection of bacteria resilient to specific pH conditions become vital considerations in the pursuit of effective dye degradation.
Effect of dye concentration
The concentration of dyes in the reaction media serves as a metabolic threshold, significantly influencing the efficiency of dye degradation or decolorization. Textile effluent presents a spectrum of dye concentrations, with documented levels ranging between 10 and 50 mg/L (Laing 1991) and others reporting concentrations between 10 and 250 mg/L (Sharma et al. 2016).
In the microbial realm, the effectiveness of bacteria in industrial applications hinges on their capacity to efficiently break down or decolorize target dyes without succumbing to inhibition at these concentrations. Studies underscore that an escalation in dye concentration exerts a diminishing effect on bacterial efficiency, primarily due to the toxicity of dyes at higher concentrations. This toxicity imposes constraints on the metabolic processes of bacteria, limiting their efficiency (Cui et al. 2014; Kapoor et al. 2021).
Several factors contribute to decreased breakdown efficiency at elevated dye concentrations, including an inadequate biomass-to-dye ratio and the impediment of enzyme active sites by excess dye molecules (Chang et al. 2001). Striking a delicate balance between dye concentration and bacterial metabolic capacity becomes paramount in optimizing dye degradation efficiency.
Impact of temperature
Temperature emerges as a pivotal determinant influencing microbial growth, metabolism, and ultimately, dye degradation. The ideal temperature creates a conducive environment for bacterial growth, survival, and metabolic processes. The rate of color loss exhibits a positive correlation with temperature, with the optimal growth temperature aligning with the temperature conducive to the highest rate of color removal.
However, the ballet of temperature influence is nuanced. A rise in temperature fosters an increase in the rate of color removal, but a subsequent decline is attributed to the loss of cell viability or denaturation of azoreductase enzymes. Microorganisms, governed by enzymes, display varied temperature adaptations, directly impacting the pace of enzymatic reactions and microbial growth (Çetin et al. 2006).
Conversely, low or excessively high temperatures can impede the degradation rate of bacteria, either due to cell inactivation induced by low temperatures or denaturation at higher temperatures (Lalnunhlimi et al. 2016). Thus, temperature functions as both an accelerant and a potential deterrent in the microbial theater of dye degradation.
Effect of dye structure
Microbial efficiency in removing azo dyes is intricately linked to the structural complexity of the dyes. While certain azo colors pose greater challenges for microbial removal, those with simpler structures and lower molecular weights are more frequently subjected to successful color removal.
Microbial enzymes, particularly azo reductases, exhibit remarkable selectivity toward specific dye structures. For instance, the azo groups in orange I and its derivatives are selectively broken down by orange I azoreductase when hydroxy groups are positioned in the para position. Another enzyme, orange II azoreductase, exclusively acts on compounds of the orange II type with the hydroxy group in the ortho position.
Structural nuances play a significant role in the microbial breakdown of azo phenol. Electron-donating methyl and methoxy substituents accelerate enzymatic breakdown, while electron-withdrawing chloro, fluoro, and nitro substituents impede oxidation (Verma et al. 2017).
The pH, dye concentration, temperature, and dye structure intricately choreograph the microbial ballet of dye degradation. The mastery of these parameters is essential for crafting efficient and sustainable strategies in combating dye pollution, paving the way for environmentally conscious wastewater treatment.
Effect of agitating and non-agitating conditions
The efficiency of bacterial biodegradation encounters yet another variable—the rhythmic dance between agitating and non-agitating conditions. This nuanced interplay, as highlighted by Cui et al. (2014), significantly shapes the efficiency of bacterial degradation processes.
Under the spotlight of agitating conditions, a notable transformation occurs—the augmentation of dissolved oxygen levels. This surge in oxygen becomes the fuel for microbial enzyme activity, fostering an environment conducive to heightened bacterial metabolic activity (Benkhaya et al. 2020). The result is an enhanced capacity of bacteria to degrade dyes, a phenomenon intricately linked to the high metabolic activity of bacterial enzymes.
However, this seemingly beneficial symphony of agitation is not without its caveats. While shaking conditions elevate the degradation rate, a delicate balance must be maintained. Excessive oxygen in the reaction medium, a consequence of vigorous agitation, can hinder the functionality of reductive enzymes, such as azo reductase. The heightened oxygen levels create a stronger redox potential, steering the reductive enzymes toward the reduction of the redox mediator rather than the targeted dye. This unintended diversion diminishes the degradation rate of bacterial species, underscoring the need for a judicious balance in agitation dynamics (Chang et al. 2001).
In the dynamic realm of microbial dye degradation, the choice between agitation and stillness becomes a strategic consideration. Striking the right balance is akin to orchestrating a symphony, where each note—whether stirred or tranquil—contributes to the harmonious efficiency of bacterial biodegradation.
Mechanisms of degradation of azo dyes degradation
Mechanisms of degradation of azo dyes degradation with the help of bacteria
Understanding the intricate mechanisms employed by bacteria in the degradation of azo dyes unveils a symphony of biochemical transformations, shedding light on the dual processes of direct decolorization and indirect/mediated decolorization. These processes, governed by a repertoire of enzymes, hold significant promise for environmentally friendly and effective azo dye treatment (Verma et al. 2017).
In the realm of azo dye degradation, bacteria employ two distinct approaches: direct decolorization and indirect/mediated decolorization. These methodologies rely on a suite of enzymes to dismantle the intricate structures of refractory substances within microbial systems (Verma et al. 2017). The arsenal of enzymes includes laccase, azoreductase, lignin peroxidase, NADH-DCIP reductase, and hexane oxidase, each contributing to the breakdown of complex chemical compounds.
The dichotomy of oxygenic and non-oxygenic environments serves as the stage for bacterial experiments in azo dye degradation. While both environments have been explored, anaerobic reductive cleavage of azo bonds by the azoreductase enzyme takes center stage in most bacterial dye degradation processes. This anaerobic reductive cleavage, occurring in two stages, involves the transfer of four electrons, resulting in the decolorization of the dye and the production of colorless amines. The subsequent aerobic breakdown of these aromatic amines in the second stage completes the microbial degradation cycle (Hsu et al. 2012).
A prelude to the biodegradation spectacle is bacterial adsorption, a physical technique involving the efficient adsorption of dye molecules onto biomass. However, the limitations of this method emerge as dye molecules saturate the biomass quickly, necessitating a more robust and sustainable approach for long-term treatment. Yet, the significance of this initial adsorption step lies in its role as the launchpad for the subsequent stages of microbial dye degradation (Adenan et al. 2021).
Following physical adsorption, the biodegradation of azo dyes unfolds in a captivating two-step dance within bacterial cells. In the first stage, the azo bond (–N=N–) of the dye undergoes reductive cleavage, facilitated by azoreductase enzymes. This transformative step yields aromatic amines, typically colorless but potentially hazardous. The second stage, set in an aerobic environment, orchestrates the degradation of these aromatic amines, ensuring a comprehensive and environmentally benign breakdown of azo dyes (Maniyam et al. 2020).
In the fungal realm, laccase takes center stage, playing a pivotal role in the asymmetric cleavage of azo dye structures. The fungus strain Lentinus sp. showcases the prowess of laccase in the biodegradation of anthraquinone and azo dyes. Additionally, Brevibacillus sp. (Z1) and Anoxybacillus gonensis P39127 contribute to the removal of various azo dyes, showcasing the diverse enzymatic repertoire harnessed by microorganisms in dye degradation (Singh, 2014).
The efficiency of azo dye degradation is further heightened by the strategic use of redox mediators. Studies reveal the accelerated biodeterioration of azo dyes upon the addition of redox mediators, such as artificial electron carriers, anthraquinone-2-sulfonate, or quinone molecules. These mediators act as catalysts, expediting the breakdown of azo dyes. Notably, alternative redox mediators like activated carbon, carbon black, and carbon nanotubes emerge as cost-effective options, ensuring the application of bacteria-mediated decoloration (Verma et al. 2017).
Bacteria exhibit a dual role in the decolorization of azo dyes—a symphony of biosorption and biodeterioration. Biosorption involves the adsorption of azo dye onto bacterial cells, initiating a transformative process where bacterial cells alter the color of adsorbed dye molecules. This alteration is perceptible through a decrease in the dye's absorption spectrum. On the other hand, biodeterioration breaks down the dye structure to its fundamental components, as evidenced by the disappearance of dye absorption peaks or the emergence of new peaks indicative of metabolite production (Adenan et al. 2021).
A noteworthy discovery in the realm of azo dye degradation is the strategic use of dead bacterial cells. Studies on malachite green (MG), methyl violet (MV), crystal violet (CV), and cotton blue (CB) demonstrate that both dead and living Streptomyces bacillaris bacterial cells are integral to the decolorization process. While living cells actively engage in dye degradation, dead cells play a crucial role in adsorption, providing insights into the synergy between different microbial states in the removal of azo dyes (Mishra et al. 2020).
In conclusion, the mechanism of azo dye degradation by bacteria is a multifaceted symphony, encompassing enzymatic transformations, adsorption, and biodeterioration. This orchestrated interplay unfolds in oxygenic and non-oxygenic environments, showcasing the adaptability of bacteria in tackling diverse dye structures. The strategic use of enzymes, redox mediators, and the dual role of biosorption and biodeterioration highlight the intricate strategies employed by bacteria in their quest for environmentally benign and effective azo dye treatment. Figure 15 serves as a visual representation, encapsulating the essence of this biochemical symphony, where bacteria emerge as virtuosos in the figure.
Mechanism of azo dye degradation by white rot fungi
In the realm of wastewater treatment, White Rot Fungi stand as nature's virtuosos, showcasing an impressive mechanism for the degradation of azo dyes. These fungi have garnered significant attention for their remarkable capacity to tackle industrial wastewater laden with intricate contaminants (Kumar et al., 2020).
At the heart of White Rot Fungi's prowess lies the production of extracellular lignin-degrading enzymes, primarily lignin peroxidase (LiP) and manganese peroxidase (MnP). These enzymes serve as the maestros orchestrating the biochemical symphony within the fungal kingdom. Through redox reactions, LiP and MnP generate reactive radicals, potent oxidizing agents with the ability to degrade a diverse array of complex and persistent pollutants present in industrial wastewater (Morsi et al. 2020; Sun et al. 2023).
The reactive radicals generated by LiP and MnP play a pivotal role in the degradation process. These radicals act as highly effective oxidizing agents, initiating a cascade of oxidative transformations within the pollutants. The mechanisms include the removal of hydrogen atoms, breakdown of chemical bonds, and other oxidative processes that dismantle recalcitrant pollutants with finesse (Morsi et al. 2020; Sun et al. 2023). The enzyme-based degradation orchestrated by White Rot Fungi is akin to a precision orchestra. LiP and MnP, as enzymatic virtuosos, target and dismantle the complex structures of pollutants, ensuring a comprehensive and efficient breakdown. This mechanism highlights the adaptability of White Rot Fungi in tackling a wide range of contaminants encountered in industrial wastewater (Morsi et al. 2020). White Rot Fungi don't rely solely on enzymatic prowess; they also harness biosorption and bioprecipitation processes as part of their cleanup repertoire. The fungal cell wall, fortified with chitin and other polymers, becomes an efficient tool for binding and storing metal ions and pollutants through electrostatic interactions, complexation, and ion exchange (Javanbakht et al. 2014).
In a fascinating display of fungal alchemy, White Rot Fungi induce the precipitation of metal ions as salts or hydroxides. This process is triggered by the fungi altering the pH or redox conditions in wastewater treatment systems. The ability to manipulate these environmental factors underscores the multifaceted nature of White Rot Fungi's approach to pollutant removal (Chandra et al., 2019).
Figure 16 serves as a visual gateway into the intricate mechanism of azo dye degradation by White Rot Fungi. The figure encapsulates the enzymatic precision, radical-driven oxidative transformations, biosorption capabilities, and the fungal alchemy of metal ion precipitation. It's a visual testament to the versatile and effective nature of White Rot Fungi in addressing the complex challenges posed by industrial wastewater laden with azo dyes.
In essence, the mechanism of azo dye degradation by White Rot Fungi is a harmonious interplay of enzymatic brilliance, radical-driven transformations, and nature's cleanup strategies. As guardians of environmental balance, these fungi showcase a repertoire of tools to dismantle pollutants, offering a sustainable and effective approach to wastewater treatment. Figure 16 encapsulates this symphony, where White Rot Fungi emerge as conductors of nature's orchestra, orchestrating the breakdown of azo dyes with finesse.
Other strategies used for the degradation of azo dyes
In the relentless pursuit of sustainable solutions for environmental challenges, cutting-edge scientific technologies have emerged as potent tools in the degradation of azo dyes. This technological symphony encompasses system biology, omics (proteomics and glycomics), nanotechnology, and gene editing tools, pushing the boundaries of bioremediation efficiency.
System biology stands out as a beacon of promise in unraveling the intricacies of microbial communities across diverse environmental settings. This approach provides a holistic understanding of the dynamic interactions within these communities, offering insights crucial for efficient bioremediation strategies (Chakraborty et al. 2022).
The omics revolution, featuring proteomics and genomics, has revolutionized our ability to analyze genetic and protein-level regulations governing bioremediation processes. Techniques such as sequencing, MALDI-TOF, and the exploration of new functional genes have become indispensable tools in deciphering the pathways involved in pollutant degradation (Chakraborty et al. 2022).
As genetic engineering strides forward, the role of genetically modified organisms, metagenomics, and metabolomics is taking center stage. These advancements aim to deliver cost-effective and reliable methods for azo dye degradation (Gaur et al. 2018). The exploration of genomic methods, including PCR-based techniques, isotope distribution analysis, DNA hybridization, and metabolic engineering, further enhances our understanding of the intricate biodegradation processes (Hakeem et al. 2020).
Polymerase chain reaction (PCR)-based techniques play a pivotal role in genotypic fingerprinting. These techniques, including amplified ribosomal DNA restriction analysis (ARDRA), amplified fragment length polymorphisms (AFLP), randomly amplified polymorphic DNA analysis (RAPD), terminal-restriction fragment length polymorphism (T-RFLP), single strand conformation polymorphism (SSCP), and length heterogeneity, empower researchers to analyze bacterial species, construct functional structural models, and generate genetic fingerprints in the exploration of soil microbial communities (Domínguez-Rodríguez et al., 2022).
Figure 17 serves as a visual testament to the diverse array of technologies employed in the removal of azo dyes. From system biology to advanced genetic engineering tools, this image encapsulates the scientific prowess harnessed to combat environmental challenges. It signifies a collaborative effort across disciplines, portraying the convergence of innovation for a cleaner and more sustainable future.
The technological symphony in azo dye degradation is an evolving narrative, where each innovation adds a unique note to the harmonious orchestration of environmental stewardship. As we delve deeper into the intricacies of microbial communities and molecular regulations, these technologies promise not only effective solutions but also a deeper understanding of our role as custodians of the planet.
Conclusion and future perspectives
The widespread utilization of azo dyes within the textile industry has ignited a growing environmental predicament, characterized by the release of dye-laden effluents into ecosystems. These electron-deficient xenobiotic chemicals, featuring azo (–N=N–) groups and aromatic functionality, not only bestow vibrant hues upon textiles but also raise serious environmental concerns due to their inherent toxicity, mutagenicity, and carcinogenicity. The imperative to address the environmental repercussions of dye-containing wastewater is evident, necessitating effective solutions to mitigate the impact of textile industry effluents.
Biological methods have emerged as a promising avenue, surmounting the limitations of conventional physicochemical approaches. Microorganisms such as bacteria, fungi, yeast, algae, and plants offer a sustainable, environmentally benign approach to the removal of azo dyes from effluents. The scope of these biological processes, elucidated in this study, lies in their affordability and adaptability to a diverse array of dye structural types. Among these microorganisms, bacteria stand out as preferred agents for dye removal from textile effluents, owing to their ease of cultivation, resilience in challenging environments, and rapid decolorization capabilities in comparison to other microorganisms.
Looking toward the future, the amalgamation of nanotechnology with traditional biological processes heralds a paradigm shift in environmental protection strategies. Nanotechnologies, when synergized with biological methods, hold the promise of significantly enhancing the efficiency of azo dye remediation. This integration opens new frontiers for sustainable solutions to the challenges posed by colored wastewater in textile dyeing industries.
The path forward in research necessitates a deeper exploration of enzyme profiles, intermediate metabolites, and the intricate mechanisms governing simultaneous dye and metal-dye complex removal. Genome and proteome studies are poised to play a pivotal role in unraveling the complexities of microbial processes, guiding the optimization of bioreactors, and shedding light on the (non)toxicity and biodegradation processes of azo dye compounds.
Moving forward, the research agenda involves a concerted effort to streamline variables hindering microbial activity, amplify the effectiveness of biodegradation, and optimize the operating conditions of bioreactors. Advanced molecular biology approaches will be instrumental in delving into the genetic and enzymatic intricacies of azo dye treatment. This holistic understanding will provide researchers with novel perspectives on biodegradation mechanisms, propelling the field toward innovative and sustainable solutions.
In conclusion, as we navigate the intricate terrain of azo dye remediation, the seamless integration of biological methods with cutting-edge technological innovations holds the key to a cleaner, more sustainable future. The strides made in this research underscore the potential for transformative change, where environmental stewardship aligns seamlessly with scientific progress. This synergy promises not only the remediation of azo dye pollutants but also the establishment of a blueprint for sustainable environmental practices in the broader context of industrial processes.
Data availability
All data collected and reviewed are included in this article and properly referenced.
References
Abdullah MM, Baker JH, Albargi HB, Jagnandan A, Ahmad MZ, Jebahi S, Algethami JS (2023) Environmental remediation via solution combustion route synthesis of single-phase ferromagnetic nickel doped zinc oxide (Ni-ZnO) nanostructure for enhanced photocatalytic activity. Sci Adv Mater 15(2):169–175
Adenan NH, Lim YY, Ting ASY (2021) Identification and optimization of triphenylmethane dyes removal by Streptomyces sp. from forest soil. Sustain Environ Res 31(1):1–15. https://doi.org/10.1186/s42834-021-00081-z
Ahlström LH, Eskilsson CS, Björklund E (2005) Determination of banned azo dyes in consumer goods. TrAC Trends Anal Chem 24(1):49–56. https://doi.org/10.1016/j.trac.2004.09.004
Ajaz M, Rehman A, Khan Z et al. (2019) Degradation of azo dyes by Alcaligenes aquatilis 3c and its potential use in the wastewater treatment. AMB Expr 9:64. https://doi.org/10.1186/s13568-019-0788-3
Ajaz M, Shakeel S, Rehman A (2020) Microbial use for azo dye degradation—a strategy for dye bioremediation. Int Microbiol 23:149–159. https://doi.org/10.1007/s10123-019-00103-2
Akpomie KG, Conradie J (2020) Advances in application of cotton-based adsorbents for heavy metals trapping, surface modifications and future perspectives. Ecotoxicol Environ Saf 201:110825. https://doi.org/10.1016/j.ecoenv.2020.110825
Ali SS, Al-Tohamy R, Sun J, Wu J, Huizi L (2019) Screening and construction of a novel microbial consortium SSA-6 enriched from the gut symbionts of wood-feeding termite, Coptotermes formosanus and its biomass-based biorefineries. Fuel 236:1128–1145. https://doi.org/10.1016/j.fuel.2018.08.117
Ali MY, Hassan GM, Hassan AMS, Mohamed ZA, Ramadan MF (2020) In vivo genotoxicity assessment of sunset yellow and sodium benzoate in female rats. Drug Chem Toxicol 43(5):504–513. https://doi.org/10.1080/01480545.2018.1510416
Ambrosio ST, Vilar JC, da Silva CAA, Okada K, Nascimento AE, Longo RL (2012) A biosorption isotherm model for the removal of reactive azo dyes by inactivated mycelia of Cunninghamella elegans UCP542. Molecules 17:452–462. https://doi.org/10.3390/molecules17010452
Ameen F, Dawoud TM, Alshehrei F, Alsamhary K, Almansob A (2021) Decolorization of acid blue 29, disperse red 1 and congo red by different indigenous fungal strains. Chemosphere 271:129532. https://doi.org/10.1016/j.chemosphere.2021.129532
Anjaneyulu Y, Sreedhara Chary N, Samuel Suman D (2005) Decolourization of industrial effluents–available methods and emerging technologies–a review. Rev Environ Sci Bio/Technol 4:245–273. https://doi.org/10.1007/s11157-005-1246-z
Anliker R, Butler GC, Clarke EA, Förstner U, Funke W, Hyslop C, Anliker R (1980) Organic dyes and pigments. Anthropogenic Compd. https://doi.org/10.1007/978-3-540-38522-6_7
Arulazhagan P (2016) A study on microbial decolorization of Reactive Red M8B by Bacillus subtilis isolated from dye contaminated soil samples. Int J Curr Res Biol Med 1:1–13. https://doi.org/10.1080/10826068.2013.772063
Asad S, Amoozegar MA, Pourbabaee A, Sarbolouki MN, Dastgheib SMM (2007) Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour Technol 98(11):2082–2088. https://doi.org/10.1016/j.biortech.2006.08.020
Asgher M, Bhatti HN, Ashraf M, Legge RL (2008) Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19:771–783. https://doi.org/10.1007/s10532-008-9185-3
Asgher M, Yasmeen Q, Iqbal HMN (2013) Enhanced decolorization of Solar Brilliant Red 80 textile dye by an indigenous white rot fungus Schizophyllum commune IBL-06. Saudi J Biol Sci 20:347–352. https://doi.org/10.1016/j.sjbs.2013.03.004
Atul K, Pratibha C, Poonam V (2012) A ccomparative study on the treatment methods of textile dye effluents. J Chem Pharmaceutic Res 4(1):763–771
Ayed L, Mahdhi A, Cheref A, Bakhrouf A (2011a) Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimoboilis: biotoxicity and metabolites characterization. Desalination 274:272–277. https://doi.org/10.1016/j.desal.2011.02.024
Ayed L, Mahdhi A, Cheref A, Bakhrouf A (2011b) Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimobilis: Biotoxicity and metabolites characterization. Desalination 274:272–277. https://doi.org/10.1016/j.desal.2011.02.024
Banerjee M, Basu RK, Das SK (2018) Cr (VI) adsorption by a green adsorbent walnut shell: adsorption studies, regeneration studies, scale-up design and economic feasibility. Process Saf Environ Prot 116:693–702. https://doi.org/10.1016/j.psep.2018.03.037
Bawazeer TM (2022) Investigating the photocatalytic degradation of N-chloropiperidine in aqueous solution by utilizing TiO2 under blacklight illumination. Sci Adv Mater 14(4):772–778
Bayramoğlu G, Arıca MY (2007) Biosorption of benzidine based textile dyes “Direct Blue 1 and Direct Red 128” using native and heat-treated biomass of Trametes versicolor. J Hazard Mater 143(1–2):135–143. https://doi.org/10.1016/j.jhazmat.2006.09.002
Behera M, Nayak J, Banerjee S, Chakrabortty S, Tripathy SK (2021) A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: an integrated system design approach. J Environ Chem Eng 9(4):105277. https://doi.org/10.1016/j.jece.2021.105277
Bell J, Plumb JJ, Buckley CA, Stuckey DC (2000) Treatment and decolorization of dyes in an anaerobic baffled reactor. J Environ Eng 126(11):1026–1032. https://doi.org/10.1061/(ASCE)0733-9372(2000)126:11(1026)
Benkhaya S, M’rabet S, El Harfi A (2020) Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 6(1):e03271. https://doi.org/10.1016/j.heliyon.2020.e03271
Bharathi D, Nandagopal JGT, Ranjithkumar R, Gupta PK, Djearamane S (2022) Microbial approaches for sustainable remediation of dye-contaminated wastewater: a review. Archiv Microbiol 204(3):169. https://doi.org/10.1007/s00203-022-02767-3
Bhatia D, Kanwar RS, Singh J, Sharma NR, Khandare RV (2022) Degradation and decolorization of Disperse red 167 dye with an in-situ isolated azo-reductase enzyme producing bacterium Paenochrobactrum glaciei. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-022-04163-2
Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, Gupta VK (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Saf 148:702–712. https://doi.org/10.1016/j.ecoenv.2017.11.034
Carliell CM, Godefroy SJ, Naidoo N, Buckley CA, Senior E, Mulholland D, Martineigh BS (1994). Anaerobic decolourisation of azo dyes. In: Seventh international symposium on anaerobic digestion (pp. 303–306). RSA Litho (Pty) Ltd Goodwood, Cape Town, South Africa. https://doi.org/10.1016/S0960-8524(99)00098-X
Carmen Z, Daniela S (2012) Textile organic dyes-characteristics, polluting effects and separation/elimination procedures from industrial effluents-a critical overview (Vol. 3, pp. 55–86). Rijeka: IntechOpen.https://doi.org/10.5772/32373
Carvalho MC, Pereira C, Goncalves IC, Pinheiro HM, Santos AR, Lopes A, Ferra MI (2008) Assessment of the biodegradability of a monosulfonated azo dye and aromatic amines. Int Biodeteriorat Biodegradation 62(2):96–103. https://doi.org/10.1016/j.ibiod.2007.12.008
Celik L, Ozturk A, Abdullah MI (2012) Biodegradation of Reactive Red 195 azo dye by the bacterium Rhodopseudomonas palustris 51ATA. Afr J Microbiol Res 6:120–126. https://doi.org/10.5897/AJMR11.1059
Çetin D, Dönmez G (2006) Decolorization of reactive dyes by mixed cultures isolated from textile effluent under anaerobic conditions. Enzyme Microbial Technol 38(7):926–930. https://doi.org/10.1016/j.enzmictec.2005.08.020
Chakraborty R, Karmakar S, Ansar W (2022) Advances and applications of bioremediation: network of omics, system biology, gene editing and nanotechnology. Environ Inform Challenges Solut. https://doi.org/10.1007/978-981-19-2083-7_10
Chandra P, Enespa (2019) Mycoremediation of environmental pollutants from contaminated soil. In: Varma A, Choudhary DK (eds) Mycorrhizosphere and Pedogenesis. Springer Singapore, Singapore, pp 239–274. https://doi.org/10.1007/978-981-13-6480-8_15
Chang JS, Chou C, Lin YC, Lin PJ, Ho JY, Hu TL (2001) Kinetic characteristics of bacterial azo-dye decolorization by Pseudomonas luteola. Water Res 35(12):2841–2850. https://doi.org/10.1016/S0043-1354(00)00581-9
Chaudhary S, Kaur Y, Jayee B, Chaudhary GR, Umar A (2018) NiO nanodisks: highly efficient visible-light driven photocatalyst, potential scaffold for seed germination of vigna radiata and antibacterial properties. J Clean Product 190:563–576. https://doi.org/10.1016/j.jclepro.2018.04.110
Chen KC, Huang WT, Wu JY, Houng JY (1999) Microbial decolorization of azo dyes by Proteus mirabilis. J Ind Microbiol Biotechnol 23:686–690. https://doi.org/10.1038/sj.jim.2900689
Chen KC, Wu JY, Liou DJ, Hwang SCJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68. https://doi.org/10.1016/S0168-1656(02)00303-6
Chen G, An X, Li H, Lai F, Yuan E, Xia X, Zhang Q (2021) Detoxification of azo dye Direct Black G by thermophilic Anoxybacillus sp. PDR2 and its application potential in bioremediation. Ecotoxicology and Environmental Safety 214:112084. https://doi.org/10.1016/j.ecoenv.2021.112084
Chen YG, Huang JH, Luo R, Ge HZ, Wołowicz A, Wawrzkiewicz M, Chen SH (2021) Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol Environ Saf 219:112336. https://doi.org/10.1016/j.ecoenv.2021.112336
Chung KT (2016) Azo dyes and human health: a review. J Environ Sci Health, Part C 34(4):233–261. https://doi.org/10.1080/10590501.2016.1236602
Cotillas S, Llanos J, Cañizares P, Clematis D, Cerisola G, Rodrigo MA, Panizza M (2018) Removal of Procion Red MX-5B dye from wastewater by conductive-diamond electrochemical oxidation. Electrochimica Acta 263:1–7. https://doi.org/10.1016/j.electacta.2018.01.0524
Cui D, Li G, Zhao M, Han S (2014) Decolourization of azo dyes by a newly isolated Klebsiella sp. strain Y3, and effects of various factors on biodegradation. Biotechnol. Biotechnol. Equipment 28(3):478–486. https://doi.org/10.1080/13102818.2014.926053
Das A, Mishra S (2016) Decolorization of Different Textile Azo Dyes using an Isolated Bacterium Enterococcus durans GM13. Int J Curr Microbiol App Sci. 5(7):676–686. https://doi.org/10.20546/ijcmas.2016.507.077
Dasgupta J, Mondal D, Chakraborty S, Sikder J, Curcio S, Arafat HA (2015) Nanofiltration based water reclamation from tannery effluent following coagulation pretreatment. Ecotoxicol Environ Saf 121:22–30. https://doi.org/10.1016/j.ecoenv.2015.07.006
Dilarri G, Corso CR (2018) Saccharomyces cerevisiae immobilized onto cross-linked chitosan beads: Application of a novel material for the removal of dye toxicity. Environ Technol 39(14):1851–1867. https://doi.org/10.1080/09593330.2017.1340351
Domínguez-Rodríguez G, Marina ML, Plaza M (2022) Rapid fingerprinting of extractable and non-extractable polyphenols from tropical fruit peels using direct analysis in real time coupled to orbitrap mass spectrometry. Food Chem 371:131191. https://doi.org/10.1016/j.foodchem.2021.131191
El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009a) Biodegradation of dyes by some green algae and cyanobacteria. Int Biodeterior Biodegrad 63:699–704. https://doi.org/10.1016/j.ibiod.2009.04.010
El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009) Biodegradation of dyes by some green algae and cyanobacteria. Int Biodeteriorat Biodegradat 63(6):699–704. https://doi.org/10.1016/j.ibiod.2009.04.010
Erden E, Kaymaz Y, Pazarlioglu NK (2011) Biosorption kinetics of a direct azo dye Sirius Blue K-CFN by Trametes versicolor. Electron J Biotechnol 14(2):3–3. https://doi.org/10.2225/vol14-issue2-fulltext-8
Evangelista-Barreto NS, Albuquerque CD, Vieira RHSF, Campos-Takaki GM (2009) Co-metabolic decolorization of the reactive azo dye Orange II by Geobacillus stearothermophilus UCP 986. Text Res J 79:1266–1273. https://doi.org/10.1177/0040517508087858
Gao J, Fan H, Liu W-W, Yang Q (2022) A new 3D Sm (III) compound: photocatalytic property and application in the treatment of osteoarthritis of the foot and ankle. Sci Adv Mater 14(2):344–349
Gao Y, Yang B, Wang Q (2018) Biodegradation and decolorization of dye wastewater: a review. In: IOP Conference Series: Earth and Environmental Science (Vol. 178, No. 1, p. 012013). IOP Publishing.https://doi.org/10.1088/1755-1315/178/1/012013
Gaur N, Narasimhulu K, PydiSetty Y (2018) Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J Clean Product 198:1602–1631. https://doi.org/10.1016/j.jclepro.2018.07.076
Gayathri PV, Yesodharan S, Yesodharan EP (2019) Microwave/persulphate assisted ZnO mediated photocatalysis (MW/PS/UV/ZnO) as an efficient advanced oxidation process for the removal of RhB dye pollutant from water. J Environ Chem Eng 7(4):103122. https://doi.org/10.1016/j.jece.2019.103122
Ghaly AE, Ananthashankar R, Alhattab MVVR, Ramakrishnan VV (2014) Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 5(1):1–19. https://doi.org/10.4172/2157-7048.1000182
Ghosh A, Ghosh Dastidar M, Sreekrishnan TR (2016) Recent advances in bioremediation of heavy metals and metal complex dyes. J Environ Eng 142(9):C4015003. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000965
Gomare SS, Tamboli DP, Kagalkar AN, Govindwar SP (2009) Eco-friendly biodegradation of a reactive textile dye Golden Yellow HER by Brevibacillus laterosporus MTCC 2298. Int Biodeter Biodegr 63:582–586. https://doi.org/10.1016/j.ibiod.2009.03.005
Gomez V, Larrechi MS, Callao MP (2007) Kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 69(7):1151–1158. https://doi.org/10.1016/j.chemosphere.2007.03.076
Grassi E, Scodeller P, Filiel N, Carballo R, Levin L (2011) Potential of Trametes trogii culture fluids and its purified laccase for the decolorization of different types of recalcitrant dyes without the addition of redox mediators. Int Biodeter Biodegr 65:635–643. https://doi.org/10.1016/j.ibiod.2011.03.007
Grinhut T, Salame TM, Chen Y, Hadar Y (2011) Involvement of ligninolytic enzymes and Fenton-like reaction in humic acid degradation by Trametes sp. Appl Microbiol Biotechnol 91:1131–1140. https://doi.org/10.1007/s00253-011-3300-9
Guo G, Hao J, Tian F, Liu C, Ding K, Xu J, Guan Z (2020) Decolorization and detoxification of azo dye by halo-alkaliphilic bacterial consortium: systematic investigations of performance, pathway and metagenome. Ecotoxicol Environ Saf 204:111073. https://doi.org/10.1016/j.ecoenv.2020.111073
Gupta V, Yadav RK, Umar A, Ibrahim AA, Singh S, Shahin R, Baskoutas S (2023) Highly efficient self-assembled activated carbon cloth-templated photocatalyst for NADH regeneration and photocatalytic reduction of 4-nitro benzyl alcohol. Catalysts 13(4):666
Hadibarata T, Yusoff ARM, Aris A, Salmiati HT, Kristanti RA (2012) Decolorization of azo, triphenylmethane and anthraquinone dyes by laccase of a newly isolated Armillaria sp. F022. Water Air Soil Poll 223:1045–1054. https://doi.org/10.1007/s11270-011-0922-6
Hadibarata T, Adnan LA, Yusoff ARM, Yuniarto A, Rubiyanto ZMMFA, Khudhair AB, Teh ZC, Naser MA (2013) Microbial decolorization of an azo dye Reactive Black 5 using white rot fungus Pleurotus eryngii F032. Water Air Soil Poll 224:1595–1604. https://doi.org/10.1007/s11270-013-1595-0
Hai FI, Yamamoto K, Nakajima F, Fukushi K (2012) Application of a GAC-coated hollow fiber module to couple enzymatic degradation of dye on membrane to whole cell biodegradation within a membrane bioreactor. J Membrane Sci 389:67–75. https://doi.org/10.1016/j.memsci.2011.10.016
Hakeem KR, Bhat RA, Qadri H (2020) Bioremediation and Biotechnology. Springer, Cham
Handayani W, Meitiniarti VI, Timotius KH (2007) Decolorization of Acid Red 27 and Reactive Red 2 by Enterococcus faecalis under a batch system. World J Microbiol Biotechnol 23:1239–1244. https://doi.org/10.1007/s11274-007-9355-1
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manag 182:351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Hsu CA, Wen TN, Su YC, Jiang ZB, Chen CW, Shyur LF (2012) Biological degradation of anthroquinone and azo dyes by a novel laccase from Lentinus sp. Environ Sci Technol 46(9):5109–5117. https://doi.org/10.1021/es2047014
Ilyas S, Rehman A (2013) Decolorization and detoxification of Synozol red HF-6BN azo dye, by Aspergillus niger and Nigrospora sp.. Iran J Environ Health Scie Eng 10:1–9. https://doi.org/10.1186/1735-2746-10-12
Imran M, Kim EB, Akhtar MS, Umar A, Kwak DH, Ameen S, Baskoutas S (2023) Catalytic oxidation of ibuprofen over bulk heterojunction photocatalysts based on conjugated donor-acceptor configured benzoselenadiazole molecule. Environ Res 216:114712
Ito T, Shimada Y, Suto T (2018) Potential use of bacteria collected from human hands for textile dye decolorization. Water Resour Ind 20:46–53. https://doi.org/10.1016/j.wri.2018.09.001
Jadhav UU, Dawkar VV, Ghodake GS, Govindwar SP (2008) Biodegradation of Direct Red 5B, a textile dyes by newly isolated Comamonas sp. UVS J Hazard Mater 158:507–516. https://doi.org/10.1016/j.jhazmat.2008.01.099
Jafari N, Soudi MR, Kasra-Kermanshahi R (2014) Biodecolorization of textile azo dyes by isolated yeast from activated sludge: Issatchenkia orientalis JKS6. Ann Microbiol 64:475–482. https://doi.org/10.1007/s13213-013-0677-y
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69:1775–1787. https://doi.org/10.2166/wst.2013.718
Jiku MAS, Singha A, Faruquee M, Rahaman MA, Alam MA, Ehsanullah M (2021) Toxic wastewater status for irrigation usage at Gazipur and Savar industrial vicinity of Bangladesh. Acta Ecologica Sinica 41(4):358–364. https://doi.org/10.1016/j.chnaes.2021.07.001
Kalyani DC, Telke AA, Dhanve RS, Jadhav JP (2009) Ecofriendly biodegradation and detoxification of Reactive Red 2 textile dye by newly isolated Pseudomonas sp. SUK1. J Hazard Mater 163(2–3):735–742. https://doi.org/10.1016/j.jhazmat.2008.07.020
Kamalam M, Menaka J, Inbanathan SSR, Sethuraman K, Shahid A, Fouad Hassan, Hussain S (2023) Tin oxide-graphene oxide (SnO2/GO) nanocomposite: a promising photocatalyst for rhodamine-b dye degradation. Sci Adv Mater 15:648–654
Kapoor RT, Danish M, Singh RS, Rafatullah M, Abdul Khalil HPS (2021) Exploiting microbial biomass in treating azo dyes contaminated wastewater: mechanism of degradation and factors affecting microbial efficiency. J Water Process Eng 43:102255. https://doi.org/10.1016/j.jwpe.2021.102255
Kaur M, Umar A, Mehta SK, Kansal SK (2019) Reduced graphene oxide-CdS heterostructure: an efficient fluorescent probe for the sensing of Ag(I) and sunset yellow and a visible-light responsive photocatalyst for the degradation of levofloxacin drug in aqueous phase. Appl Catal B: Environ 245:143–158. https://doi.org/10.1016/j.apcatb.2018.12.042
Kaur R, Kaur A, Kaur R, Singh S, Bhatti MS, Ahmad U, Baskoutas S, Kansal SK (2021) Cu-BTC metal organic framework (MOF) derived Cu-doped TiO2 nanoparticles and their use as visible light active photocatalyst for the decomposition of ofloxacin (OFX) antibiotic and antibacterial activity. Adv Powder Technol 32(5):1350–1361. https://doi.org/10.1016/j.apt.2021.02.037
Kaur M, Singh S, Mehta SK, Kansal SK, Umar A, Ibrahim AA, Baskoutas S (2023) CeO2 quantum dots decorated g-C3N4 nanosheets: a potential scaffold for fluorescence sensing of heavy metals and visible-light driven photocatalyst. J Alloys Compod 960:170637. https://doi.org/10.1016/j.jallcom.2023.170637
Khan S, Malik A (2014) Environmental and health effects of textile industry wastewater. Environ Deteriorat Hum Health Nat Anthropogenic Determinants. https://doi.org/10.1007/978-94-007-7890-0_4
Khan S, Malik A (2016) Degradation of Reactive Black 5 dye by a newly isolated bacterium Pseudomonas entomophila BS1. Can J Microbiol 62:220–232. https://doi.org/10.1139/cjm-2015-0552
Khataee AR, Dehghan G (2011) Optimization of biological treatment of a dye solution by macroalgae Cladophora sp. using response surface methodology. J Taiwan Inst Chem Eng 42(1): 26–33. https://doi.org/10.1016/j.jtice.2010.03.007
Khouni I, Marrot B, Moulin P, Amar RB (2011) Decolourization of the reconstituted textile effluent by different process treatments: enzymatic catalysis, coagulation/flocculation and nanofiltration processes. Desalination 268(1–3):27–37. https://doi.org/10.1016/j.desal.2010.09.046
Kim H-J, Yoon J-W, Choi K-I, Jang HW, Umar A, Lee J-H (2013) Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 5(15):7066–7073
Kishor R, Purchase D, Saratale GD, Saratale RG, Ferreira LFR, Bilal M, Bharagava RN (2021) Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng 9(2):105012. https://doi.org/10.1016/j.jece.2020.105012
Kolekar YM, Kodam KM (2012) Decolorization of textile dyes by Alishewanella sp. KMK6. Appl Microbiol Biotechnol 95:521–529. https://doi.org/10.1007/s00253-011-3698-0
Kumar A, Chandra R (2020) Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6:e03170. https://doi.org/10.1016/j.heliyon.2020.e03170
Kumar R, Umar A, Rajesh Kumar D, Rana MSC (2021) Low-temperature synthesis of cadmium-doped zinc oxide nanosheets for enhanced sensing and environmental remediation applications. J Alloys Compd 863:158649. https://doi.org/10.1016/j.jallcom.2021.158649
Kumar N, Yadav RK, Umar A, Ibrahim AA, Singh S, Shahin R, Singh AP et al. (2023) Selective aerobic coupling of amines to imines using solar spectrum-responsive flower-like Nen-graphene quantum dots (GQDs) decorated with 2, 4-dinitrophenylhydrazine (PH) as a photocatalyst. Chemosphere 341:139697
Laing IG (1991) The impact of effluent regulations on the dyeing industry. Rev Progress Coloration Related Topics 21(1):56–71. https://doi.org/10.1111/j.1478-4408.1991.tb00081.x
Lalnunhlimi S, Krishnaswamy V (2016) Decolorization of azo dyes (Direct Blue 151 and Direct Red 31) by moderately alkaliphilic bacterial consortium. Brazil J Microbiol 47:39–46. https://doi.org/10.1016/j.bjm.2015.11.013
Lamba R, Ahmad U, Mehta SK, Kansal SK (2015) Well-crystalline porous ZnO–SnO2 nanosheets: an effective visible-light driven photocatalyst and highly sensitive smart sensor material. Talanta 131:490–498
Lark D, Buzzo AJD, Garcia JAA, Côrrea VG, Helm CV, Corrêa RCG, Peralta RA, Peralta Muniz Moreira RF, Bracht A, Peralt RM (2019) Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.121655
Li T, Liu Y, Lin S, Liu Y, Xie Y (2019) Soil pollution management in China: a brief introduction. Sustainability 11(3):556. https://doi.org/10.3390/su11030556
Li F, Zhao K, Ng TSA, Dai Y, Wang CH (2022) Sustainable production of bio-oil and carbonaceous materials from biowaste co-pyrolysis. Chem Eng J 427:131821. https://doi.org/10.1016/j.cej.2021.131821
Li P-X, Yang A-Y, Xin L, Xue B, Yin C-H (2022b) Photocatalytic activity and mechanism of Cu2+ doped ZnO nanomaterials. Sci Adv Mater 14(10):1599–1604
Liang CZ, Sun SP, Li FY, Ong YK, Chung TS (2014) Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J Membrane Sci 469:306–315. https://doi.org/10.1016/j.memsci.2014.06.057
Liang C, Wei D, Zhang S, Ren Q, Shi J, Liu L (2021) Removal of antibiotic resistance genes from swine wastewater by membrane filtration treatment. Ecotoxicol Environ Saf 210:111885. https://doi.org/10.1016/j.ecoenv.2020.111885
Lim SL, Chu WL, Phang SM (2010) Use of Chlorella vulgaris for bioremediation of textile wastewater. Bioresour Technol 101(19):7314–7322. https://doi.org/10.1016/j.biortech.2010.04.092
Lin YH, Leu JY (2008) Kinetics of reactive azo-dye decolorization by Pseudomonas luteola in a biological activated carbon process. Biochem Eng J 39(3):457–467. https://doi.org/10.1016/j.bej.2007.10.015
Lin CC, Chiu CC, Lee PY, Chen KJ, He CX, Hsu SK, Cheng KC (2022) The adverse effects of air pollution on the eye: a review. Int J Environ Res Public Health 19(3):1186. https://doi.org/10.3390/ijerph19031186
Lin D, Liu Z, Wang B, Han Y (2022b) Recovery of tungsten and vanadium from spent selective catalytic reduction catalysts by soda roasting: a mechanistic study. Sci Adv Mater 14(9):1494–1502
Liu S-l, Yi-fu Bu, Tian C, Jing Wu (2022a) Synthesis of g-C3N4/TiO2/RGO ternary composites and performance for catalytic fructose dehydration to 5-hydroxymethylfurfural. Sci Adv Mater 14(7):1242–1248
Liu X, Jing Y, She Y, Liu J, Wenwei Hu, Yin D (2022b) Bifunctional oxidation catalysis of new titanium-silicon molecular sieve (HTS-1) based on the reaction of allyl alcohol and hydrogen peroxide. Sci Adv Mater 14(6):1144–1149
Ma M, Zheng L, Yin X, Gao W, Han B, Li Q, Yang H (2021) Reconstruction and evaluation of oil-degrading consortia isolated from sediments of hydrothermal vents in the South Mid-Atlantic Ridge. Sci Rep 11(1):1456. https://doi.org/10.1038/s41598-021-80991-5
Man LW, Kumar P, Teng TT, Wasewar KL (2012) Design of experiments for malachite green dye removal from wastewater using thermolysis–coagulation– flocculation. Desalin Water Treat 40(1–3):260–271. https://doi.org/10.1080/19443994.2012.671257
Mandal B, Purkayastha A, Prabhu AA., Dasu VV (2020) Development in wastewater treatment plant design. In: Emerging technologies in environmental bioremediation. Elsevier. pp 311–321. https://doi.org/10.1016/B978-0-12-819860-5.00013-4
Manickam P, Vijay D (2021) Chemical hazards in textiles. In: Chemical management in textiles and fashion. Woodhead Publishing, pp 19–52. https://doi.org/10.1016/B978-0-12-820494-8.00002-2
Maniyam MN, Ibrahim AL, Cass AE (2020) Decolourization and biodegradation of azo dye methyl red by Rhodococcus strain UCC 0016. Environ Technol 41(1):71–85. https://doi.org/10.1080/09593330.2018.1491634
Meng R, Li F, Li X-N, Dong J-Z, Wei Y-J, Yi Z (2022) Two New coordination polymers on the basis of Cu (II) applied as photocatalysts and the treatment activity against coronary heart disease by regulating matrix metalloprotein expression. Sci Adv Mater 14(1):98–104
Mishra V, Mishra M, Chaudhari BP, Khanna R, Das M (2014) Argemone oil and butter yellow induced toxicity in hepatic and extra hepatic tissues. Bioenergetics 3(1):2–7. https://doi.org/10.4172/2167-7662.1000111
Mishra S, Nayak JK, Maiti A (2020) Bacteria-mediated bio-degradation of reactive azo dyes coupled with bio-energy generation from model wastewater. Clean Technol Environ Policy 22:651–667. https://doi.org/10.1007/s10098-020-01809-y
Mokri HG, Modirshahla N, Behnajady MA, Vahid B (2015) Adsorption of CI Acid Red 97 dye from aqueous solution onto walnut shell: kinetics, thermodynamics parameters, isotherms. Int J Environ Sci Technol 12(4):1401–1408. https://doi.org/10.1007/s13762-014-0725-6
Montiague EN, Guzman JPMD, Unciano NM, Panerio EG, Bigol UG, Castro IJL, Jose JPG, Mantaring SDA (2020) Biodecolourization of textile dye and wastewaters by crude laccase from pleurotus florida ITDI 6003 cultivated in wheat grains. Curr Res Environ Appl Mycol (J Fungal Biol) 10(1):167–175. https://doi.org/10.5943/cream/10/1/17
Morsi R, Bilal M, Iqbal HMN, Ashraf SS (2020) Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci Total Environ 714:136572. https://doi.org/10.1016/j.scitotenv.2020.136572
Mudhoo A, Ramasamy DL, Bhatnagar A, Usman M, Sillanpää M (2020) An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol Environ Saf 197:110587. https://doi.org/10.1016/j.ecoenv.2020.110587
Munari FM, Gaio TA, Calloni R, Dillon AJP (2008) Decolorization of textile dyes by enzymatic extract and submerged cultures of Pleurotus sajor-caju. World J Microbiol Biotechnol 24:1383–1392. https://doi.org/10.1007/s11274-007-9621-2
Nikulina GL, Deveĭkis DN, Glu P (1995) Toxicity dynamics of anionic dyes in the air of a work place and long-term effects after absorption through the skin. Med Tr Prom Ekol 6:25–28
Ning X, Yang C, Wang Y, Yang Z, Wang J, Li R (2014) Decolorization and biodegradation of the azo dye Congo Red by an isolated Acinetobacter baumannii YNWH 226. Biotechnol Bioprocess Eng 19:687–695. https://doi.org/10.1007/s12257-013-0729-y
Noreen S, Bhatti HN, Iqbal M, Hussain F, Sarim FM (2020) Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int J Biol Macromol 147:439–452. https://doi.org/10.1016/j.ijbiomac.2019.12.257
Ola IO, Akintokun AK, Akpan I, Omomowo IO, Areo VO (2010) Aerobic decolorization of two reactive azo dyes under varying carbon and nitrogen source by Bacillus cereus. Afr J Biotechnol 9:672–677. https://doi.org/10.5897/AJB09.1374
Olisah C, Adams JB, Rubidge G (2021) The state of persistent organic pollutants in South African estuaries: a review of environmental exposure and sources. Ecotoxicol Environ Saf 219:112316. https://doi.org/10.1016/j.ecoenv.2021.112316
Olukanni OD, Osuntoki AA, Gbenle GO (2009) Decolourization of azo dyes by a strain of Micrococcus isolated from a refuse dump soil. Biotechnology 8(4):442–448. https://doi.org/10.3923/biotech.2009.442.448
Oon YS, Ong SA, Ho LN, Wong YS, Oon YL, Lehl HK, Nordin N (2017) Microbial fuel cell operation using monoazo and diazo dyes as terminal electron acceptor for simultaneous decolourisation and bioelectricity generation. J Hazard Mater 325:170–177. https://doi.org/10.1016/j.jhazmat.2016.11.074
Pan H, Feng J, Cerniglia CE, Chen H (2011) Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol 38:1729–1738. https://doi.org/10.1007/s10295-011-0962-3
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeter Biodegr 59:73–84. https://doi.org/10.1016/j.ibiod.2006.08.006
Parmar S, Daki S, Bhattacharya S, Shrivastav A (2022) Microorganism: an ecofriendly tool for waste management and environmental safety. In: Development in wastewater treatment research and processes. Elsevier. pp. 175–193, https://doi.org/10.1016/B978-0-323-85657-7.00001-8
Patil R, Zahid M, Govindwar S, Khandare R, Vyavahare G, Gurav R, Jadhav J (2022) Constructed wetland: a promising technology for the treatment of hazardous textile dyes and effluent. In: Development in wastewater treatment research and processes. Elsevier. pp 173–198, https://doi.org/10.1016/B978-0-323-85583-9.00016-8
Pinheiro LRS, Gradíssimo DG, Xavier LP, Santos AV (2022) Degradation of azo dyes: bacterial potential for bioremediation. Sustainability 14(3):1510. https://doi.org/10.3390/su14031510
Popli S, Patel UD (2015) Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: a review. Int J Environ Sci Technol 12:405–420. https://doi.org/10.1007/s13762-014-0499-x
Porri A, Baroncelli R, Guglielminetti L, Sarrocco S, Guazzelli L, Forti M (2011) Fusarium oxysporum degradation and detoxification of a new textile glyco-conjugate azo dye (GAD). Fungal Biol 115:30–37. https://doi.org/10.1016/j.funbio.2010.10.001
Puvaneswari N, Muthukrishnan J, Gunasekaran P (2006) Toxicity assessment and microbial degradation of azo dyes.http://nopr.niscpr.res.in/handle/123456789/6554
Radhika NP, Selvin R, Kakkar R, Umar A (2019) Recent advances in nano-photocatalysts for organic synthesis. Arab J Chem 12(8):4550–4578
Rafii FATEMEH, Franklin WIRT, Cerniglia CE (1990) Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol 56(7):2146–2151. https://doi.org/10.1128/aem.56.7.2146-2151.1990
Rehman K, Shahzad T, Sahar A, Hussain S, Mahmood F, Siddique MH, Rashid MI (2018) Effect of reactive black 5 azo dye on soil processes related to C and N cycling. PeerJ 6:e4802. https://doi.org/10.7717/peerj.4802
Ritika MK, Umar A, Mehta S, Singh S, Sushil Kansal H, Fouad OA (2018) Rapid solar-light driven superior photocatalytic degradation of methylene blue using MoS2-ZnO heterostructure nanorods photocatalyst. Materials 11(11):2254. https://doi.org/10.3390/ma11112254
Robinson T, Chandran B, Nigam P (2001) Studies on the production of enzymes by white-rot fungi for the decolourisation of textile dyes. Enzyme Microbial Technol 29(8–9):575–579. https://doi.org/10.1016/S0141-0229(01)00430-6
Rodríguez A, Castrejón-Godínez ML; Anchez-Salinas E, Mussali-Galante P, Tovar-Sánchez E, Ortiz-Hernández M (2022) Pesticide bioremediation: OMICs technologies for understanding the processes. In: Pesticides bioremediation; Springer: Cham, pp 197–242. https://doi.org/10.1007/978-3-030-97000-0_8
Saharan Y, Singh J, Goyat R, Umar A, Ibrahim AA, Akbar S, Baskoutas S (2023) Recent advances in soil cleanup technologies for oil spills: a systematic review. Water Air Soil Pollut 234(8):503
Sahasrabudhe MM, Saratale RG, Saratale GD, Pathade GR (2014) Decolorization and detoxification of sulfonated toxic diazo dye C.I. Direct Red 81 by Enterococcus faecalis YZ66. J Environ Health Sci Eng 12:151–163. https://doi.org/10.1186/s40201-014-0151-1
Samsami S, Mohamadizaniani M, Sarrafzadeh MH, Rene ER, Firoozbahr M (2020) Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf Environ Protect 143:138–163. https://doi.org/10.1016/j.psep.2020.05.034
Saravanan A, Kumar PS, Rangasamy G (2022a) Removal of toxic pollutants from industrial effluent: sustainable approach and recent advances in metal organic framework. Ind Eng Chem Res 61(43):15754–15765. https://doi.org/10.1021/acs.iecr.2c02945
Saravanan A, Kumar PS, Hemavathy RV, Jeevanantham S, Harikumar P, Priyanka G, Devakirubai DRA (2022) A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci Total Environ 812:152456. https://doi.org/10.1016/j.scitotenv.2021.152456
Sedighi M, Karimi A, Vahabzadeh F (2009) Involvement of ligninolytic enzymes of Phanerochaete chrysosporium in treating the textile effluent containing Astrazon Red FBL in a packed-bed bioreactor. J Hazard Mater 169:88–93. https://doi.org/10.1016/j.jhazmat.2009.03.070
Sen K, Pakshirajan K, Santra SB (2012) Modelling the biomass growth and enzyme secretion by the white rot fungus Phanerochaete chrysosporium: a stochastic based approach. Appl Biochem Biotechnol 167:705–713. https://doi.org/10.1007/s12010-012-9720-x
Shah M (2014) Effective treatment systems for azo dye degradation: a joint venture between physico-chemical & microbiological process. Int J Environ Bioremed Biodegrad 2(5):231–242. https://doi.org/10.12691/ijebb-2-5-4
Sharma SCD, Sun Q, Li J, Wang Y, Suanon F, Yang J, Yu CP (2016) Decolorization of azo dye methyl red by suspended and co-immobilized bacterial cells with mediators anthraquinone-2, 6-disulfonate and Fe3O4 nanoparticles. Int Biodeteriorat Biodegrad 112:88–97. https://doi.org/10.1016/j.ibiod.2016.04.035
Sharma S, Ibhadon AO, Grazia Francesconi M, Mehta SK, Elumalai S, Kansal SK, Umar A, Baskoutas S (2020) Bi2WO6/C-Dots/TiO2: a novel Z-scheme photocatalyst for the degradation of fluoroquinolone levofloxacin from aqueous medium. Nanomaterials 10(5):910. https://doi.org/10.3390/nano10050910
Shen Y, Sun P, Ye L, Dong Xu (2023) Progress of anaerobic membrane bioreactor in municipal wastewater treatment. Sci Adv Mater 15(10):1277–1298
Sherwani S, Alshammari EM, Alqahtani FO, Khan M, Khan S, Khan WA, Khan MWA, Umar A (2022) Lanthanum doped zinc molybdate: antibacterial and photo-catalysis properties. Sci Adv Mater 14(8):1320–1330. https://doi.org/10.1166/sam.2022.4327
Shi B, Li G, Wang D, Feng C, Tang H (2007) Removal of direct dyes by coagulation: the performance of preformed polymeric aluminum species. J Hazard Mater 143(1–2):567–574. https://doi.org/10.1016/j.jhazmat.2006.09.076
Shi Y, Yang Z, Xing L et al (2021) Recent advances in the biodegradation of azo dyes. World J Microbiol Biotechnol 37:137. https://doi.org/10.1007/s11274-021-03110-6
Shindhal T, Rakholiya P, Varjani S, Pandey A, Ngo HH, Guo W, Taherzadeh MJ (2021) A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 12(1):70–87. https://doi.org/10.1080/21655979.2020.1863034
Silva AC, Silvestre AJ, Freire CS, Vilela C (2021) Modification of textiles for functional applications. In: Fundamentals of natural fibres and textiles. Woodhead Publishing, pp 303–365. https://doi.org/10.1016/B978-0-12-821483-1.00010-3
Singh SN (ed) (2015) Microbial degradation of synthetic dyes in wastewaters. Springer International Publishing, Cham
Singh G, Dwivedi SK (2020) Decolorization and degradation of Direct Blue-1 (Azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environ Technol Innov 18:100751. https://doi.org/10.1016/j.eti.2020.100751
Singh L, Singh VP (2015) Textile dyes degradation: a microbial approach for biodegradation of pollutants. Microbial Degrad Synthetic Dyes Wastewaters. https://doi.org/10.1007/978-3-319-10942-8_9
Singh RP, Singh PK, Singh RL (2014) Bacterial decolorization of textile azo dye Acid Orange by Staphylococcus hominis RMLRT03. Toxicol Int 21:160–166. https://doi.org/10.4103/0971-6580.139797
Singh R, Yadav RK, Shukla RK, Singh S, Singh AP, Dwivedi DK, Umar A, Gupta NK (2023) Highly selective nitrogen-doped graphene quantum dots/eriochrome cyanine composite photocatalyst for NADH regeneration and coupling of benzylamine in aerobic condition under solar light. Catalysts 13(1):199
Sojobi AO, Zayed T (2022) Impact of sewer overflow on public health: a comprehensive scientometric analysis and systematic review. Environ Res 203:111609. https://doi.org/10.1016/j.envres.2021.111609
Solovchenko, A., Lukyanov, A., Aswathanarayana, R. G., Pleissner, D., & Ambati, R. R. (2020). Recent developments in microalgal conversion of organic-enriched waste streams. Current Opinion in Green and Sustainable Chemistry, 24, 61–66.https://doi.org/10.1016/j.cogsc.2020.03.006
Sood S, Umar A, Mehta SK, Kansal SK (2015) Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J Coll Interface Sci 450:213–223. https://doi.org/10.1016/j.jcis.2015.03.018
Sood S, Umar A, Mehta SK, Kansal SK (2015) α-Bi2O3 nanorods: an efficient sunlight active photocatalyst for degradation of Rhodamine B and 2, 4, 6-trichlorophenol. Ceram Int 41(3):3355–3364
Su Fu, Huang J, Yanhua Xu (2023) Facile fabrication of Bi2MoO6/g-C3N4 heterojunction nanosheets: facile synthesis and enhanced visible light photocatalytic property. Sci Adv Mater 15(7):905–914
Sun S, Liu P, Ullah M (2023) Efficient azo dye biodecolorization system using lignin-co-cultured white-rot fungus. J Fungi 9:91. https://doi.org/10.3390/jof9010091
Suo S, Gao J, Li J, Yuxia Hu, Wang C, Deng R, Chen S (2022) 3D Cd (II)-based complex with photocatalytic property and treatment activity on endometrial cancer. Sci Adv Mater 14(2):338–343
Tan IAW, Ahmad AL, Hameed BH (2009) Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 164(2–3):473–482. https://doi.org/10.1016/j.jhazmat.2008.08.025
Tang S-L, Liu Y-L, Li X-M, Chen Q-W, Chen H-Y, Zhou J-L, Chen L et al. (2022) A Novel core-shell structure TiO2 nanolayer sphere preparation and electrocatalytic degradation study. Sci Adv Mater 14(3):576–580
Ukaogo PO, Ewuzie U, Onwuka CV (2020) Environmental pollution: causes, effects, and the remedies. In: Microorganisms for sustainable environment and health, Elsevier. pp 419–429, https://doi.org/10.1016/B978-0-12-819001-2.00021-8
Varjani S, Rakholiya P, Ng HY, You S, Teixeira JA (2020) Microbial degradation of dyes: an overview. Bioresour Technol 314:123728. https://doi.org/10.1016/j.biortech.2020.123728
Varjani S, Rakholiya P, Shindhal T, Shah AV, Ngo HH (2021) Trends in dye industry effluent treatment and recovery of value added products. J Water Process Eng 39:101734. https://doi.org/10.1016/j.jwpe.2020.101734
Verma A, Dhiman K, Shirkot P (2017) Biodegradation of Malachite green by extracellular bacterial laccase and its phytotoxicity studies. Int J Pure App Biosci 5(2):913–922
Vinitnantharat S, Chartthe W, Pinisakul A (2008) Toxicity of reactive red 141 and basic red 14 to algae and waterfleas. Water Sci Technol 58(6):1193–1198. https://doi.org/10.2166/wst.2008.476
Walker R (1970) The metabolism of azo compounds: a review of the literature. Food Cosmet Toxicol 8(6):659–676. https://doi.org/10.1016/S0015-6264(70)80455-2
Wang Ke (2023) Application of green pollution-free materials in environmental art design. Sci Adv Mater 15(1):41–49
Wang J, Liu GF, Lu H, Jin RF, Zhou JT, Lei TM (2012) Biodegradation of Acid Orange 7 and its auto-oxidative decolorization product in membrane-aerated biofilm reaction. Int Biodeter Biodegr 67:73–77. https://doi.org/10.1016/j.ibiod.2011.12.003
Wang AJ, Wang HC, Cheng HY, Liang B, Liu WZ, Han JL, Wang SS (2020) Electrochemistry-stimulated environmental bioremediation: Development of applicable modular electrode and system scale-up. Environ Sci Ecotechnol 3:100050. https://doi.org/10.1016/j.ese.2020.100050
Wang X, Xia J, Ding S, Zhang S, Li M, Shang Z, Ding J (2020) Removing organic matters from reverse osmosis concentrate using advanced oxidation-biological activated carbon process combined with Fe3+/humus-reducing bacteria. Ecotoxicol Environ Saf 203:110945. https://doi.org/10.1016/j.ecoenv.2020.110945
Wang F, Li L, Iqbal J, Yang Z, Du Y (2022) Preparation of magnetic chitosan corn straw biochar and its application in adsorption of amaranth dye in aqueous solution. Int J Biological Macromol 199:234–242. https://doi.org/10.1016/j.ijbiomac.2021.12.195
Wang W (1990) Toxicity assessment of pretreated industrial wastewaters using higher plants. Res J Water Pollu Contr Feder 853–860.
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22(1–2):161–187. https://doi.org/10.1016/j.biotechadv.2003.08.011
Wu JS, Liu CH, Chu KH, Suen SY (2008) Removal of cationic dye methyl violet 2B from water by cation exchange membranes. J Membr Sci 309(1–2):239245. https://doi.org/10.1016/j.memsci.2007.10.035
Wu K, Shi M, Pan X, Zhang J, Zhang X, Shen T, Tian Y (2022) Decolourization and biodegradation of methylene blue dye by a ligninolytic enzyme-producing Bacillus thuringiensis: degradation products and pathway. Enzyme Microbial Technol 156:109999. https://doi.org/10.1016/j.enzmictec.2022.109999
Wuhrmann K, Mechsner KL, Kappeler TH (1980) Investigation on rate—Determining factors in the microbial reduction of azo dyes. Eur J Appl Microbiol Biotechnol 9:325–338. https://doi.org/10.1007/BF00508109
Xue F, Tang B, Bin L, Ye J, Huang S, Fu F, Cui J (2019) Residual micro organic pollutants and their biotoxicity of the effluent from the typical textile wastewater treatment plants at Pearl River Delta. Sci Total Environ 657:696–703. https://doi.org/10.1016/j.scitotenv.2018.12.008
Yan C, Qu Z, Wang J, Cao L, Han Q (2022) Microalgal bioremediation of heavy metal pollution in water: recent advances, challenges, and prospects. Chemosphere 286:131870. https://doi.org/10.1016/j.chemosphere.2021.131870
Zanoni TB, Lizier TM, das Dores Assis M, Zanoni MVB, De Oliveira DP (2013) CYP-450 isoenzymes catalyze the generation of hazardous aromatic amines after reaction with the azo dye Sudan III. Food Chem Toxicol 57:217–226. https://doi.org/10.1016/j.fct.2013.03.035
Zhang S, Song S, Gu P, Ma R, Wei D, Zhao G, Wang X (2019) Visible-light-driven activation of persulfate over cyano and hydroxyl group co-modified mesoporous gC3N4 for boosting bisphenol A degradation. J Mater Chem A 7(10):5552–5560. https://doi.org/10.1039/C9TA00339H
Zhang J, Liu R, Kuang M, Wang J, Ji Z (2023) Effect of calcination temperature on surface acidity and photocatalytic activity of nano-TiO2/diatomite composite photocatalyst. Sci Adv Mater 15(6):781–790
Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang X (2018) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem 9(26):3562–3582. https://doi.org/10.1039/C8PY00484F
Zheng J, Tang X, Zhang S, Huang T, Zheng H, Sun B (2020) Relationship between the structure of chitosan-based flocculants and their performances in the treatment of model azo dyeing wastewater. Chemosphere 247:125920. https://doi.org/10.1016/j.chemosphere.2020.125920
Zhiqiang C, Wenjie Z, Jiangtao M, Jinyan C, Shanshan L, Xiaolin Z, Guanghua Y, Xiyue Z (2015) Biodegradation of azo dye Disperse Orange S-RL by a newly isolated strain Acinetobacter sp. SRL8. Water Environ Res 87:516–523. https://doi.org/10.2175/106143014X13975035526068
Zhuo R, Ma L, Fan F, Gong Y, Wan X, Jiang M (2011) Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene. J Hazard Mater 192:855–873. https://doi.org/10.1016/j.jhazmat.2011.05.106
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University, Najran, Kingdom of Saudi Arabia for funding under the Research Group funding program grant no. NU/RG/MRC/12/2. The authors are grateful to all research staff who contributed to the data collection required for this review article. We also acknowledge to bioRendor tool for designing the template of figures.
Funding
The authors are thankful to the Deanship of Scientific Research at Najran University, Najran, Kingdom of Saudi Arabia for funding under the Research Group funding program grant no. NU/RG/MRC/12/2.
Author information
Authors and Affiliations
Contributions
MS, SS, MSA, RK, AU, and AAMA were involved in the investigation, methodology, validation, formal analysis, writing—original draft, and visualization. AU and SB contributed to writing—review and editing and data curation. All authors commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
We certify that the manuscript titled “Microorganisms-Assisted Degradation of Acid Orange 7 Dye: A Review” (hereinafter referred to as “the Paper”) is entirely our original work, and it does not infringe the copyright of any third party. The submission of the Paper to Environmental Science and Pollution Research implies that the paper has not been published previously. This work is not under consideration for publication elsewhere. Its publication is approved by all authors and, if accepted, it will not be published elsewhere in the same form, either in English or in any other language, without the written consent of the Publisher. Copyrights for articles published in Environmental Science and Pollution Research are retained by the authors, with first publication rights granted to Environmental Science and Pollution Research.
Consent to participate
We affirm that all authors have participated in the research work and are fully aware of ethical responsibilities.
Consent for publication
We affirm that all authors have agreed for submission of the Paper to ESPR and are fully aware of ethical responsibilities.
Additional information
Editorial responsibility: S. Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, M., Sharma, S., Akhtar, M.S. et al. Microorganisms-assisted degradation of Acid Orange 7 dye: a review. Int. J. Environ. Sci. Technol. 21, 6133–6166 (2024). https://doi.org/10.1007/s13762-023-05438-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05438-y